1. Introduction
Numerous cellular studies that utilized microfluidic devices have been conducted over the last decade [
1]. The findings have afforded a precise understanding of cell behavior that could not have been obtained by conventional in vitro experiments. Microfluidic techniques, especially those that utilize microvalves, are employed in the development of microfluidic devices, which enable precise fluid control [
2]. For example, a microvalve facilitates precise volumetric control [
3] and rapid mixing [
4] of biochemical reagents. The combination enables the development of on-chip biochemical processing devices [
5].
The microvalves employed in microfluidic devices are classified based on their control and flow damming methods. The control methods include translation by electromagnetic actuators [
6], variation of the fluid viscosity by an electric field [
7], and displacement by pneumatic pressure [
8]. Owing to their simplicity and compactness, pneumatic pressure-controlled microvalves are widely used. In the case of flow damming methods, examples include the deformation of the channel [
8,
9] and insertion of a partition [
10]. Microvalves that utilize the deformation technique are quite common, primarily because of their simplicity and ease of fabrication. Hence, microvalves that utilize pneumatic pressure and channel deformation are often employed in microfluidic devices. A microvalve of this type is fabricated by placing a thin polydimethylsiloxane (PDMS) membrane between two microchannels [
11]. Application of pneumatic pressure to one of the microchannels causes the thin membrane to bend, resulting in the other microchannel being closed [
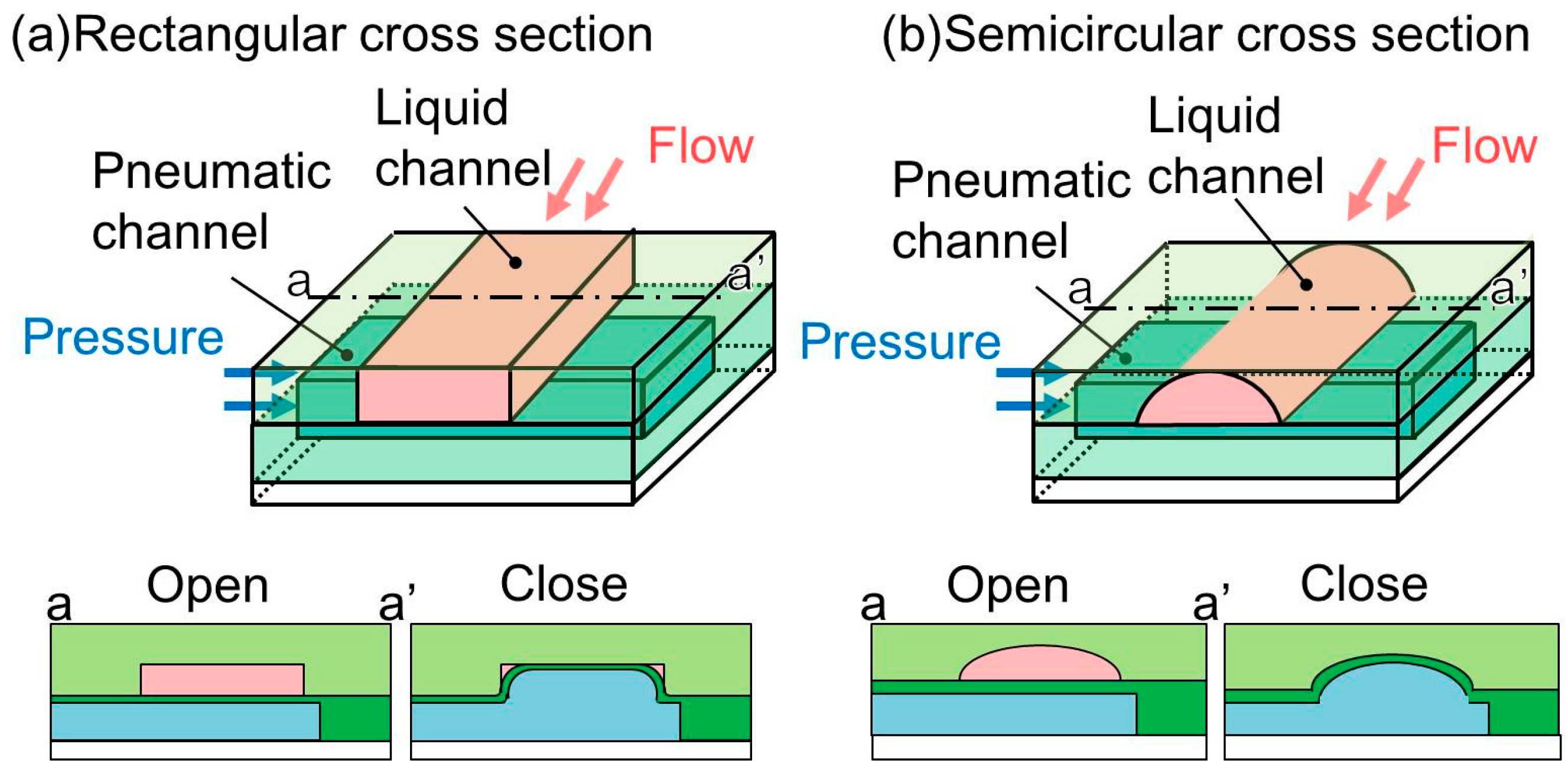
12]. However, such a microchannel with a rectangular cross section (
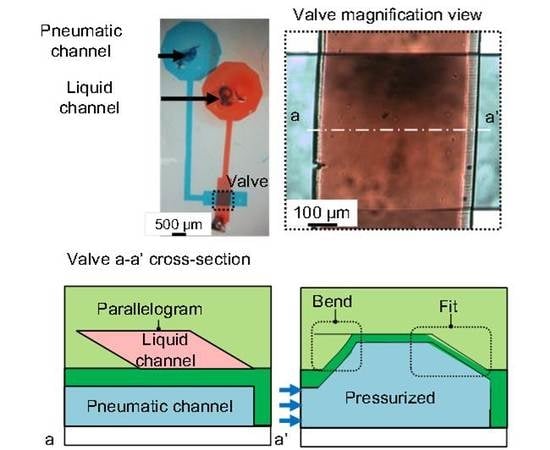
Figure 1a), a replica of a photoresist mold, is incapable of completely shutting its corners to liquid flow. This is because of the inability of the membrane to fit into the right-angled corners of the microchannel. It may cause cross-contamination when microvalves are used for biological experiments with multiple cell types. Therefore, the leakage of the microvalves, even though it is very small, is not acceptable.
To solve this problem, a microchannel with a semicircular cross section has been proposed. This microchannel (
Figure 1b), which is used to convey a liquid, has no corners and the membrane is able to fit securely against the wall, resulting in complete closure. The mold that is used to produce this type of microchannel is fabricated by a reflow process using a patterned photoresist. The patterned photoresist is melted at a high temperature and used to form a curved surface by the application of a surface tension. The height of the semicircular cross-sectional microchannel is commonly less than 30 μm [
13]. To the best of our knowledge, we found only one situation where the semicircular cross-sectional microchannel of the pneumatic microvalve achieved 65 μm in height [
14]. The process is thus not suitable for a pneumatic microvalve for a tall microchannel. The small cross-sectional area of a microchannel equipped with a microvalve produced by the reflow process cannot accommodate large cells such as egg and skeletal muscle cells. For these large cells, microvalves for tall microchannels, whose cross-sectional areas are large, have been developed. For example, a vertical membrane microvalve is fabricated using double-sided molding [
15]. This microvalve could close a tall microchannel, though the three-dimensional channel configurations are complex. A microvalve for a tall semicircular cross-sectional microchannel is fabricated by grayscale lithography of SU-8 (thick negative photoresist) [
16]. However, the technique requires a digital micro-mirror device, which is expensive. A more simple and inexpensive technique is thus required.
In the present study, we used an easy method to fabricate a pneumatic microvalve that enables complete closure of a tall microchannel. A single inclined lithography process was used to achieve the adopted parallelogram-shaped cross section of the microchannelis used. The effectiveness of the developed microvalve was verified by applying it to a cellular process.
2. Operating Principal of Pneumatic Microvalve for Tall Microchannel
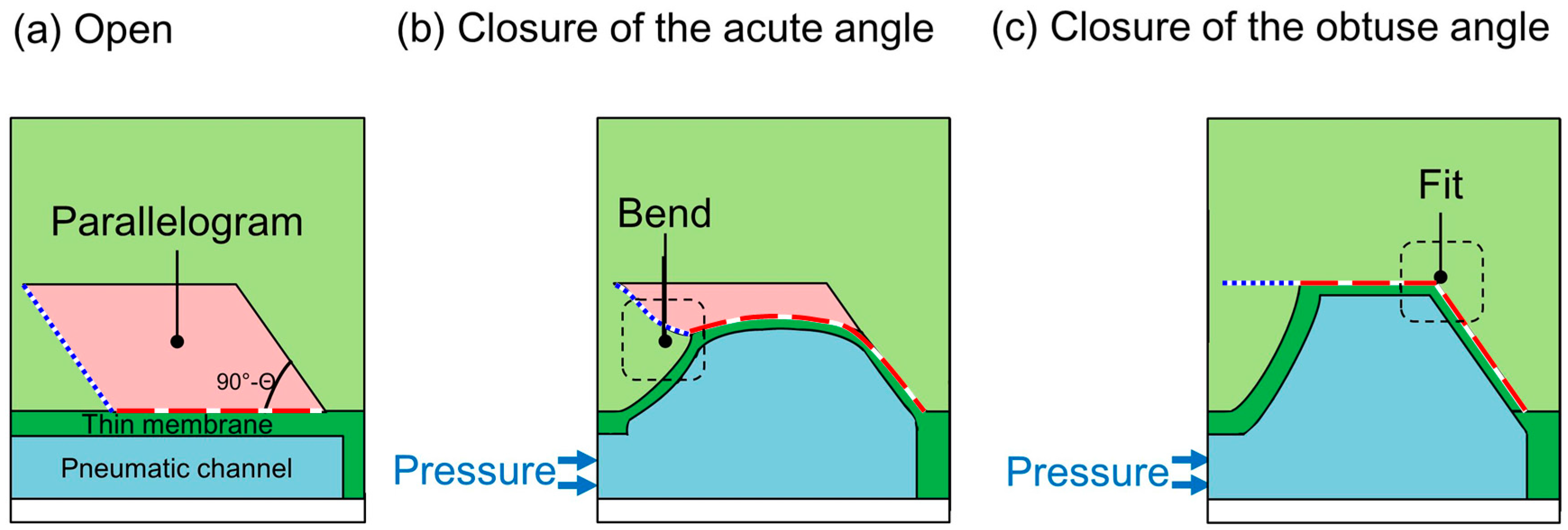
The operation of the proposed microvalve is based on the expansion of a pneumatic microchannel (
Figure 2). The microvalve is composed of three layers. The first layer is the actual liquid channel, which has a parallelogram-shaped cross section. The second layer consists of a thin membrane in between the first layer and the third layer, which is the pneumatic microchannel (
Figure 2a and
Figure 3). The three layers are bonded, with the two microchannels orthogonally aligned to each other. When the pneumatic microchannel is pressurized, the thin membrane (red dash line) is bent and fits well against the wall of the liquid microchannel. An overhanging structure with a sharp edge at the acute-angled corner of the liquid microchannel (blue dot line) also bends to shut the corner (
Figure 2b), enabling the membrane to easily fit into the obtuse-angled corner (
Figure 2c). By this means, the liquid microchannel is completely shut.
This type of liquid microchannel with a parallelogram-shaped cross section can be made taller than one with a semicircular cross section because its fabrication does not involve the reflow process. The employed fabrication mold can be produced by inclined lithography [
17] using thick-patterned photoresists. The achieved pneumatic microvalve is applicable to microfluidic devices equipped with tall microchannels, such as those used to handle large cells. Further, the microvalve with a tall microchannel might collaterally reduce shear stress applied to the cells.
3. Fabrication of Pneumatic Microvalve for Tall Microchannel
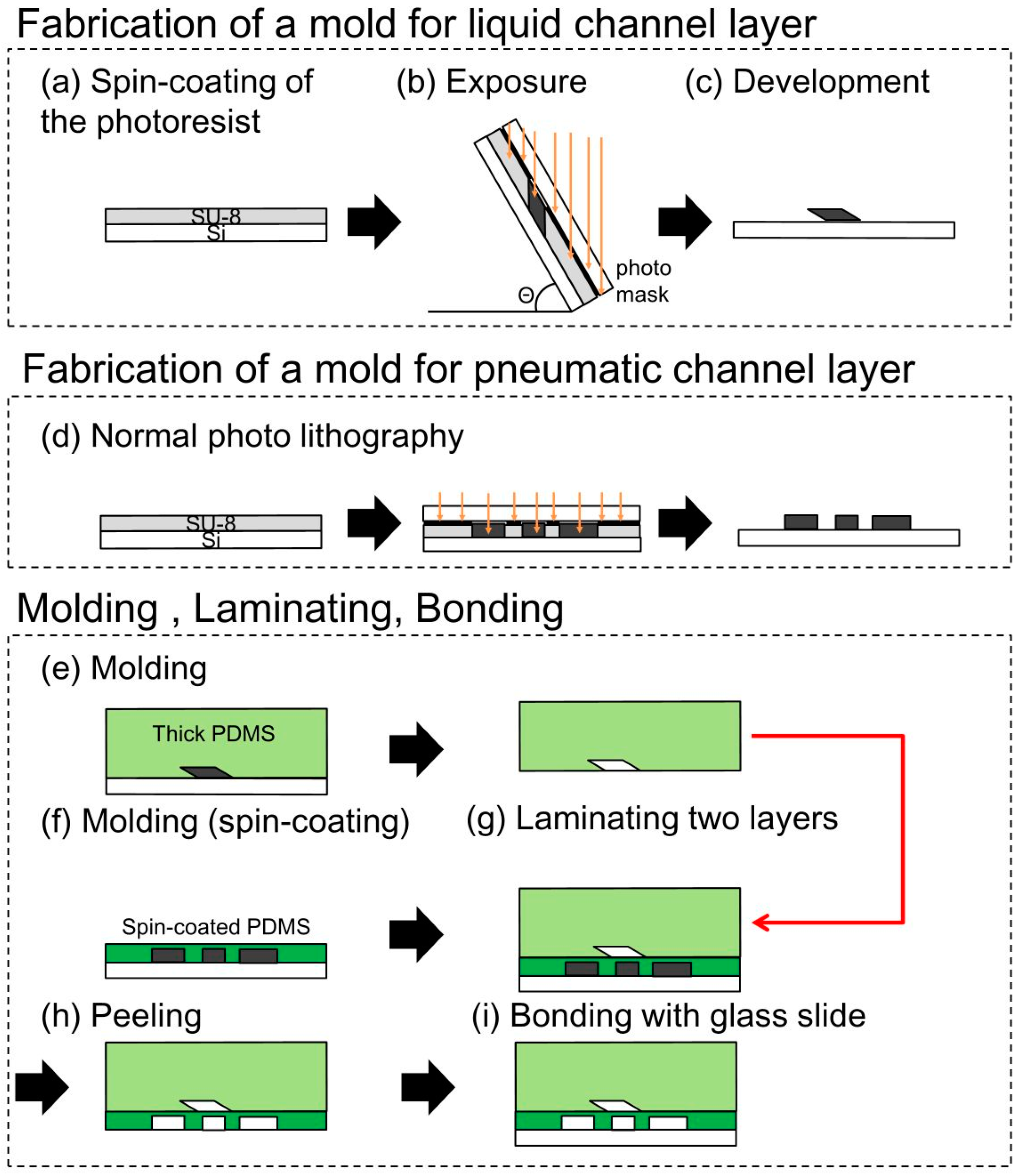
In the present study, the pneumatic microchannel was placed under the liquid microchannel. Both microchannels were fabricated by casting PDMS on molds produced by photolithography (
Figure 4). The mold for the liquid microchannel, which had a parallelogram-shaped cross section, was produced by inclined lithography. The complete fabrication process was as follows: (a) SU-8 (SU-8 3025, Microchem, Westborough, MA, USA) of 50 or 100 μm in thickness was spin-coated on a Si substrate; (b) A photomask was aligned with and fixed to the Si substrate. The mask and the Si substrate were subsequently inclined at an angle θ (0°, 30°, and 60° were respectively considered in our experiment) and exposed to ultra violet (UV) light; (c) The patterned SU-8 for the mold of the liquid microchannel was developed; (d) The mold for the pneumatic microchannel was fabricated by normal lithography; (e) PDMS (Silpot 184 W/C, Dow Corning Toray, Midland, MI, USA) was cast on the mold of the liquid microchannel fabricated in (c); (f) The thin membrane between the liquid and pneumatic microchannel was fabricated by PDMS spin-coating on the mold of the pneumatic microchannel. The membranes of 30, 40, 80 μm in thickness were cured on the hotplate of 100 °C; (g) Before completing the cure process of the spin-coated PDMS, the PDMS layer of the liquid microchannel was detached from the mold and was attached to the spin-coated PDMS with an orthogonal alignment of the liquid and pneumatic microchannels. The attached PDMS layers are baked on the hotplate of 100 °C for the good bond between the PDMS layers; (h) The bonded PDMS layers were then detached from the mold of the liquid microchannel; (i) The bonded PDMS layers were then bonded to a glass substrate using the irradiation of vacuum UV/O
3 using excimer lamp (SUS713, Ushio Inc., Tokyo, Japan). The PDMS used in all processes was a mixture of an elastomer and a cross-linker with a weight ratio of 10:1.
Figure 5 shows the fabricated device. In the experiments performed in the present study, the liquid and pneumatic microchannels were respectively filled with red- and blue-dyed water to improve visibility.
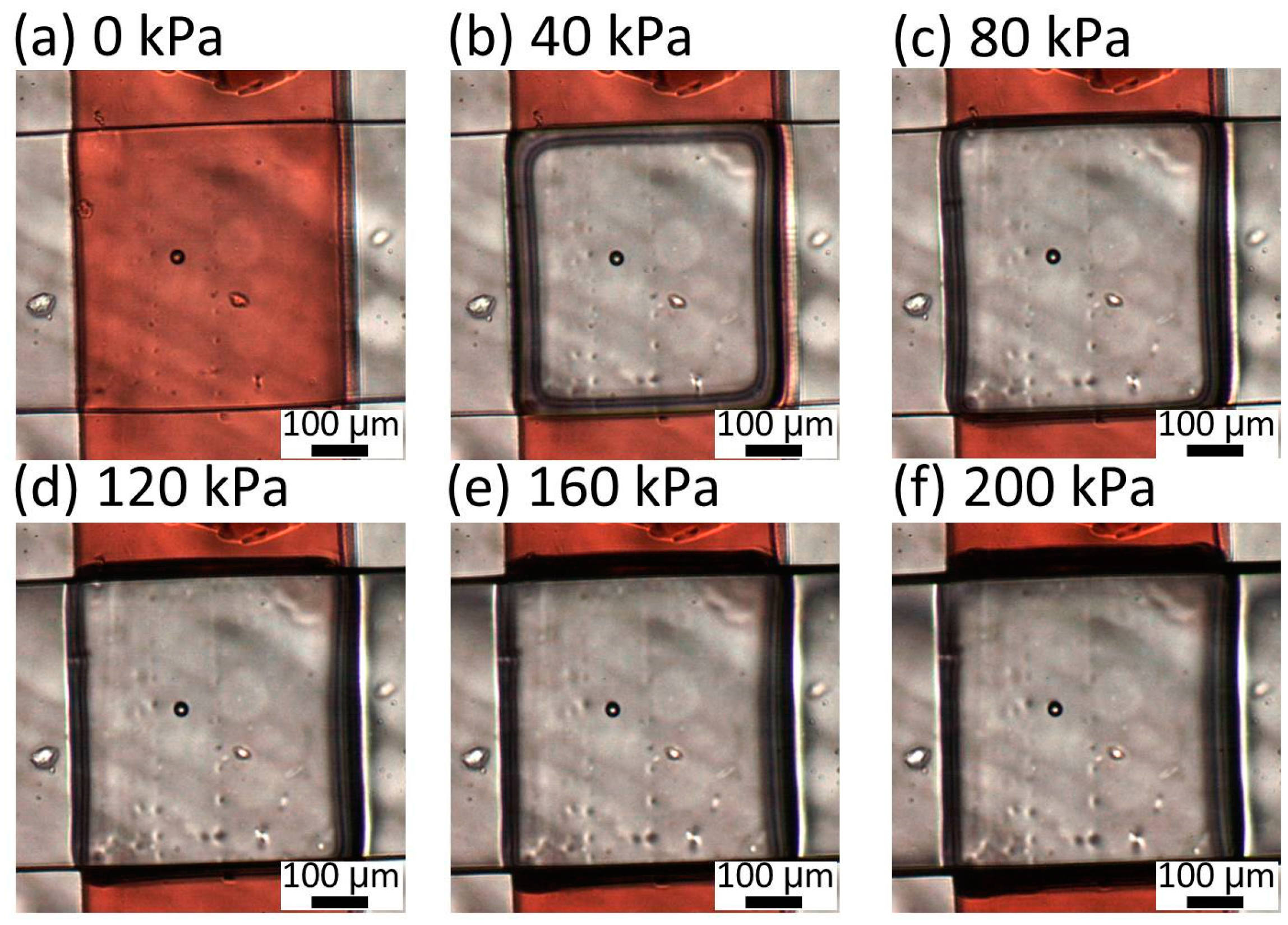
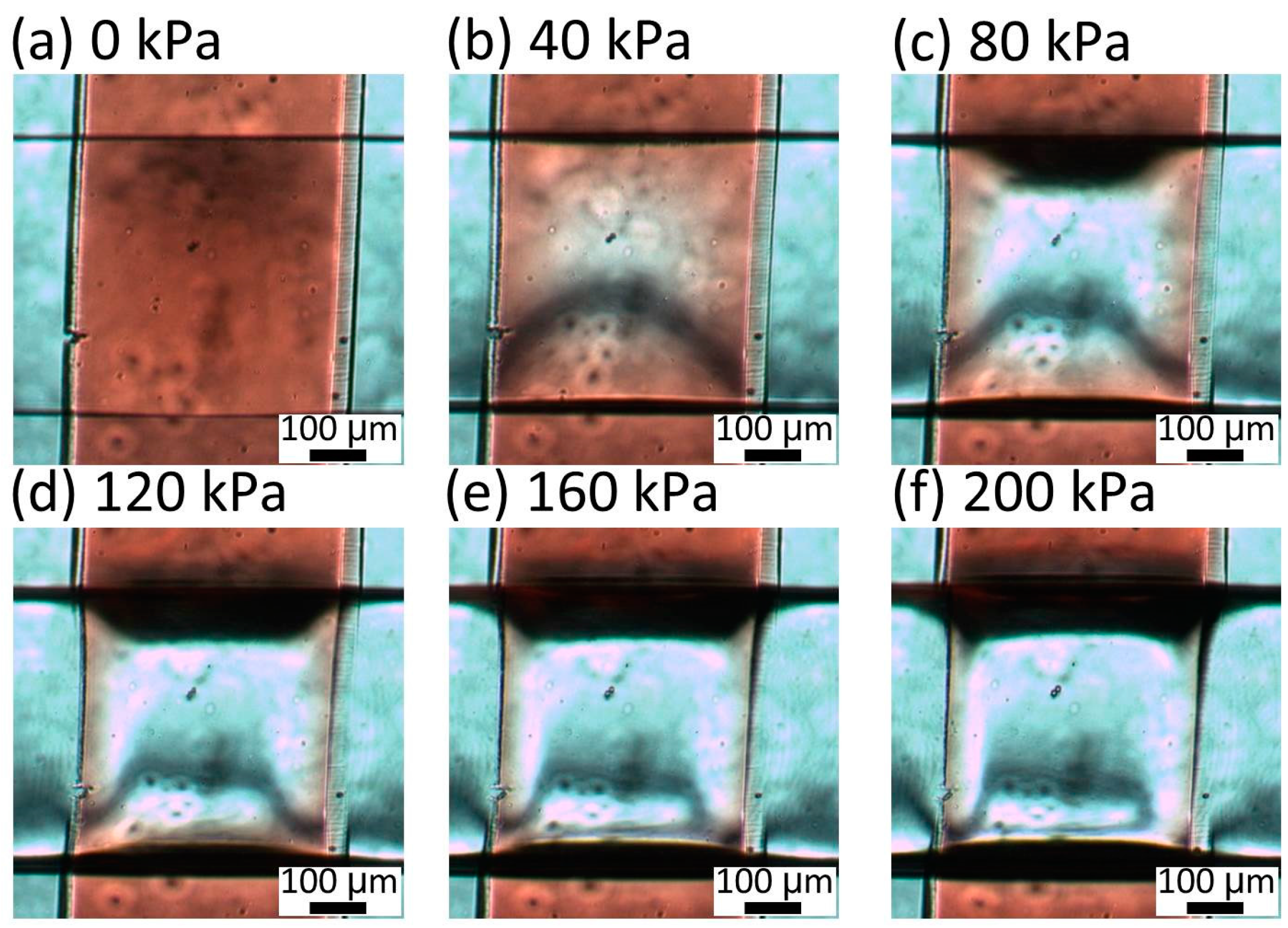
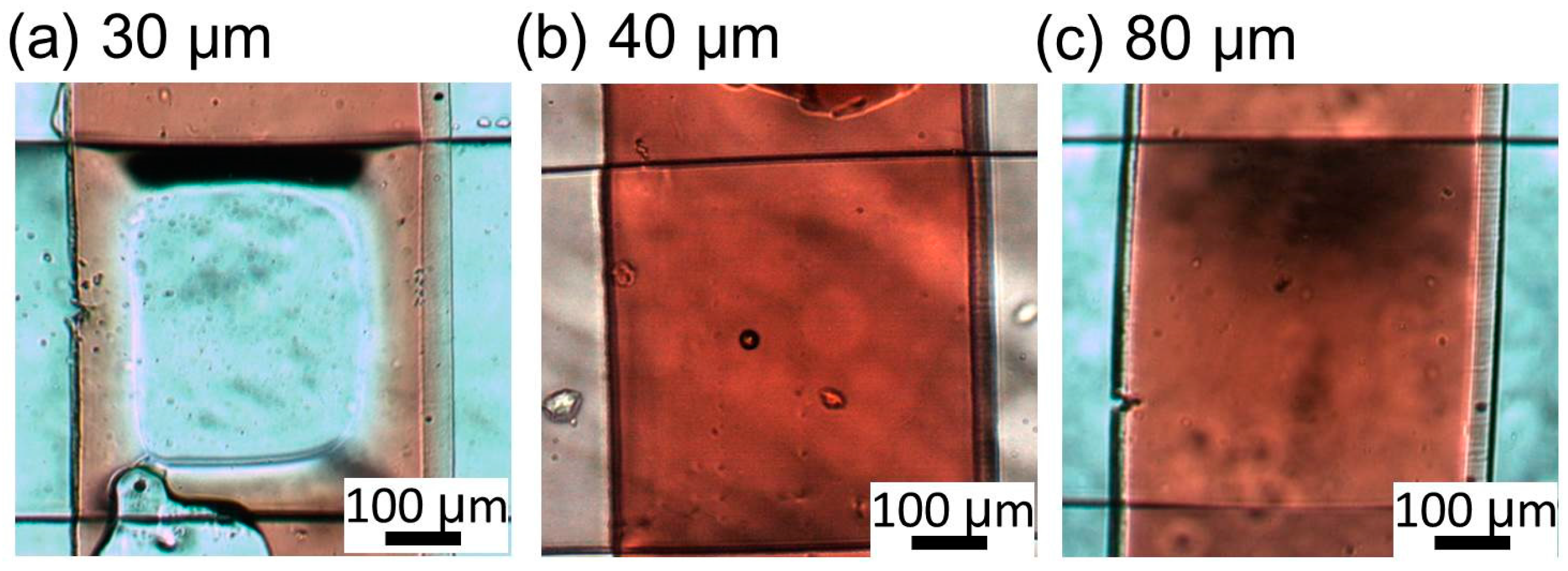
Figure 5b is the zoom-in image of an intersection of liquid and pneumatic microchannels. The intersection dimension is 500 μm in width (width of the liquid microchannel) and 500 μm in length (width of the pneumatic microchannel). Though we can scale down the dimensions, the mold of the liquid microchannel for a narrow intersection is difficult to fabricate due to a high aspect ratio. Moreover, the narrow pneumatic microchannel increases the response time of the microvalve. The both sides of the liquid microchannel are gradient in red, which shows the acute- and obtuse-angled structures. The cross section (a-a′ in
Figure 5b) of the liquid channel before bonded to the pneumatic channel is shown in
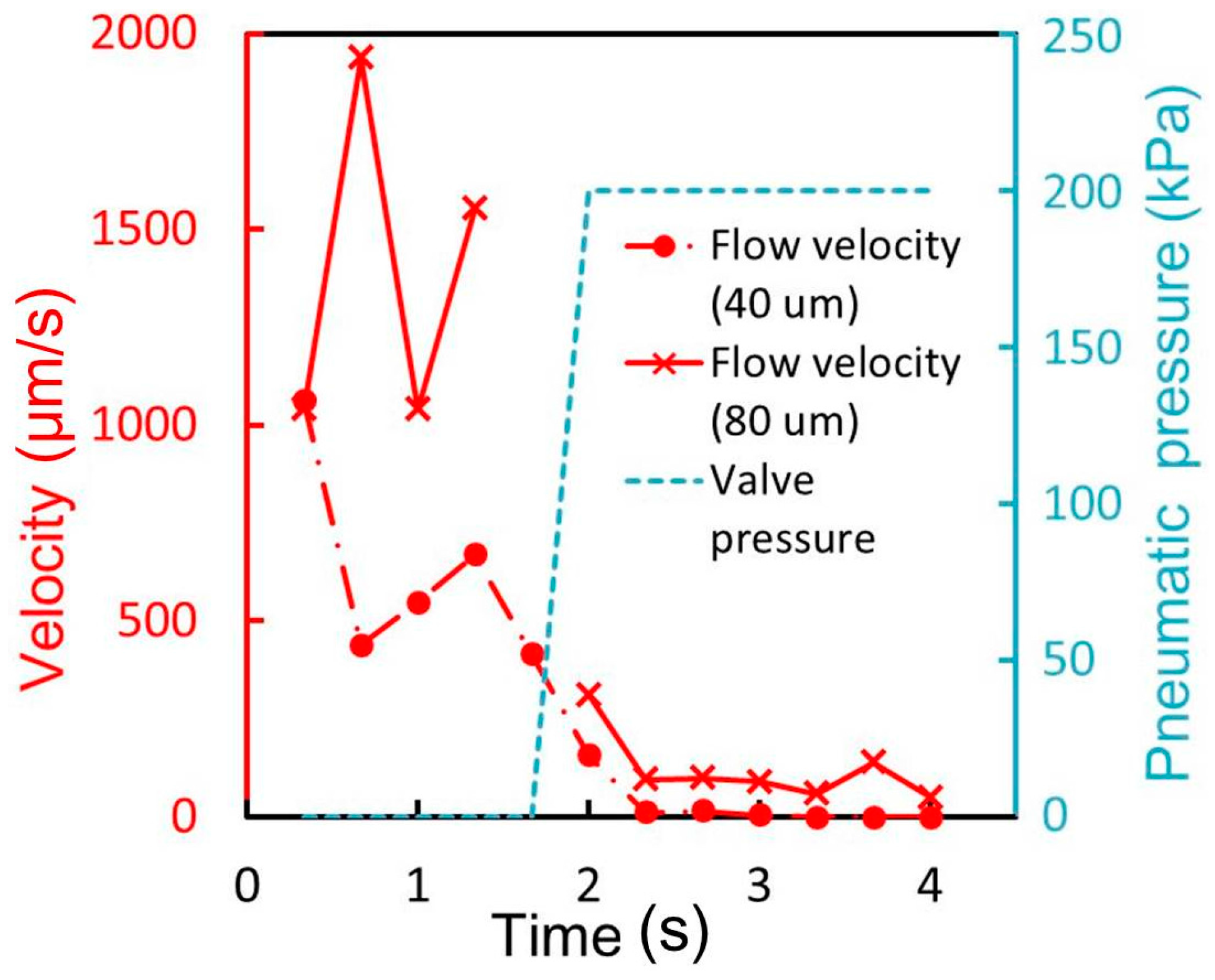
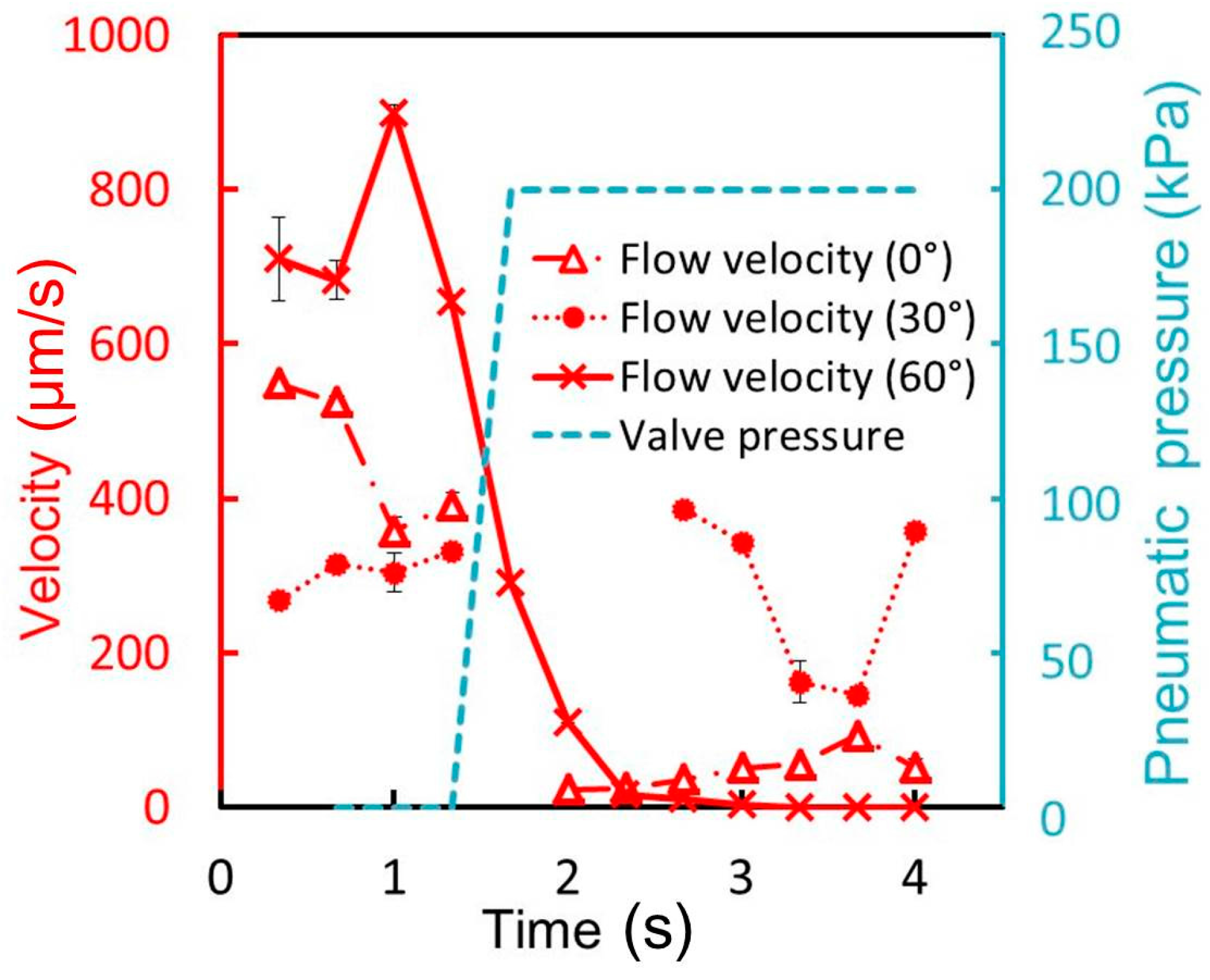
Figure 5c. The pneumatic and liquid microchannels both had a width of 500 μm and height of 50 μm. The acute angle of the parallelogram cross section of the liquid channel is measured to be 88.4°, 64.7°, and 53.6°, corresponding to incline angles θ of 0°, 30°, and 60° (90° − θ = 90°, 60°, and 30°), respectively. The difference is due to the refractive index of SU-8, which is 1.67 at the UV exposure wavelength [
18]. According to Snell’s law [
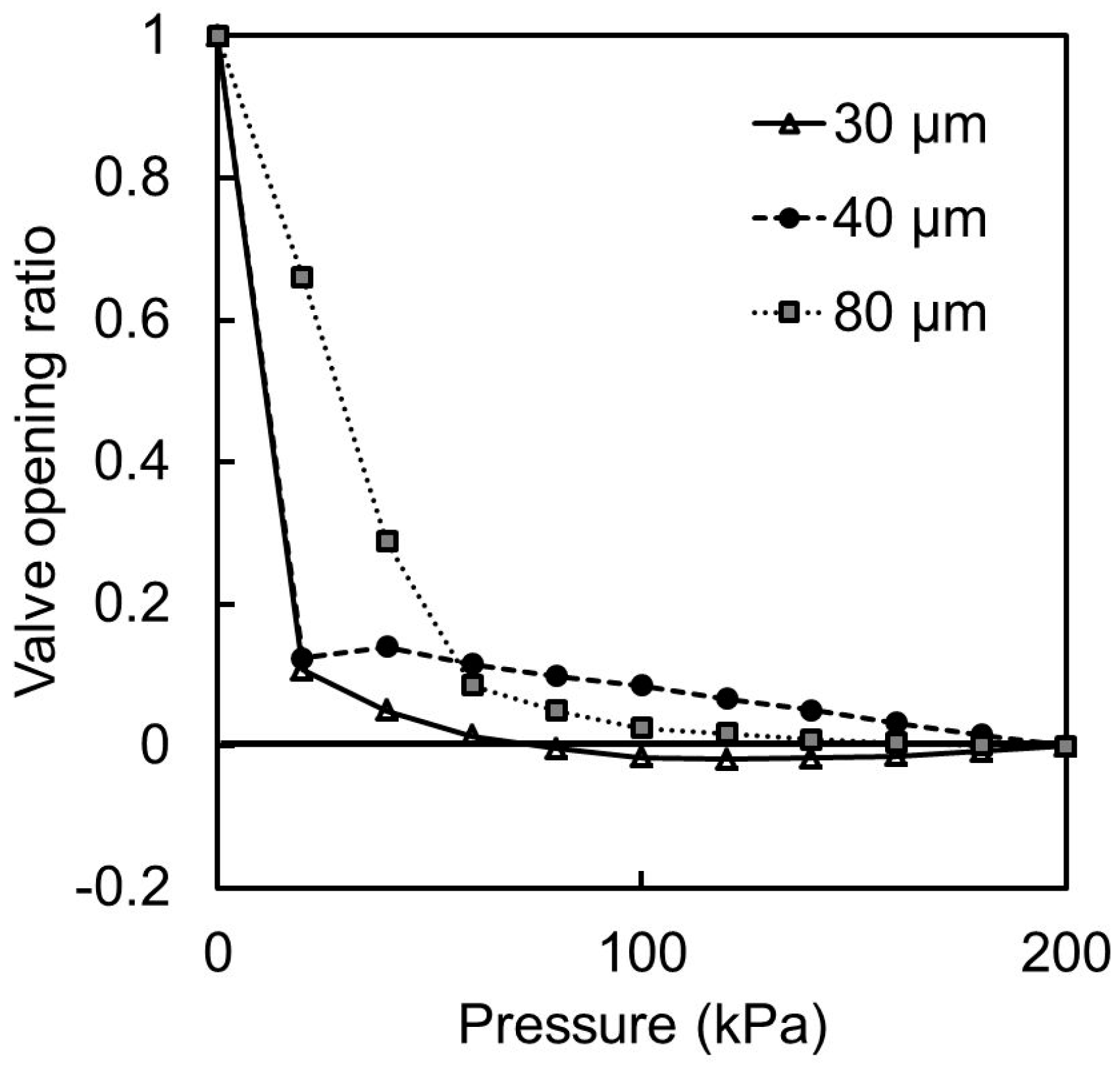
19], the acute angle of the parallelogram cross section of the liquid channel is given by θ′ = 90° − arcsin(sinθ/1.67). Thus, the calculated acute angle θ′ is: 90°, 72.5°, and 58.8° when θ becomes 0°, 30°, and 60° respectively. The measured acute angles are in good agreement with those theoretically anticipated, considering the wavelength of UV light. The minimum acute angle in SU-8 microstructures during inclined UV exposure is calculated to be 53.2° (= 90° − arcsin(1/1.67)). The operable inclination angle for inclined lithography and the operable membrane thickness were 60° and 40 μm, respectively, as determined by the experiments described in
Section 4.2,
Section 4.3,
Section 4.4 and
Section 4.5.
5. Demonstration of Use of Microvalve for Cellular Experiment
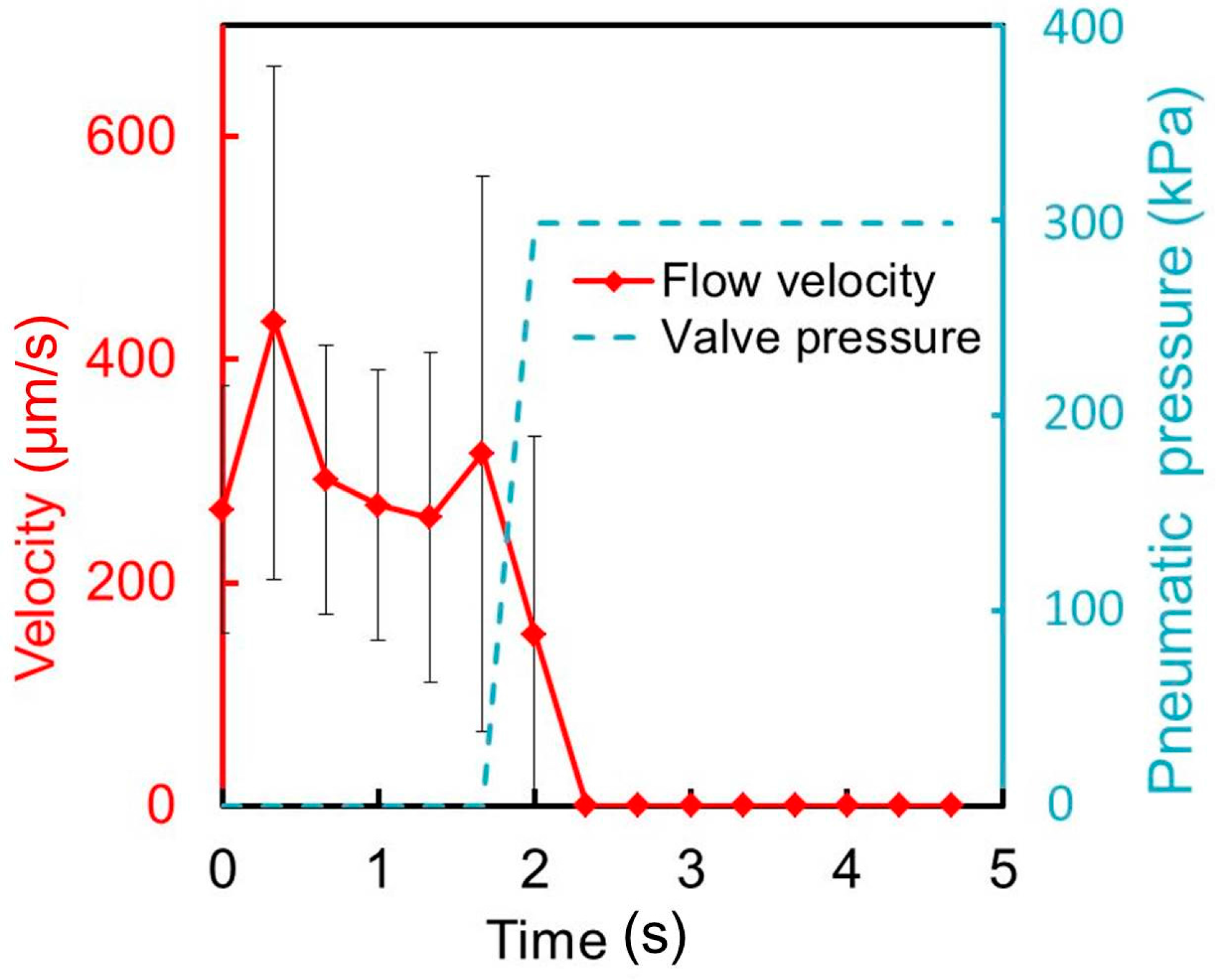
For its practical application, the microvalve was used to perform a cellular experiment in which a HeLa cell suspension was passed through the liquid microchannel of height 50 μm. The effect of the microvalve on the cells was examined in terms of the cell proliferation ratio and morphology as major factors of cellular status. The microvalve was randomly opened and closed 30 times over 10 min while the cell suspension, which contained 4.0 × 106 cells/mL, was injected into the liquid microchannel. The cells were collected after passing through the microvalve and cultured in dishes. The cultured cells were counted and observed daily. As a control, cultured cells that had not been passed through the microvalve were counted and observed daily.
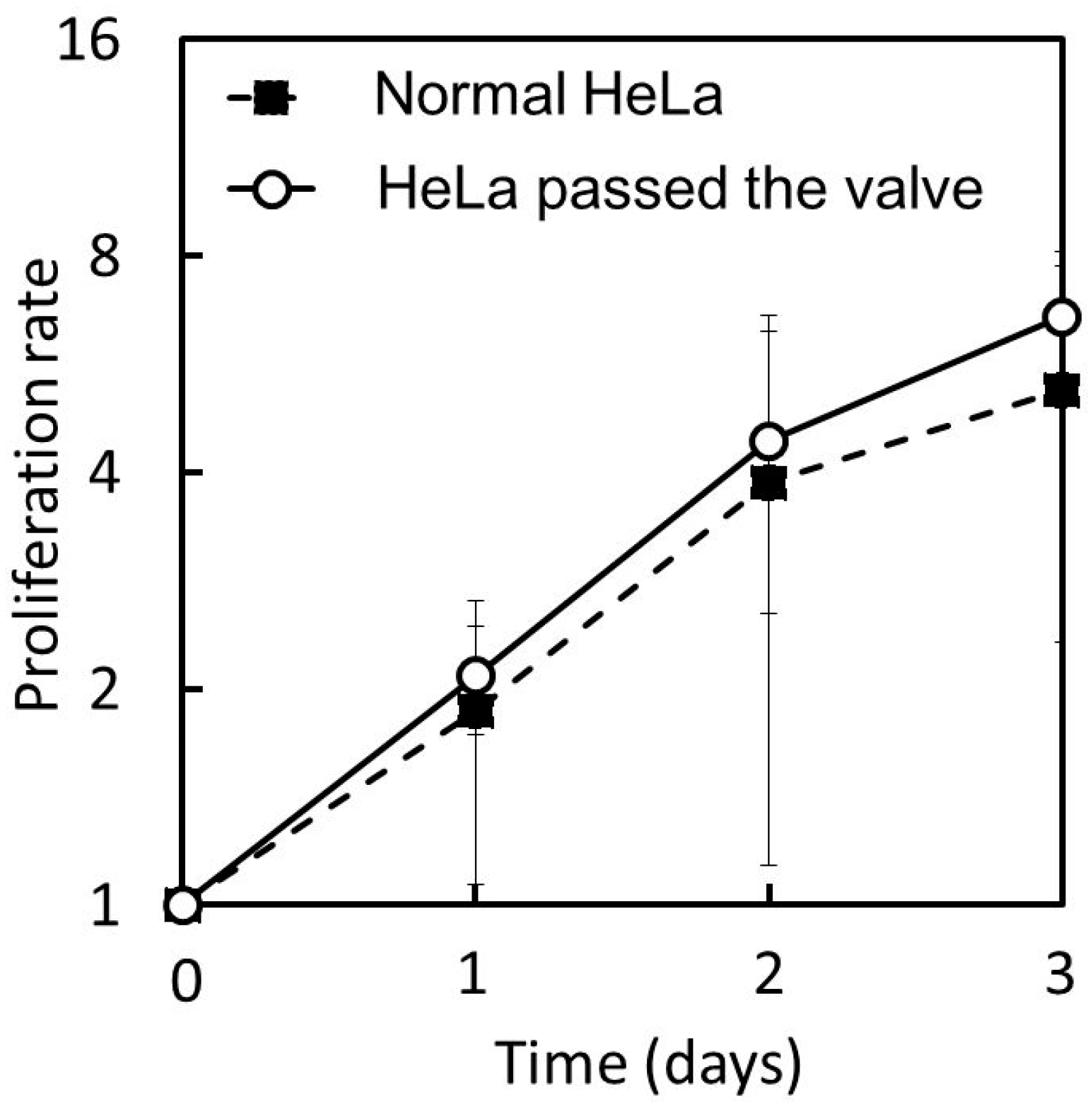
Figure 16 shows the proliferation curves of the HeLa cells that were passed through the microvalve and those that were not passed. The cell proliferation rate was normalized by the initial number of cells and plotted on a binary logarithmic scale, considering that HeLa cells are known to be doubled daily. The proliferation curves for the two groups of cells can be observed to be approximately the same. Both cells approximately doubled in one day, and increased four-fold two days after cell seeding. The average proliferation ratio over three days of the cells passed through the microvalve was 6.6 (
n = 3, S.D. = 1.15), while that of the cells that were not passed was 5.2 (
n = 3, S.D. = 2.89). The cell morphologies for the two groups of cells were also examined, as shown in
Figure 17a,b. No significant difference was observed. These observations suggest that the operation of the proposed microvalve did not largely affect the proliferation and morphologies in the HeLa cells.