Light-Activated Metal Oxide Gas Sensors: A Review
Abstract
:1. Introduction
2. Pure Metal Oxide Materials for Gas Sensing
2.1. Pure Metal Oxide Nanoparticles
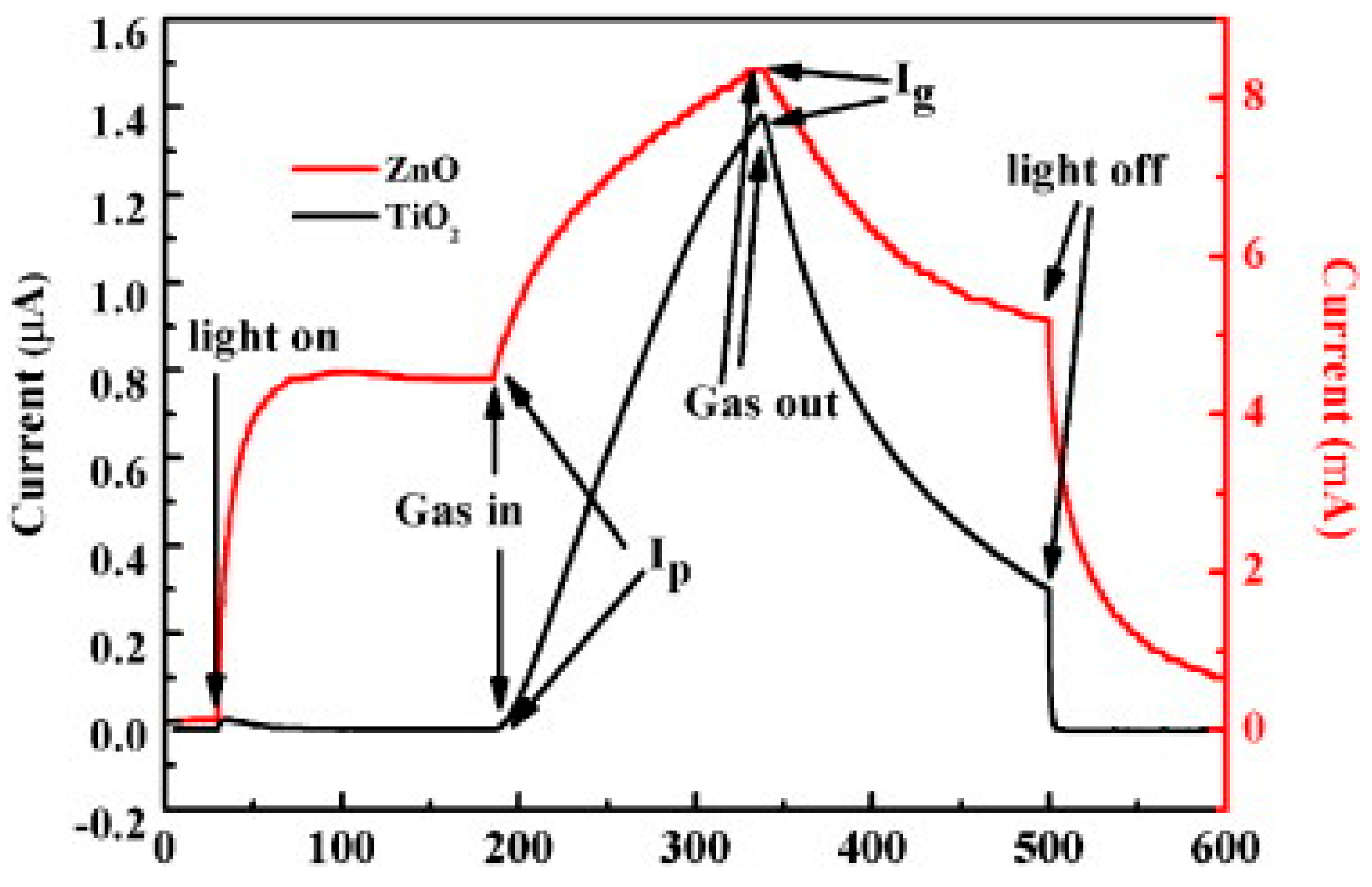
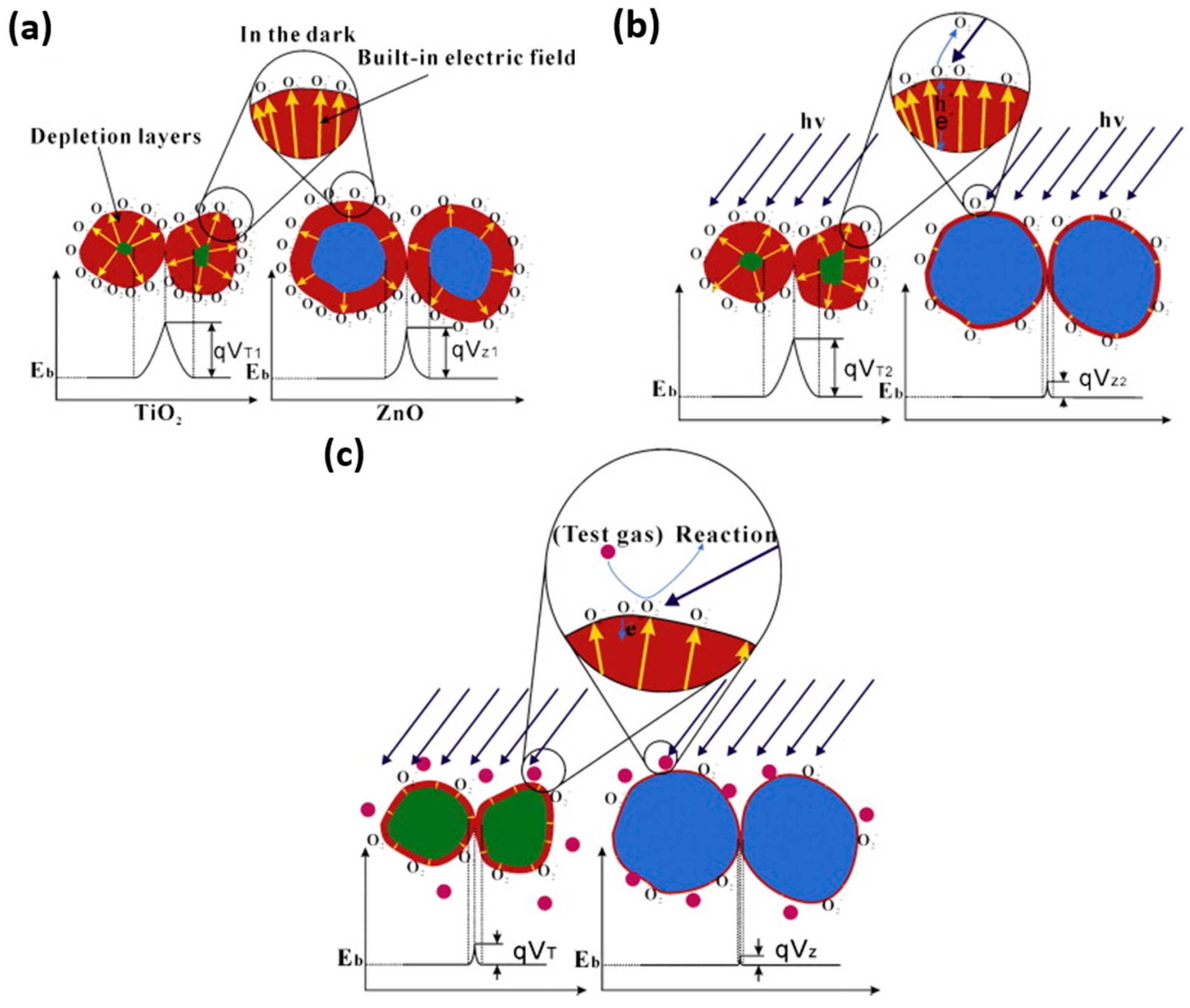
2.1.1. ZnO
2.1.2. SnO2
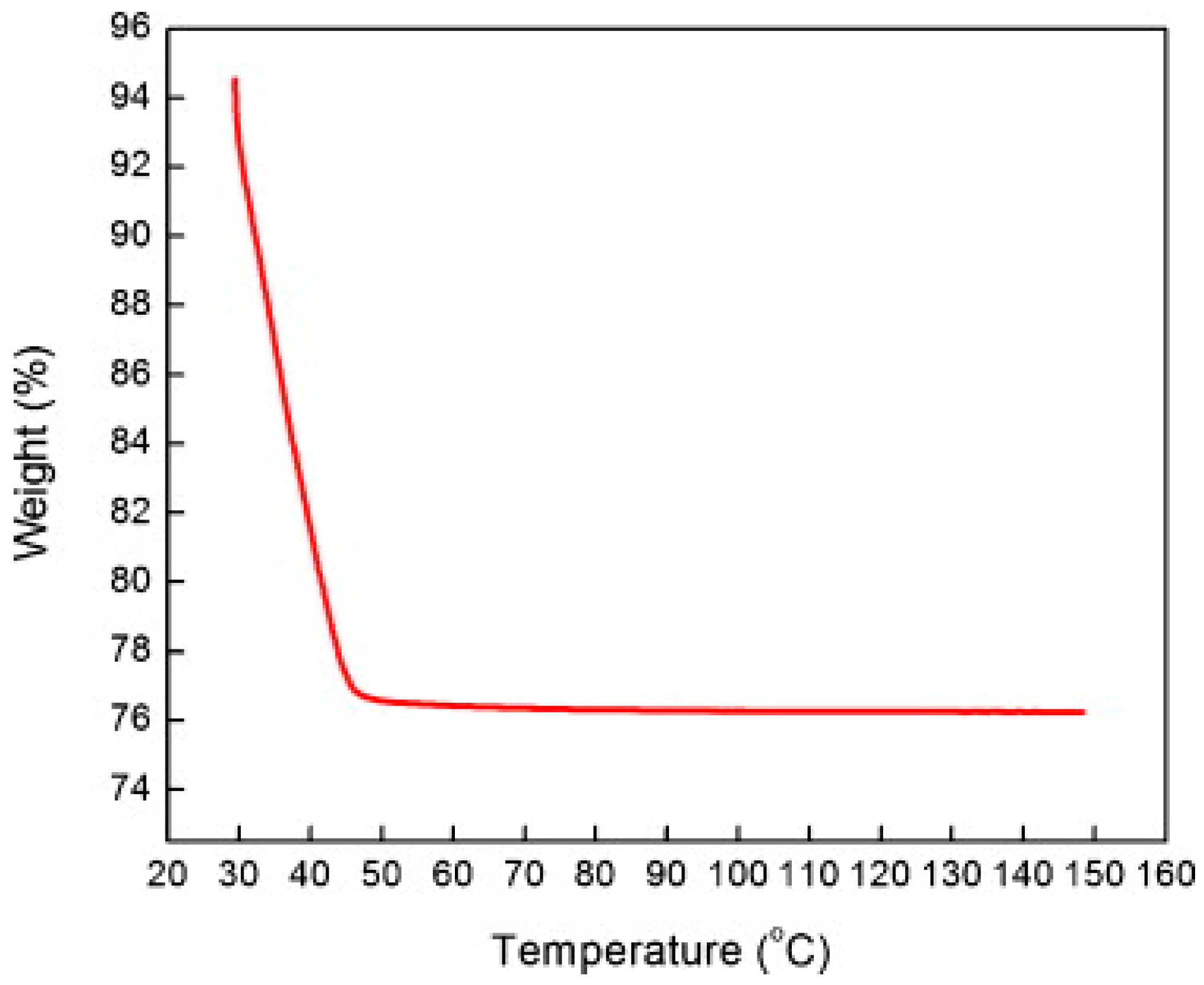
2.1.3. TiO2
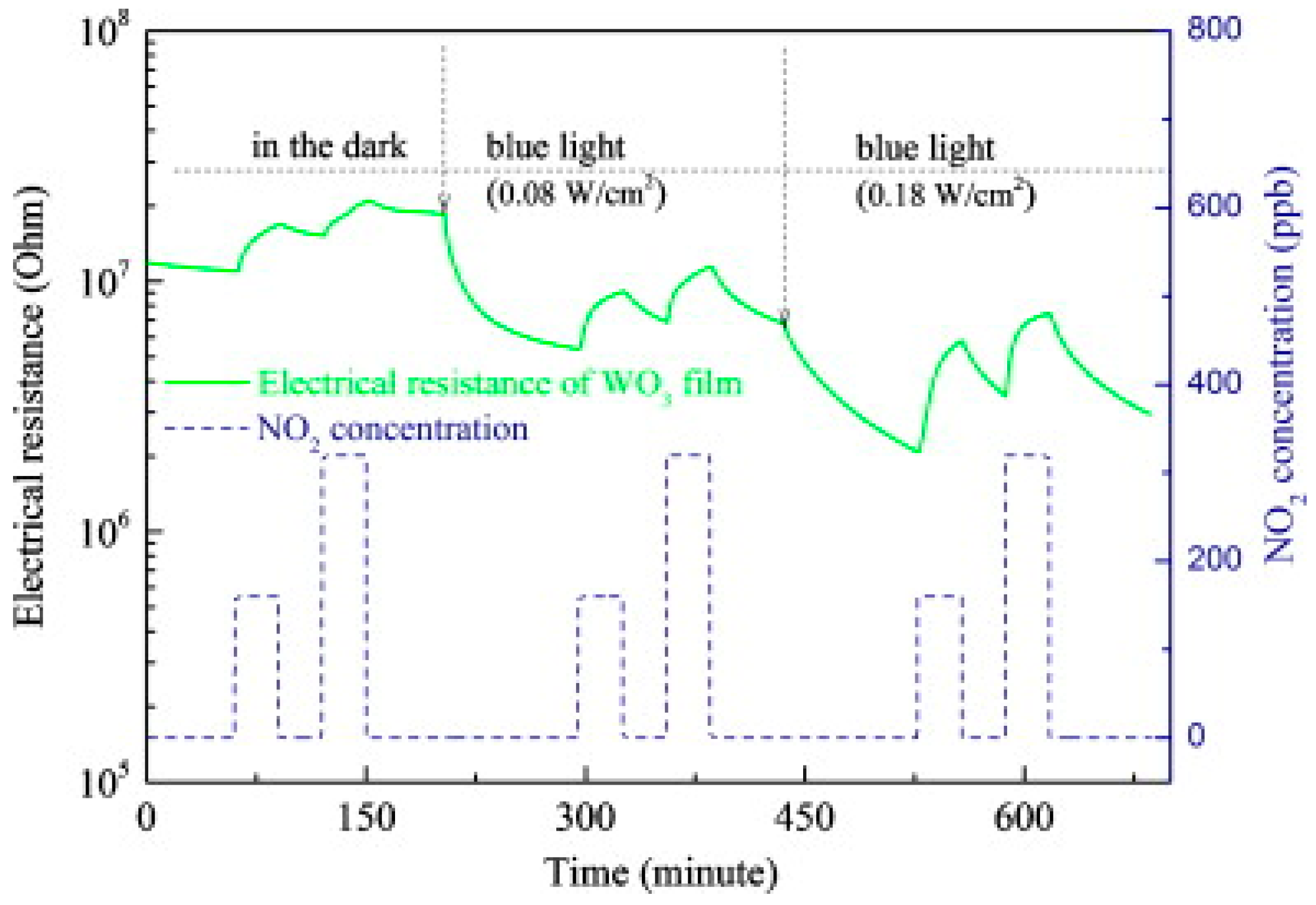
2.1.4. WO3
2.1.5. In2O3
2.1.6. Summary
2.2. Metal Oxides with One-Dimensional Nanostructures
2.2.1. Nanowires
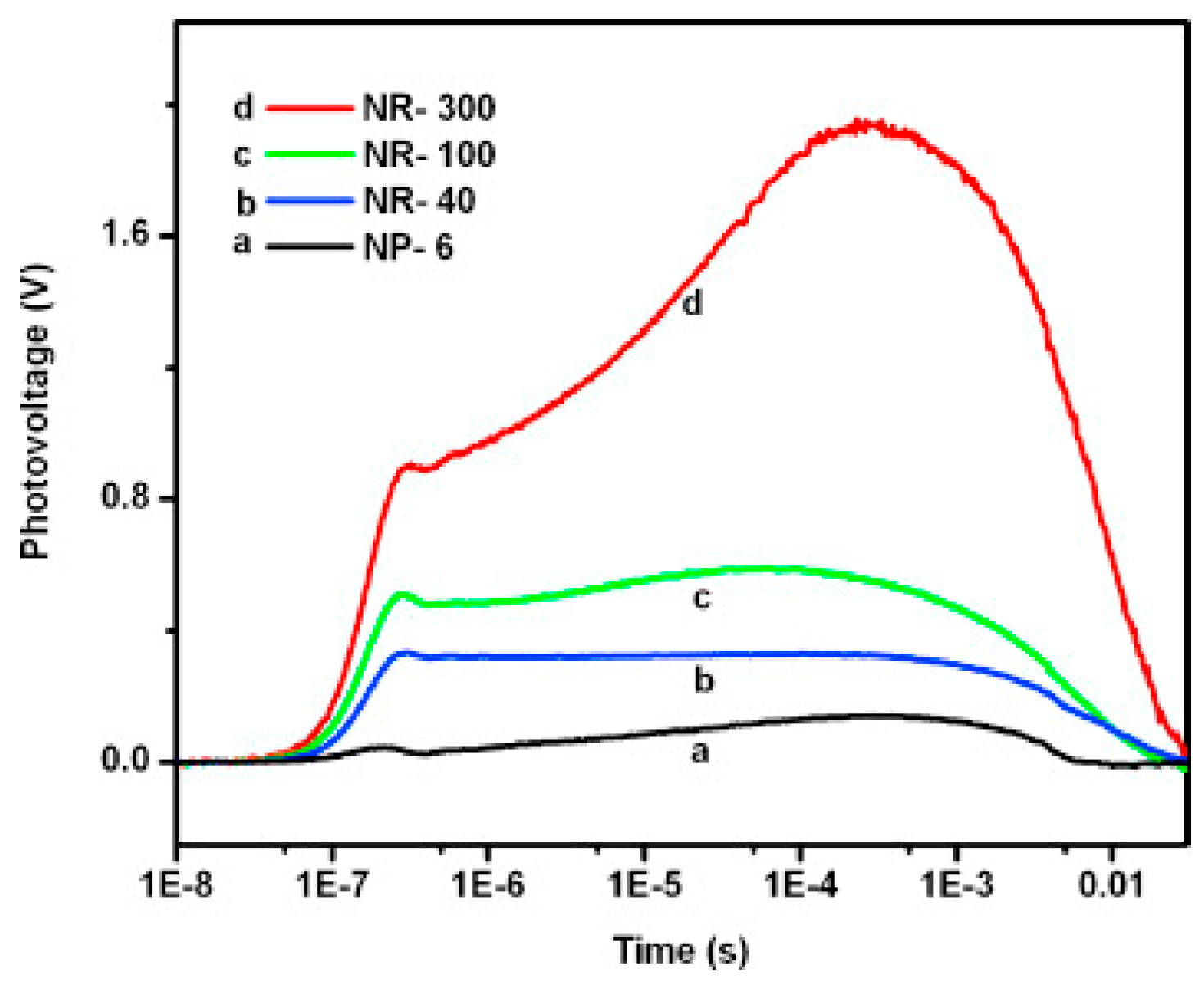
2.2.2. Nanorods
2.2.3. Nanofibers
2.2.4. Summary
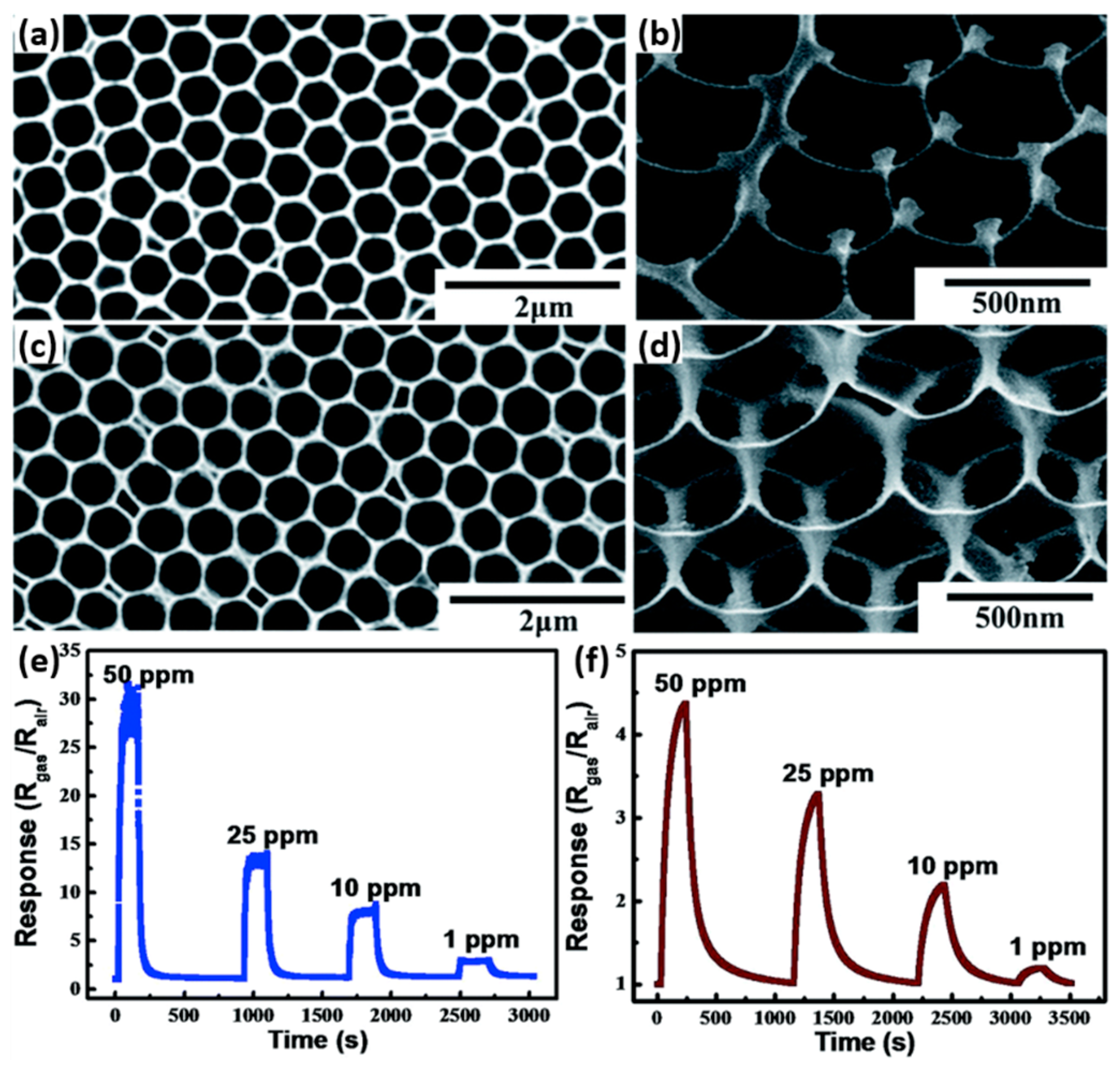
2.3. Metal Oxides with Porous Nanostructures
3. Metal Oxide Materials Composites and Metal Oxide with Doping for Gas Sensing
3.1. Heterostructures
3.1.1. Heterostructures Consisted of Inorganic Semiconductors and Metal Oxide Semiconductors
Simple Coalesced Nanostructure
Core-Shell Nanostructure
3.1.2. Heterostructures Containing Metal and Metal Oxide Semiconductors
3.1.3. Summary
3.2. Metal Oxides with Doping
Summary
4. Challenges and Outlook
- (1)
- LSPR absorption of noble metal nanoparticles extends the absorption spectrum to the visible range;
- (2)
- Hot electrons emerged in noble metal nanoparticles may result in carrier injection into the conduction band of the semiconductor, hence leading to an increase in the concentration of energetic electrons;
- (3)
- Schottky barrier formed at the interface between the noble metal and metal oxides can suppress recombination of electron-hole pairs;
- (4)
- Localized electric filed enhancement can increase the separation of election-hole pairs on the semiconductor side.
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Volanti, D.P.; Felix, A.A.; Orlandi, M.O.; Whitfield, G.; Yang, D.J.; Longo, E.; Tuller, H.L.; Varela, J.A. The role of hierarchical morphologies in the superior gas sensing performance of CuO-based chemiresistors. Adv. Funct. Mater. 2013, 23, 1759–1766. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.J.; Lee, I.; Youn, D.Y.; Park, C.O.; Lee, J.H.; Tuller, H.L.; Kim, I.D. Thin-wall assembled SnO2 fibers functionalized by catalytic Pt nanoparticles and their superior exhaled-breath-sensing properties for the diagnosis of diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Katwal, G.; Paulose, M.; Rusakova, I.A.; Martinez, J.E.; Varghese, O.K. Rapid growth of zinc oxide nanotube-nanowire hybrid architectures and their use in breast cancer-related volatile organics detection. Nano Lett. 2016, 16, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Choi, S.J.; Jang, J.S.; Kim, N.H.; Hakim, M.; Tuller, H.L.; Kim, I.D. Mesoporous WO3 nanofibers with protein-templated nanoscale catalysts for detection of trace biomarkers in exhaled breath. ACS Nano 2016, 10, 5891–5899. [Google Scholar] [CrossRef] [PubMed]
- Andringa, A.M.; Piliego, C.; Katsouras, I.; Blom, P.W.; de Leeuw, D.M. NO2 detection and real-time sensing with field-effect transistors. Chem. Mat. 2014, 26, 773–785. [Google Scholar] [CrossRef]
- Hagedorn, K.; Li, W.; Liang, Q.; Dilger, S.; Noebels, M.; Wagner, M.R.; Reparaz, J.S.; Dollinger, A.; auf der Günne, J.S.; Dekorsy, T.; et al. Catalytically doped semiconductors for chemical gas sensing: aerogel-like aluminum-containing zinc oxide materials prepared in the gas phase. Adv. Funct. Mater. 2016, 26, 3424–3437. [Google Scholar] [CrossRef]
- Dong, X.; Li, T.; Liu, Y.; Li, Y.; Zhao, C.L.; Chan, C.C. Polyvinyl alcohol-coated hybrid fiber grating for relative humidity sensing. J. Biomed. Opt. 2011, 16, 077001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dong, X.; Li, T.; Chan, C.C.; Shum, P.P. Simultaneous measurement of relative humidity and temperature with PCF-MZI cascaded by fiber Bragg grating. Opt. Commun. 2013, 303, 42–45. [Google Scholar] [CrossRef]
- Fabbri, B.; Gaiardo, A.; Giberti, A.; Guidi, V.; Malagù, C.; Martucci, A.; Sturaro, M.; Zonta, G.; Gherardi, S.; Bernardoni, P. Chemoresistive properties of photo-activated thin and thick ZnO films. Sens. Actuator B 2016, 222, 1251–1256. [Google Scholar] [CrossRef]
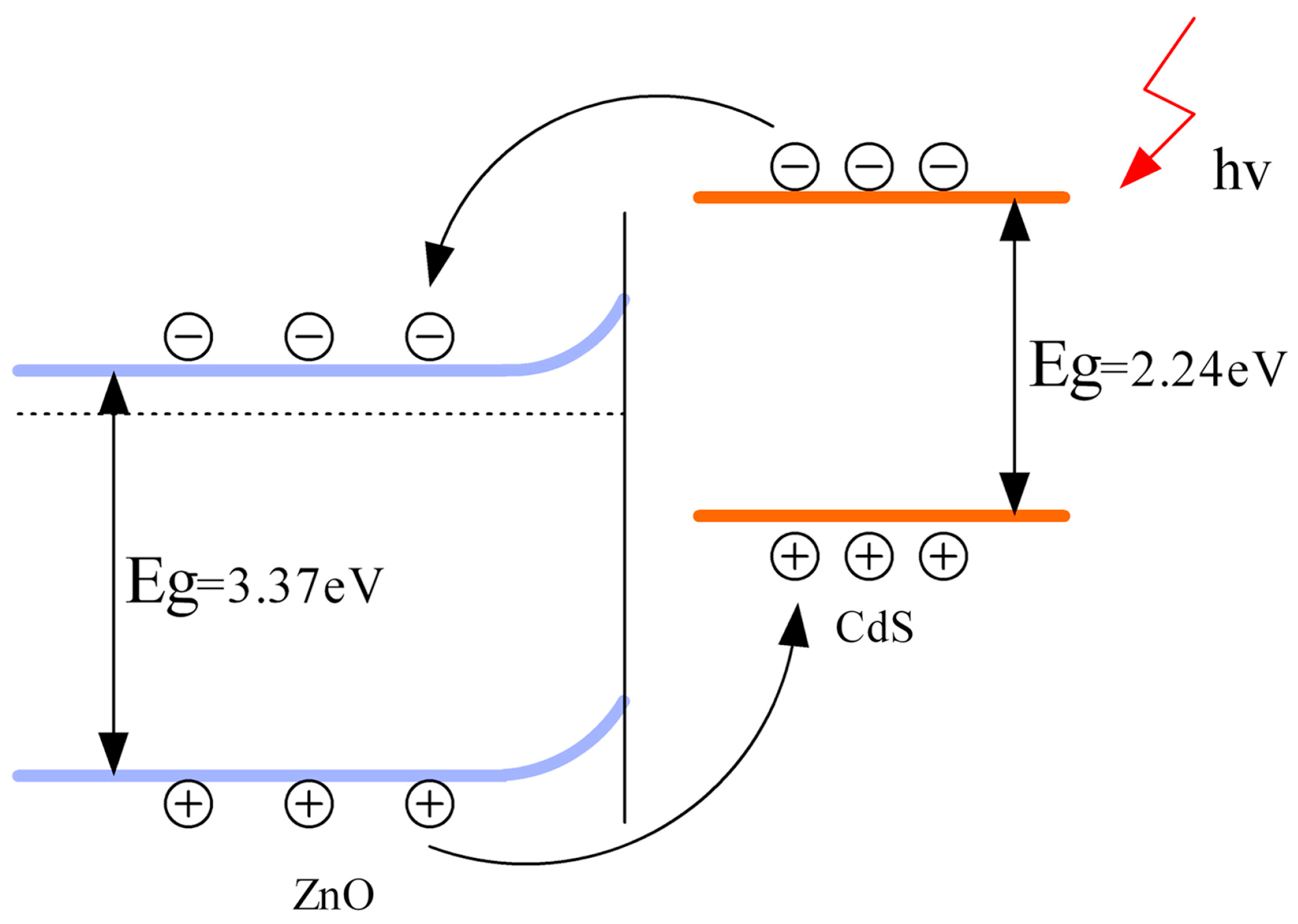
- Zhai, J.; Wang, D.; Peng, L.; Lin, Y.; Li, X.; Xie, T. Visible-light-induced photoelectric gas sensing to formaldehyde based on CdS nanoparticles/ZnO heterostructures. Sens. Actuator B 2010, 147, 234–240. [Google Scholar] [CrossRef]
- Peng, L.; Xie, T.-F.; Yang, M.; Wang, P.; Xu, D.; Pang, S.; Wang, D.-J. Light induced enhancing gas sensitivity of copper-doped zinc oxide at room temperature. Sens. Actuator B 2008, 131, 660–664. [Google Scholar] [CrossRef]
- Şahin, Y.; Öztürk, S.; Kılınç, N.; Kösemen, A.; Erkovan, M.; Öztürk, Z.Z. Electrical conduction and NO2 gas sensing properties of ZnO nanorods. Appl. Surf. Sci. 2014, 303, 90–96. [Google Scholar] [CrossRef]
- Peng, L.; Zhai, J.; Wang, D.; Zhang, Y.; Wang, P.; Zhao, Q.; Xie, T. Size- and photoelectric characteristics-dependent formaldehyde sensitivity of ZnO irradiated with UV light. Sens. Actuator B 2010, 148, 66–73. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.; Chai, X.; Hu, Z.; Deng, Y. UV-light-activated ZnO fibers for organic gas sensing at room temperature. J. Phys. Chem. C 2009, 114, 1293–1298. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Li, X.; Wang, J.; Tang, Z. UV activated hollow ZnO microspheres for selective ethanol sensors at low temperatures. Sens. Actuator B 2016, 232, 158–164. [Google Scholar] [CrossRef]
- Comini, E.; Cristalli, A.; Faglia, G.; Sberveglieri, G. Light enhanced gas sensing properties of indium oxide and tin dioxide sensors. Sens. Actuator B 2000, 65, 260–263. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Yuan, Z.; Sun, Y.; Chang, F.; Deng, H.; Xie, L.; Li, H. A highly sensitive gas sensor based on CuO nanoparticles synthetized via a sol–gel method. RSC Adv. 2016, 6, 79343–79349. [Google Scholar] [CrossRef]
- Liu, M.; Ren, S.P.; Zhang, R.Y.; Xue, Z.Y.; Ma, C.R.; Yin, M.L.; Xu, X.; Bao, S.Y.; Chen, C.L. Gas sensing properties of epitaxial LaBaCo2O5.5+delta thin films. Sci. Rep. 2015, 5, 10784. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.L.; Qiu, Y.; Yu, J.J.; Yin, B.; Cao, G.Y.; Zhang, H.Q.; Hu, L.Z. Ethanol sensing with Au-modified ZnO microwires. Sens. Actuator B 2016, 227, 65–72. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Song, J.; Yu, T.; Liu, J.; Zou, Z. Versatile nanobead-scaffolded N-SnO2 mesoporous microspheres: One-step synthesis and superb performance in dye-sensitized solar cell, gas sensor, and photocatalytic degradation of dye. J. Mater. Chem. A 2013, 1, 524–531. [Google Scholar] [CrossRef]
- Saboor, F.H.; Ueda, T.; Kamada, K.; Hyodo, T.; Mortazavi, Y.; Khodadadi, A.A.; Shimizu, Y. Enhanced NO2 gas sensing performance of bare and Pd-loaded SnO2 thick film sensors under UV-light irradiation at room temperature. Sens. Actuator B 2016, 223, 429–439. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Quasi-one dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Prog. Mater. Sci. 2009, 54, 1–67. [Google Scholar] [CrossRef]
- Lee, J.-H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuator B 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Tiemann, M. Porous metal oxides as gas sensors. Chem. Eur. J. 2007, 13, 8376–8388. [Google Scholar] [CrossRef] [PubMed]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and metal oxide nanoparticles in chemiresistors: Does the nanoscale matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuator B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Smulko, J.M.; Trawka, M.; Granqvist, C.G.; Ionescu, R.; Annanouch, F.; Llobet, E.; Kish, L.B. New approaches for improving selectivity and sensitivity of resistive gas sensors: A review. Sens. Rev. 2015, 35, 340–347. [Google Scholar] [CrossRef]
- Arya, S.K.; Krishnan, S.; Silva, H.; Jean, S.; Bhansali, S. Advances in materials for room temperature hydrogen sensors. Analyst 2012, 137, 2743–2756. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kan, K.; Song, W.; Zhang, G.; Dang, L.; Xie, Y.; Shen, P.; Li, L.; Shi, K. Controllable synthesis of Co3O4/polyethyleneimine-carbon nanotubes nanocomposites for CO and NH3 gas sensing at room temperature. J. Alloy. Compd. 2015, 639, 187–196. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, L.; Yao, L.; Chai, L.; Li, L.; Zhang, G.; Shi, K. One-pot reflux method synthesis of cobalt hydroxide nanoflake-reduced graphene oxide hybrid and their NOx gas sensors at room temperature. J. Alloy Compd. 2014, 612, 126–133. [Google Scholar] [CrossRef]
- Zhang, M.; Zhen, Y.; Sun, F.; Xu, C. Hydrothermally synthesized SnO2-graphene composites for H2 sensing at low operating temperature. Mater. Sci. Eng. B 2016, 209, 37–44. [Google Scholar] [CrossRef]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Fan, S.W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- Geng, Q.; He, Z.; Chen, X.; Dai, W.; Wang, X. Gas sensing property of ZnO under visible light irradiation at room temperature. Sens. Actuator B 2013, 188, 293–297. [Google Scholar] [CrossRef]
- De Lacy Costello, B.P.; Ewen, R.J.; Ratcliffe, N.M.; Richards, M. Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens. Actuator B 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Prades, J.D.; Jimenez-Diaz, R.; Hernandez-Ramirez, F.; Barth, S.; Cirera, A.; Romano-Rodriguez, A.; Mathur, S.; Morante, J.R. Equivalence between thermal and room temperature UV light-modulated responses of gas sensors based on individual SnO2 nanowires. Sens. Actuator B 2009, 140, 337–341. [Google Scholar] [CrossRef]
- Prades, J.D.; Jimenez-Diaz, R.; Manzanares, M.; Hernandez-Ramirez, F.; Andreu, T.; Cirera, A.; Romano-Rodriguez, A.; Morantea, J.R. Photoexcited individual nanowires: key elements in room temperature detection of oxidizing gases. AIP Conf. Proc. 2009, 1137, 400–403. [Google Scholar]
- Yang, Z.; Guo, L.; Zu, B.; Guo, Y.; Xu, T.; Dou, X. CdS/ZnO core/shell nanowire-built films for enhanced photodetecting and optoelectronic gas-sensing applications. Adv. Opt. Mater. 2014, 2, 738–745. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Nandhini, V.; Jeyaprakash, B.G. Improved sensing response of photo activated ZnO thin film for hydrogen peroxide detection. J. Colloid Interface Sci. 2016, 482, 81–88. [Google Scholar] [CrossRef] [PubMed]
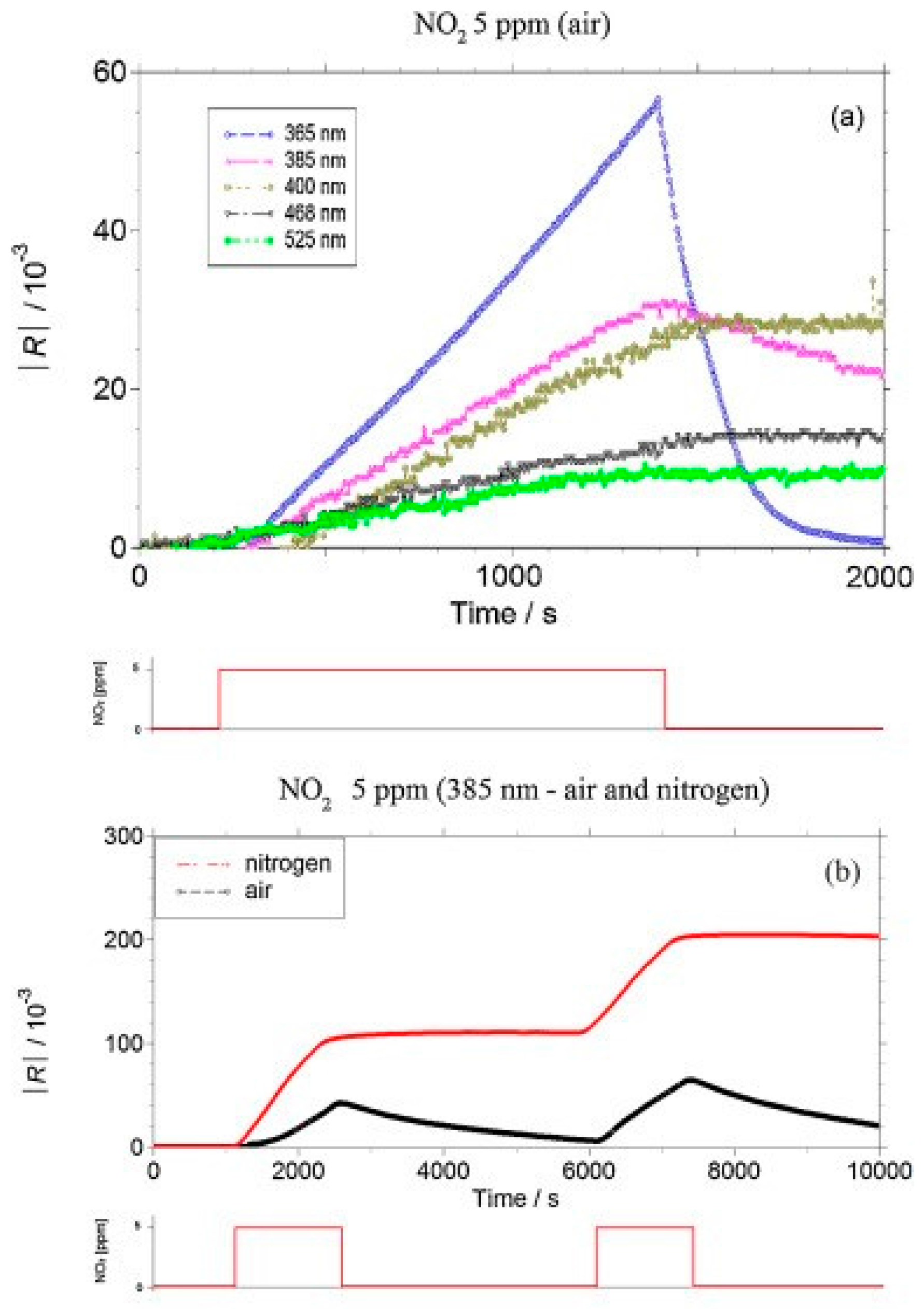
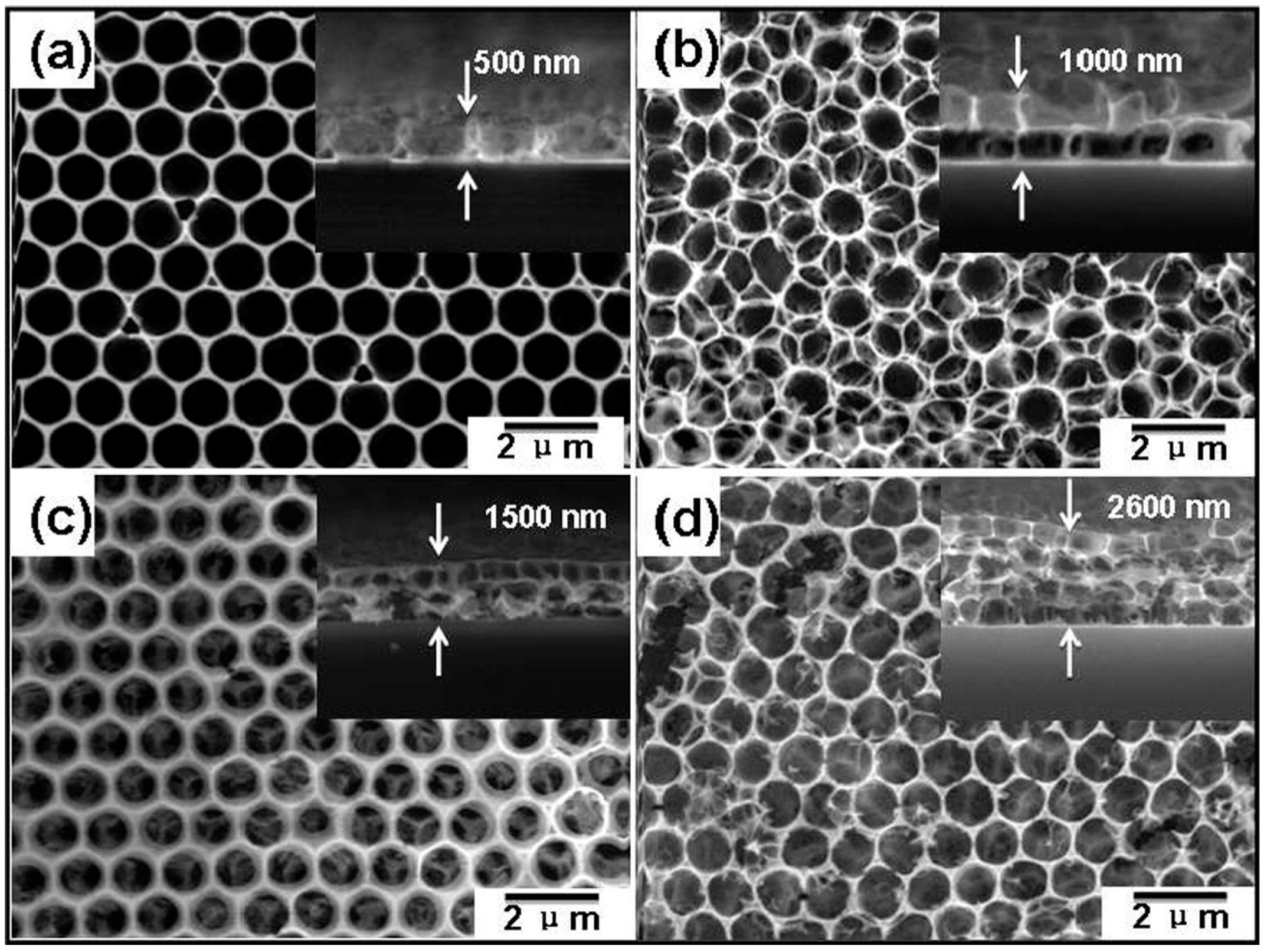
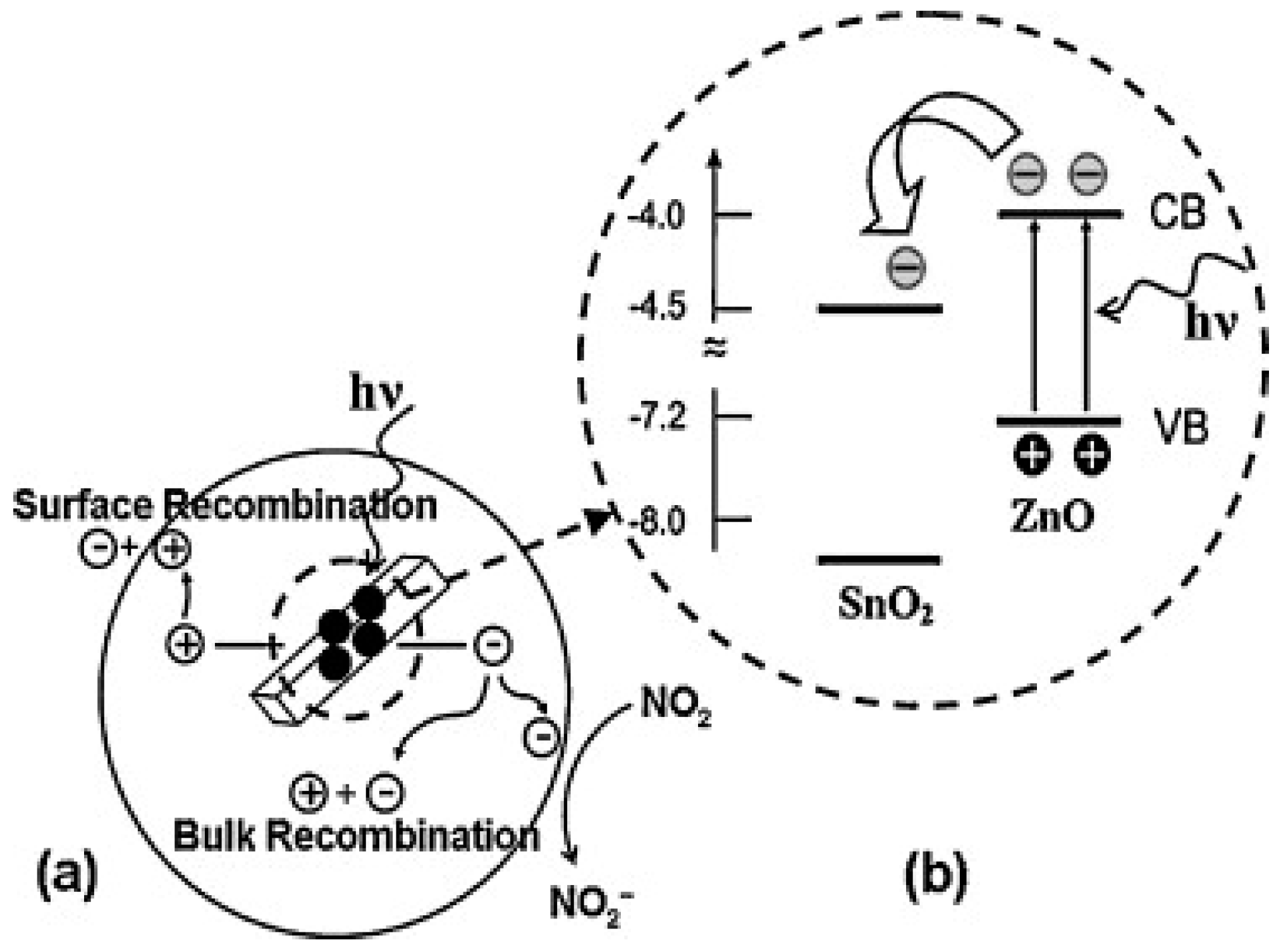
- Su, X.; Duan, G.; Xu, Z.; Zhou, F.; Cai, W. Structure and thickness-dependent gas sensing responses to NO2 under UV irradiation for the multilayered ZnO micro/nanostructured porous thin films. J. Colloid Interface Sci. 2017, 503, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Procek, M.; Pustelny, T.; Stolarczyk, A. Influence of External Gaseous Environments on the Electrical Properties of ZnO Nanostructures Obtained by a Hydrothermal Method. Nanomaterials 2016, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-C.; Hsu, Y.-T.; Lan, W.-H.; Shih, M.-C.; Hong, J.-H.; Huang, K.-F.; Huang, C.-J. UV enhanced oxygen response resistance ratio of ZnO prepared by thermally oxidized zn on sapphire substrate. J. Nanomater. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Faglia, G.; Baratto, C.; Comini, E.; Sberveglieri, G. A selective semiconductor gas sensor based on surface photovoltage. Proc. SPIE 2002, 4936, 186–193. [Google Scholar]
- Ao, D.; Ichimura, M. UV irradiation effects on hydrogen sensors based on SnO2 thin films fabricated by the photochemical deposition. Solid-State Electron. 2012, 69, 1–3. [Google Scholar] [CrossRef]
- Jeng, C.C.; Chong, P.J.H.; Chiu, C.C.; Jiang, G.J.; Lin, H.J.; Wu, R.J.; Wu, C.H. A dynamic equilibrium method for the SnO2-based ozone sensors using UV-LED continuous irradiation. Sens. Actuator B 2014, 195, 702–706. [Google Scholar] [CrossRef]
- Saura, J. Gas-sensing properties of SnO2 pyrolytic films subjected toultraviolet radiation. Sens. Actuator B 1994, 17, 211–214. [Google Scholar] [CrossRef]
- Anothainart, K.; Burgmair, M.; Karthigeyan, A.; Zimmer, M.; Eisele, I. Light enhanced NO2 gas sensing with tin oxide at room temperature: Conductance and work function measurements. Sens. Actuator B 2003, 93, 580–584. [Google Scholar] [CrossRef]
- Zhang, S.; Lei, T.; Li, D.; Zhang, G.; Xie, C. UV light activation of TiO2 for sensing formaldehyde: How to be sensitive, recovering fast, and humidity less sensitive. Sens. Actuator B 2014, 202, 964–970. [Google Scholar] [CrossRef]
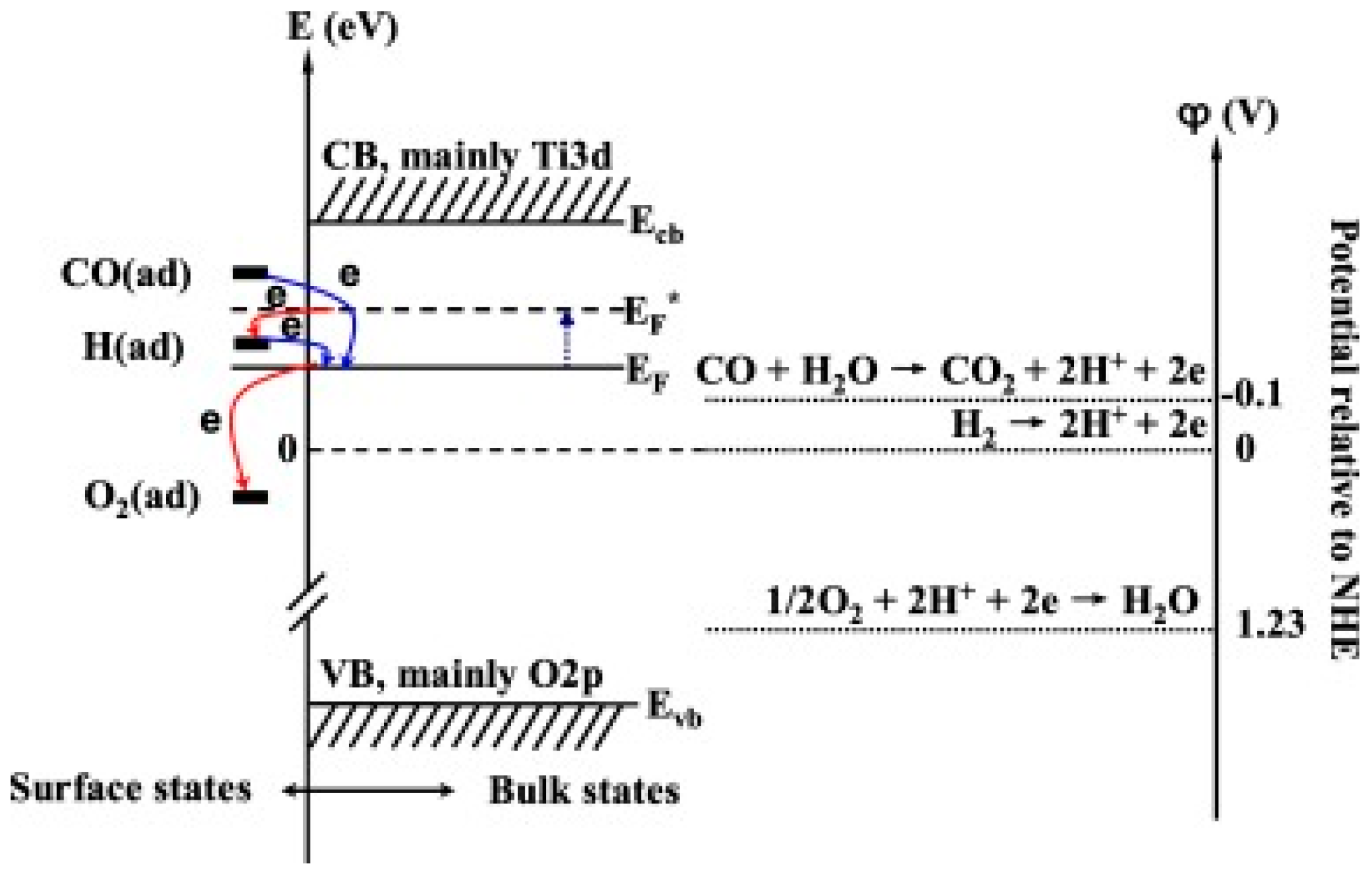
- Peng, X.; He, Z.; Yang, K.; Chen, X.; Wang, X.; Dai, W.; Fu, X. Correlation between donating or accepting electron behavior of the adsorbed CO or H2 and its oxidation over TiO2 under ultraviolet light irradiation. Appl. Surf. Sci. 2016, 360, 698–706. [Google Scholar] [CrossRef]
- Wang, C.Y.; Kinzer, M.; Youn, S.K.; Ramgir, N.; Kunzer, M.; Köhler, K.; Zacharias, M.; Cimalla, V. Oxidation behaviour of carbon monoxide at the photostimulated surface of ZnO nanowires. J. Phys. D 2011, 44, 305302. [Google Scholar] [CrossRef]
- Trawka, M.P.; Smulko, J.M.; Hasse, L.Z.; Granqvist, C.G.; Ionescu, R.; Llobet, E.; Annanouch, F.E.; Kish, L.B. UV-light-induced fluctuation enhanced sensing by WO3-based gas sensors. IEEE Sens. J. 2016, 16, 5152–5159. [Google Scholar] [CrossRef]
- Trawka, M.; Smulko, J.; Hasse, L.; Granqvist, C.G.; Annanouch, F.E.; Ionescu, R. Fluctuation enhanced gas sensing with WO3-based nanoparticle gas sensors modulated by UV light at selected wavelengths. Sens. Actuator B 2016, 234, 453–461. [Google Scholar] [CrossRef]
- Zhang, C.; Boudiba, A.; de Marco, P.; Snyders, R.; Olivier, M.G.; Debliquy, M. Room temperature responses of visible-light illuminated WO3 sensors to NO2 in sub-ppm range. Sens. Actuator B 2013, 181, 395–401. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cimalla, V.; Kups, T.; Röhlig, C.C.; Stauden, T.; Ambacher, O.; Kunzer, M.; Passow, T.; Schirmacher, W.; Pletschen, W.; et al. Integration of In2O3 nanoparticle based ozone sensors with GaInN/GaN light emitting diodes. Appl. Phys. Lett. 2007, 91, 103509. [Google Scholar] [CrossRef]
- Wang, C.Y.; Becker, R.W.; Passow, T.; Pletschen, W.; Köhler, K.; Cimalla, V.; Ambacher, O. Photon stimulated sensor based on indium oxide nanoparticles I: Wide-concentration-range ozone monitoring in air. Sens. Actuator B 2011, 152, 235–240. [Google Scholar] [CrossRef]
- Kiasari, N.M.; Soltanian, S.; Gholamkhass, B.; Servati, P. Environmental gas and light sensing using ZnO nanowires. IEEE Trans. Nanotechnol. 2014, 13, 368–374. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Z.X.; Wang, W.H.; Kuo, C.L.; Hwang, K.C.; Hong, C.C. Self-regenerating photocatalytic sensor based on dielectrophoretically assembled TiO2 nanowires for chemical vapor sensing. Sens. Actuator B 2014, 194, 1–9. [Google Scholar] [CrossRef]
- Hansen, B.J.; Kouklin, N.; Lu, G.; Lin, I.K.; Chen, J.; Zhang, X. Transport, analyte detection, and opto-electronic response of p-type CuO nanowires. J. Phys. Chem. C 2010, 114, 2440–2447. [Google Scholar] [CrossRef]
- Zampetti, E.; Macagnano, A.; Bearzotti, A. Gas sensor based on photoconductive electrospun titania nanofibres operating at room temperature. J. Nanopart. Res. 2013, 15, 1566. [Google Scholar] [CrossRef]
- Su, X.; Gao, L.; Zhou, F.; Cai, W.; Duan, G. “Close network” effect of a ZnO micro/nanoporous array allows high UV-irradiated NO2 sensing performance. RSC Adv. 2017, 7, 21054–21060. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Xie, C.; Wu, J.; Zeng, D.; Liao, Y. A comparative study on UV light activated porous TiO2 and ZnO film sensors for gas sensing at room temperature. Ceram. Int. 2012, 38, 503–509. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Dutta, P.K.; Wang, J. Room temperature impedance spectroscopy-based sensing of formaldehyde with porous TiO2 under UV illumination. Sens. Actuator B 2013, 185, 1–9. [Google Scholar] [CrossRef]
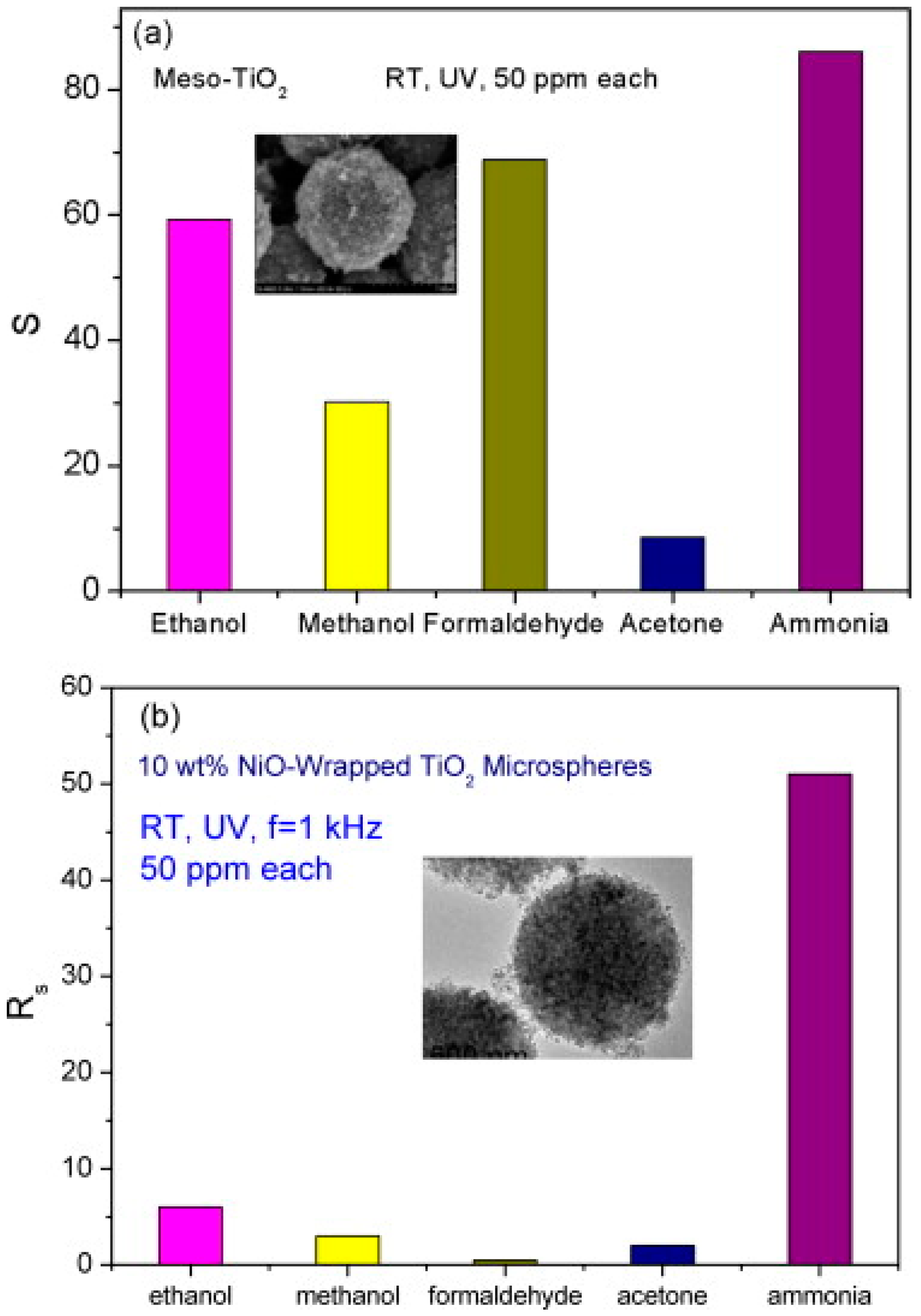
- Li, X.; Chen, N.; Lin, S.; Wang, J.; Zhang, J. NiO-wrapped mesoporous TiO2 microspheres based selective ammonia sensor at room temperature. Sens. Actuator B 2015, 209, 729–734. [Google Scholar] [CrossRef]
- Wagner, T.; Kohl, C.D.; Morandi, S.; Malagu, C.; Donato, N.; Latino, M.; Neri, G.; Tiemann, M. Photoreduction of mesoporous In2O3: Mechanistic model and utility in gas sensing. Chem. Eur. J. 2012, 18, 8216–8223. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Kohl, C.D.; Malagù, C.; Donato, N.; Latino, M.; Neri, G.; Tiemann, M. UV light-enhanced NO2 sensing by mesoporous In2O3: Interpretation of results by a new sensing model. Sens. Actuator B 2013, 187, 488–494. [Google Scholar] [CrossRef]
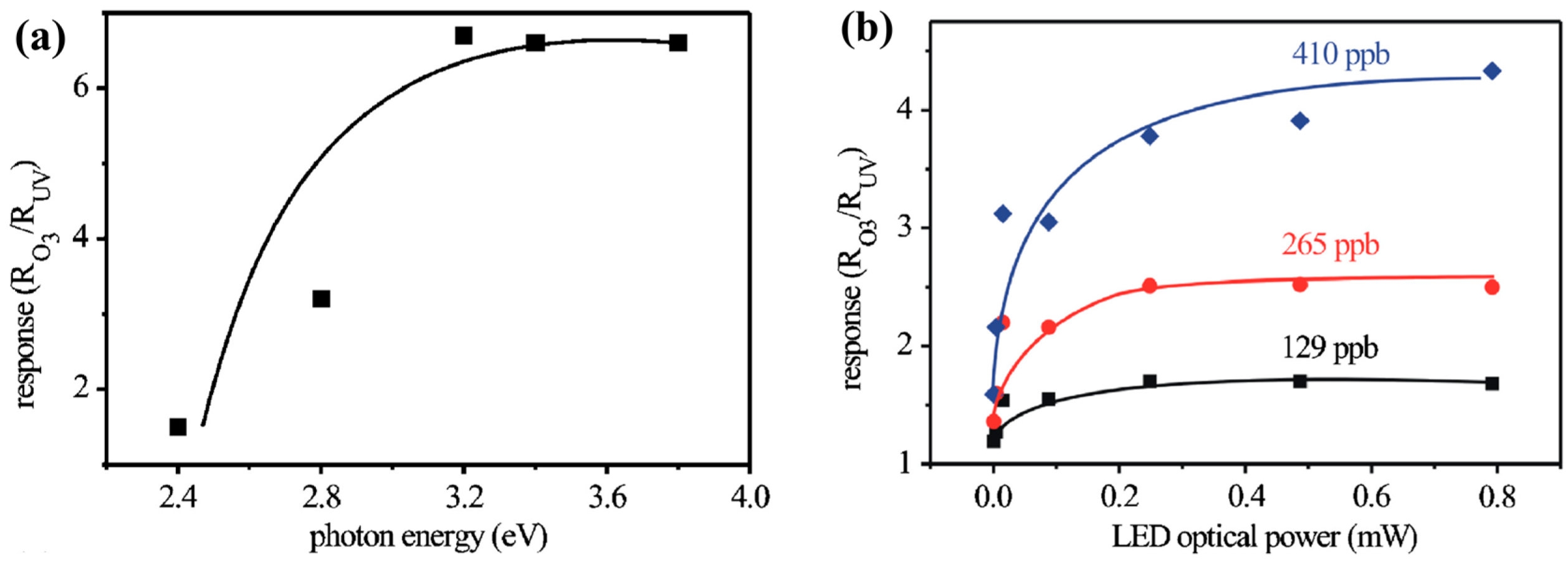
- Klaus, D.; Klawinski, D.; Amrehn, S.; Tiemann, M.; Wagner, T. Light-activated resistive ozone sensing at room temperature utilizing nanoporous In2O3 particles: Influence of particle size. Sens. Actuator B 2015, 217, 181–185. [Google Scholar] [CrossRef]
- Deng, L.; Ding, X.; Zeng, D.; Tian, S.; Li, H.; Xie, C. Visible-light activate mesoporous WO3 sensors with enhanced formaldehyde-sensing property at room temperature. Sens. Actuator B 2012, 163, 260–266. [Google Scholar] [CrossRef]
- Wu, B.; Lin, Z.; Sheng, M.; Hou, S.; Xu, J. Visible-light activated ZnO/CdSe heterostructure-based gas sensors with low operating temperature. Appl. Surf. Sci. 2016, 360, 652–657. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.; Cui, J.; Chen, L.; Jiang, T.; Lin, Y. Study on formaldehyde gas-sensing of In2O3-sensitized ZnO nanoflowers under visible light irradiation at room temperature. J. Mater. Chem. 2012, 22, 12915–12920. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, C.; Debliquy, M. Cadmium sulfide activated zinc oxide coatings deposited by liquid plasma spray for room temperature nitrogen dioxide detection under visible light illumination. Ceram. Int. 2016, 42, 4845–4852. [Google Scholar] [CrossRef]
- Lu, G.; Xu, J.; Sun, J.; Yu, Y.; Zhang, Y.; Liu, F. UV-enhanced room temperature NO2 sensor using ZnO nanorods modified with SnO2 nanoparticles. Sens. Actuator B 2012, 162, 82–88. [Google Scholar] [CrossRef]
- Hoffmann, M.W.; Gad, A.E.; Prades, J.D.; Hernandez-Ramirez, F.; Fiz, R.; Shen, H.; Mathur, S. Solar diode sensor: Sensing mechanism and applications. Nano Energy 2013, 2, 514–522. [Google Scholar] [CrossRef]
- Yang, M.; Wang, D.; Peng, L.; Zhao, Q.; Lin, Y.; Wei, X. Surface photocurrent gas sensor with properties dependent on Ru(dcbpy)2(NCS)2-sensitized ZnO nanoparticles. Sens. Actuator B 2006, 117, 80–85. [Google Scholar] [CrossRef]
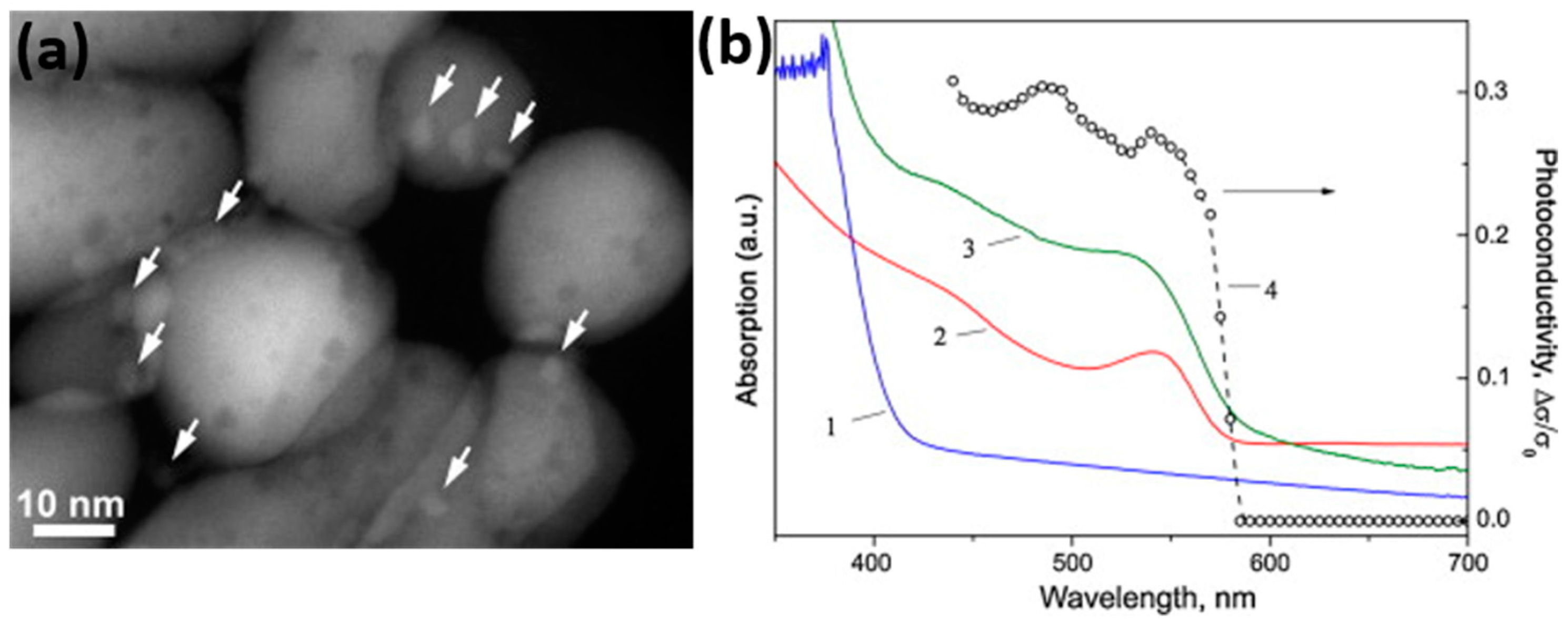
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Abakumov, A.M.; Gaskov, A.M. Visible light activated room temperature gas sensors based on nanocrystalline ZnO sensitized with CdSe quantum dots. Sens. Actuator B 2014, 205, 305–312. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Marchevsky, A.V.; Karakulina, O.M.; Abakumov, A.M.; Gaskov, A.M. Visible light activation of room temperature NO2 gas sensors based on ZnO, SnO2 and In2O3 sensitized with CdSe quantum dots. Thin Solid Films 2016, 618, 253–262. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Ko, H.; Lee, C. Light Assisted Room Temperature Ethanol Gas Sensing of ZnO–ZnS Nanowires. J. Nanosci. Nanotechnol. 2014, 14, 9025–9028. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ko, H.; Lee, S.; Kim, H.; Lee, C. Light-activated gas sensing of Bi2O3-core/ZnO-shell nanobelt gas sensors. Thin Solid Films 2014, 570, 298–302. [Google Scholar] [CrossRef]
- Karaduman, I.; Yıldız, D.E.; Sincar, M.M.; Acar, S. UV light activated gas sensor for NO2 detection. Mater. Sci. Semicond. Process. 2014, 28, 43–47. [Google Scholar] [CrossRef]
- Han, C.H.; Hong, D.W.; Han, S.D.; Gwak, J.; Singh, K.C. Catalytic combustion type hydrogen gas sensor using TiO2 and UV-LED. Sens. Actuator B 2007, 125, 224–228. [Google Scholar] [CrossRef]
- Deng, Q.; Gao, S.; Lei, T.; Ling, Y.; Zhang, S.; Xie, C. Temperature & light modulation to enhance the selectivity of Pt-modified zinc oxide gas sensor. Sens. Actuator B 2017, 247, 903–915. [Google Scholar]
- Comini, E.; Ottini, L.; Faglia, G.; Sberveglieri, G. SnO/sub 2/RGTO UV activation for CO monitoring. IEEE Sens. J. 2004, 4, 17–20. [Google Scholar] [CrossRef]
- Chen, M.H.; Lu, C.S.; Wu, R.J. Novel Pt/TiO2–WO3 materials irradiated by visible light used in a photoreductive ozone sensor. J. Taiwan Inst. Chem. Eng. 2014, 45, 1043–1048. [Google Scholar] [CrossRef]
- Cui, J.; Wang, D.; Xie, T.; Lin, Y. Study on photoelectric gas-sensing property and photogenerated carrier behavior of Ag–ZnO at the room temperature. Sens. Actuator B 2013, 186, 165–171. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; el Mir, L.; Bonavita, A.; Iannazzo, D.; Latino, M.; Donato, N.; Leonardi, S.G.; Neri, G. Gas sensing properties of Al-doped ZnO for UV-activated CO detection. J. Phys. D 2016, 49, 135502. [Google Scholar] [CrossRef]
- Wongrat, E.; Chanlek, N.; Chueaiarrom, C.; Samransuksamer, B.; Hongsith, N.; Choopun, S. Low temperature ethanol response enhancement of ZnO nanostructures sensor decorated with gold nanoparticles exposed to UV illumination. Sens. Actuator A 2016, 251, 188–197. [Google Scholar] [CrossRef]
- Fraters, B.D.; Amrollahi, R.; Mul, G. How Pt nanoparticles affect TiO2-induced gas-phase photocatalytic oxidation reactions. J. Catal. 2015, 324, 119–126. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, O.S.; Shin, D.H.; Jang, J. WO3 nanonodule-decorated hybrid carbon nanofibers for NO2 gas sensor application. J. Mater. Chem. A 2013, 1, 9099–9106. [Google Scholar] [CrossRef]
- Yang, J.; Li, R.; Huo, N.; Ma, W.L.; Lu, F.; Fan, C.; Yang, S.; Wei, Z.; Li, J.; Li, S.S. Oxygen-induced abnormal photoelectric behavior of a MoO3/graphene heterocomposite. RSC Adv. 2014, 4, 49873–49878. [Google Scholar] [CrossRef]
- Zhao, M.; Yan, L.; Zhang, X.; Xu, L.; Song, Z.; Chen, P.; Dong, F.; Chu, W. Room temperature NH3 detection of Ti/graphene devices promoted by visible light illumination. J. Mater. Chem. C 2017, 5, 1113–1120. [Google Scholar] [CrossRef]
- Ueda, T.; Takahashi, K.; Mitsugi, F.; Ikegami, T. Preparation of single-walled carbon nanotube/TiO2 hybrid atmospheric gas sensor operated at ambient temperature. Diam. Relat. Mater. 2009, 18, 493–496. [Google Scholar] [CrossRef]
- Gad, A.; Hoffmann, M.W.; Casals, O.; Mayrhofer, L.; Fàbrega, C.; Caccamo, L.; Hernández-Ramírez, F.; Mohajerani, M.S.; Moseler, M.; Shen, H.; et al. Integrated strategy toward self-powering and selectivity tuning of semiconductor gas sensors. ACS Sens. 2016, 1, 1256–1264. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Olivier, M.G.; Debliquy, M. Room temperature nitrogen dioxide sensors based on N719-dye sensitized amorphous zinc oxide sensors performed under visible-light illumination. Sens. Actuator B 2015, 209, 69–77. [Google Scholar] [CrossRef]
- Hara, T.; Ishiguro, T.; Wakiya, N.; Shinozaki, K. Oxygen Sensing Properties of SrTiO3 Thin Films. Jpn. J. Appl. Phys. 2008, 47, 7486–7489. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; Mir, L.E.; Bonavita, A.; Iannazzo, D.; Leonardi, S.G.; Neri, G. CO sensing properties under UV radiation of Ga-doped ZnO nanopowders. Appl. Surf. Sci. 2015, 355, 1321–1326. [Google Scholar] [CrossRef]
- Sturaro, M.; della Gaspera, E.; Michieli, N.; Cantalini, C.; Emamjomeh, S.M.; Guglielmi, M.; Martucci, A. Degenerately doped metal oxide nanocrystals as plasmonic and chemoresistive gas sensors. ACS Appl. Mater. Interfaces 2016, 8, 30440–30448. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, D.; Lu, Y.; Jiang, T.; Liu, B.; Lin, Y. Visible-light-assisted HCHO gas sensing based on Fe-doped flowerlike ZnO at room temperature. J. Phys. Chem. C 2011, 115, 22939–22944. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.; Lu, Y.; Jiang, T.; Chen, L.; Xie, T.; Lin, Y. Influence of annealing temperature on the photoelectric gas sensing of Fe-doped ZnO under visible light irradiation. Sens. Actuator B 2013, 177, 34–40. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, L.; Wang, D.; Lin, Y.; He, D.; Xie, T. UV-illumination room-temperature gas sensing activity of carbon-doped ZnO microspheres. Sens. Actuator B 2012, 161, 292–297. [Google Scholar] [CrossRef]
- Hien, V.X.; Heo, Y.W. Effects of violet-, green-, and red-laser illumination on gas-sensing properties of SnO thin film. Sens. Actuator B 2016, 228, 185–191. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Yao, J.D.; Wang, B.; Yang, G.W. Light-controlling, flexible and transparent ethanol gas sensor based on ZnO nanoparticles for wearable devices. Sci. Rep. 2015, 5, 11070. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chang, S.J.; Chen, W.S.; Hsueh, T.J. Transparent ZnO-nanowire-based device for UV light detection and ethanol gas sensing on c-Si solar cell. RSC Adv. 2016, 6, 11146–11150. [Google Scholar] [CrossRef]
- Bajpai, R.; Motayed, A.; Davydov, A.V.; Oleshko, V.P.; Aluri, G.S.; Bertness, K.A.; Rao, M.V.; Zaghloul, M.E. UV-assisted alcohol sensing using SnO2 functionalized GaN nanowire devices. Sens. Actuator B 2012, 171–172, 499–507. [Google Scholar] [CrossRef]
- Alenezi, M.R.; Alshammari, A.S.; Jayawardena, K.D.; Beliatis, M.J.; Henley, S.J.; Silva, S.R. Role of the exposed polar facets in the performance of thermally and UV activated ZnO nanostructured gas sensors. J. Phys. Chem. C 2013, 117, 17850–17858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; de Arquer, F.P.; Li, Y.; Lan, X.; Kim, G.H.; Voznyy, O.; Jagadamma, L.K.; Abbas, A.S.; Hoogland, S.; Lu, Z.; et al. Double-sided junctions enable high-performance colloidal-quantum-dot photovoltaics. Adv. Mater. 2016, 28, 4142–4148. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Jin, Y.; Liang, X.; Ye, Z.; Wu, Z.; Sun, B.; Ma, Z.; Tang, Z.; Wang, J.; Würfel, U.; Gao, F.; Zhang, F. Ethanedithiol treatment of solution-processed ZnO thin films: Controlling the intragap states of electron transporting interlayers for efficient and stable inverted organic photovoltaics. Adv. Energy Mater. 2015, 5, 1401606. [Google Scholar] [CrossRef]
- Chen, X.; Lin, P.; Yan, X.; Bai, Z.; Yuan, H.; Shen, Y.; Liu, Y.; Zhang, G.; Zhang, Z.; Zhang, Y. Three-dimensional ordered ZnO/Cu2O nanoheterojunctions for efficient metal-oxide solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.Q.; Trung, T.Q.; Kim, D.I.; Duy, L.T.; Hwang, B.U.; Lee, D.W.; Kim, B.Y.; Toan, L.D.; Lee, N.E. Ultrahigh responsivity in graphene-ZnO nanorod hybrid uv photodetector. Small 2015, 11, 3054–3065. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Bo, R.; Wang, F.; Fu, L.; Tricoli, A. Ultraporous electron-depleted ZnO nanoparticle networks for highly sensitive portable visible-blind UV photodetectors. Adv Mater. 2015, 27, 4336–4343. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, C.; Dai, J.; Li, J.; Wang, Y.; Lin, Y.; Li, P. Improved UV photoresponse of ZnO nanorod arrays by resonant coupling with surface plasmons of Al nanoparticles. Nanoscale 2015, 7, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhou, Q.; Chen, Y.; Mao, P.; Li, H.; Liu, H.; Wang, J.; Li, Y. Graphdiyne: ZnO nanocomposites for high-performance UV photodetectors. Adv. Mater. 2016, 28, 3697–3702. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.U.; Kim, C.W.; Kang, M.J.; Kang, Y.S. Crystal facet engineering of ZnO photoanode for the higher water splitting efficiency with proton transferable nafion film. Nano Energy 2016, 20, 156–167. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Modi, G.; Cretu, V.; Postica, V.; Lupan, O.; Reimer, T.; Paulowicz, I.; Hrkac, V.; Benecke, W.; Kienle, L.; Adelung, R. Direct growth of freestanding ZnO tetrapod networks for multifunctional applications in photocatalysis, UV photodetection, and gas sensing. ACS Appl. Mater. Interfaces 2015, 7, 14303–14316. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Liu, S. Controlled ZnO hierarchical structure for improved gas sensing performance. Sens. Actuator B 2015, 209, 343–351. [Google Scholar] [CrossRef]
- Kim, H.W.; Na, H.G.; Kwon, Y.J.; Cho, H.Y.; Lee, C. Decoration of Co nanoparticles on ZnO-branched SnO2 nanowires to enhance gas sensing. Sens. Actuator B 2015, 219, 22–29. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Zhang, T.; Chen, G.; Zhang, F.; Liu, D.; Cai, W.; Li, Y.; Yang, X.; Li, C. Complete Au@ZnO core-shell nanoparticles with enhanced plasmonic absorption enabling significantly improved photocatalysis. Nanoscale 2016, 8, 10774–10782. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Tsai, C.Y.; Chen, H.A.; Liang, Y.T.; Chen, K.P.; Gradečak, S.; Gwo, S.; Chen, L.J. Plasmonic enhancement of Au nanoparticle—embedded single-crystalline ZnO nanowire dye-sensitized solar cells. Nano Energy 2016, 20, 264–271. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, M.; Ning, F.; Xu, S.; Li, Z.; Wei, M.; Evans, D.G.; Duan, X. Au nanoparticles sensitized ZnO nanorod@ nanoplatelet core–shell arrays for enhanced photoelectrochemical water splitting. Nano Energy 2015, 12, 231–239. [Google Scholar] [CrossRef]
- Sun, M.; Xu, Z.; Yin, M.; Lin, Q.; Lu, L.; Xue, X.; Zhu, X.; Cui, Y.; Fan, Z.; Ding, Y.; Tian, L.; Wang, H.; Chen, X.; Li, D. Broad-band three dimensional nanocave ZnO thin film photodetectors enhanced by Au surface plasmon resonance. Nanoscale 2016, 8, 8924–8930. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.L.; Liu, R.S.; Tsai, D.P. Plamonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef] [PubMed]
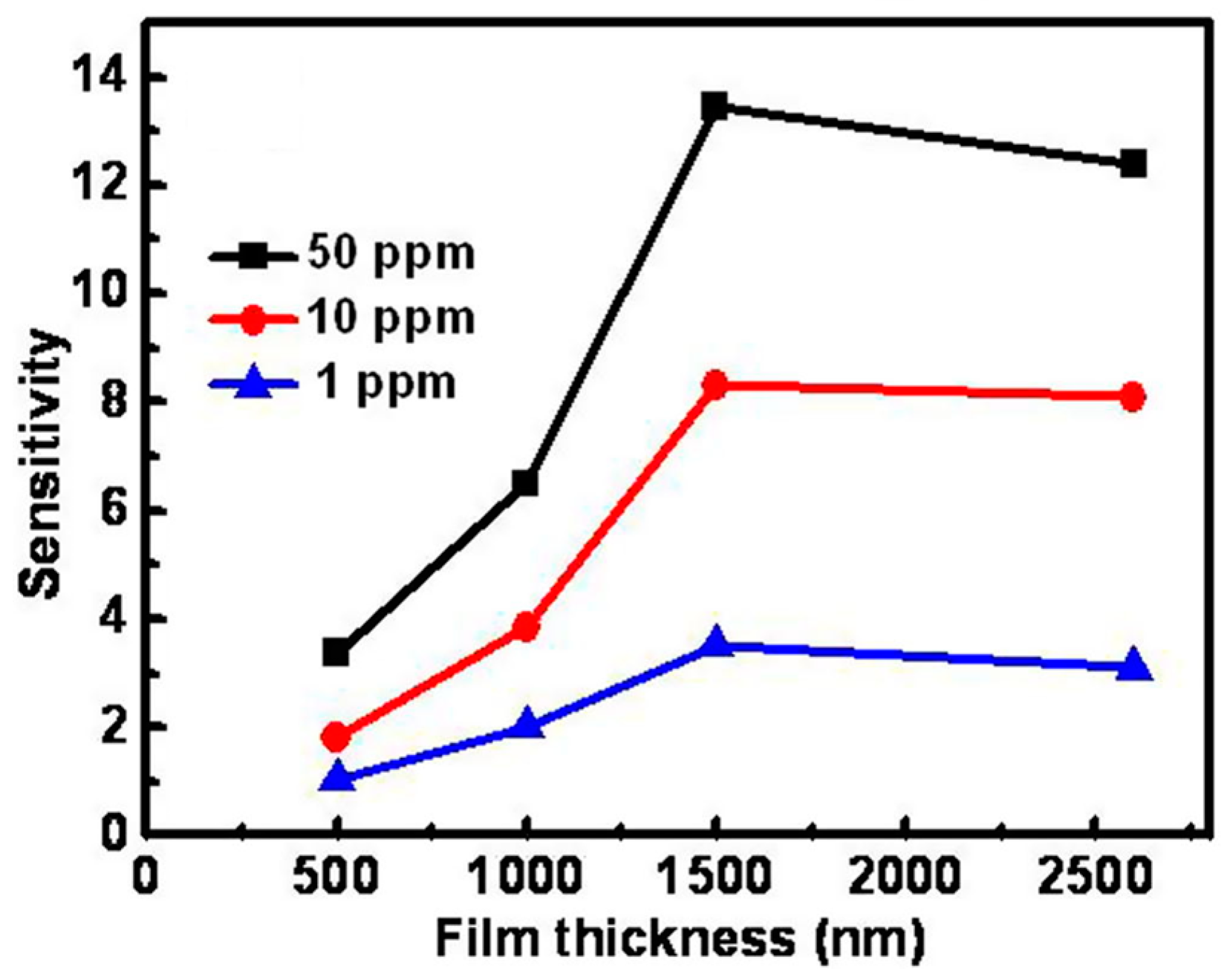
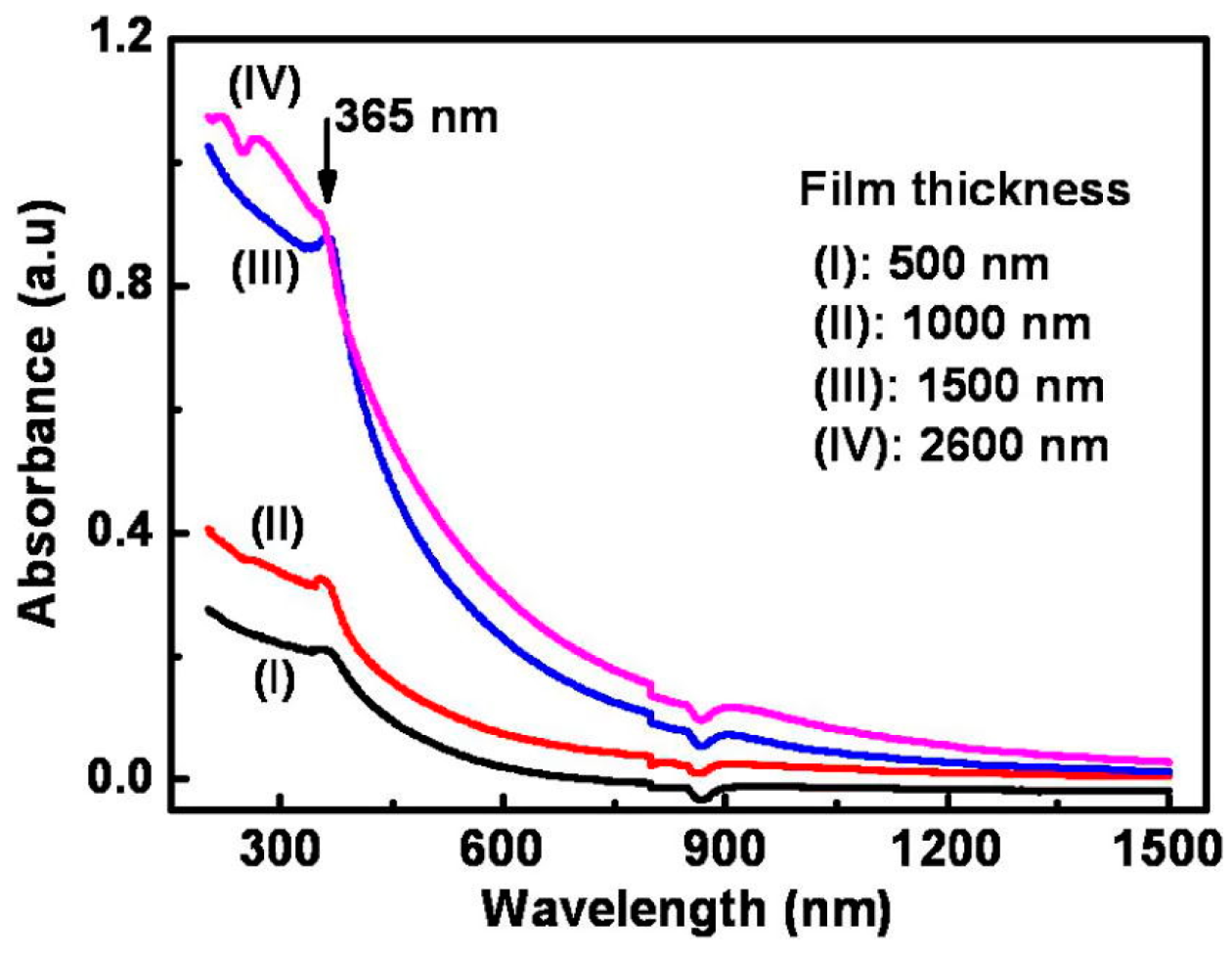
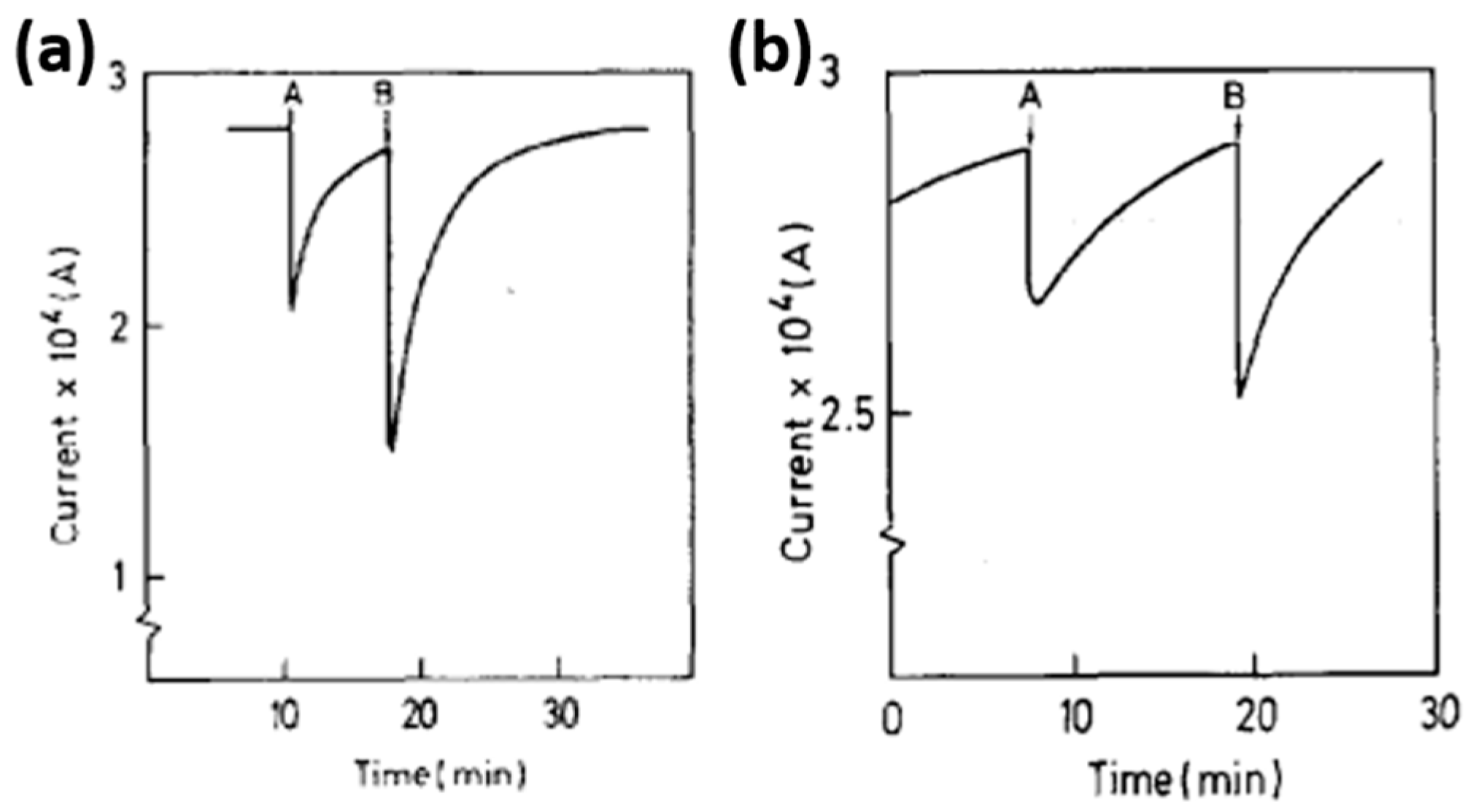
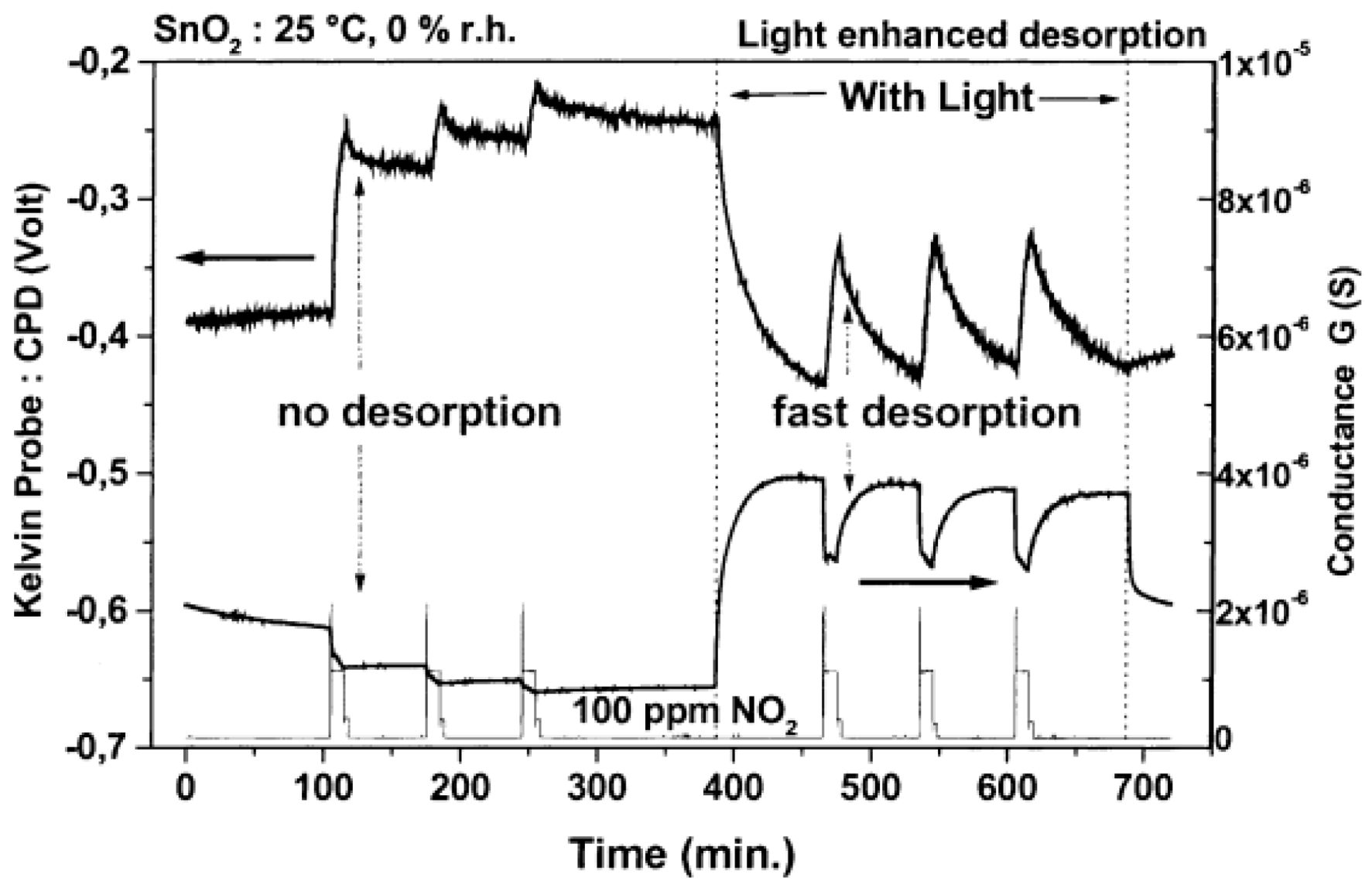
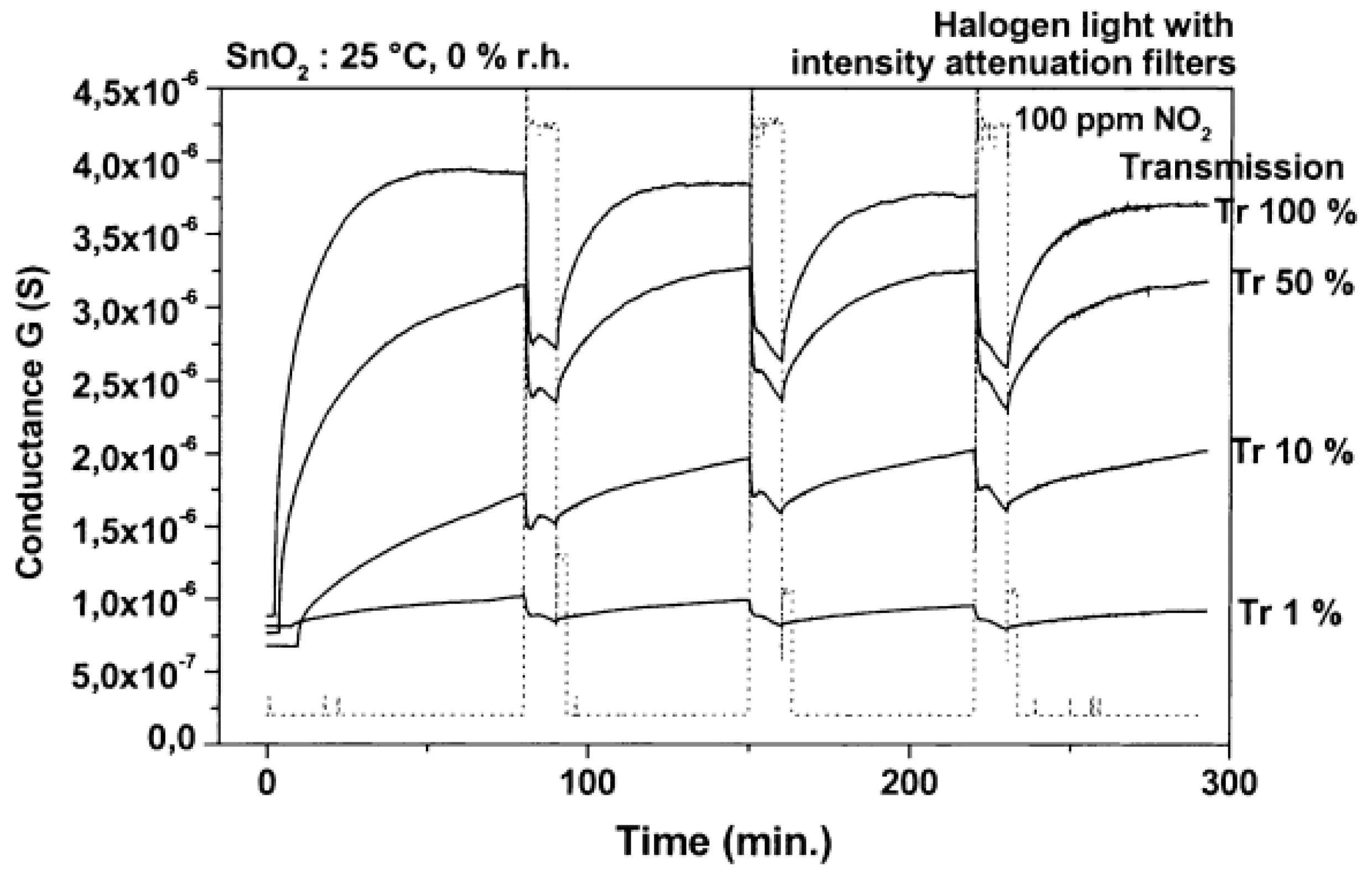
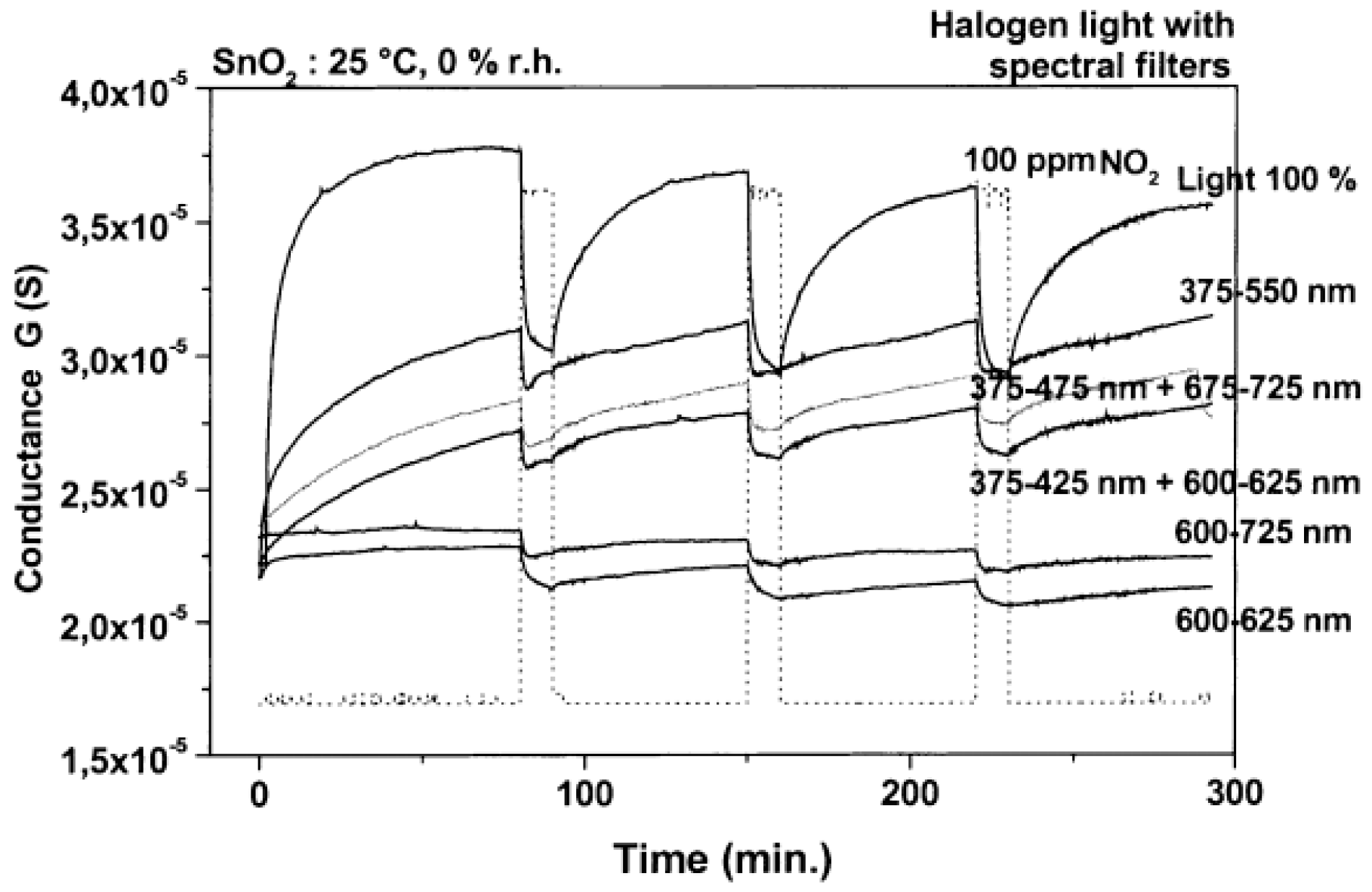
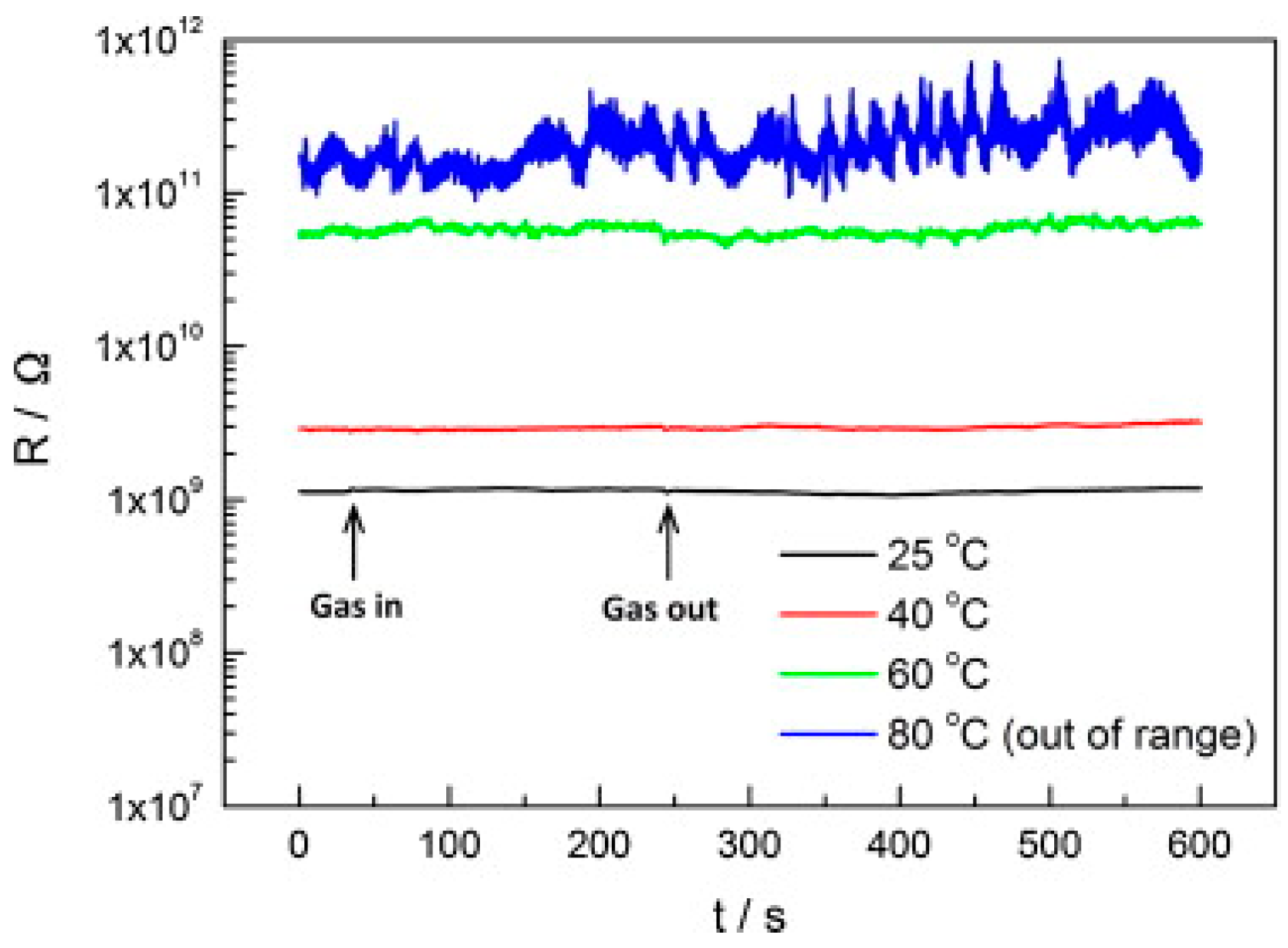
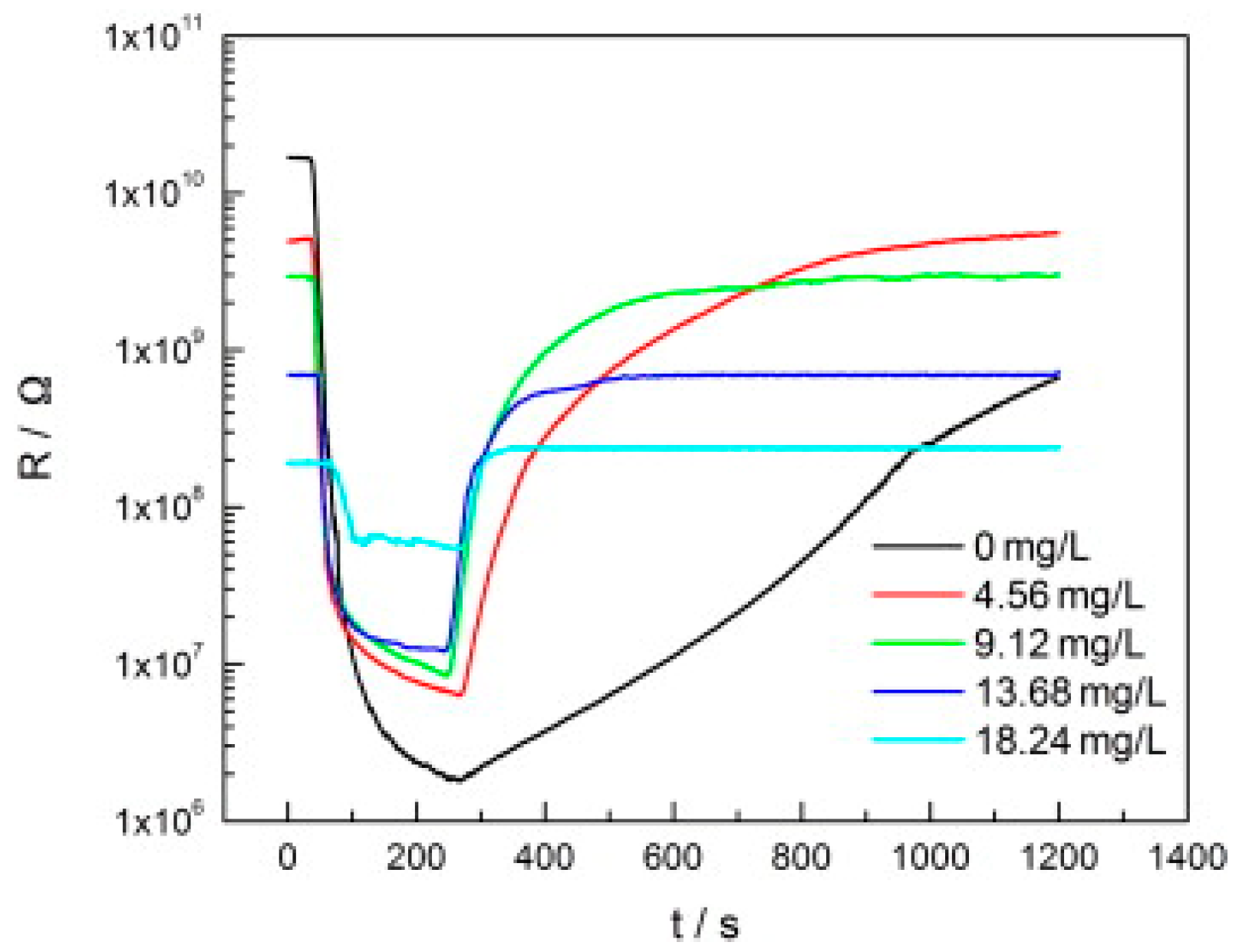

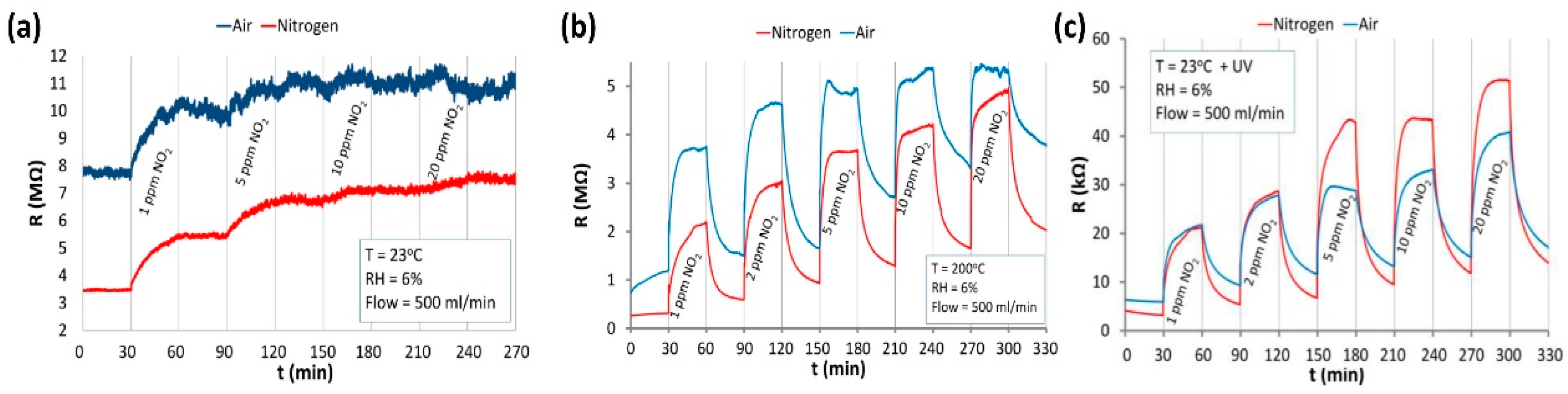
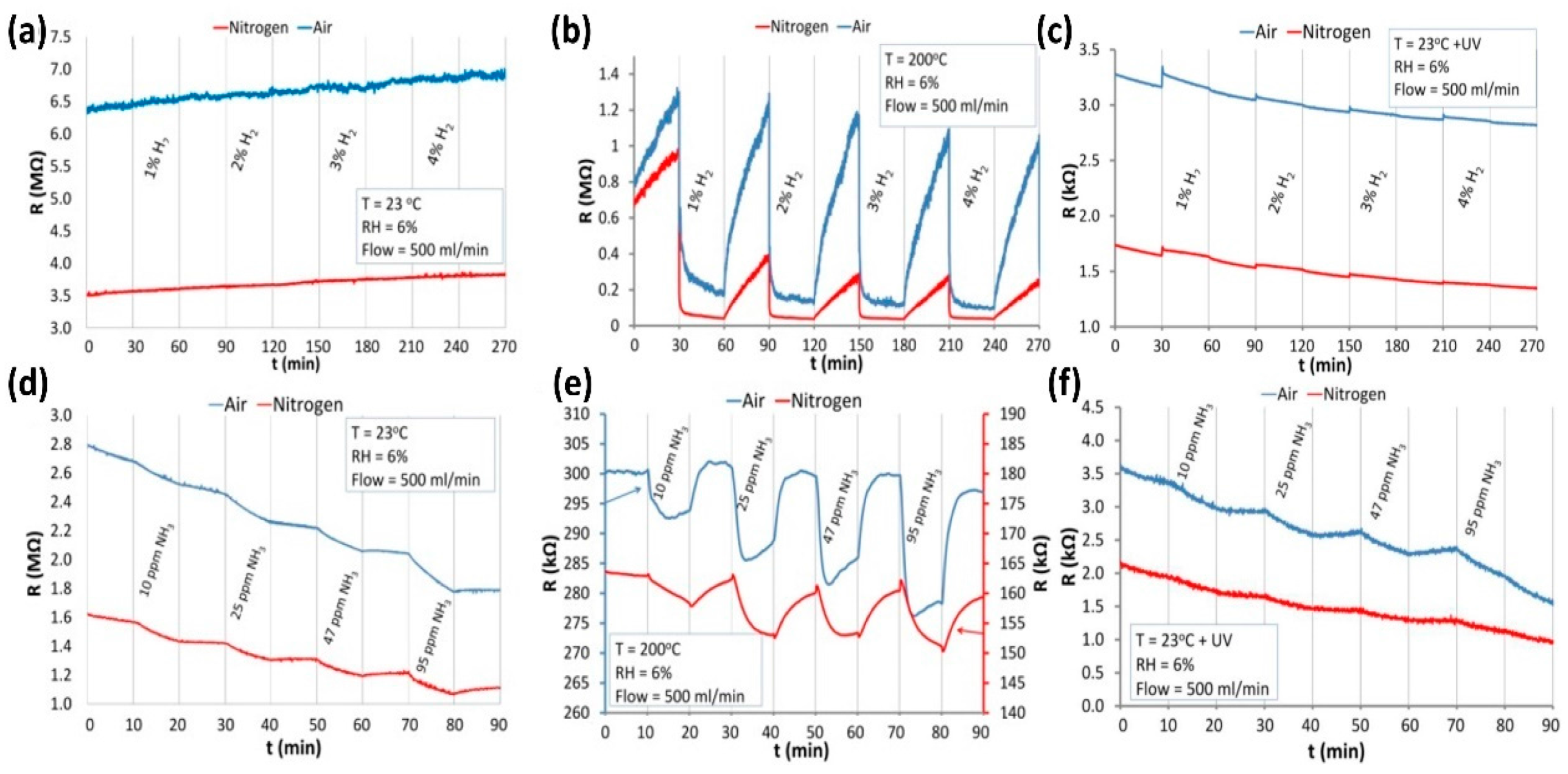

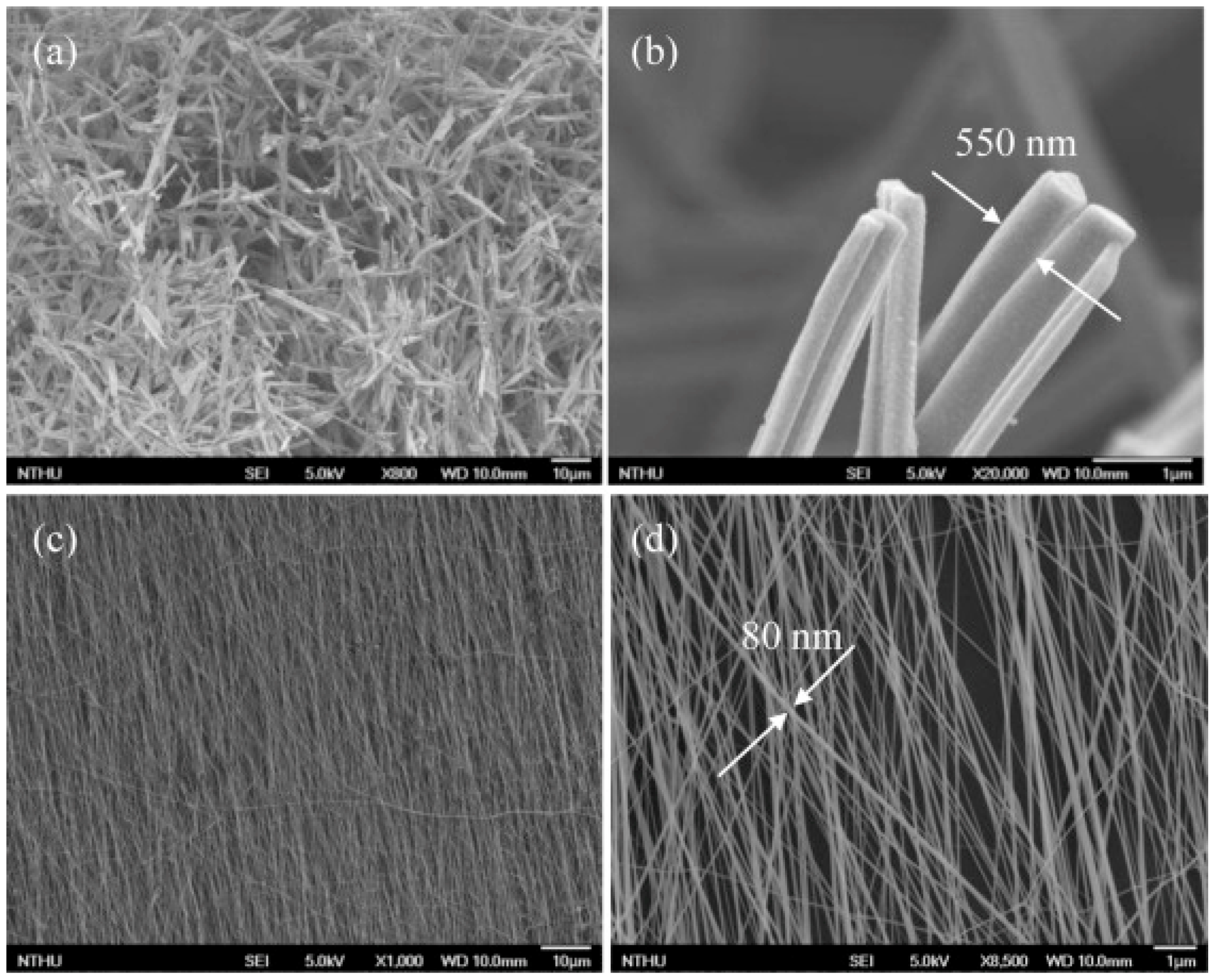
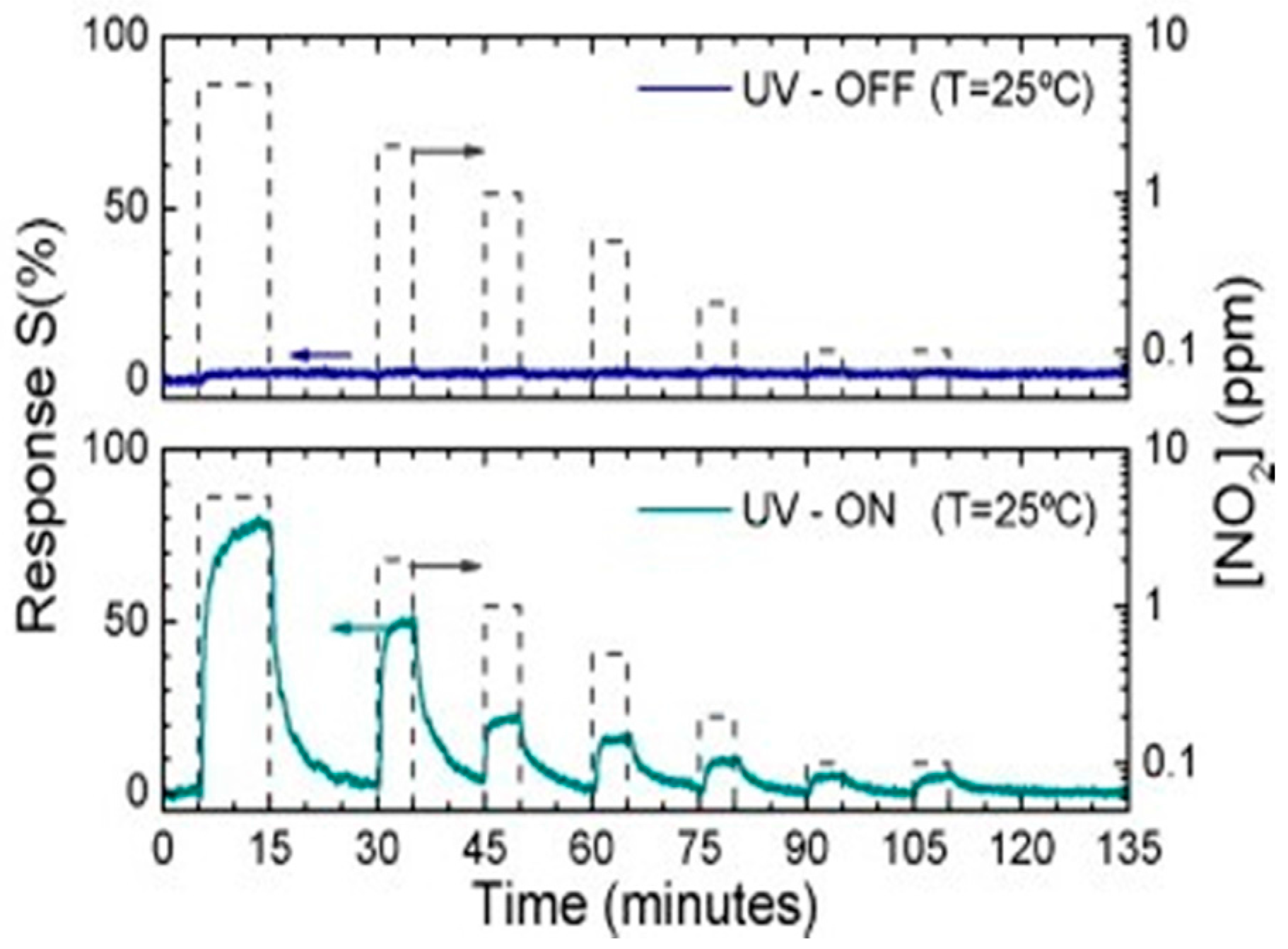
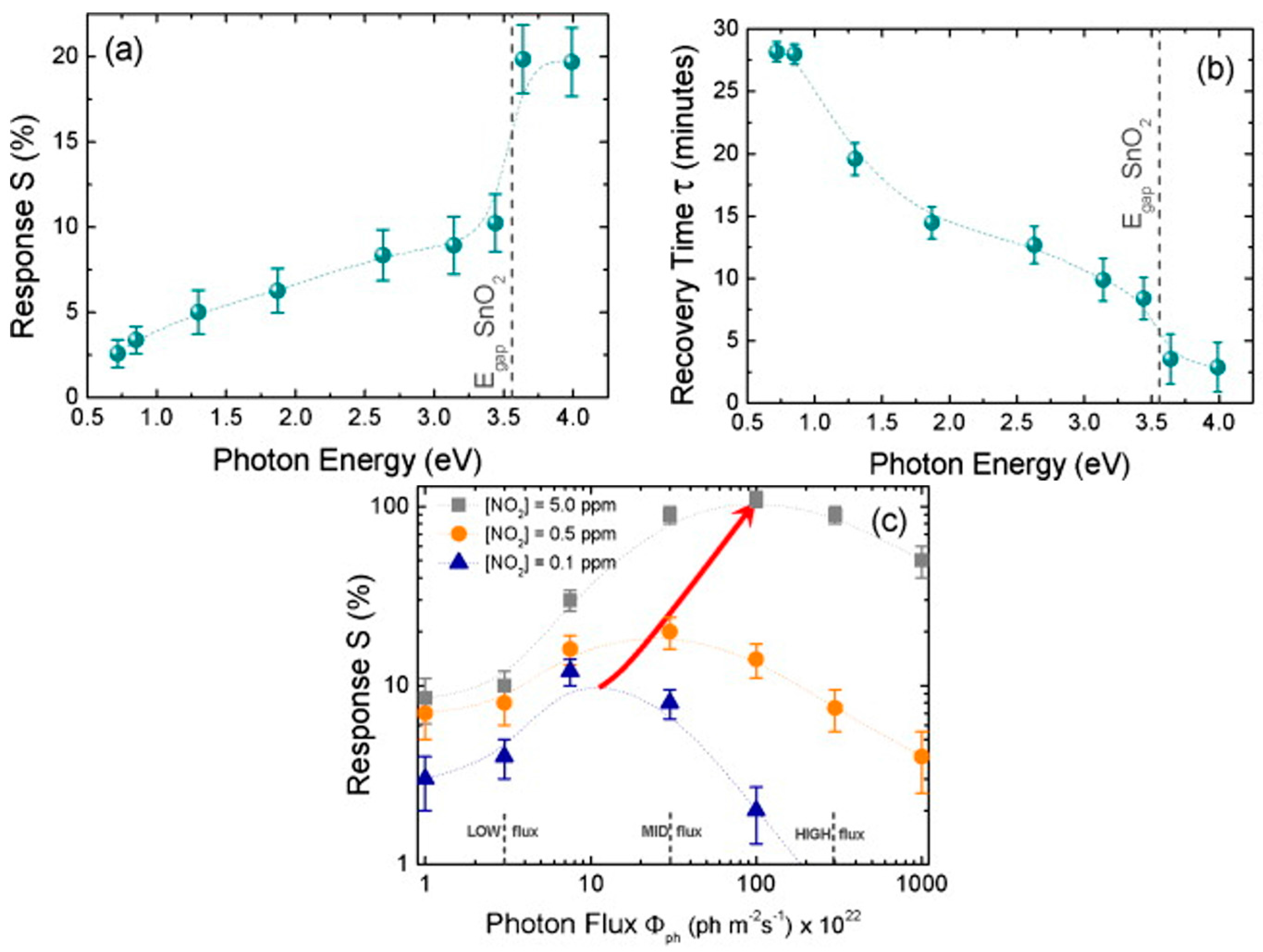
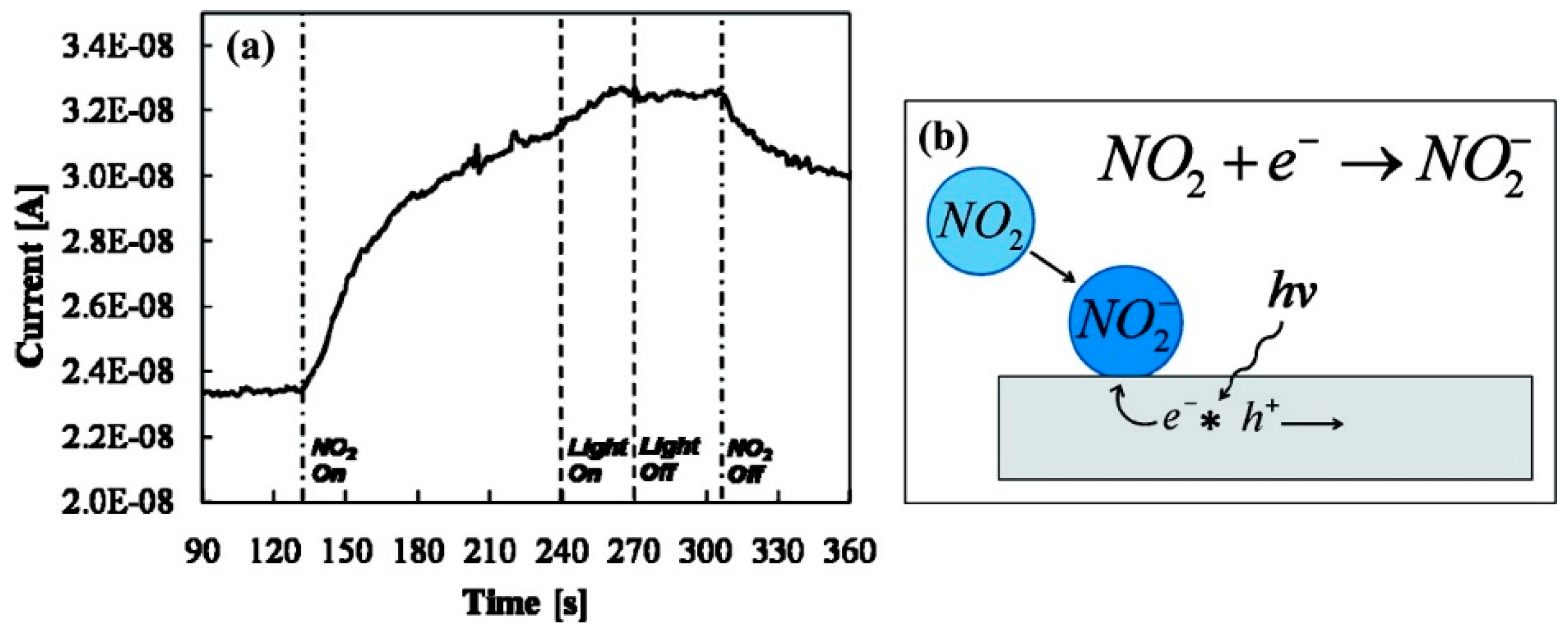
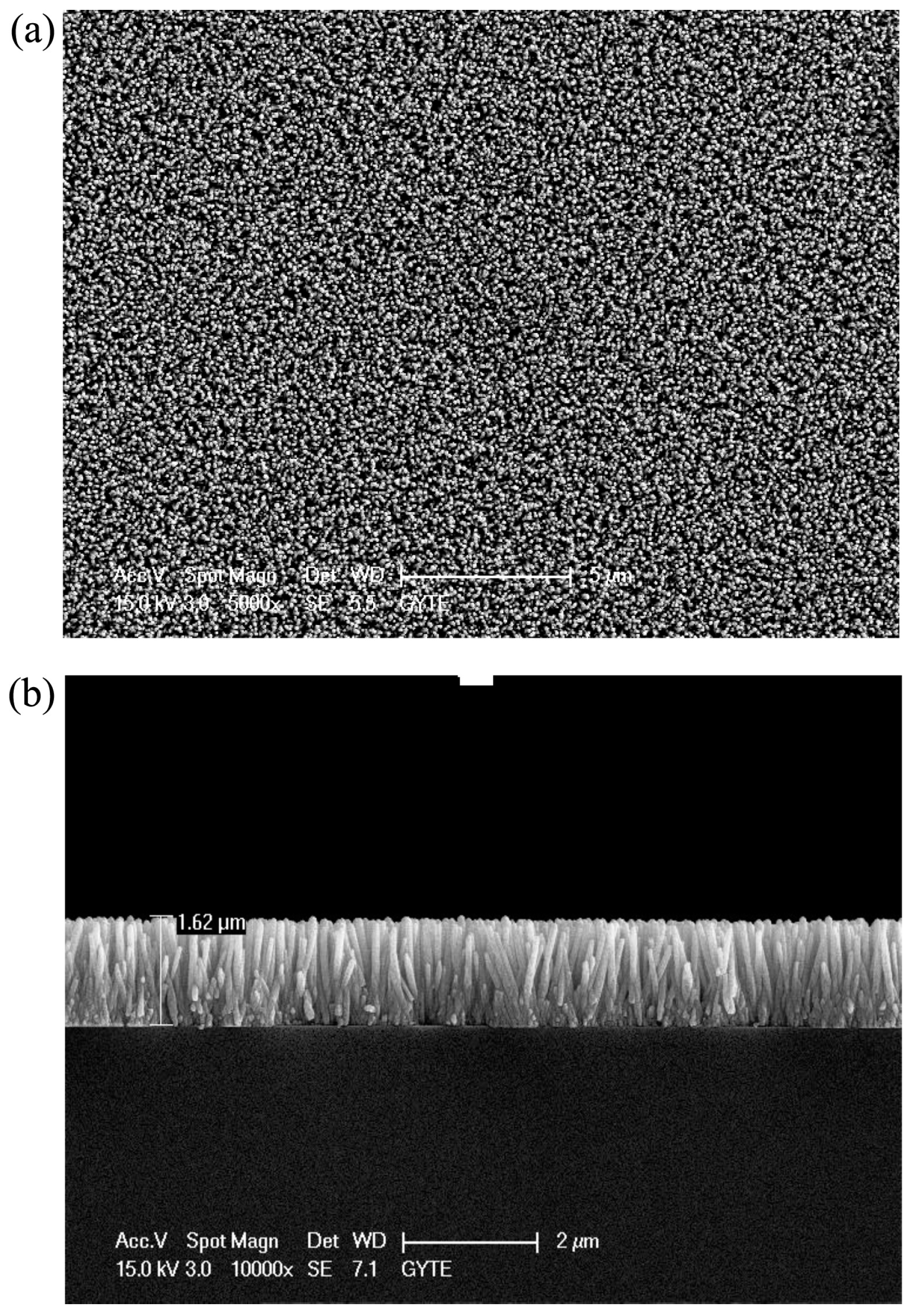

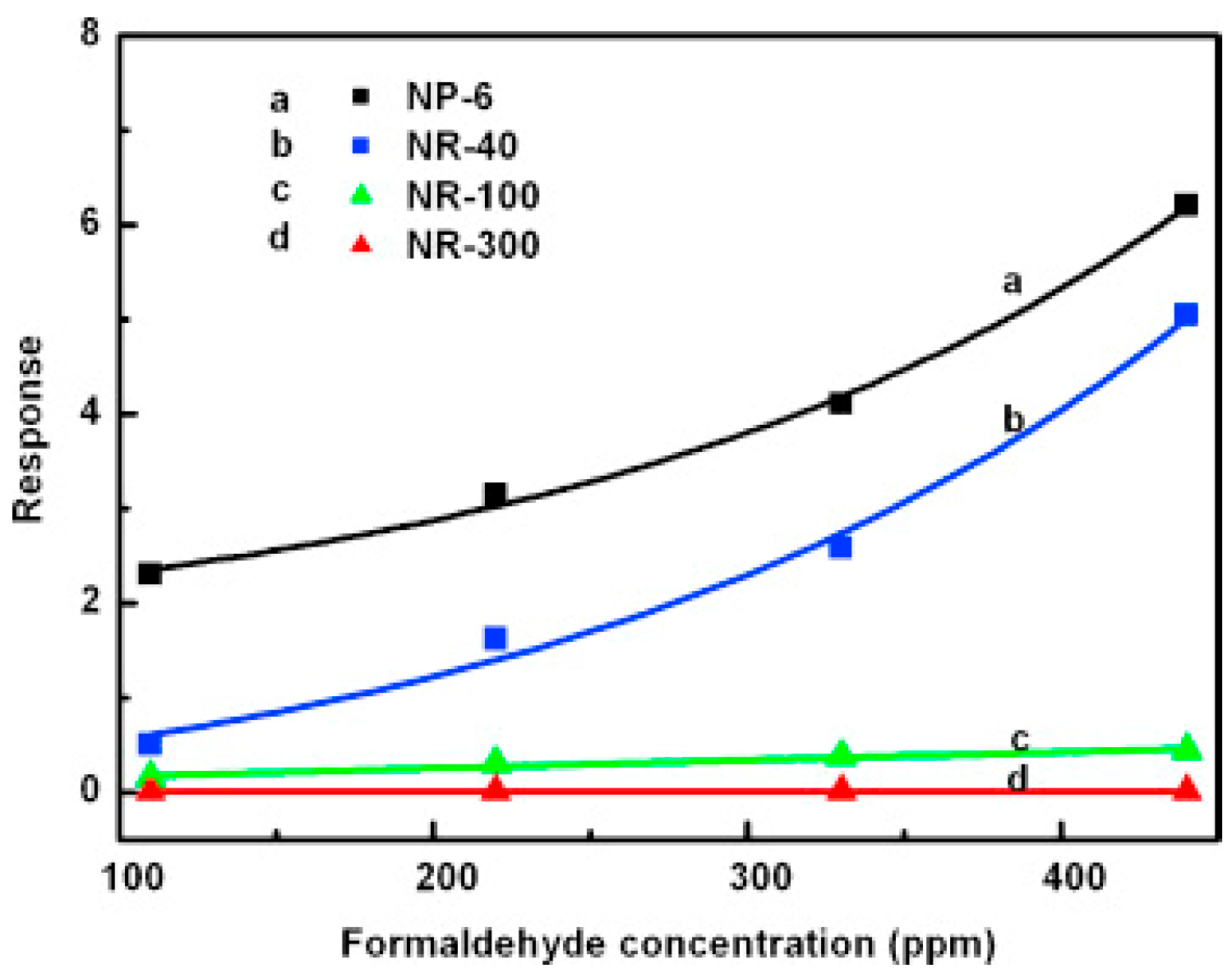
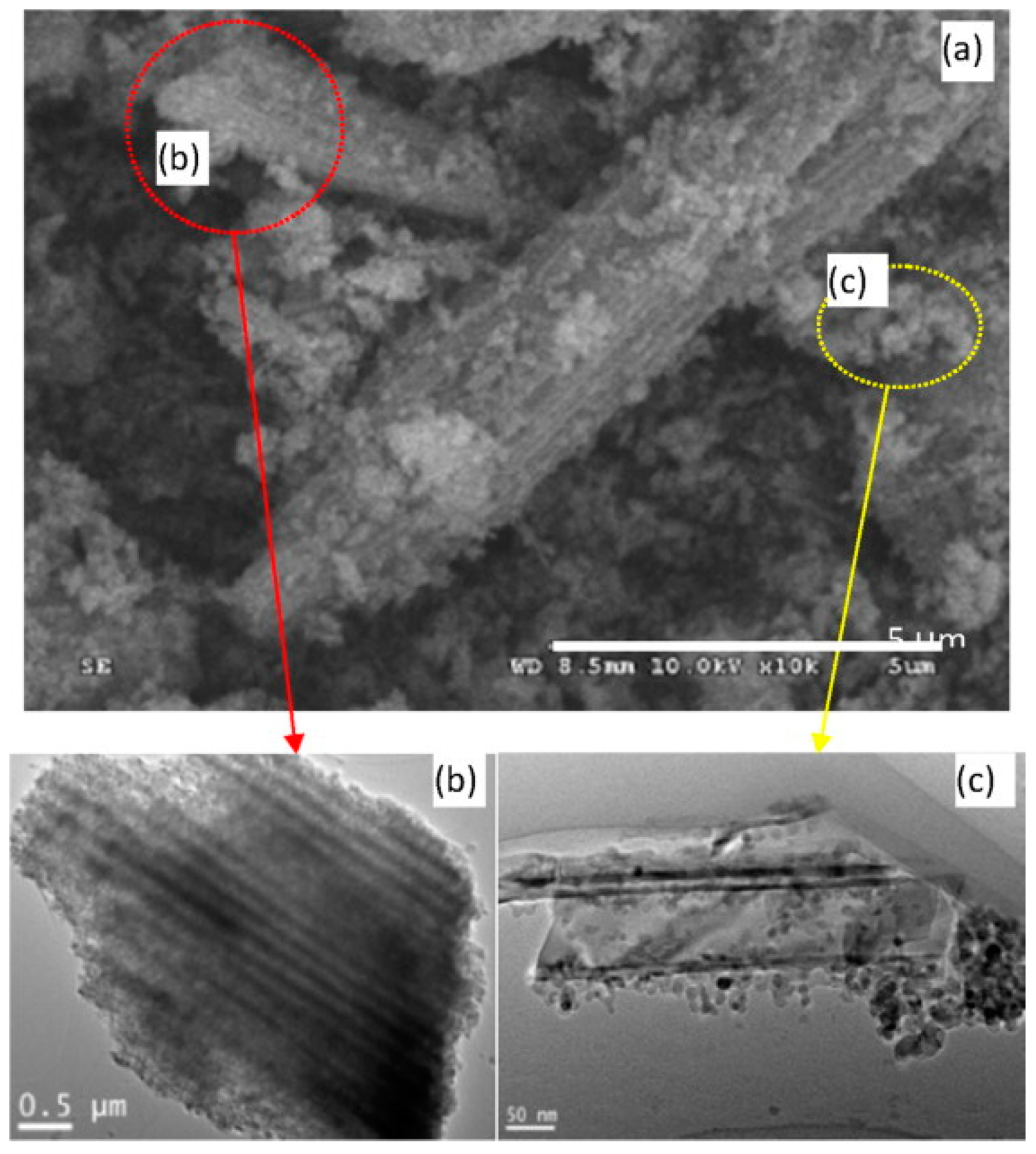
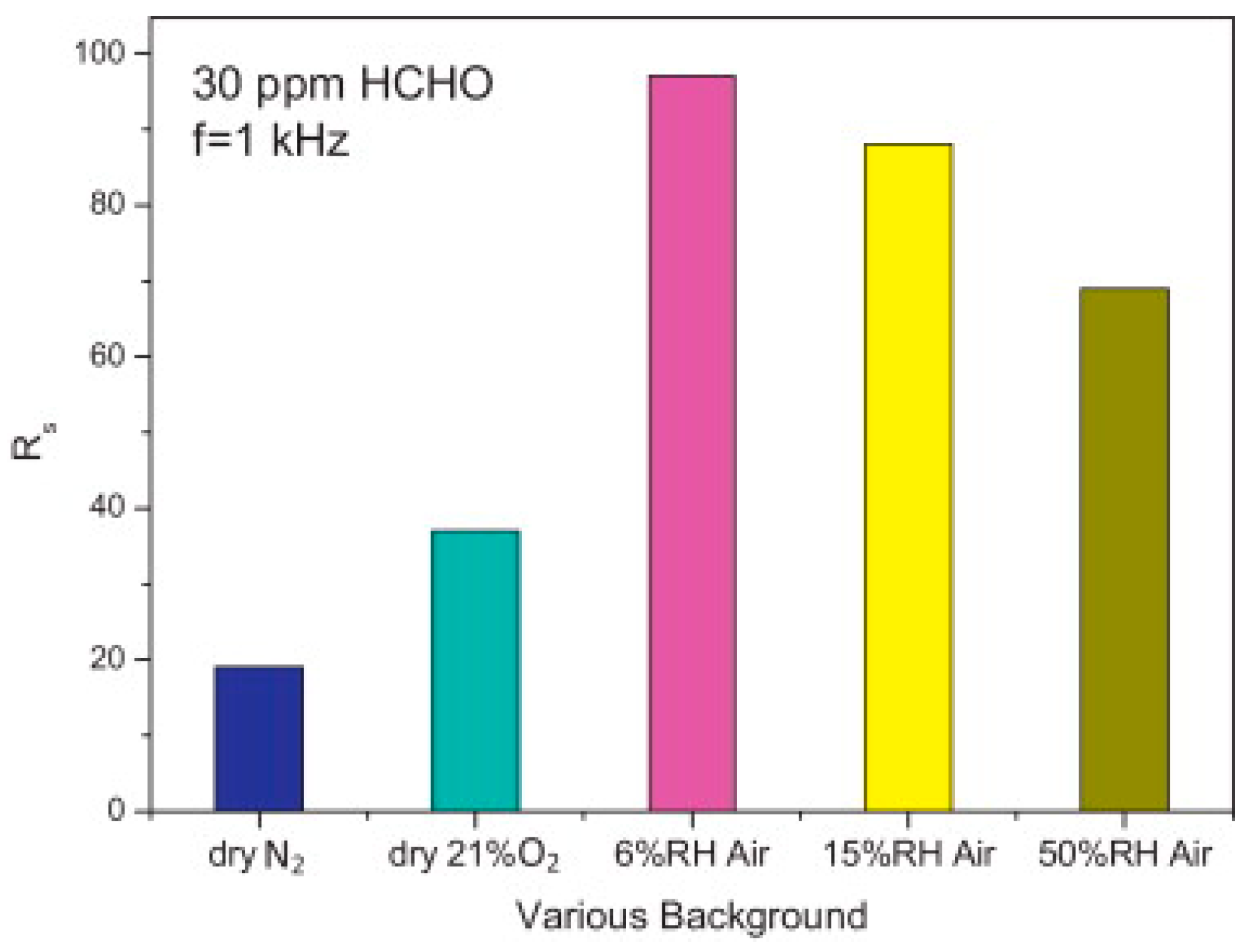
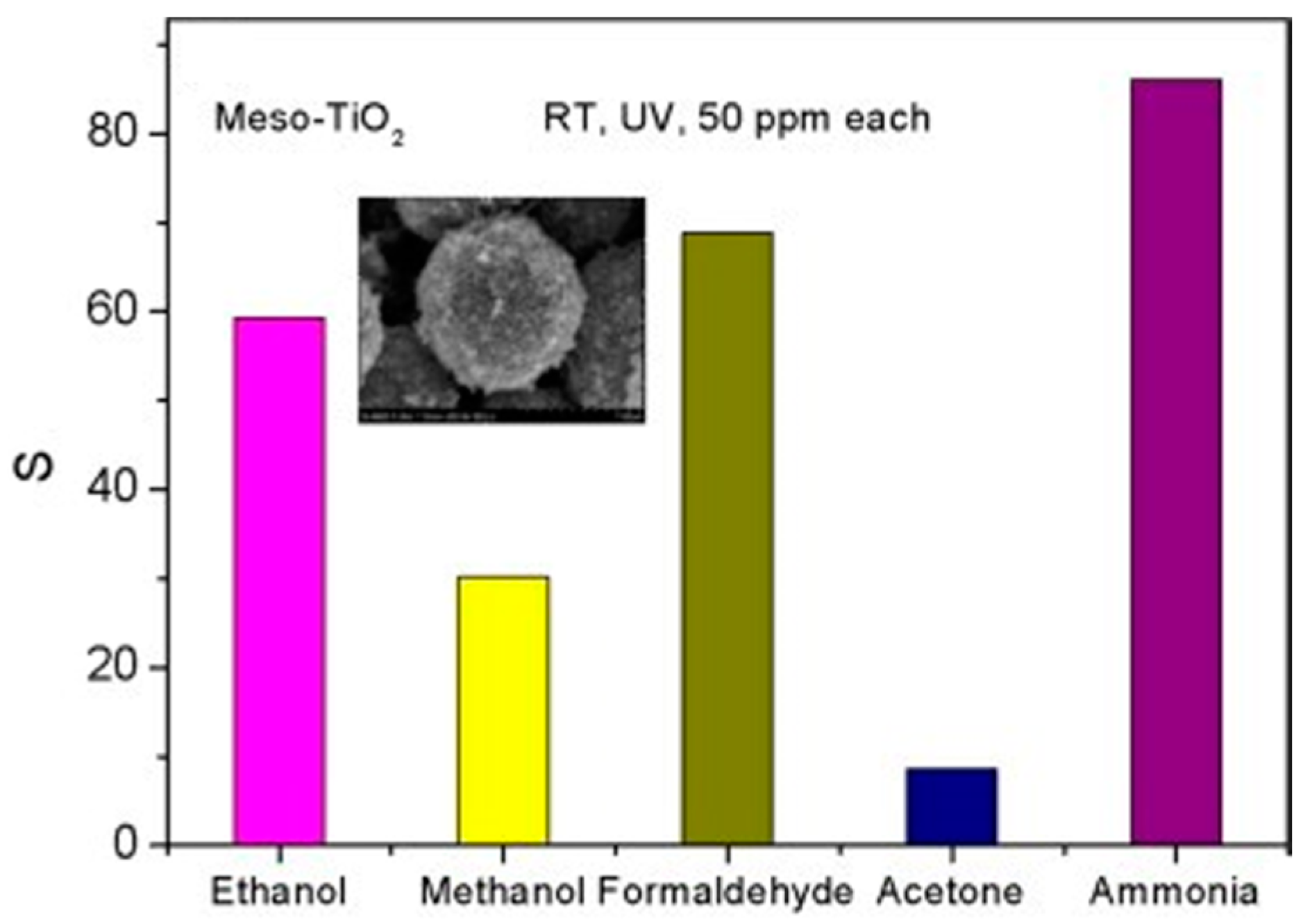
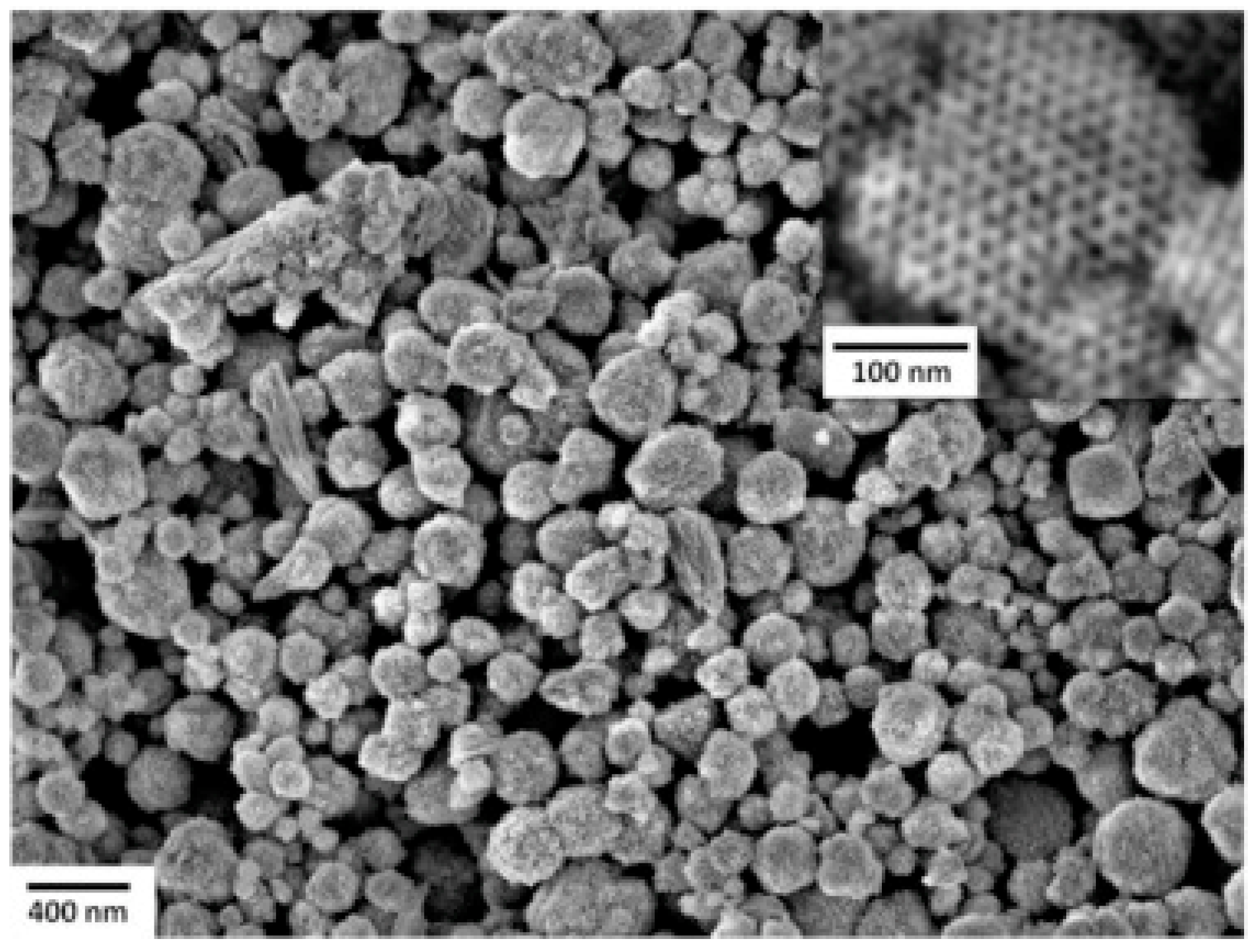
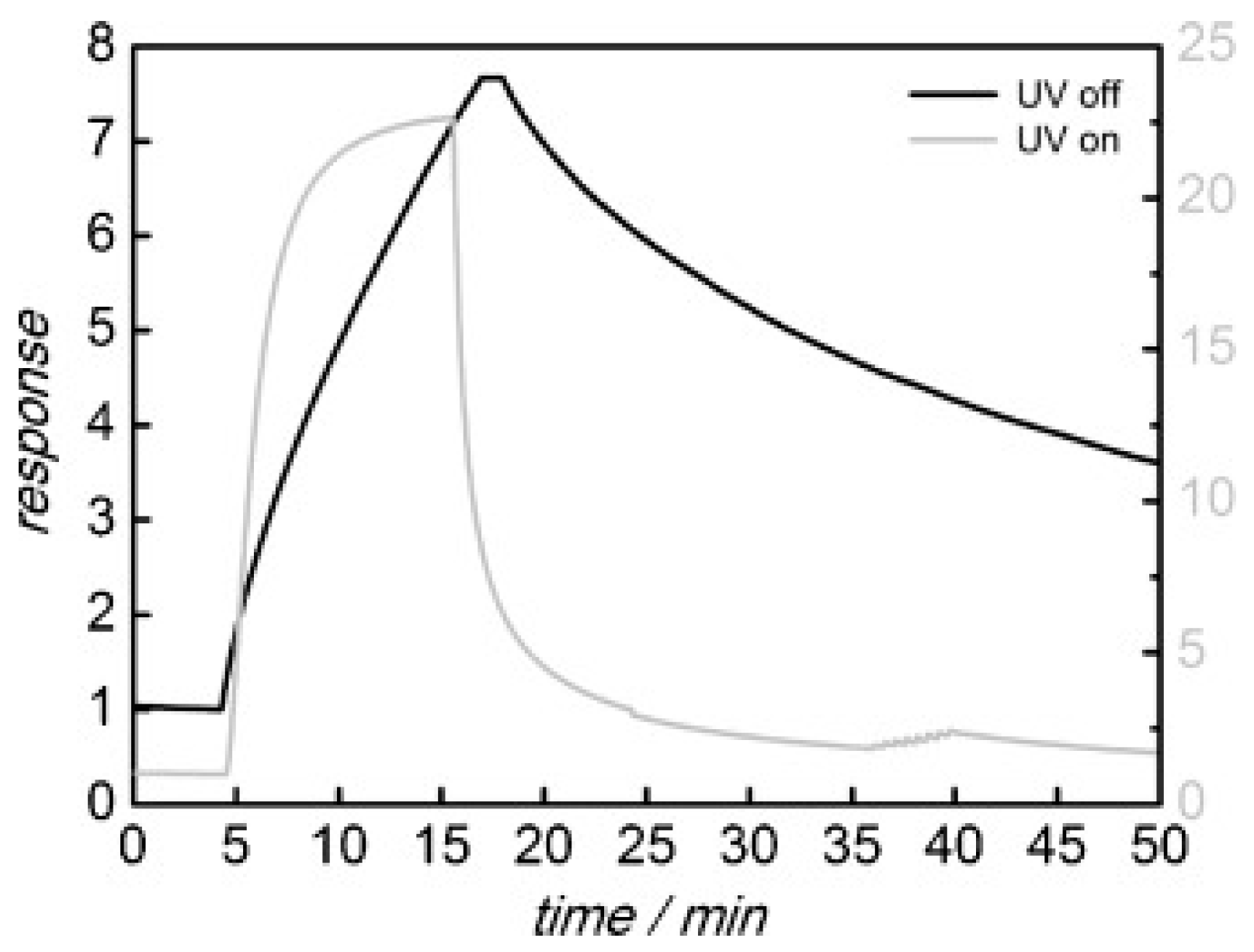
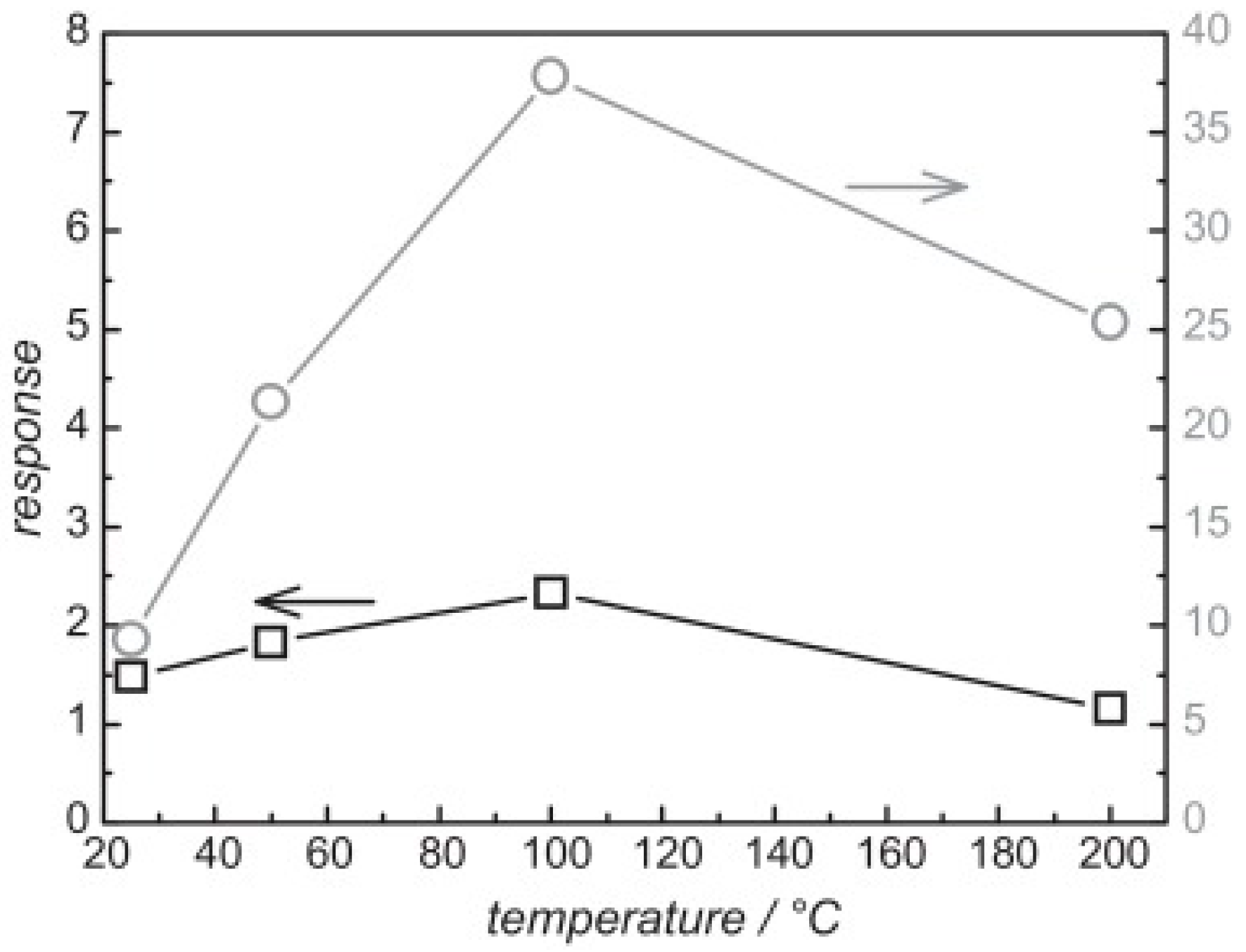
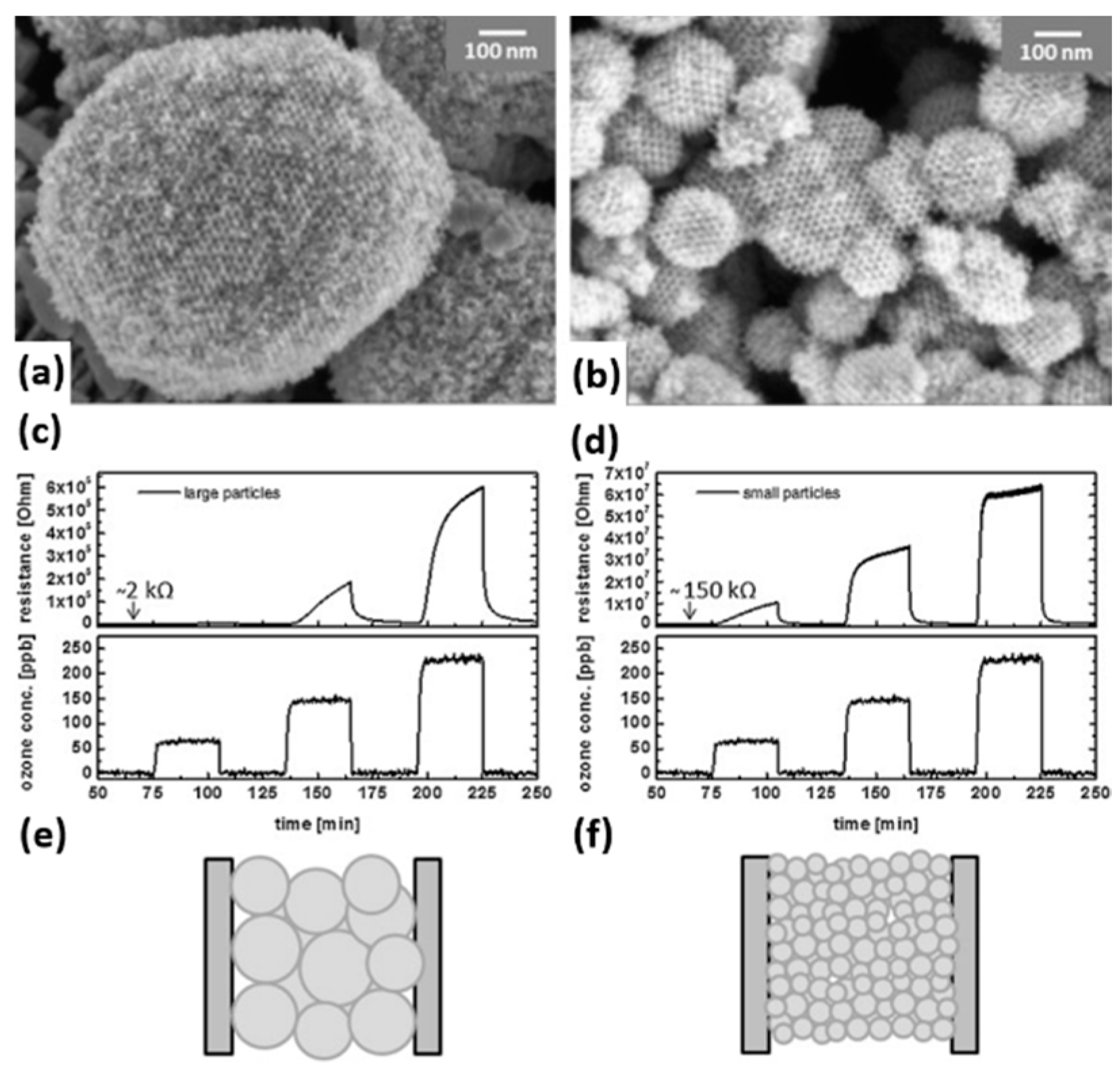

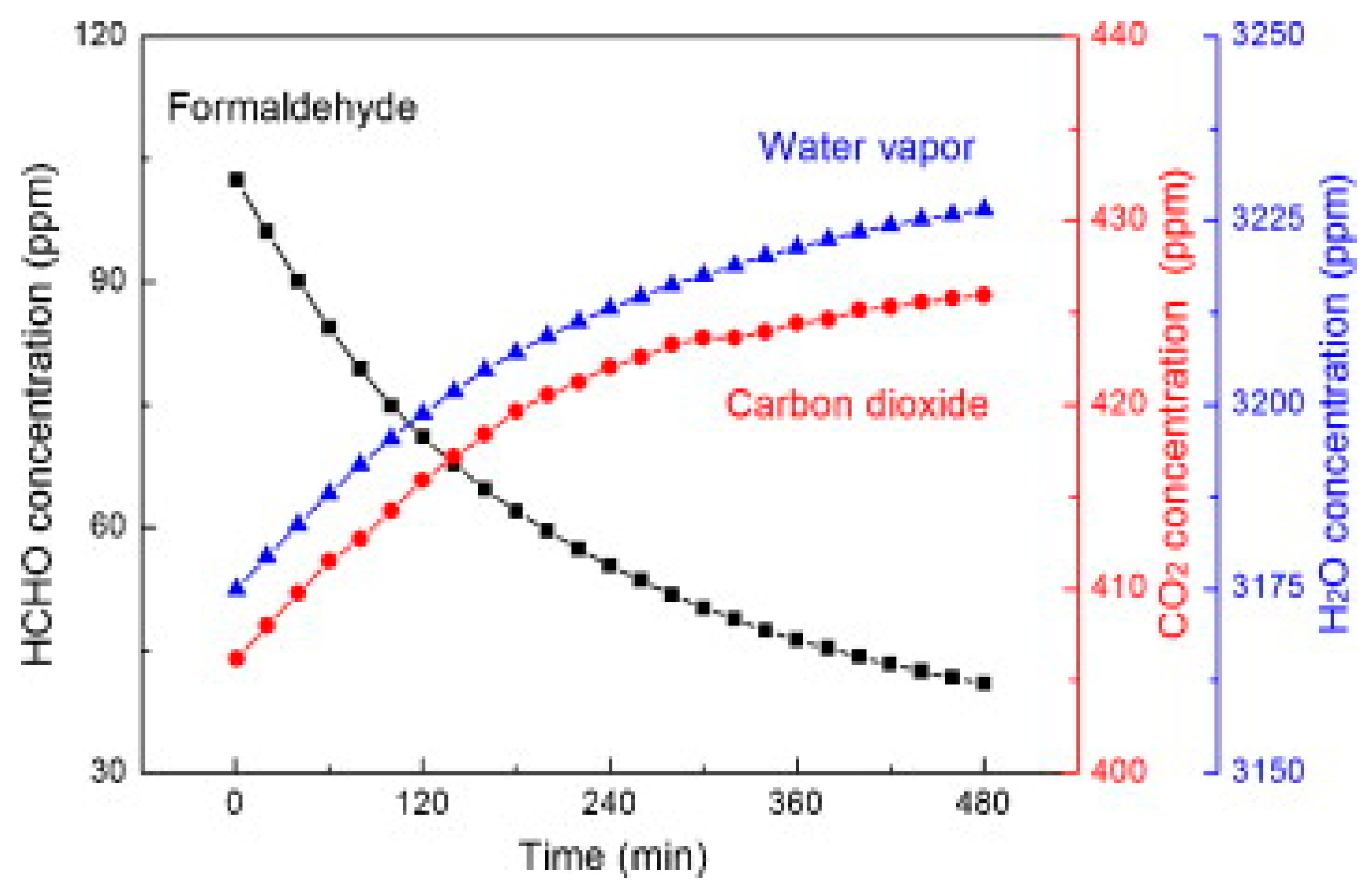
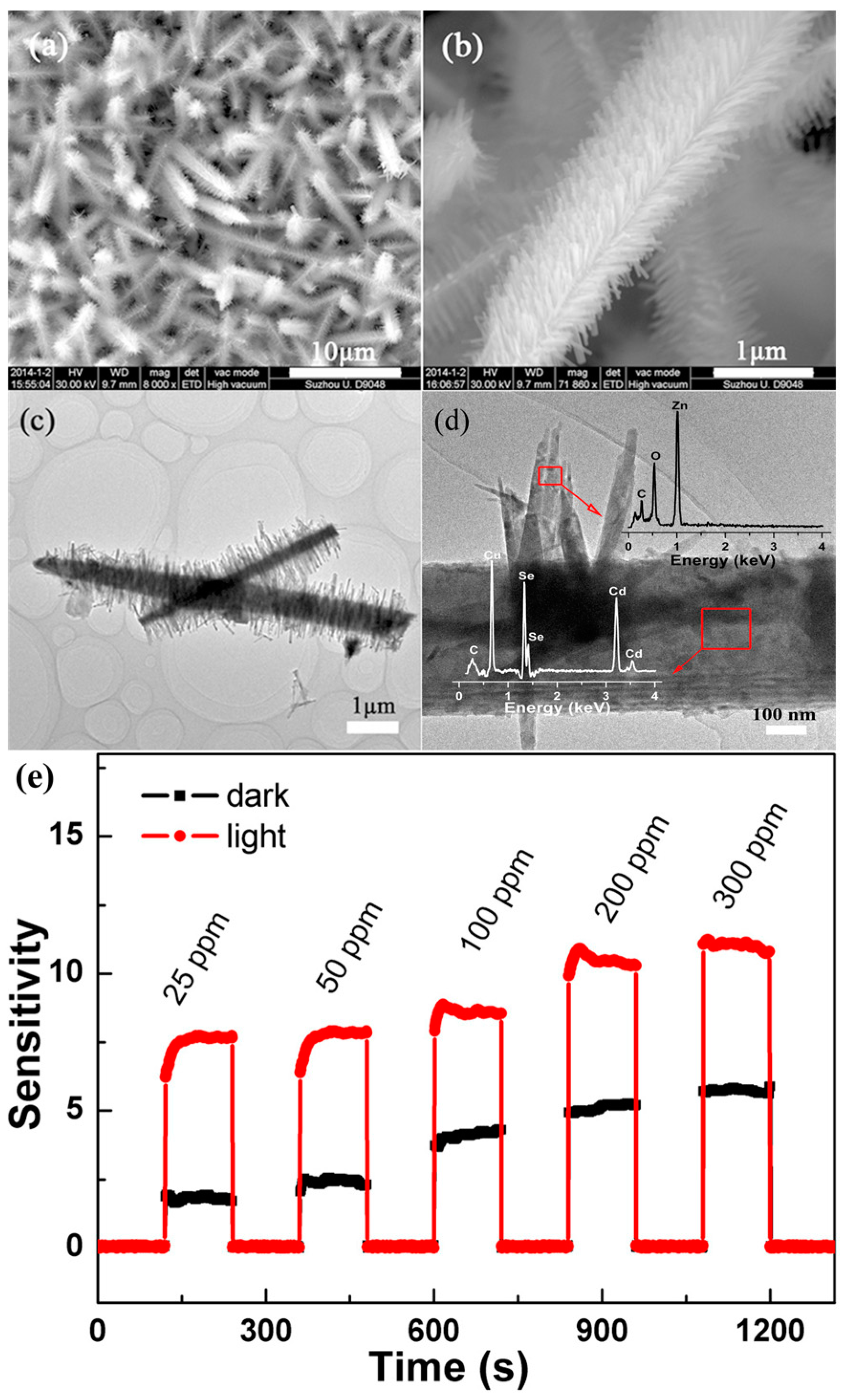
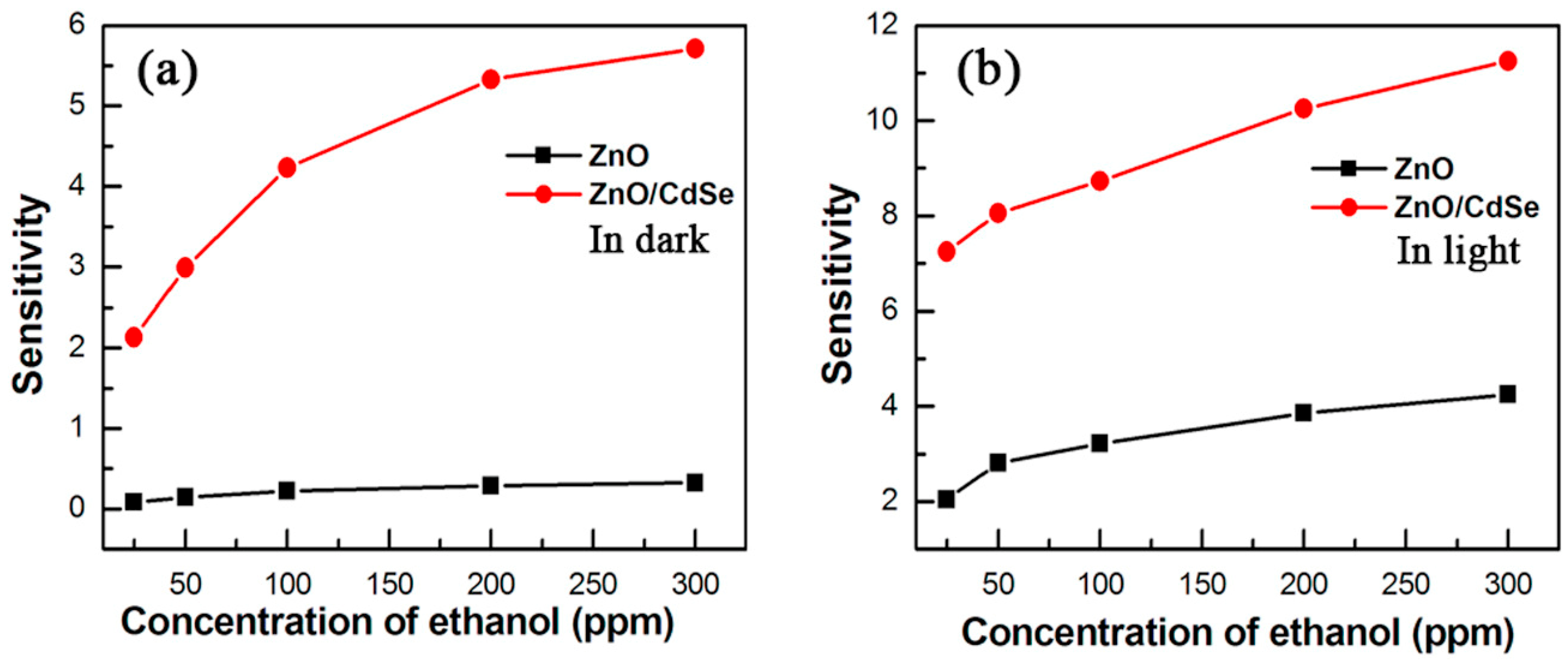
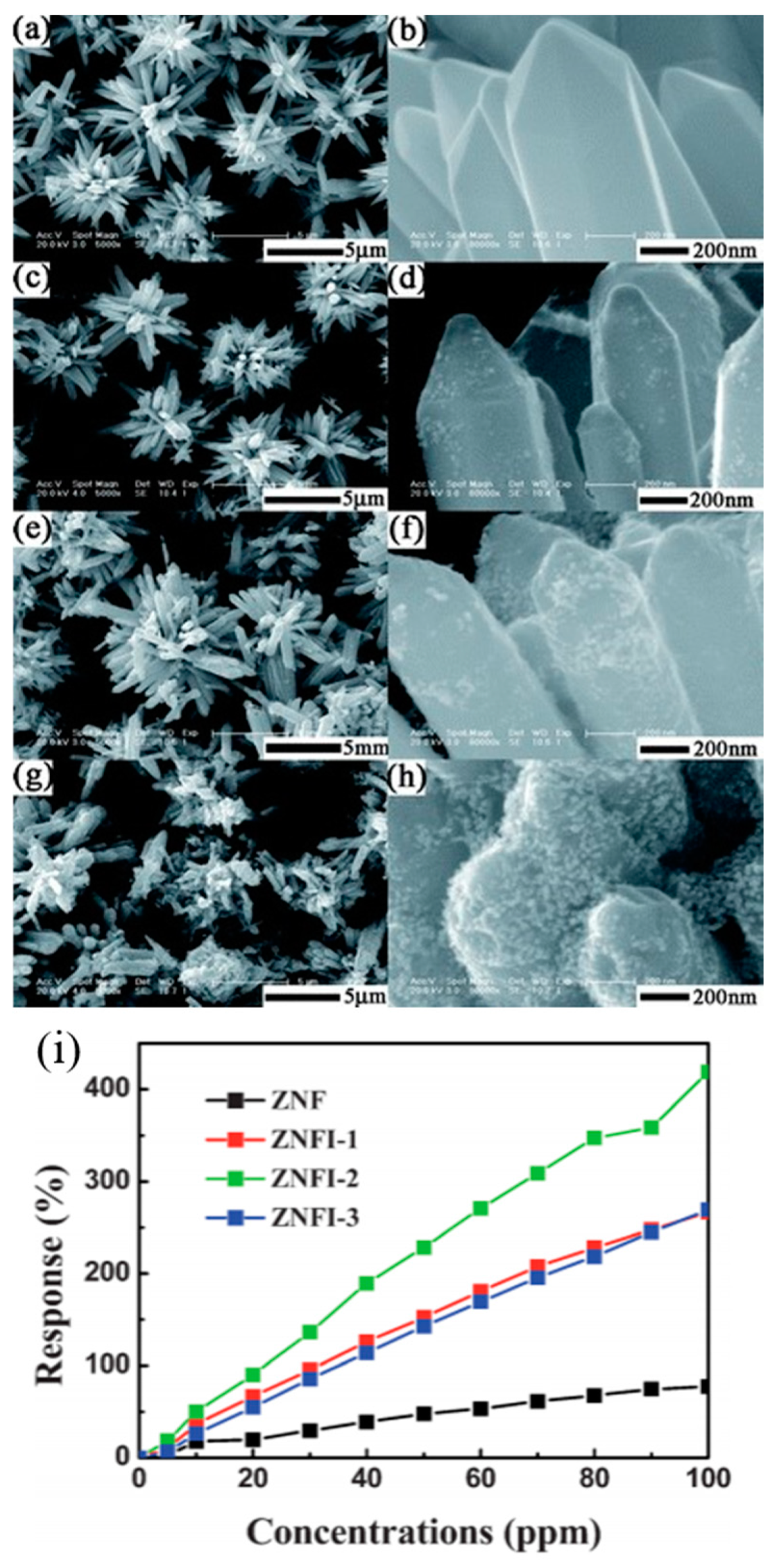
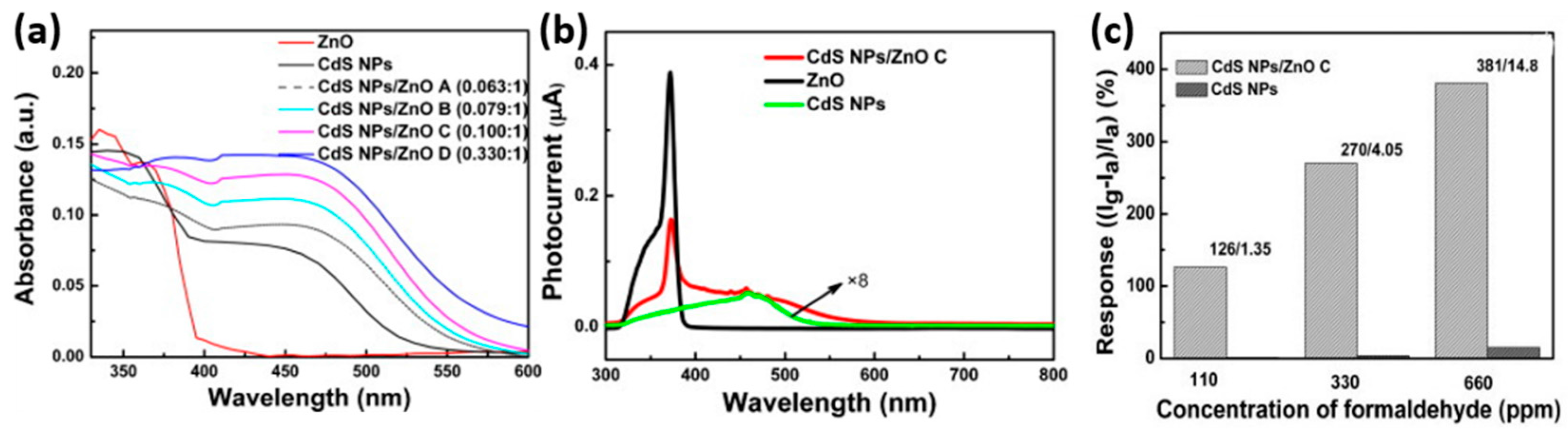



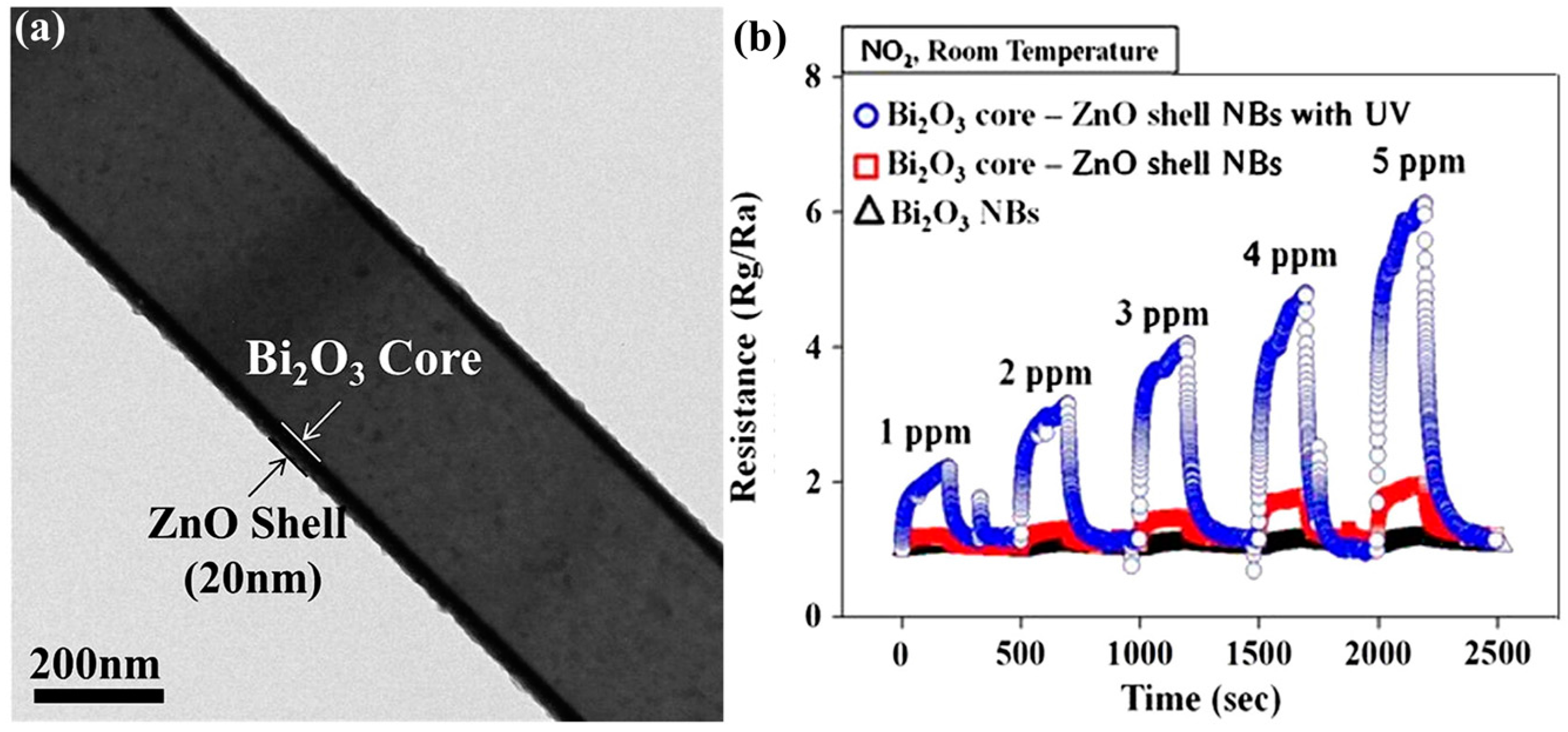

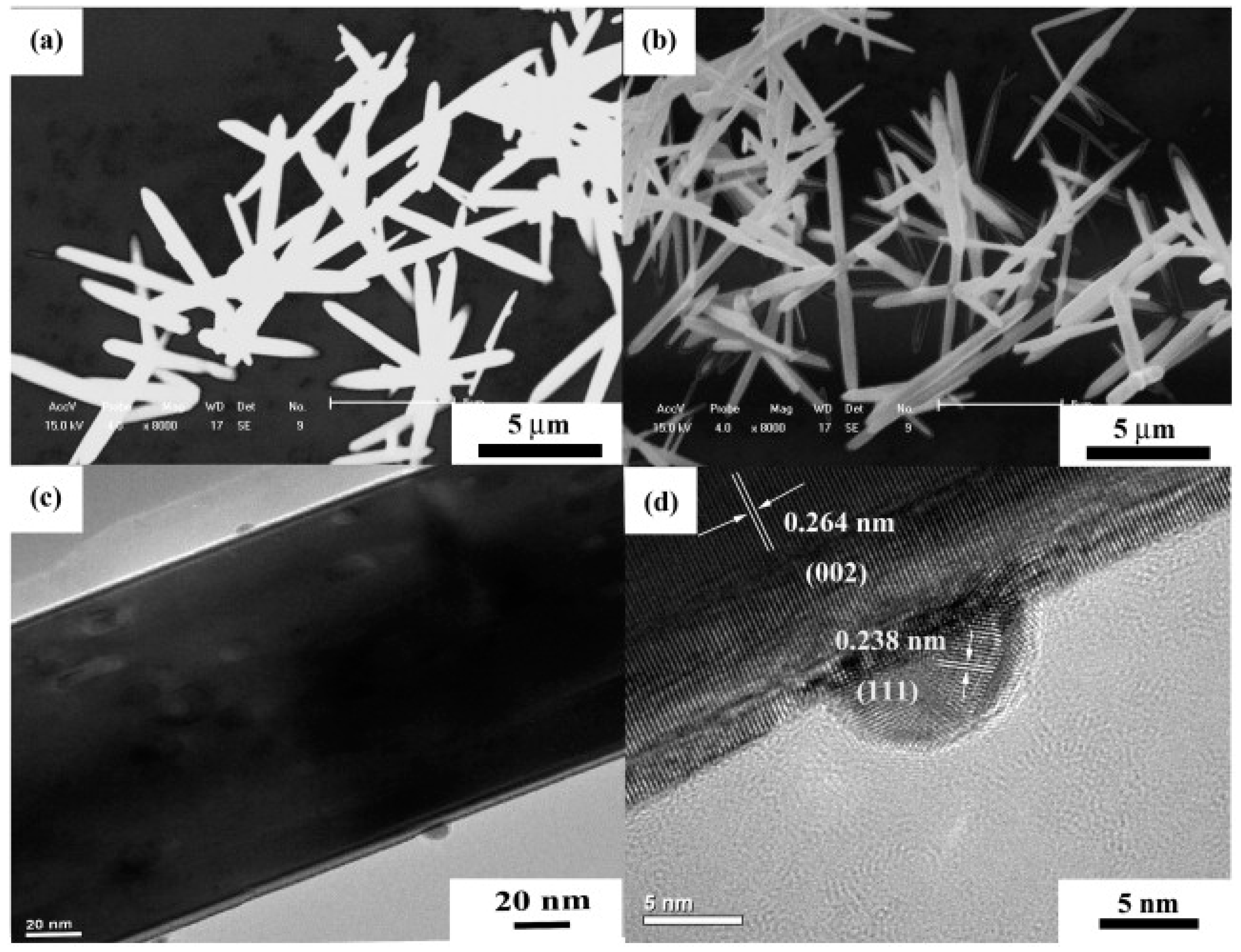
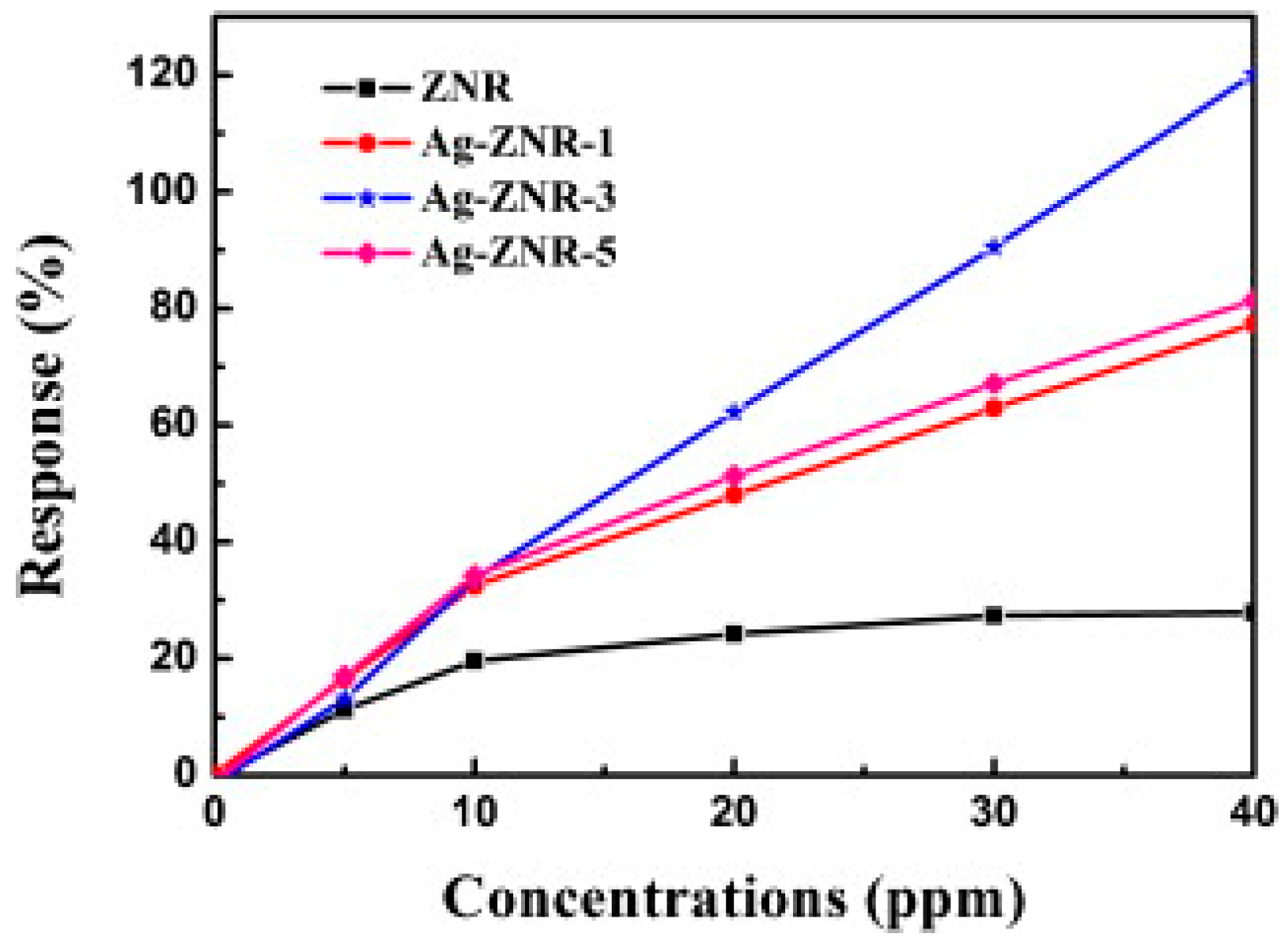
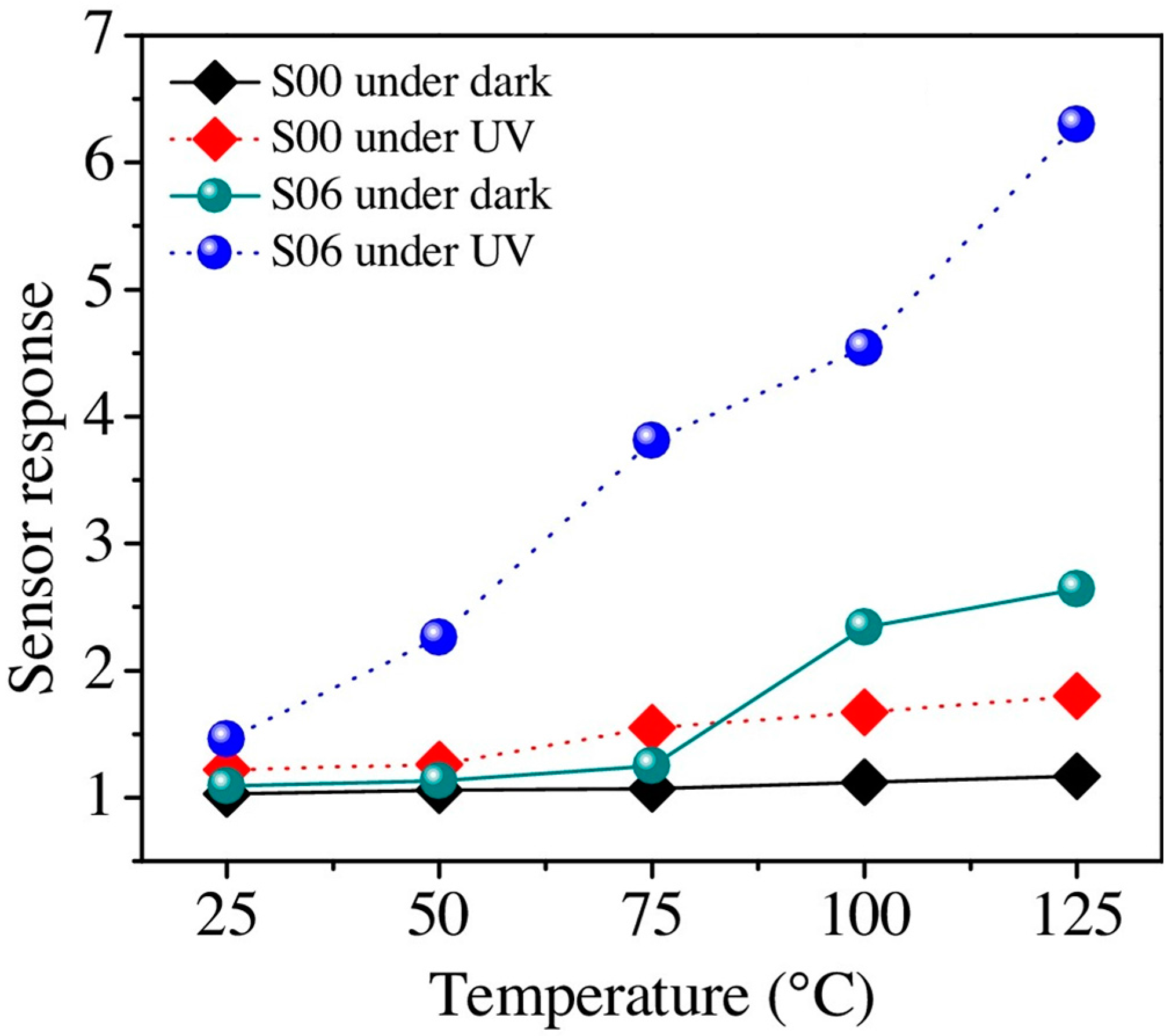
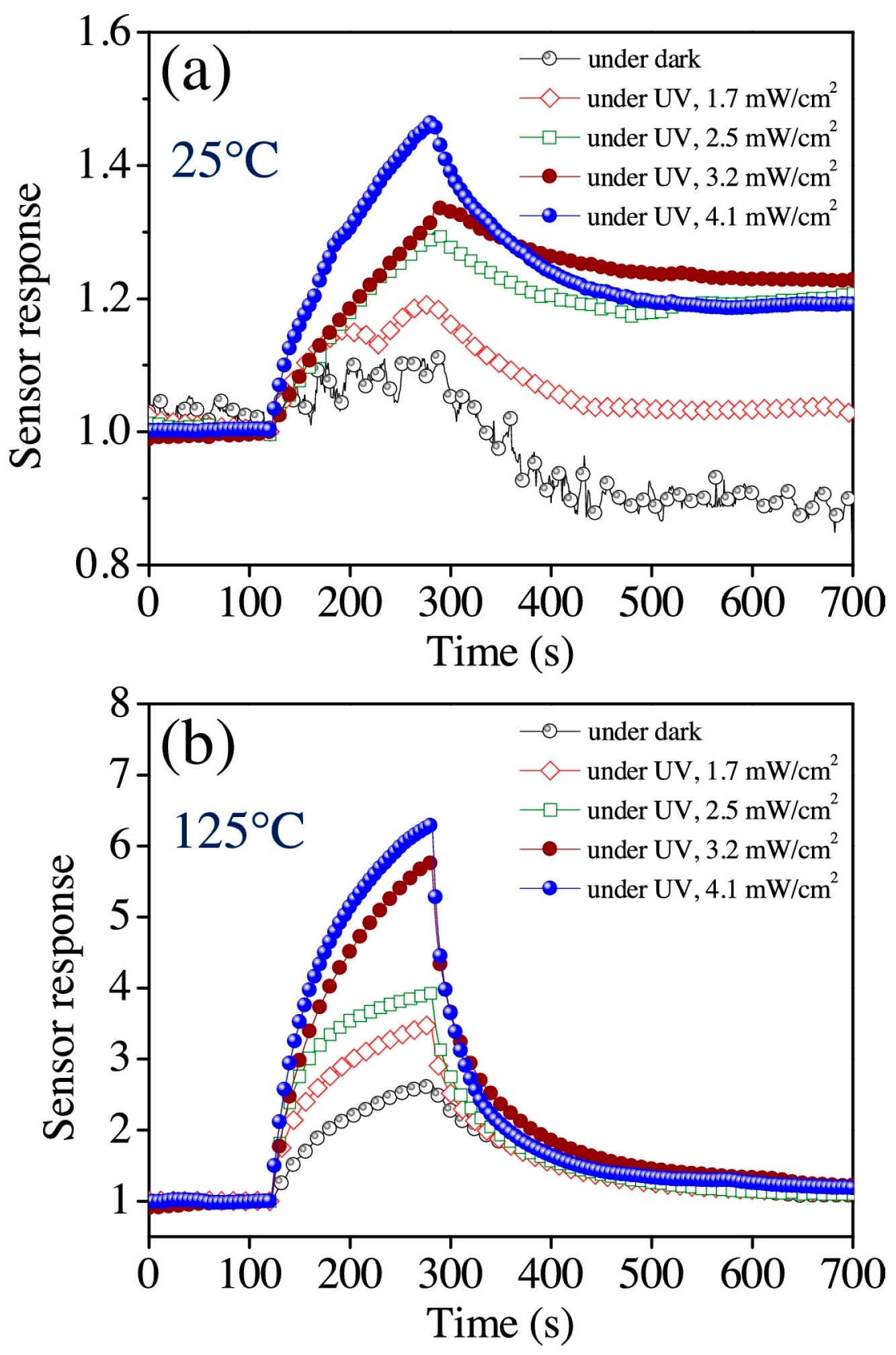
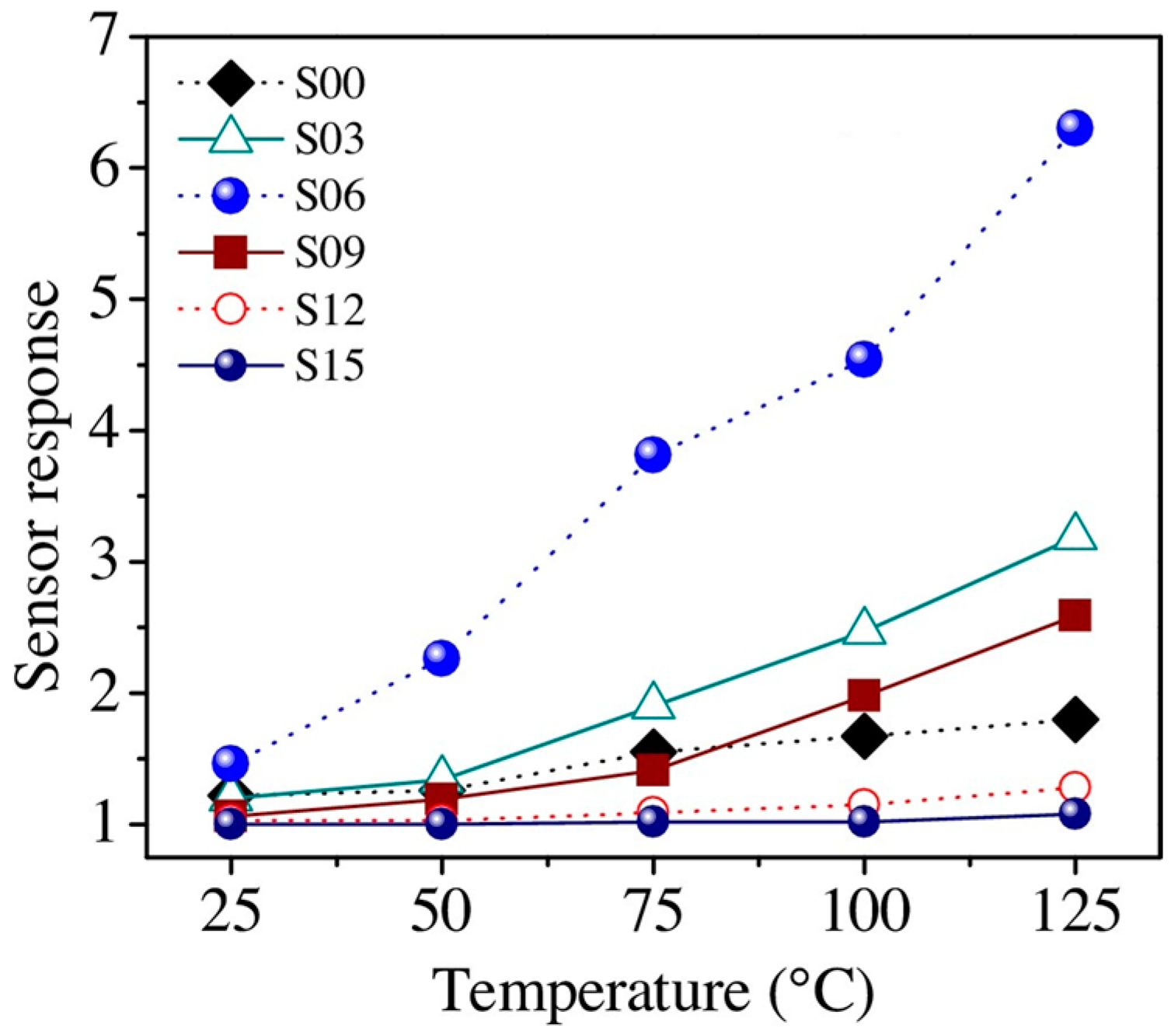
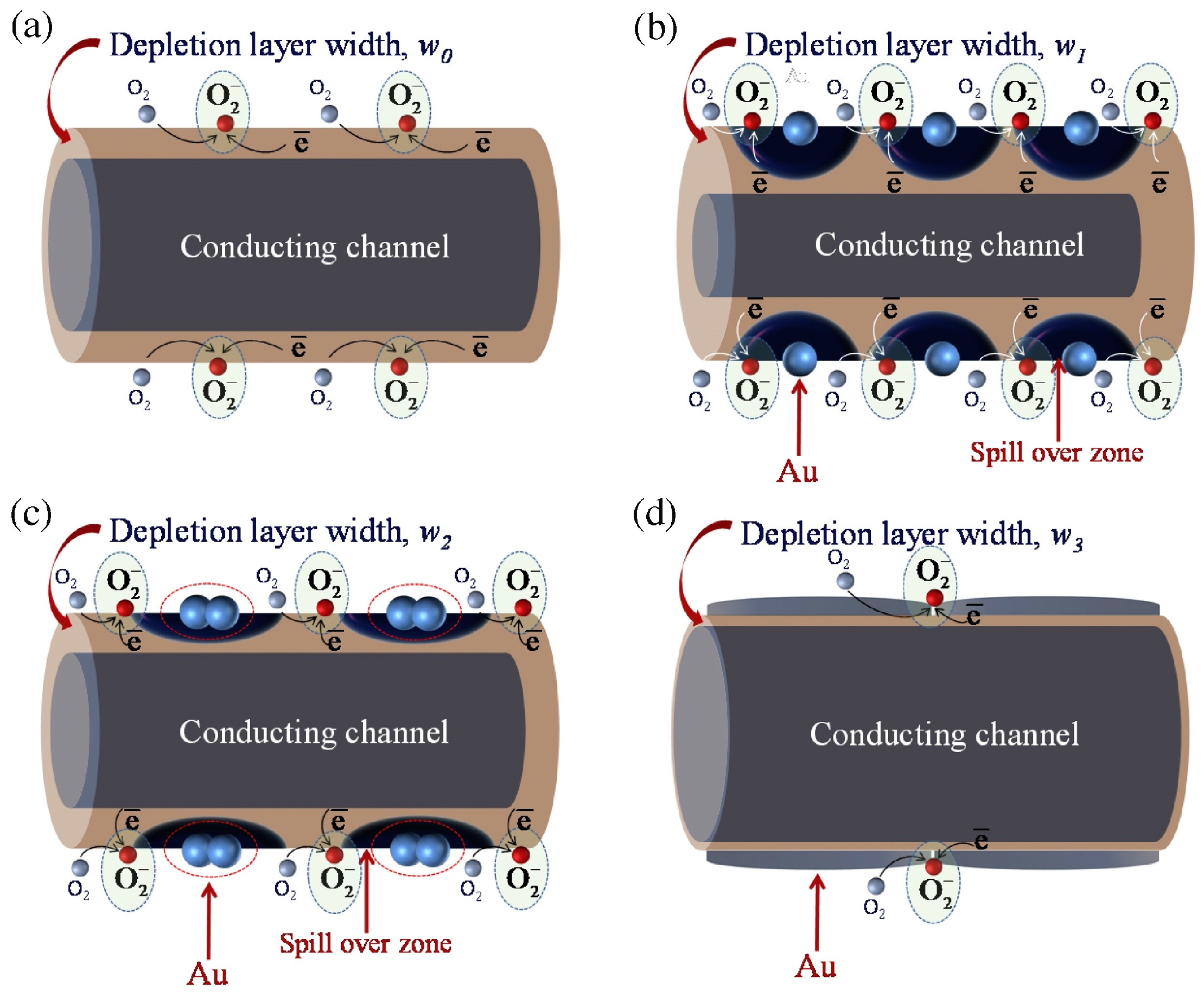
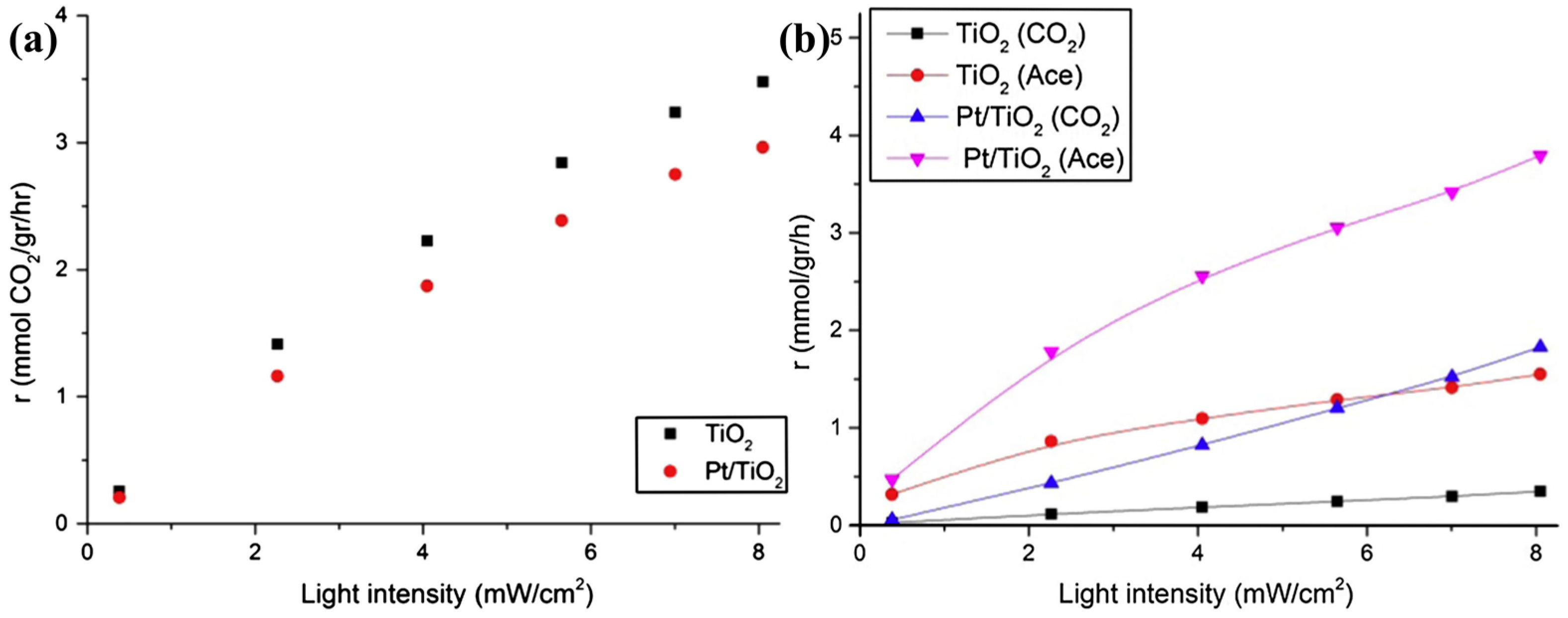
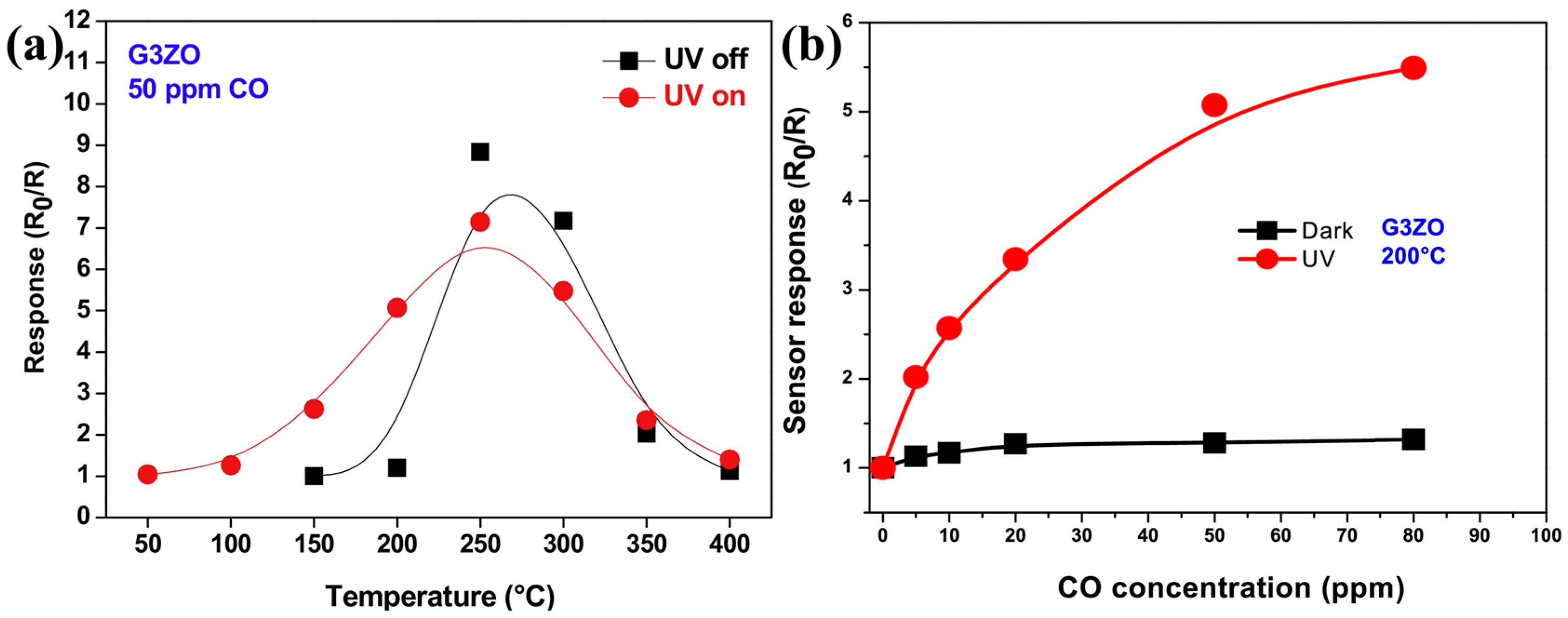
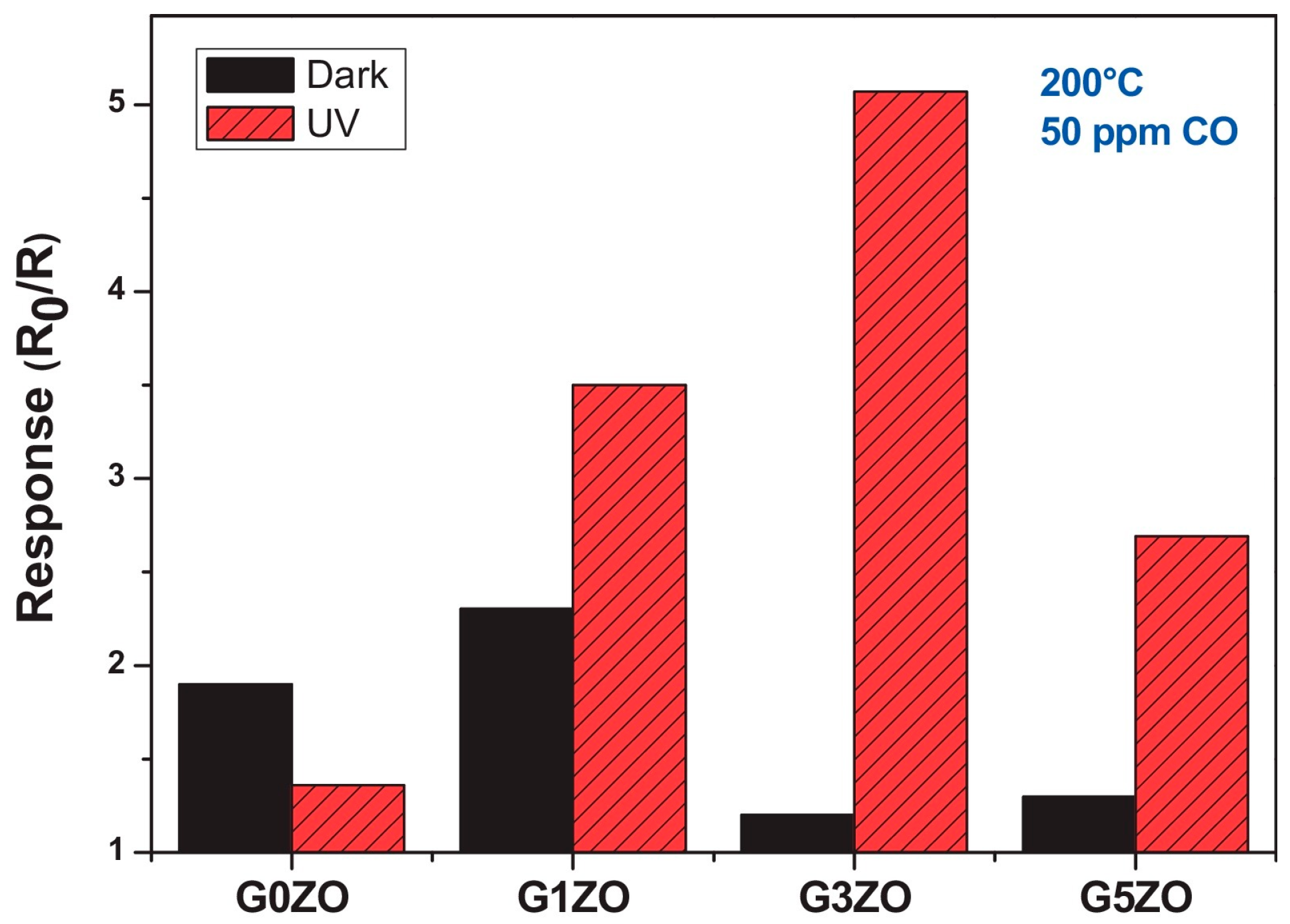

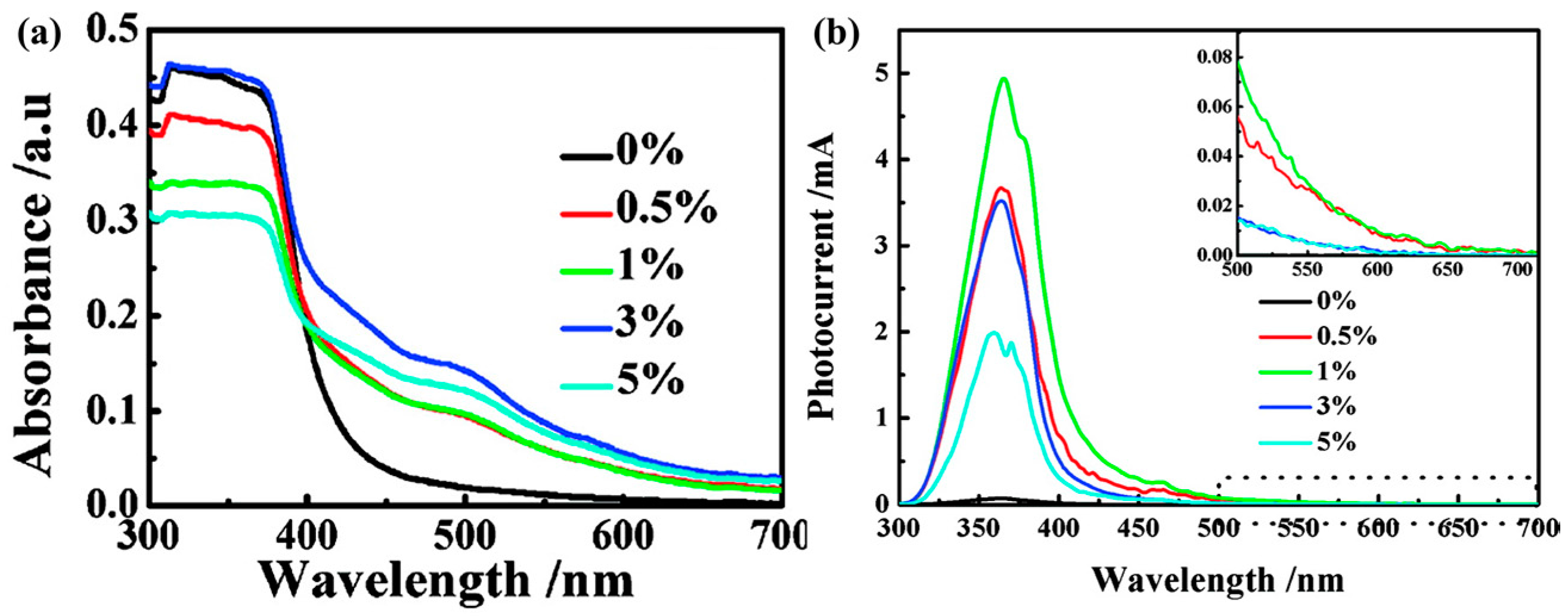
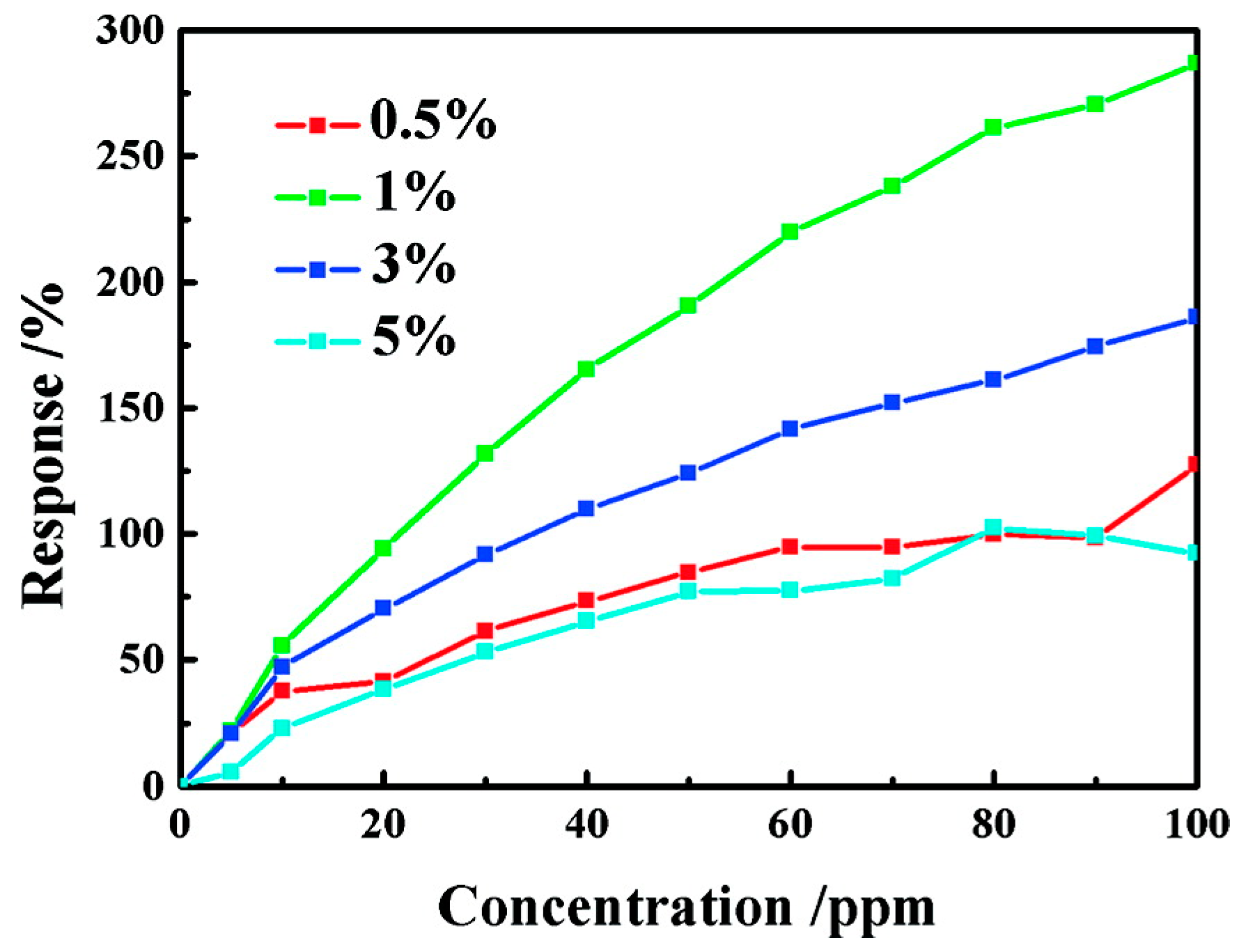

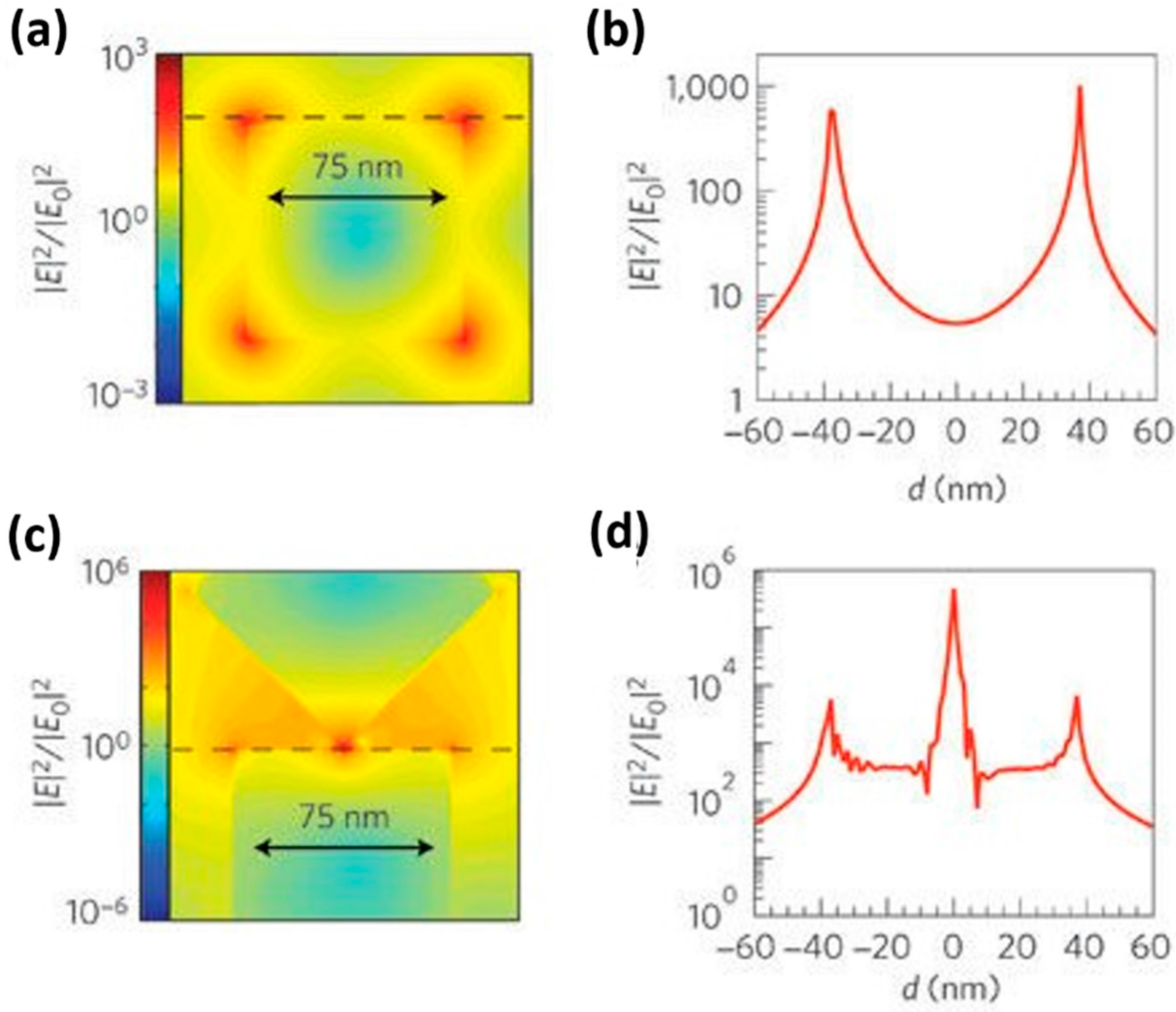
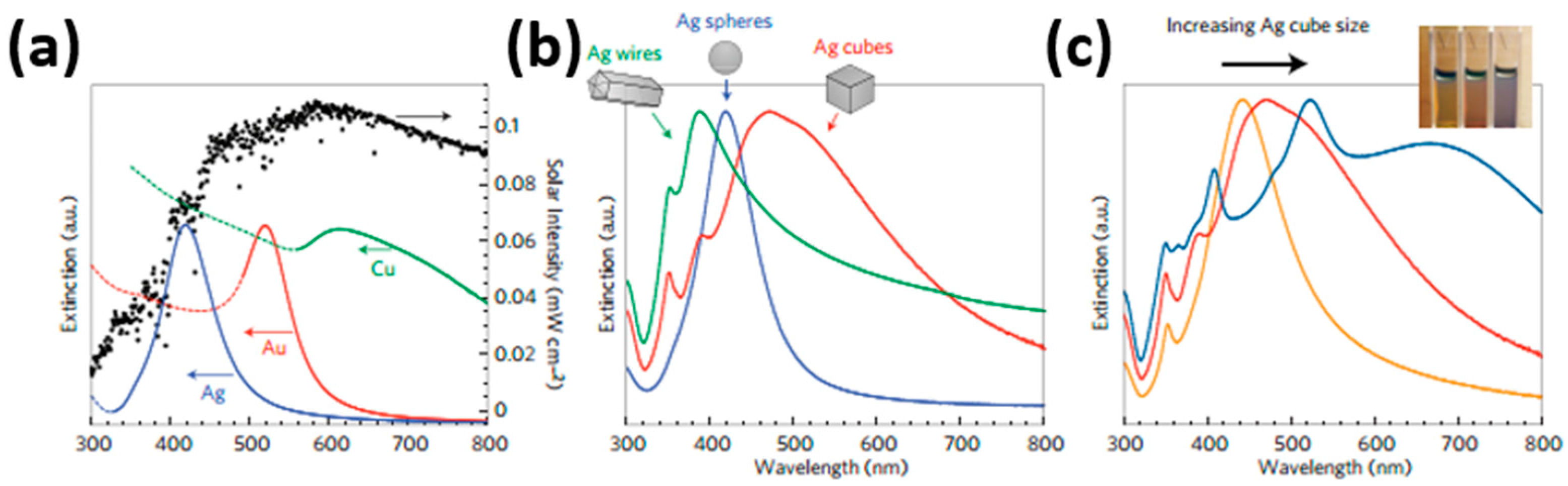

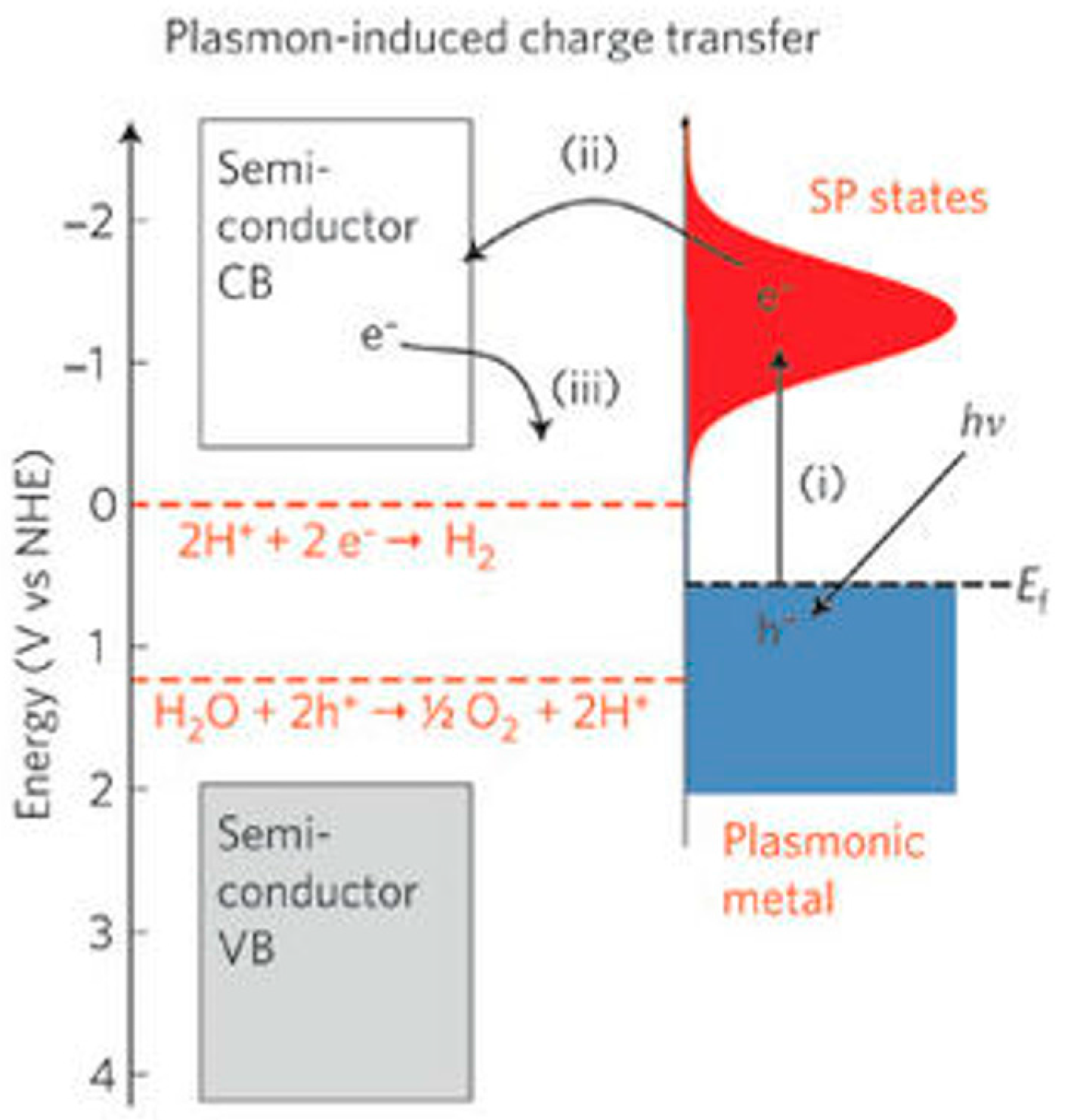
| Type of Semiconducting Material | Reductive Gas | Oxidizing Gas |
|---|---|---|
| n-type | Resistance decrease | Resistance increase |
| p-type | Resistance increase | Resistance decrease |
| Type of Semiconductor Material | Reductive Gas | Oxidizing Gas |
|---|---|---|
| n-type | Ra/Rg or (Ra − Rg)/Rg | Rg/Ra or (Rg − Ra)/Ra |
| p-type | Rg/Ra or (Rg − Ra)/Ra | Ra/Rg or (Ra − Rg)/Rg |
| Material | Light Illumination | Temperature | Vapor | Ref |
|---|---|---|---|---|
| ZnO thin film | ultravlolet (UV) | room-temperature (RT) | H2 | [36] |
| ZnO nanoline | UV | RT | H2 | [36] |
| ZnO nanoparticle | UV | RT | hexane, propane, methane, ethanol, toluene, acetadyde, acetone and pentane | [38] |
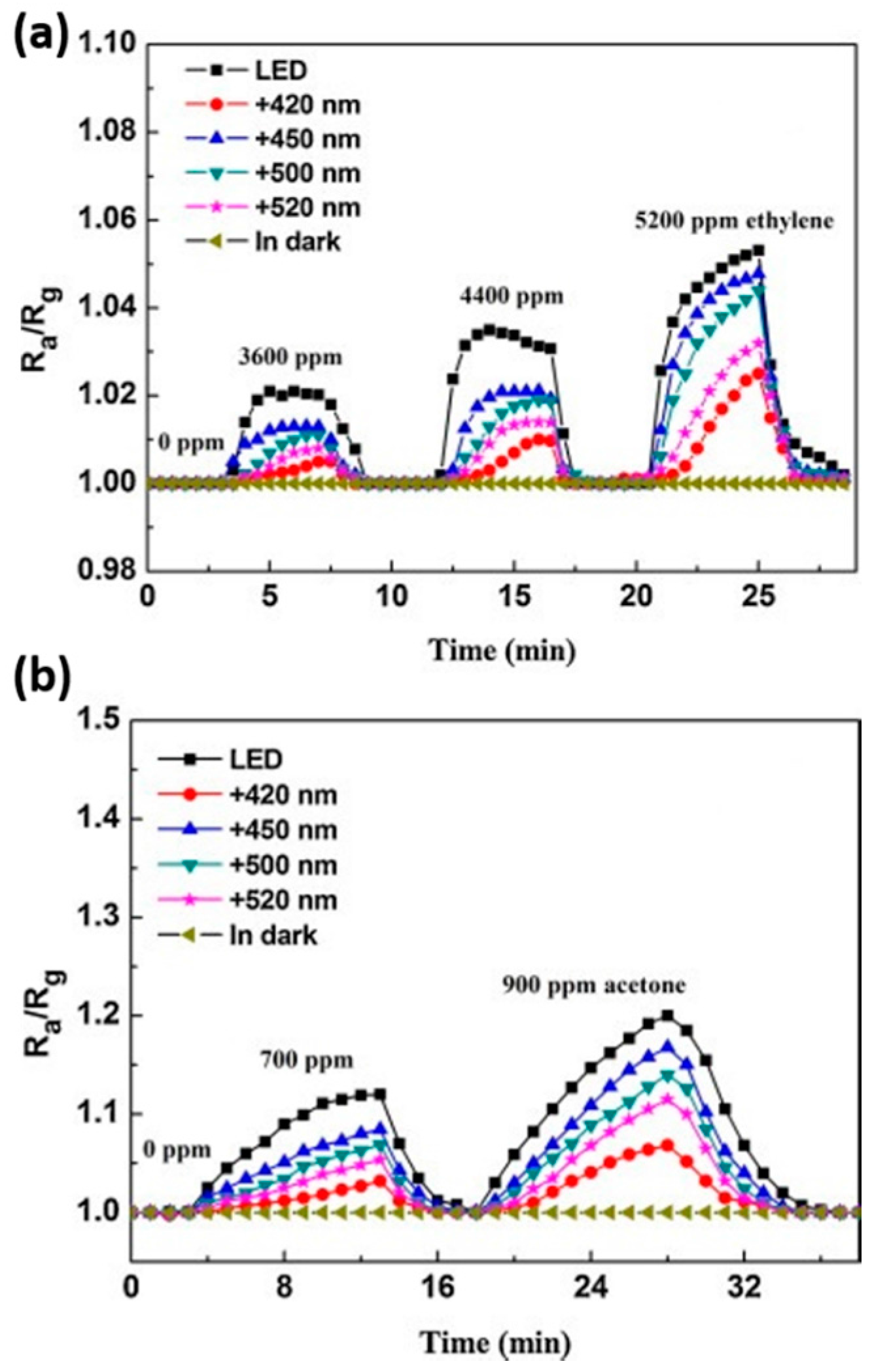
| ZnO | white LED lamp and visible light with different wavelength | RT | acetone and ethylene | [37] |
| ZnO nanospheres | white LED | RT | H2O2 | [42] |
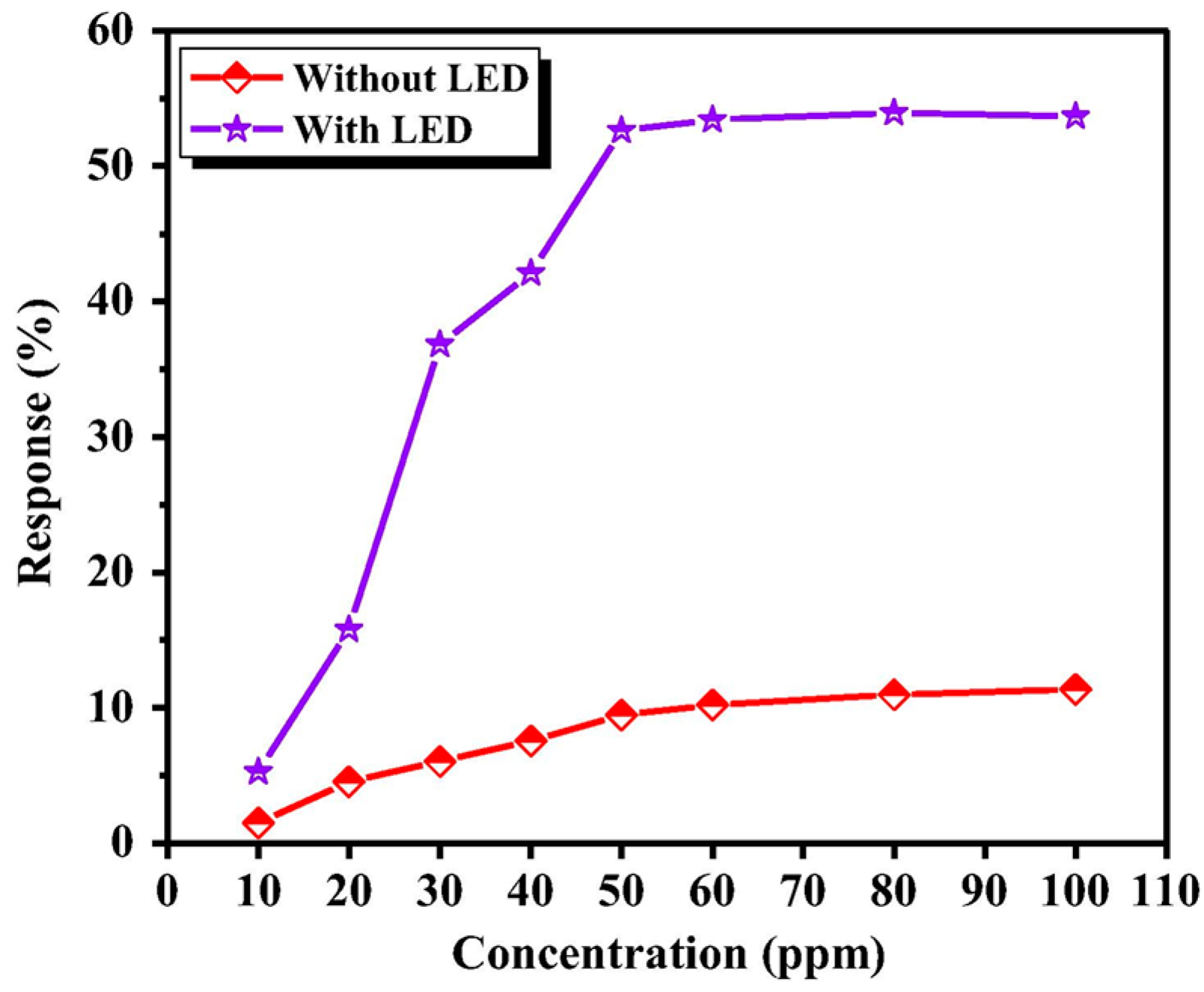
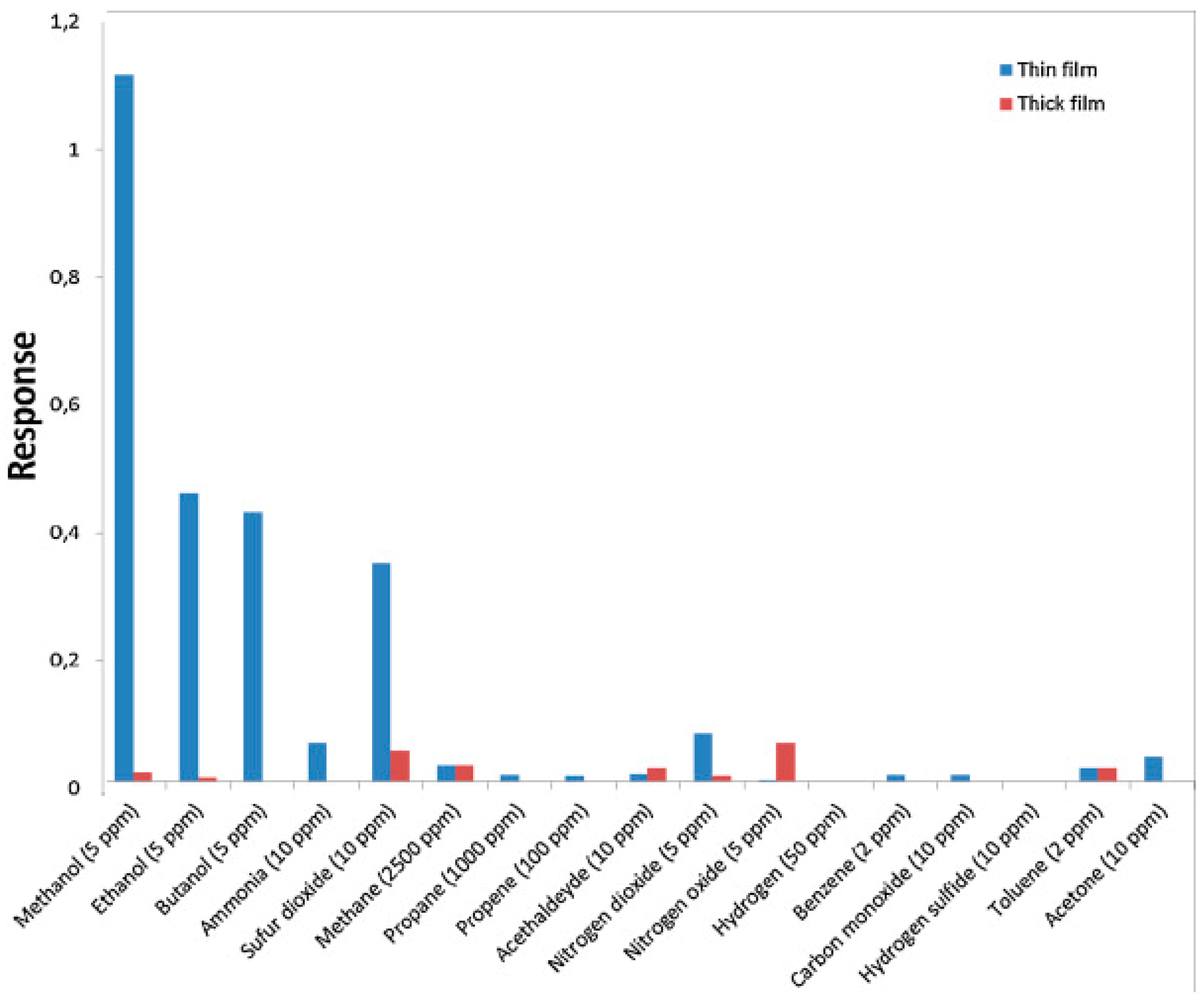
| ZnO thin film | light of different wavelength | RT | NO2, methanol, ethanol butanol, ammonia, sufur dioxide, methane, propane, etc. | [9] |
| ordered porous ZnO arrays | UV | RT | NO2 | [43] |
| SnO2 | UV LED | RT | O3 | [48] |
| SnO2 film | UV of Hg lamp | RT | H2 | [47] |
| SnO2 | monochromatic light | RT | CO, Ethyl Alcohol, NO2 | [46] |
| SnO2 pyrolytic films | UV irradiation with different wavelength | RT | acetone and trichloroethylene | [49] |
| SnO2 | halogen lamp illumination | RT | NO2 | [16] |
| TiO2 | UV | various temperatures | formaldehyde | [51] |
| TiO2 | UV lamps | not available | CO and H2 | [52] |
| WO3 | visible light | RT | NO2 | [56] |
| ultrathin layers of In2O3 nanoparticles | GaInN/GaN based LED | RT | O3 | [57] |
| 7 nm In2O3 nanoparticle and 12 nm nanoparticle | RT | RT | O3 | [58] |
| Room Temperature | ||
| Material Condition | ZnO | ZnO:Au NPs |
| Dark | No response | No response |
| UV | Low response | High response |
| High Temperature (125 °C) | ||
| Material Condition | ZnO | ZnO:Au NPs |
| dark | No response | Have response |
| UV | Have response | High response |
| Material | Light Illumination | Temperature | Vapor | Ref |
|---|---|---|---|---|
| Nb5+-doped SrTiO3 | UV | RT | O2 | [96] |
| Fe3+-doped SrTiO3 | UV | RT | O2 | [96] |
| Cr3+-doped SrTiO3 | UV | RT | O2 | [96] |
| Ga-doped ZnO nanopowder | UV | various temperatures | CO | [97] |
| Ga-doped ZnO nanocrystal | 460 nm visible light | temperatures ranging from 25 °C to 100 °C | H2, NO2 | [98] |
| Fe-doped ZnO flowers | 532 nm light | RT | HCHO | [99] |
| Fe-doped ZnO flowers | 532 nm light | RT | HCHO | [100] |
| carbon-doped ZnO microspheres | UV | RT | ethanol | [101] |
| Material | Light Illumination | Temperature | Vapor | Concentration (ppm) | Response | Ref |
|---|---|---|---|---|---|---|
| ZnO | UV 3.6 mW/cm2 (365 nm) | RT | Ethanol | 100 | 0.14 | [64] |
| SnO2-GaN nanowires | UV 3.75 mW/m2 (365 nm) | RT | Ethanol | 500 | 1.01 | [105] |
| ZnO nanodisk | UV 1.6 mW/cm2 (365 nm) | RT | Ethanol | 100 | 1.17 | [106] |
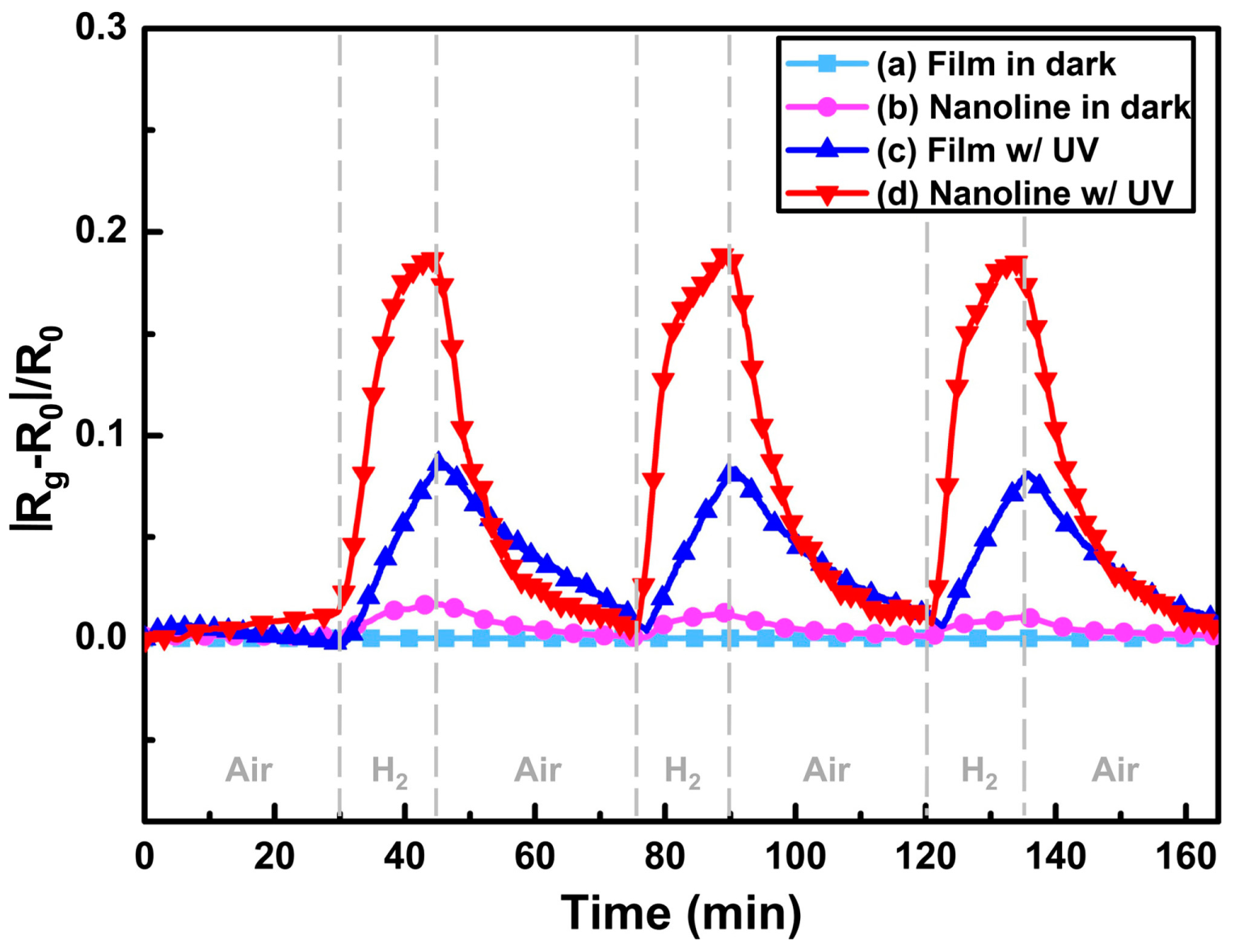
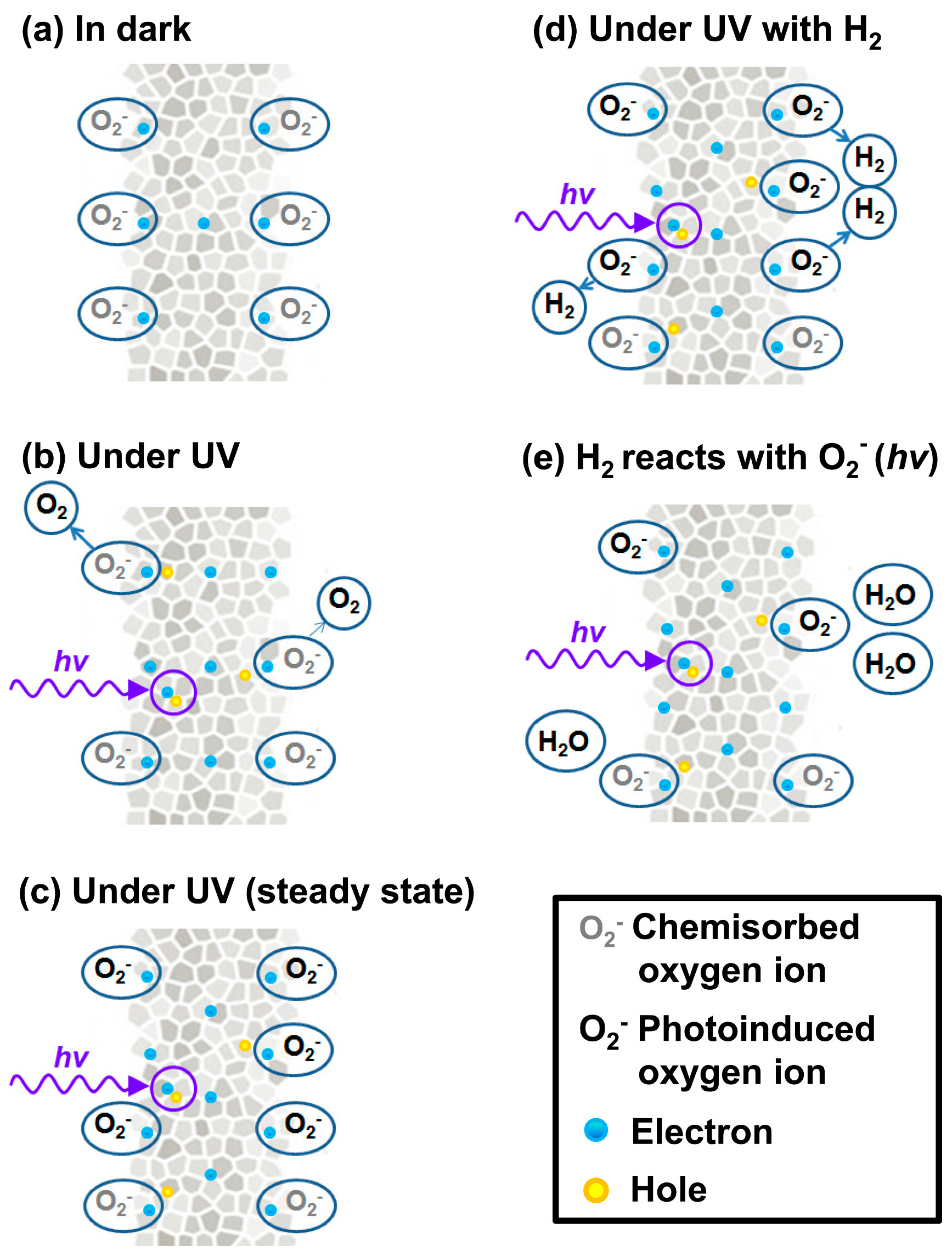
| ZnO thin film | UV | RT | H2 | 100 | 0.1 ΔR/Rair | [36] |
| ZnO nanoline | UV | RT | H2 | 100 | 0.2 | [36] |
| ZnO | White light LED | RT | Ethylene | 5200 | 1.06 | [37] |
| ZnO | White light LED | RT | H2O2 | 100 | 0.5 ΔR/Rg | [42] |
| SnO2 | UV | RT | CO | 200 | 0.84 ΔG/Gair | [16] |
| TiO2 NWs-80 nm | UV | RT | NH3 | 100 | 0.5 ΔZ/Zair | [60] |
| TiO2 NWs-550 nm | UV | RT | NH3 | 100 | 0.1 | [60] |
| ZnO | UV | RT | Ethanol | 60 | 0.8 ΔI/Iair | [14] |
| ZnO | Visible light | RT | HCHO | 110 | 1.35% ΔI/Iair | [10] |
| Cu/ZnO | 355 nm | RT | ethanol | 1120 | 64% ΔI/Iair | [11] |
| Cu/ZnO | 355 nm | RT | acetone | 1120 | 63% ΔI/Iair | [11] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; HO, H.-P. Light-Activated Metal Oxide Gas Sensors: A Review. Micromachines 2017, 8, 333. https://doi.org/10.3390/mi8110333
Xu F, HO H-P. Light-Activated Metal Oxide Gas Sensors: A Review. Micromachines. 2017; 8(11):333. https://doi.org/10.3390/mi8110333
Chicago/Turabian StyleXu, Fang, and Ho-Pui HO. 2017. "Light-Activated Metal Oxide Gas Sensors: A Review" Micromachines 8, no. 11: 333. https://doi.org/10.3390/mi8110333




