Numerical Model of Streaming DEP for Stem Cell Sorting
Abstract
:1. Introduction
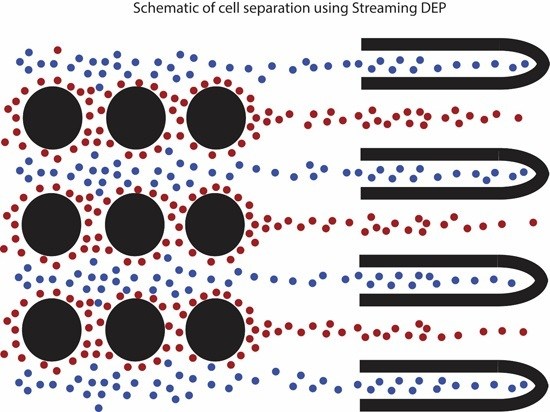
2. Operating Principle of Streaming Dielectrophoresis (DEP)
3. Materials and Methods
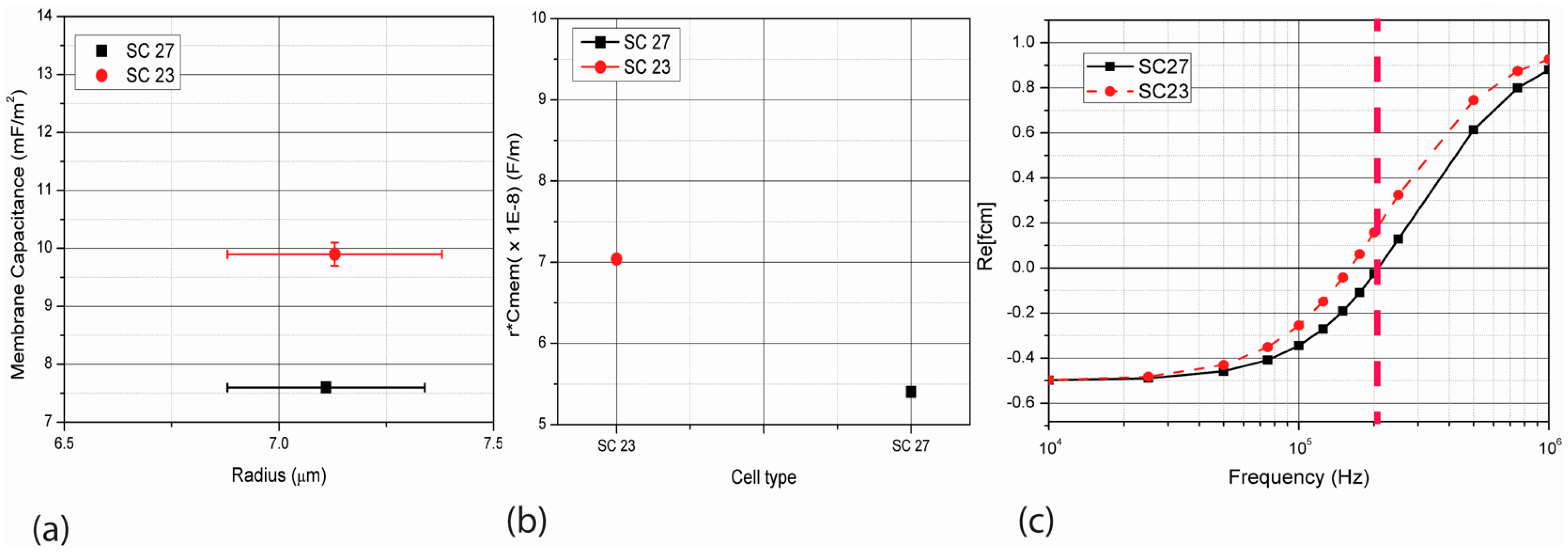
3.1. Theoretical Framework
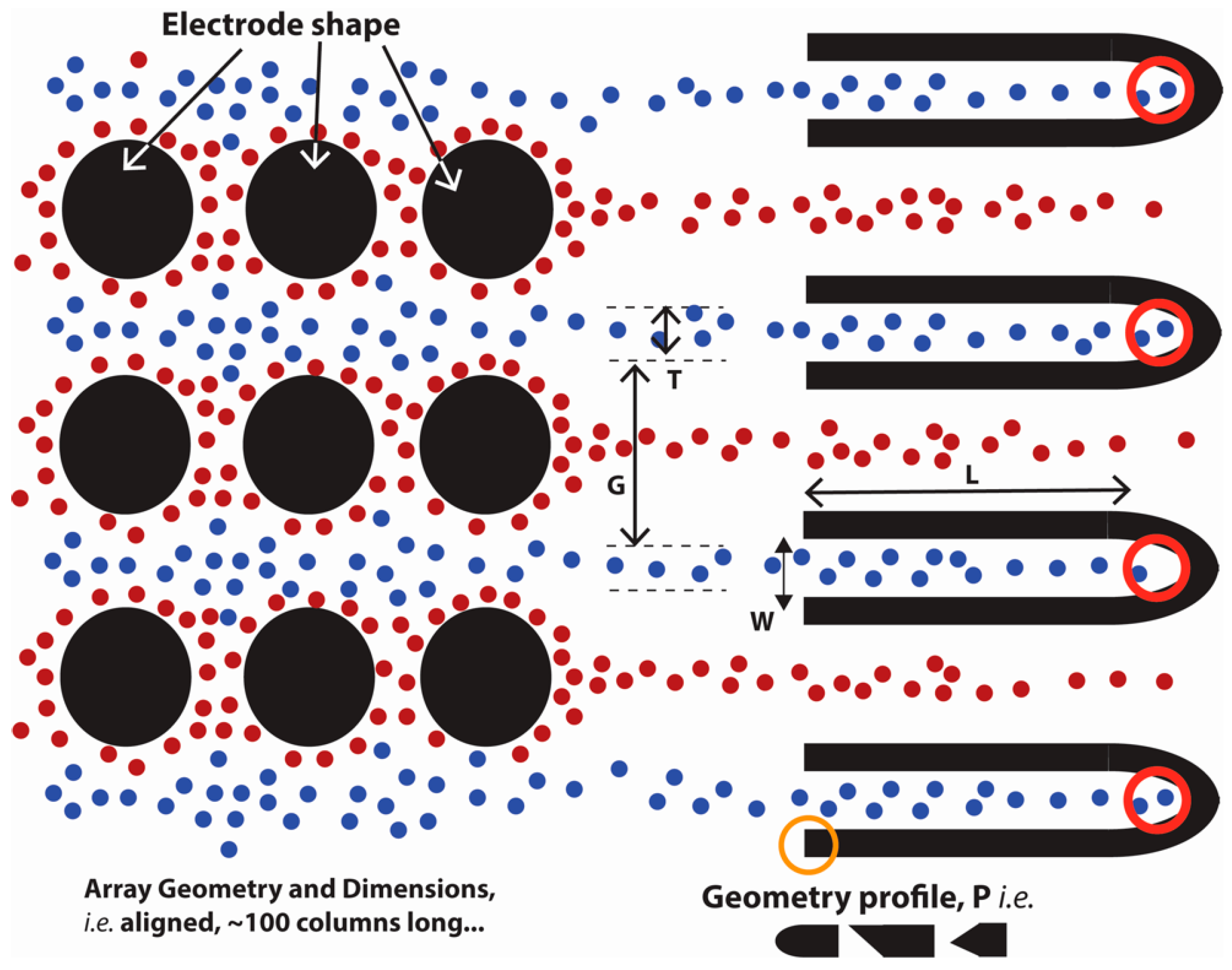
3.2. Computational Model
3.3. Data Analysis
4. Results
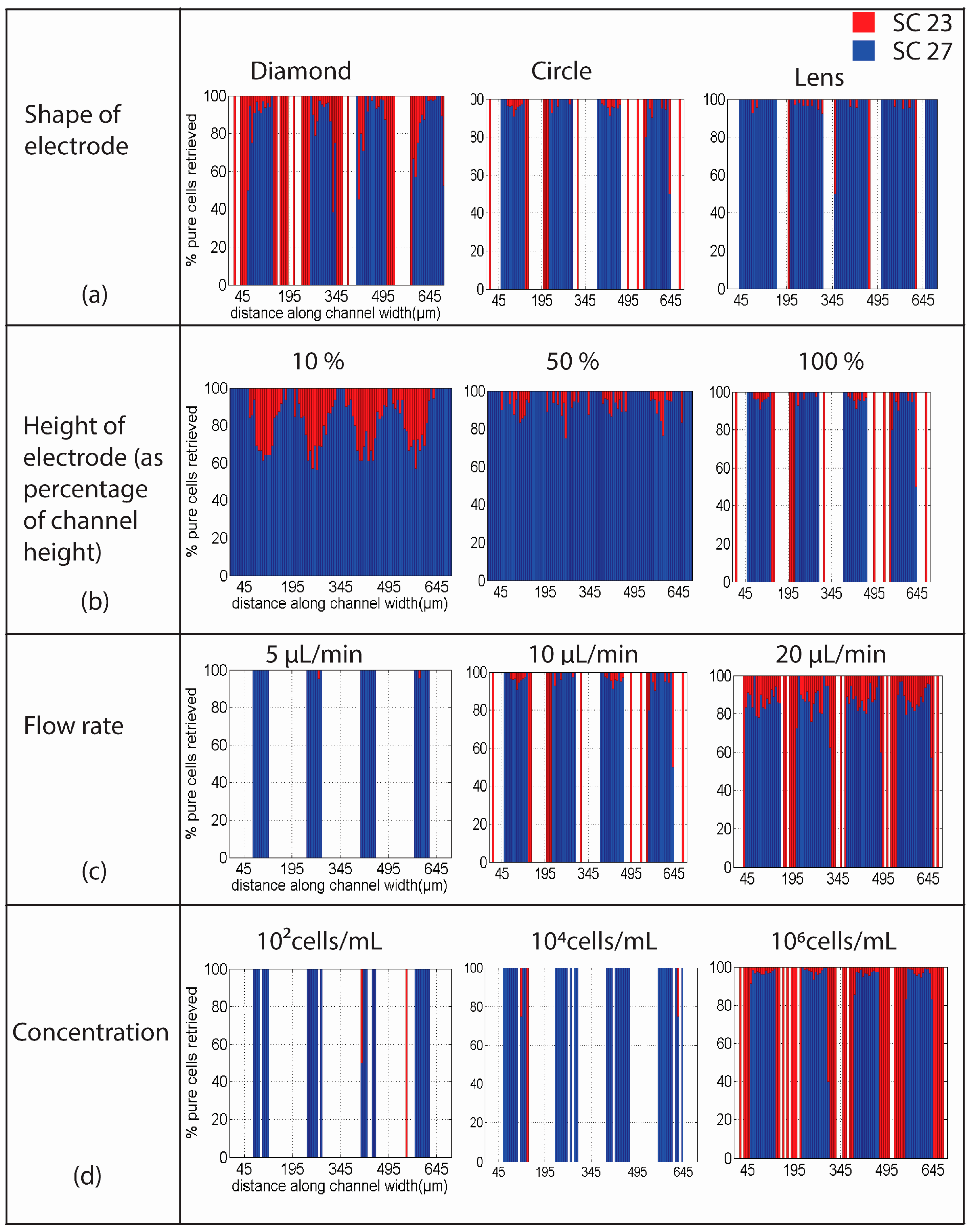
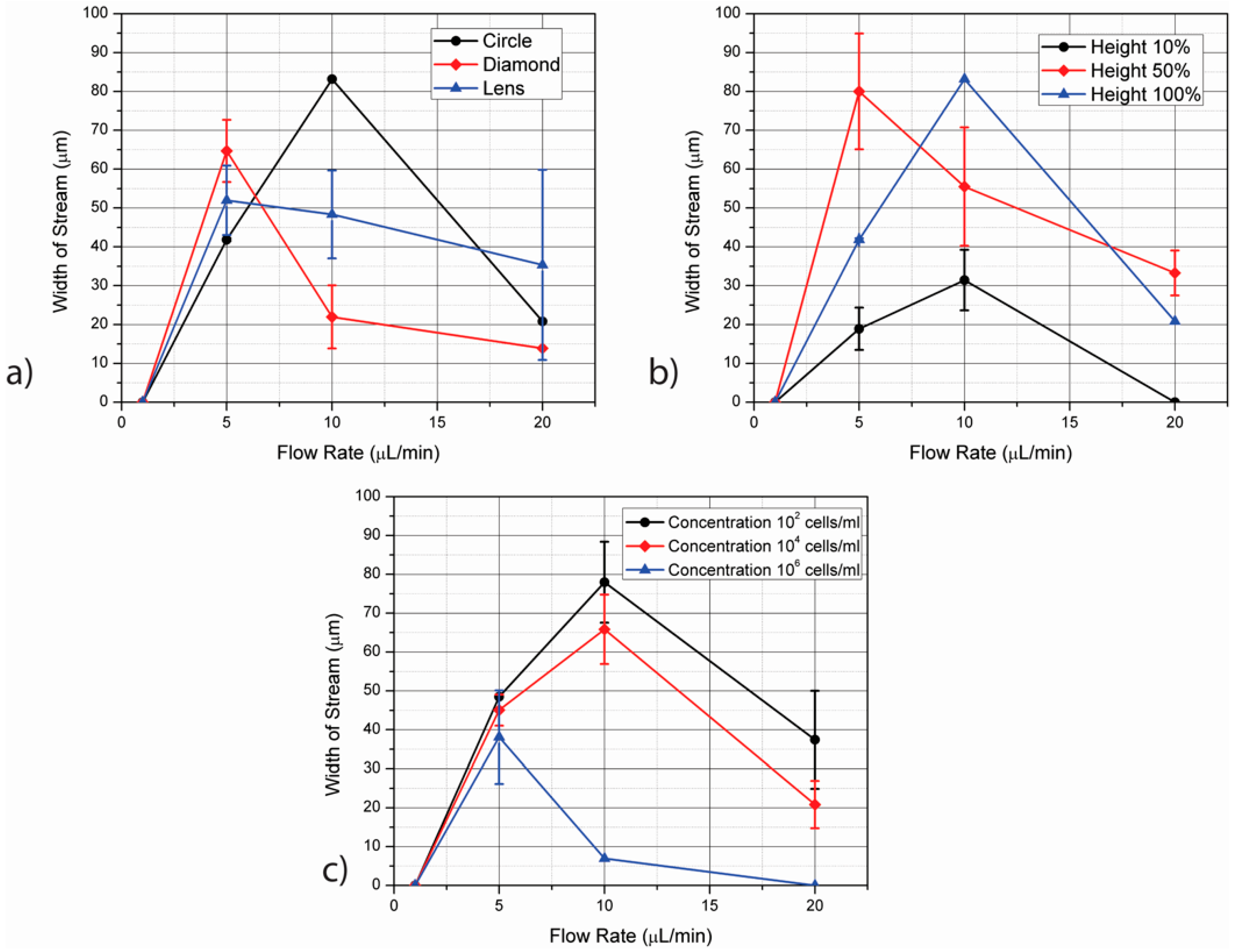
4.1. Shape of Electrodes
4.2. Height of Electrodes
4.3. Flow Rate
4.4. Cell Concentration
5. Discussion
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zandstra, P.W.; Nagy, A. Stem cell bioengineering. Annu. Rev. Biomed. Eng. 2001, 3, 275–305. [Google Scholar] [CrossRef] [PubMed]
- Koeppe, R.A.; Cortex, C.; Petrides, M.; Alivisatos, B.; Meyer, E.; Evans, A.C.; Natl, P.; Sci, A.; Owen, A.M.; Evans, A.C.; et al. Generalized potential of adult neural stem cells. Science 2000, 288, 1660–1663. [Google Scholar]
- Martino, G.; Martino, G.; Pluchino, S.; Pluchino, S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 2006, 7, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Ishii, K.; Yamane, J.; Iwanami, A.; Ikegami, T.; Katoh, H.; Iwamoto, Y.; Nakamura, M.; Miyoshi, H.; Okano, H.J.; et al. In vivo imaging of engrafted neural stem cells: Its application in evaluating the optimal timing of transplantation for spinal cord injury. FASEB J. 2005, 19, 1839–1841. [Google Scholar] [CrossRef] [PubMed]
- Kennea, N.L.; Mehmet, H. Neural stem cells. J. Pathol. 2002, 197, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S. Stem and progenitor cell-based therapy of the human central nervous system. Nat. Biotechnol. 2005, 23, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.A.; Lu, J.; Wang, L.; Marchenko, S.A.; Jeon, N.L.; Lee, A.P.; Monuki, E.S. Unique dielectric properties distinguish stem cells and their differentiated progeny. Stem Cells 2008, 26, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Buck, D.W.; He, D.; Reitsma, M.J.; Masek, M.; Phan, T.V.; Tsukamoto, A.S.; Gage, F.H.; Weissman, I.L. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. USA 2000, 97, 14720–14725. [Google Scholar] [CrossRef] [PubMed]
- Spangrude, G.J.; Heimfeld, S.; Weissman, I.L. Purification and characterization of mouse hematopoietic stem cells. Science 1988, 241, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, R.M. Micropipette aspiration of living cells. J. Biomech. 2000, 33, 15–22. [Google Scholar] [CrossRef]
- Labeed, F.H.; Lu, J.; Mulhall, H.J.; Marchenko, S.A.; Hoettges, K.F.; Estrada, L.C.; Lee, A.P.; Hughes, M.P.; Flanagan, L.A. Biophysical characteristics reveal neural stem cell differentiation potential. PLoS ONE 2011, 6, e25458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnitzler, A.C.; Verma, A.; Kehoe, D.E.; Jing, D.; Murrell, J.R.; Der, K.A.; Aysola, M.; Rapiejko, P.J.; Punreddy, S.; Rook, M.S. Bioprocessing of human mesenchymal stem/stromal cells for therapeutic use: Current technologies and challenges. Biochem. Eng. J. 2015, 108, 3–13. [Google Scholar] [CrossRef]
- Pethig, R. Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 22811. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Joo, S.; Duhon, M.; Heller, M.; Wallace, B.; Xu, X. Dielectrophoretic cell separation and gene expression profiling on microelectronic chip arrays. Anal. Chem. 2002, 74, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R.; Talary, M.S. Dielectrophoretic detection of membrane morphology changes in Jurkat T-cells undergoing etoposide-induced apoptosis. IET Nanobiotechnol. 2007, 1, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, Y.; Wang, X.; Wang, X.-B.; Becker, F.F.; Gascoyne, P.R.C. Dielectric properties of human leukocyte subpopulations determined by electrorotation as a cell separation criterion. Biophys. J. 1999, 76, 3307–3314. [Google Scholar] [CrossRef]
- Pethig, R.; Bressler, V.; Carswell-Crumpton, C.; Chen, Y.; Foster-Haje, L.; García-Ojeda, M.E.; Lee, R.S.; Lock, G.M.; Talary, M.S.; Tate, K.M. Dielectrophoretic studies of the activation of human T lymphocytes using a newly developed cell profiling system. Electrophoresis 2002, 23, 2057–2063. [Google Scholar] [CrossRef]
- Vykoukal, D.M.; Gascoyne, P.R.C.; Vykoukal, J. Dielectric characterization of complete mononuclear and polymorphonuclear blood cell subpopulations for label-free discrimination. Integr. Biol. (Camb.) 2009, 1, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Bagnaninchi, P.; Drummond, N.; Giaever, I. Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell-substrate impedance sensing. Proc. Natl. Acad. Sci. USA 2011, 108, 6462–6467. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; Talary, M.S.; Pethig, R.; Burnett, A.K.; Mills, K.I. The dielectrophoresis enrichment of CD34+ cells from peripheral blood stem cell harvests. Bone Marrow Transplant. 1996, 18, 777–782. [Google Scholar] [PubMed]
- Talary, M.S.; Mills, K.I.; Hoy, T.; Burnett, A.K.; Pethig, R. Dielectrophoretic separation and enrichment of CD34 cell subpopulations from bone marrow. Med. Biol. Eng. Comput. 1995, 33, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Vykoukal, J.; Vykoukal, D.M.; Freyberg, S.; Alt, E.U.; Gascoyne, P.R.C. Enrichment of putative stem cells from adipose tissue using dielectrophoretic field-flow fractionation. Lab Chip 2008, 8, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Natu, R.; Larraga-Martinez, M.F.; Martinez-Duarte, R. Enrichment of diluted cell populations from large sample volumes using 3D carbon-electrode dielectrophoresis. Biomicrofluidics 2016, 10, 033107. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Duarte, R.; Camacho-Alanis, F.; Renaud, P.; Ros, A. Dielectrophoresis of lambda-DNA using 3D carbon electrodes. Electrophoresis 2013, 34, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Duarte, R. Carbon-electrode dielectrophoresis for bioparticle manipulation. ECS Trans. 2014, 61, 11–22. [Google Scholar] [CrossRef]
- Martinez-Duarte, R.; Andrade-Roman, J.; Martinez, S.O.; Madou, M. A high throughput multi-stage, multi-frequency filter and separation device based on carbon dielectrophoresis. NSTI-Nanotech 2008, 3, 316–319. [Google Scholar]
- Teixidor, G.T.; Zaouk, R.B.; Park, B.Y.; Madou, M.J. Fabrication and characterization of three-dimensional carbon electrodes for lithium-ion batteries. J. Power Sources 2008, 183, 730–740. [Google Scholar] [CrossRef]
- Kralj, J.G.; Lis, M.T.W.; Schmidt, M.A.; Jensen, K.F. Continuous dielectrophoretic size-based particle sorting. Anal. Chem. 2006, 78, 5019–5025. [Google Scholar] [CrossRef] [PubMed]
- Das, C.M.; Becker, F.; Vernon, S.; Noshari, J.; Joyce, C.; Gascoyne, P.R.C. Dielectrophoretic segregation of different human cell types on microscope slides. Anal. Chem. 2005, 77, 2708–2719. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.N.G.; Turner, P.A.; Janorkar, A.V.; Zhao, F.; Minerick, A.R. Characterizing the dielectric properties of human mesenchymal stem cells and the effects of charged elastin-like polypeptide copolymer treatment. Biomicrofluidics 2014, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Kang, Y.; Xuan, X.; Li, D. Continuous separation of microparticles by size with direct current-dialectrophoresis. Electrophoresis 2006, 27, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.F., Jr.; Bussolari, S.R.; Gimbrone, M.A., Jr.; Davies, P.F. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 1981, 103, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Naveiras, O.; Wenzel, P.L.; McKinney-Freeman, S.; Mack, P.J.; Gracia-Sancho, J.; Suchy-Dicey, A.; Yoshimoto, M.; Lensch, M.W.; Yoder, M.C.; et al. Biomechanical forces promote embryonic haematopoiesis. Nature 2009, 459, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Schmid-Schönbein, G.W. Regulation of CD18 expression on neutrophils in response to fluid shear stress. Proc. Natl. Acad. Sci. USA 2003, 100, 13152–13157. [Google Scholar] [CrossRef] [PubMed]
- Gerszten, R.E.; Lim, Y.C.; Ding, H.T.; Snapp, K.; Kansas, G.; Dichek, D.A.; Cabañas, C.; Sánchez-Madrid, F.; Gimbrone, M.A.; Rosenzweig, A.; et al. Adhesion of monocytes to vascular cell adhesion molecule-1-transduced human endothelial cells: Implications for atherogenesis. Circ. Res. 1998, 82, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.P.; Ahsan, T. Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnol. Bioeng. 2013, 110, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Salmanzadeh, A.; Romero, L.; Shafiee, H.; Gallo-Villanueva, R.C.; Stremler, M.A.; Cramer, S.D.; Davalos, R.V. Isolation of prostate tumor initiating cells (TICs) through their dielectrophoretic signature. Lab Chip 2012, 12, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Warren, C.A.; Guerrant, R.L.; Swami, N.S. Dielectrophoretic monitoring and interstrain separation of intact clostridium difficile based on their S(Surface)-layers. Anal. Chem. 2014, 86, 10855–10863. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Barrios, C.A.; Dickson, A.R.; Nourse, J.L.; Lee, A.P.; Flanagan, L.A. Advancing practical usage of microtechnology: A study of the functional consequences of dielectrophoresis on neural stem cells. Integr. Biol. 2012, 4, 1223. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Duarte, R.; Renaud, P.; Madou, M.J. A novel approach to dielectrophoresis using carbon electrodes. Electrophoresis 2011, 32, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Elitas, M.; Martinez-Duarte, R.; Dhar, N.; McKinney, J.D.; Renaud, P. Dielectrophoresis-based purification of antibiotic-treated bacterial subpopulations. Lab Chip 2014, 14, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.D.C.; Martínez-Duarte, R.; Hüttener, M.; Renaud, P.; Torrents, E.; Juárez, A. Increasing PCR sensitivity by removal of polymerase inhibitors in environmental samples by using dielectrophoresis. Biosens. Bioelectron. 2013, 43, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Teissié, J.; Rols, M.P. An experimental evaluation of the critical potential difference inducing cell membrane electropermeabilization. Biophys. J. 1993, 65, 409–413. [Google Scholar] [CrossRef]
- Martinez-Duarte, R.; Rouabah, H.A.; Green, N.G.; Madou, M.; Morgan, H. Higher efficiency and throughput in particle separation with 3D dielectrophoresis with C-MEMS. In Proceedings of the 11th International Conference on Miniaturized Systems for Chemistry and Life Sciences, microTAS 2007, Paris, France, 7–11 October 2007; Volume 1, pp. 826–828.
- Xu, H.; Malladi, K.; Wang, C.; Kulinsky, L.; Song, M.; Madou, M. Carbon post-microarrays for glucose sensors. Biosens. Bioelectron. 2008, 23, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Taherabadi, L.; Jia, G.; Kassegne, S.; Zoval, J.; Madou, M. Carbon-MEMS architectures for 3D microbatteries. Proc. SPIE 2004, 5455, 295–302. [Google Scholar]
- Saucedo-Espinosa, M.A.; Lapizco-Encinas, B.H. Design of insulator-based dielectrophoretic devices: Effect of insulator posts characteristics. J. Chromatogr. A 2015, 1422, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Cummings, E.B. Streaming dielectrophoresis for continuous-flow microfluidic devices. IEEE Eng. Med. Biol. Mag. 2003, 22, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Cummings, E.B.; Singh, A.K. Dielectrophoresis in microchips containing arrays of insulating posts: Theoretical and experimental results. Anal. Chem. 2003, 75, 4724–4731. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Nuzzo, R.G. Recent progress in soft lithography. Mater. Today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Serra, M.; Brito, C.; Correia, C.; Alves, P.M. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 2012, 30, 350–359. [Google Scholar] [CrossRef] [PubMed]

| Parameter Studied | Value | Throughput for SC23 | Throughput for SC27 |
|---|---|---|---|
| Shape of electrode | Lens | 0.3% | 69.43% |
| Circle | 2.40% | 62.09% | |
| Diamond | 8.51% | 61.54% | |
| Height of electrode as percentage of channel height | 10% | 11.31% | 55.86% |
| 50% | 3.37% | 64.35% | |
| 100% | 2.40% | 62.09% | |
| Flow Rate | 1 µL/min | 0% | 0% |
| 5 µL/min | 0% | 33.33% | |
| 10 µL/min | 2.40% | 68.09% | |
| 20 µL/min | 14.48% | 81.40% | |
| Cell Concentration | 102 cells/mL | 10.61% | 41.92% |
| 104 cells/mL | 2.88% | 68.18% | |
| 106 cells/mL | 2.36% | 62.52% |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natu, R.; Martinez-Duarte, R. Numerical Model of Streaming DEP for Stem Cell Sorting. Micromachines 2016, 7, 217. https://doi.org/10.3390/mi7120217
Natu R, Martinez-Duarte R. Numerical Model of Streaming DEP for Stem Cell Sorting. Micromachines. 2016; 7(12):217. https://doi.org/10.3390/mi7120217
Chicago/Turabian StyleNatu, Rucha, and Rodrigo Martinez-Duarte. 2016. "Numerical Model of Streaming DEP for Stem Cell Sorting" Micromachines 7, no. 12: 217. https://doi.org/10.3390/mi7120217