A Microfluidic Device for Culturing an Encapsulated Ovarian Follicle
Abstract
:1. Introduction
2. Materials and Methods
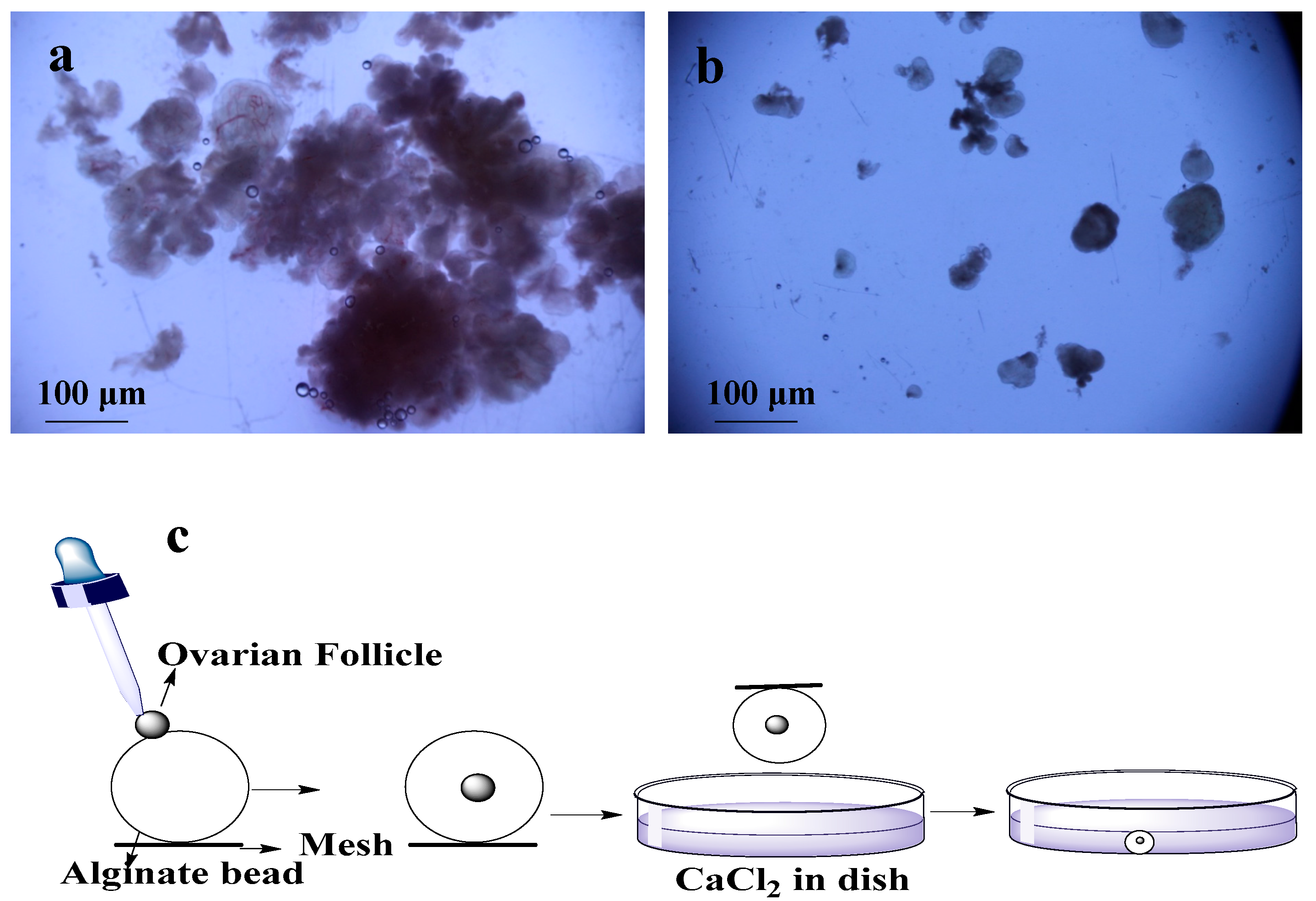
2.1. Isolation of Ovarian Follicles
2.2. Encapsulation of Ovarian Follicles
2.3. Culture of Ovarian Follicle in Dish
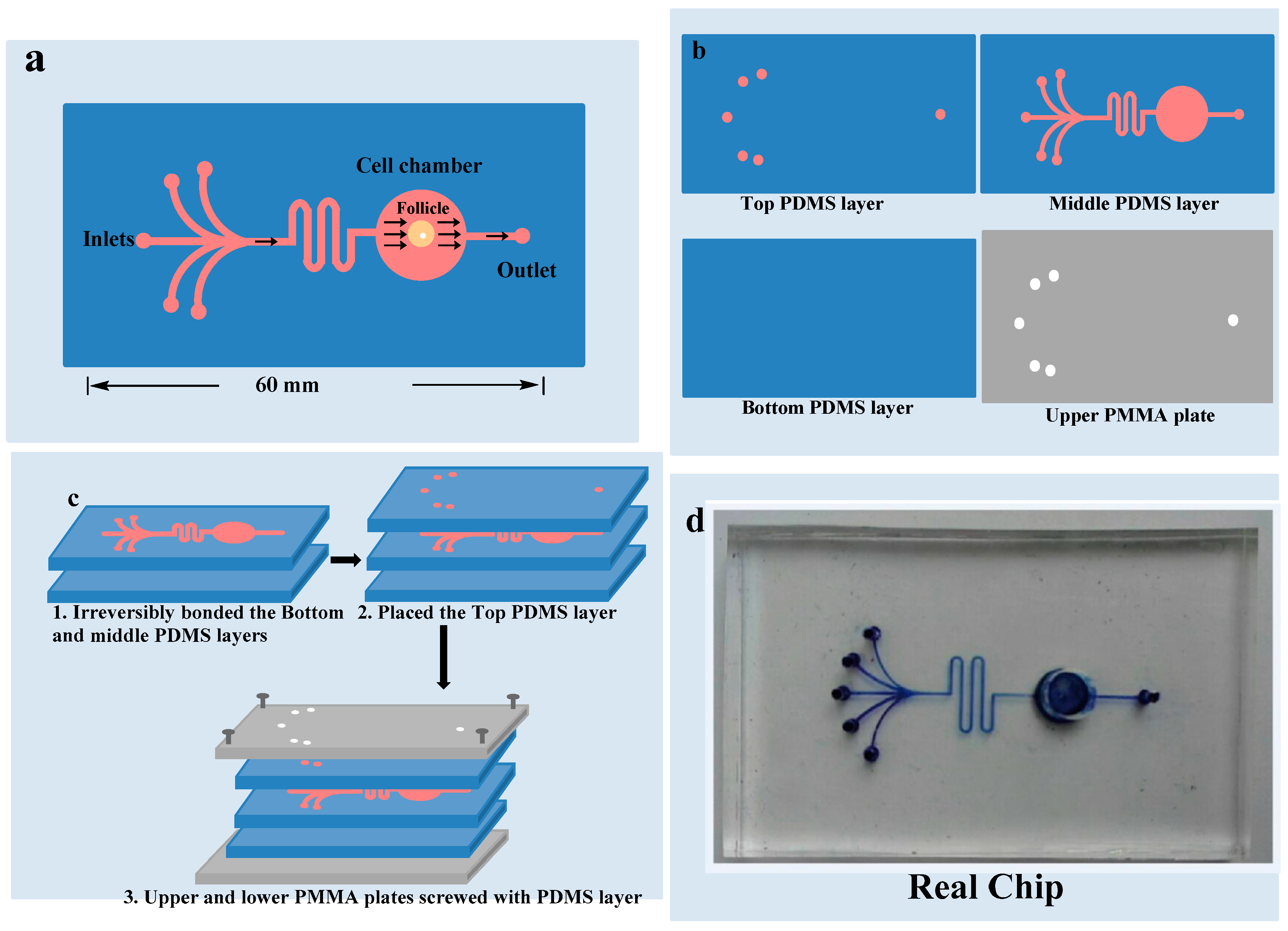
2.4. Fabrication of the Microfluidic Model
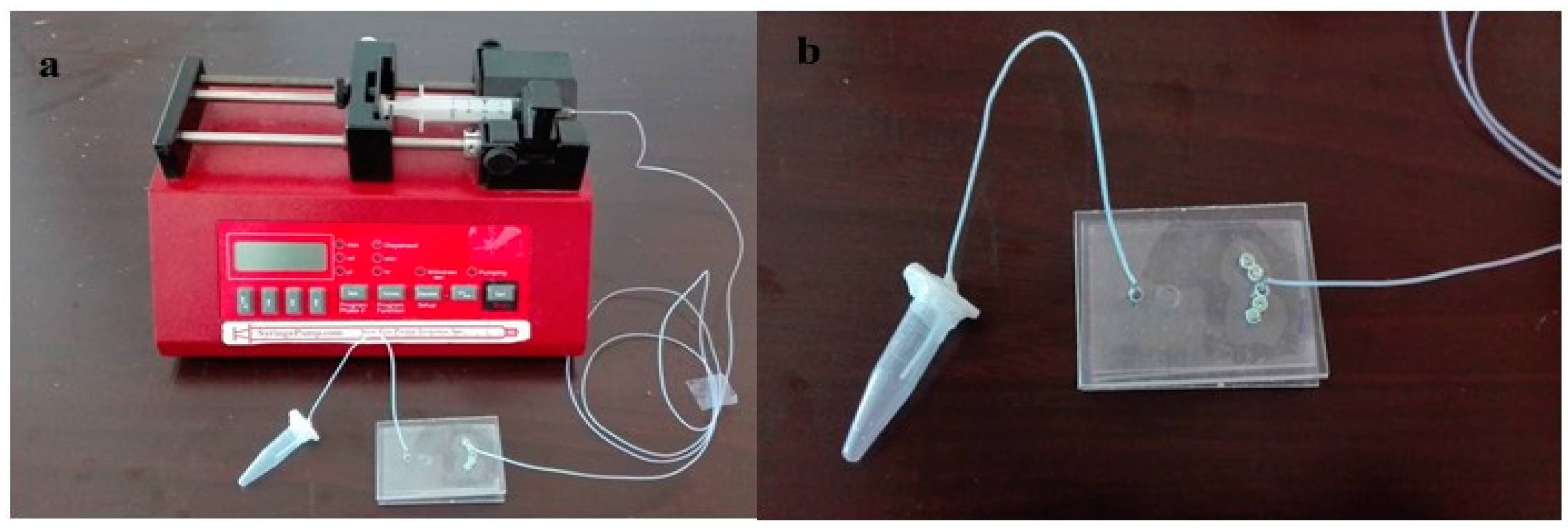
2.5. Culture of Ovarian Follicle on Chip
2.6. Follicle Measurements and Hormone Tests
2.7. Statistical Analysis
3. Results
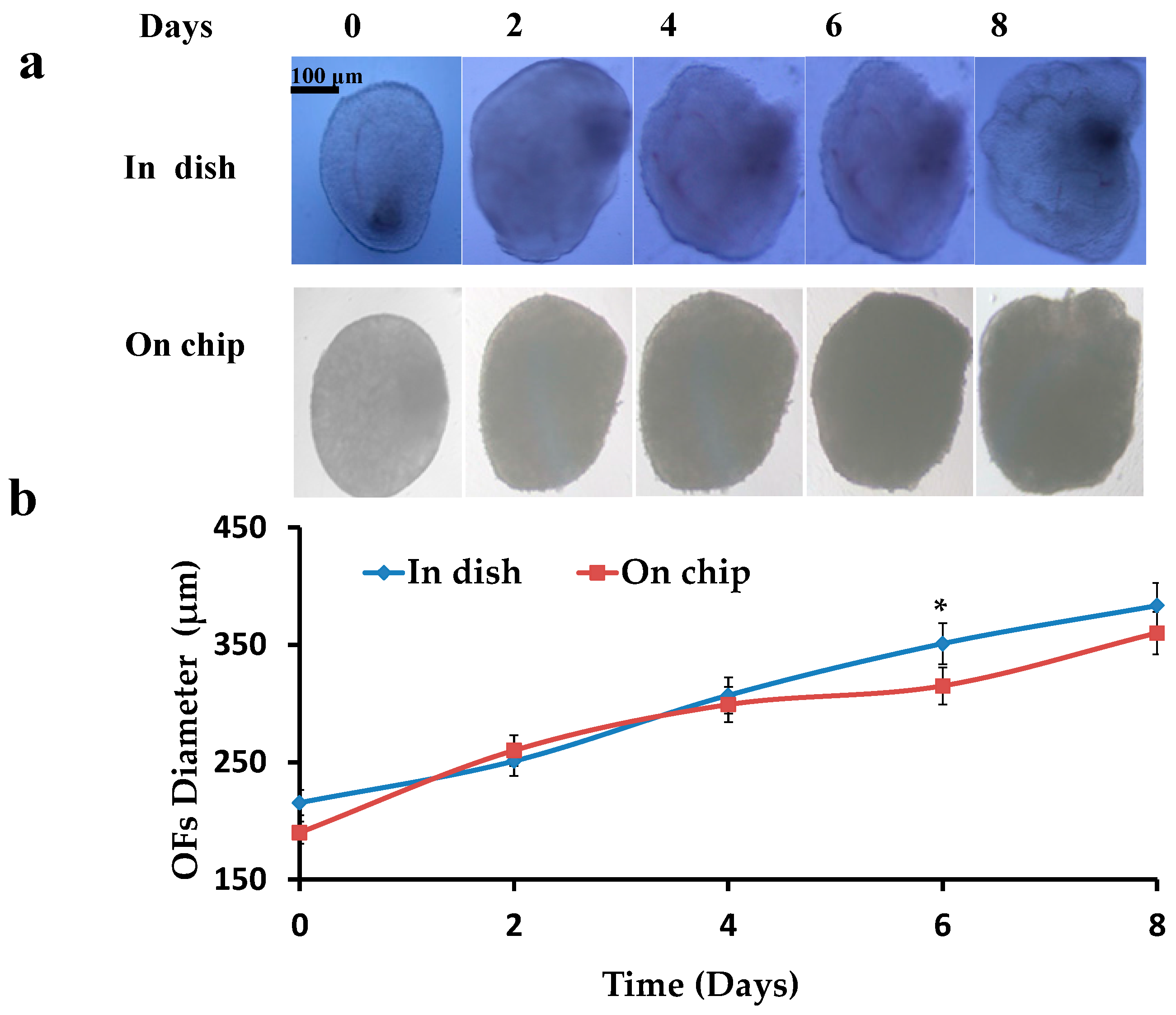
3.1. Diameter of the Follicles
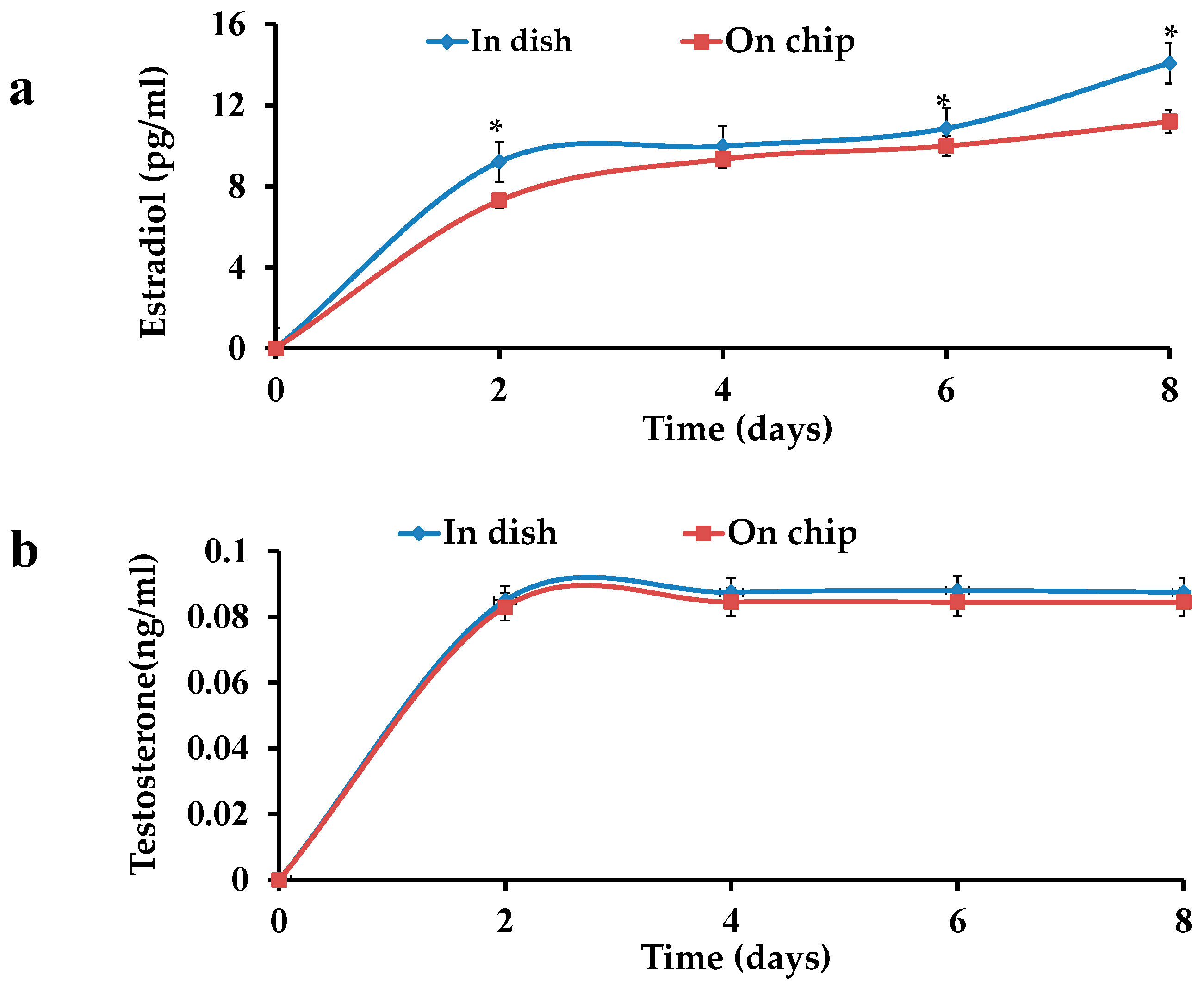
3.2. Hormone Tests for the Ovarian Follicles in Dish and on Chip
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aziz, A.U.R.; Geng, C.; Fu, M.; Yu, X.; Qin, K.; Liu, B. The role of microfluidics for organ on chip simulations. Bioengineering 2017, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Toth, T.L. In vitro culture of ovarian follicles from peromyscus. Semin. Cell Dev. Biol. 2017, 61, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Alex, A.; AbdelHafez, F.; Calabro, A.; Goldfarb, J.; Fleischman, A.; Falcone, T. Three-dimensional in vitro follicle growth: Overview of culture models, biomaterials, design parameters and future directions. Reprod. Biol. Endocrinol. 2010, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Agarwal, P.; He, X. In vitro culture of early secondary preantral follicles in hanging drop of ovarian cell-conditioned medium to obtain mii oocytes from outbred deer mice. Tissue Eng. Part A 2013, 19, 2626–2637. [Google Scholar] [CrossRef] [PubMed]
- West, E.R.; Shea, L.D.; Woodruff, T.K. Engineering the follicle microenvironment. Semin. Reprod. Med. 2007, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Shikanov, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials 2009, 30, 5476–5485. [Google Scholar] [CrossRef] [PubMed]
- Shea, L.D.; Woodruff, T.K.; Shikanov, A. Bioengineering the ovarian follicle microenvironment. Annu. Rev. Biomed. Eng. 2014, 16, 29–52. [Google Scholar] [CrossRef] [PubMed]
- He, X. Microfluidic encapsulation of ovarian follicles for 3d culture. Ann. Biomed Eng. 2017, 45, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, fsh, anti-mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Agarwal, P.; Huang, H.; Zhao, S.; He, X. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials 2014, 35, 5122–5128. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Choi, J.K.; Huang, H.; Zhao, S.; Dumbleton, J.; Li, J.; He, X. A biomimetic core-shell platform for miniaturized 3d cell and tissue engineering. Part. Part. Syst. Charact. 2015, 32, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Coppeta, J.R.; Rogers, H.B.; Isenberg, B.C.; Zhu, J.; Olalekan, S.A.; McKinnon, K.E.; Dokic, D.; Rashedi, A.S.; Haisenleder, D.J.; et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Kreeger, P.K.; Shea, L.D.; Woodruff, T.K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006, 12, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Barrett, S.L.; West-Farrell, E.; Kondapalli, L.A.; Kiesewetter, S.E.; Shea, L.D.; Woodruff, T.K. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum. Reprod. 2009, 24, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Greenwald, G.S. Methods of separation and in vitro culture of pre-antral follicles from mammalian ovaries. Hum. Reprod. Update 1996, 2, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Camboni, A.; Van Langendonckt, A.; Donnez, J.; Vanacker, J.; Dolmans, M.M.; Amorim, C.A. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology 2013, 67, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Banc, A.; Woodruff, T.K.; Shea, L.D. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol. Bioeng. 2009, 103, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, J.; Romero, M.M.; Smith, K.N.; Shea, L.D.; Woodruff, T.K. In vitro follicle growth supports human oocyte meiotic maturation. Sci. Rep. 2015, 5, 17323. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Ma, X.C.; Luo, Y.; Fang, S.M.; Xie, Z.R.; Li, X.J.; Qi, D.Y.; Zhang, F.Y.; Kong, J.; Li, J.; et al. Application of a microfluidic-based perivascular tumor model for testing drug sensitivity in head and neck cancers and toxicity in endothelium. RSC Adv. 2016, 6, 29598–29607. [Google Scholar] [CrossRef]
- Stocco, C. Aromatase expression in the ovary: Hormonal and molecular regulation. Steroids 2008, 73, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.A.; Mannan, M.A.; O’Shaughnessy, P.J. Development of cytochrome p450 aromatase mrna levels and enzyme activity in ovaries of normal and hypogonadal (hpg) mice. J. Mol. Endocrinol. 1995, 14, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, T.W.; Wright, P.; Nelson, S.M.; Anderson, R.A.; Wallace, W.H. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS ONE 2011, 6, e22024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagen, C.P.; Aksglaede, L.; Sorensen, K.; Mouritsen, A.; Andersson, A.M.; Petersen, J.H.; Main, K.M.; Juul, A. Individual serum levels of anti-mullerian hormone in healthy girls persist through childhood and adolescence: A longitudinal cohort study. Hum. Reprod. 2012, 27, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Lashen, H.; Dunger, D.B.; Ness, A.; Ong, K.K. Peripubertal changes in circulating antimullerian hormone levels in girls. Fertil. Steril. 2013, 99, 2071–2075. [Google Scholar] [CrossRef] [PubMed]
- La Marca, A.; Malmusi, S.; Giulini, S.; Tamaro, L.F.; Orvieto, R.; Levratti, P.; Volpe, A. Anti-mullerian hormone plasma levels in spontaneous menstrual cycle and during treatment with fsh to induce ovulation. Hum. Reprod. 2004, 19, 2738–2741. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.; Margalioth, E.J.; Chetrit, A.B.; Gal, M.; Goldberg, D.; Alerhand, S.; Eldar-Geva, T. The dynamics of serum anti-mullerian-hormone levels during controlled ovarian hyperstimulation with gnrh-antagonist short protocol in polycystic ovary syndrome and low responders. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 176, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Kim, S.H.; Kim, S.M.; Jee, B.C.; Ku, S.Y.; Suh, C.S.; Choi, Y.M.; Kim, J.G.; Moon, S.Y. Anti-mullerian hormone dynamics during controlled ovarian hyperstimulation and optimal timing of measurement for outcome prediction. Hum. Reprod. 2010, 25, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.Y.; Byskov, A.G. Estradiol and regulation of anti-mullerian hormone, inhibin-a, and inhibin-b secretion: Analysis of small antral and preovulatory human follicles' fluid. J. Clin. Endocrinol. Metab. 2006, 91, 4064–4069. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.Y.; Lossl, K. Increased intrafollicular androgen levels affect human granulosa cell secretion of anti-mullerian hormone and inhibin-b. Fertil. Steril. 2008, 89, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.E.; Rasmussen, I.A.; Kristensen, S.G.; Christensen, S.T.; Mollgard, K.; Wreford Andersen, E.; Byskov, A.G.; Yding Andersen, C. In human granulosa cells from small antral follicles, androgen receptor mrna and androgen levels in follicular fluid correlate with fsh receptor mrna. Mol. Hum. Reprod. 2011, 17, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Liberty, G.; Ben-Chetrit, A.; Margalioth, E.J.; Hyman, J.H.; Galoyan, N.; Eldar-Geva, T. Does estrogen directly modulate anti-mullerian hormone secretion in women? Fertil. Steril. 2010, 94, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Gnoth, C.; Roos, J.; Broomhead, D.; Schiffner, J.; Godehardt, E.; Freundl, G.; Johnson, S. Antimullerian hormone levels and numbers and sizes of antral follicles in regularly menstruating women of reproductive age referenced to true ovulation day. Fertil. Steril. 2015, 104, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Guerrier, D.; Boussin, L.; Mader, S.; Josso, N.; Kahn, A.; Picard, J.Y. Expression of the gene for anti-mullerian hormone. J. Reprod. Fertil. 1990, 88, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, M.; Pierre, A.; Rey, R.; Leclerc, A.; Arouche, N.; Hesters, L.; Catteau-Jonard, S.; Frydman, R.; Picard, J.Y.; Fanchin, R.; et al. Differential regulation of ovarian anti-mullerian hormone (amh) by estradiol through alpha- and beta-estrogen receptors. J. Clin. Endocrinol. Metab. 2012, 97, E1649–E1657. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Yates, M.M.; Deroo, B.J.; Korach, K.S. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 2005, 146, 3247–3262. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, A.U.R.; Fu, M.; Deng, J.; Geng, C.; Luo, Y.; Lin, B.; Yu, X.; Liu, B. A Microfluidic Device for Culturing an Encapsulated Ovarian Follicle. Micromachines 2017, 8, 335. https://doi.org/10.3390/mi8110335
Aziz AUR, Fu M, Deng J, Geng C, Luo Y, Lin B, Yu X, Liu B. A Microfluidic Device for Culturing an Encapsulated Ovarian Follicle. Micromachines. 2017; 8(11):335. https://doi.org/10.3390/mi8110335
Chicago/Turabian StyleAziz, Aziz Ur Rehman, Mengjie Fu, Jiu Deng, Chunyang Geng, Yong Luo, Bingcheng Lin, Xiaohui Yu, and Bo Liu. 2017. "A Microfluidic Device for Culturing an Encapsulated Ovarian Follicle" Micromachines 8, no. 11: 335. https://doi.org/10.3390/mi8110335



