Recent Progress toward Microfluidic Quality Control Testing of Radiopharmaceuticals
Abstract
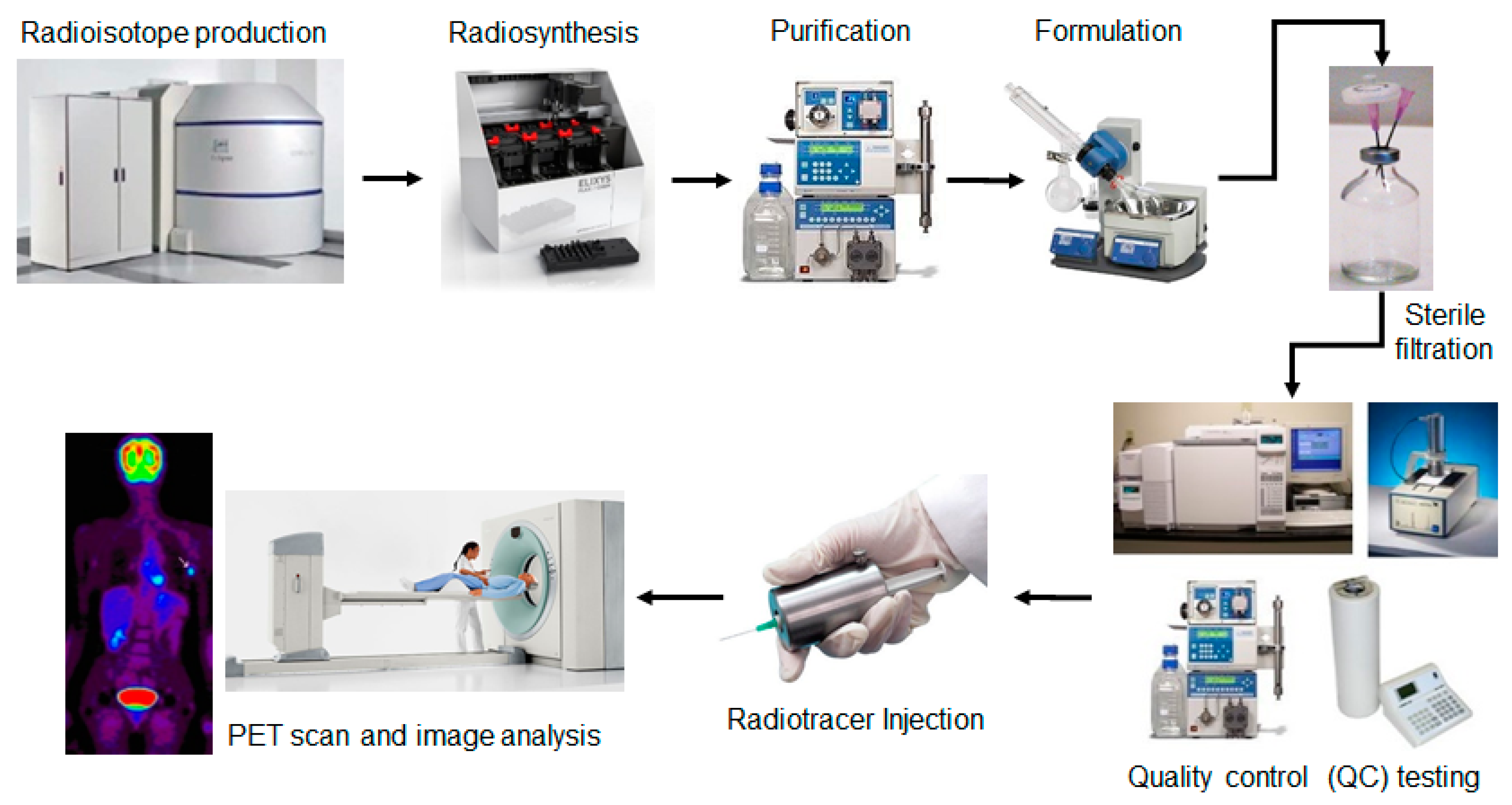
:1. Introduction
2. Miniaturization of Quality Control Tests
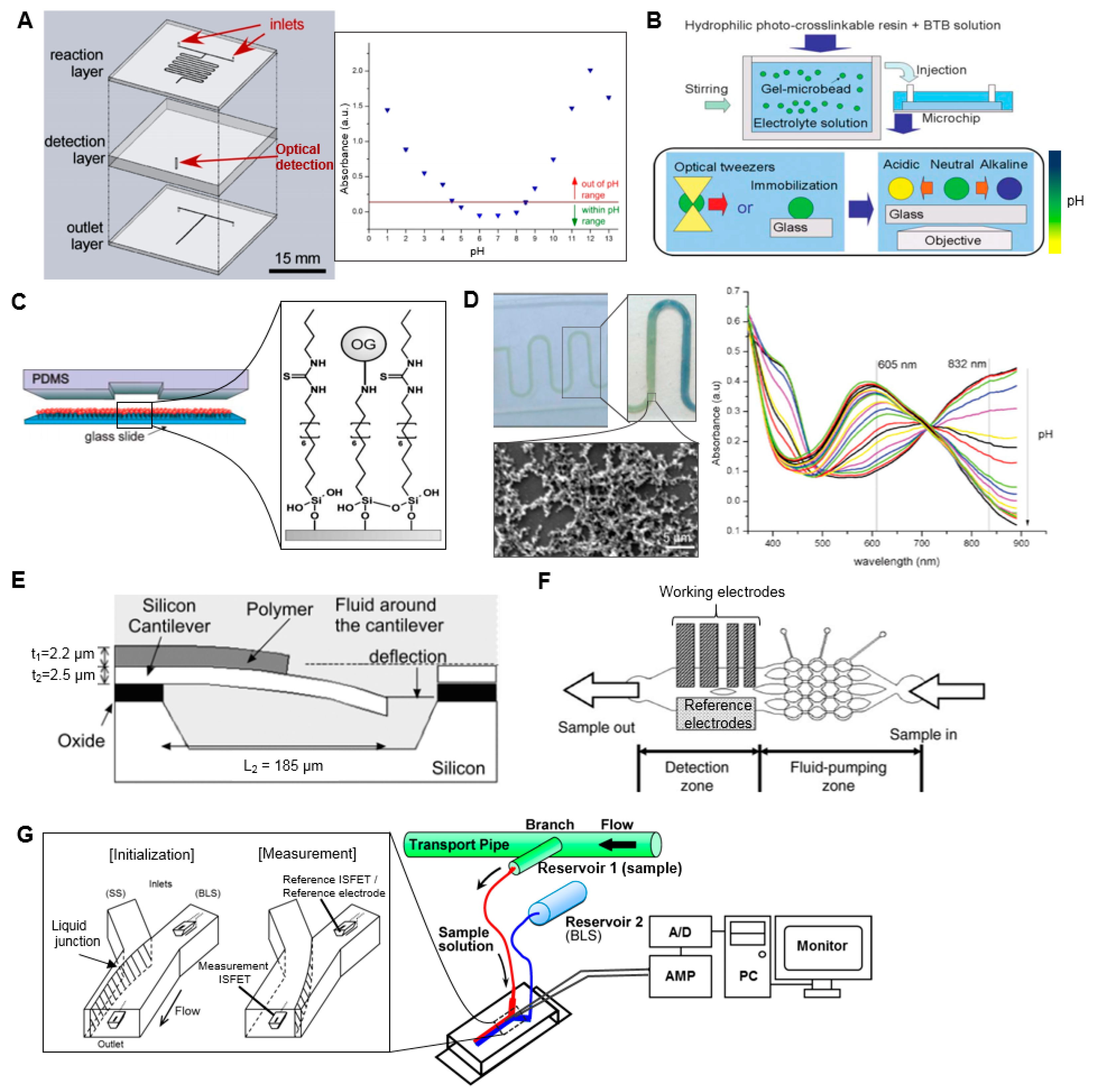
2.1. pH Test
2.2. Appearance Test (Optical Clarity Test)
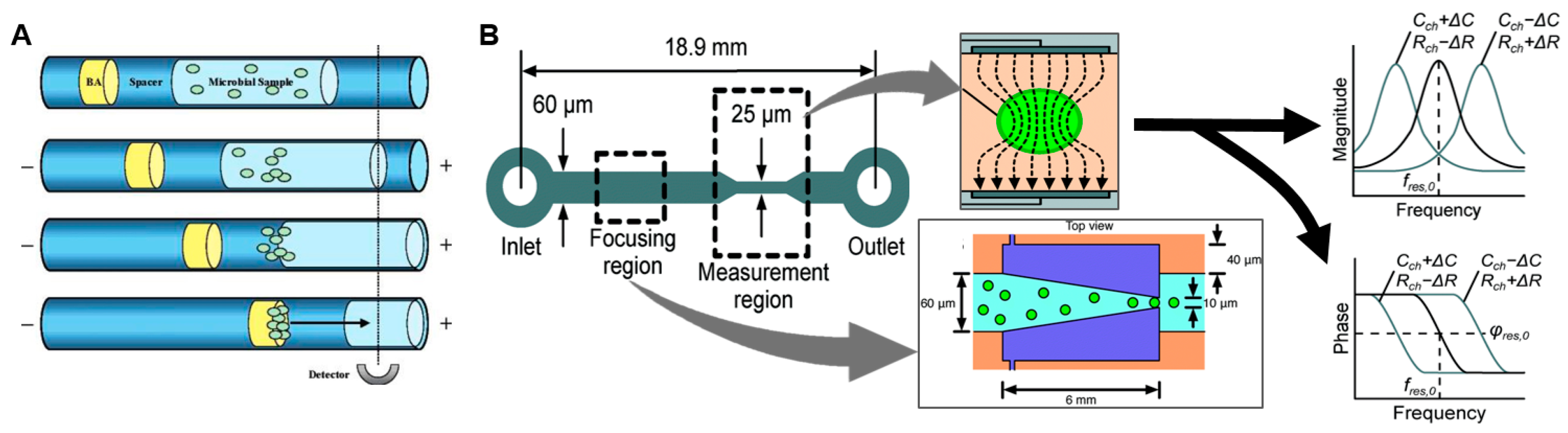
2.3. Sterility Test
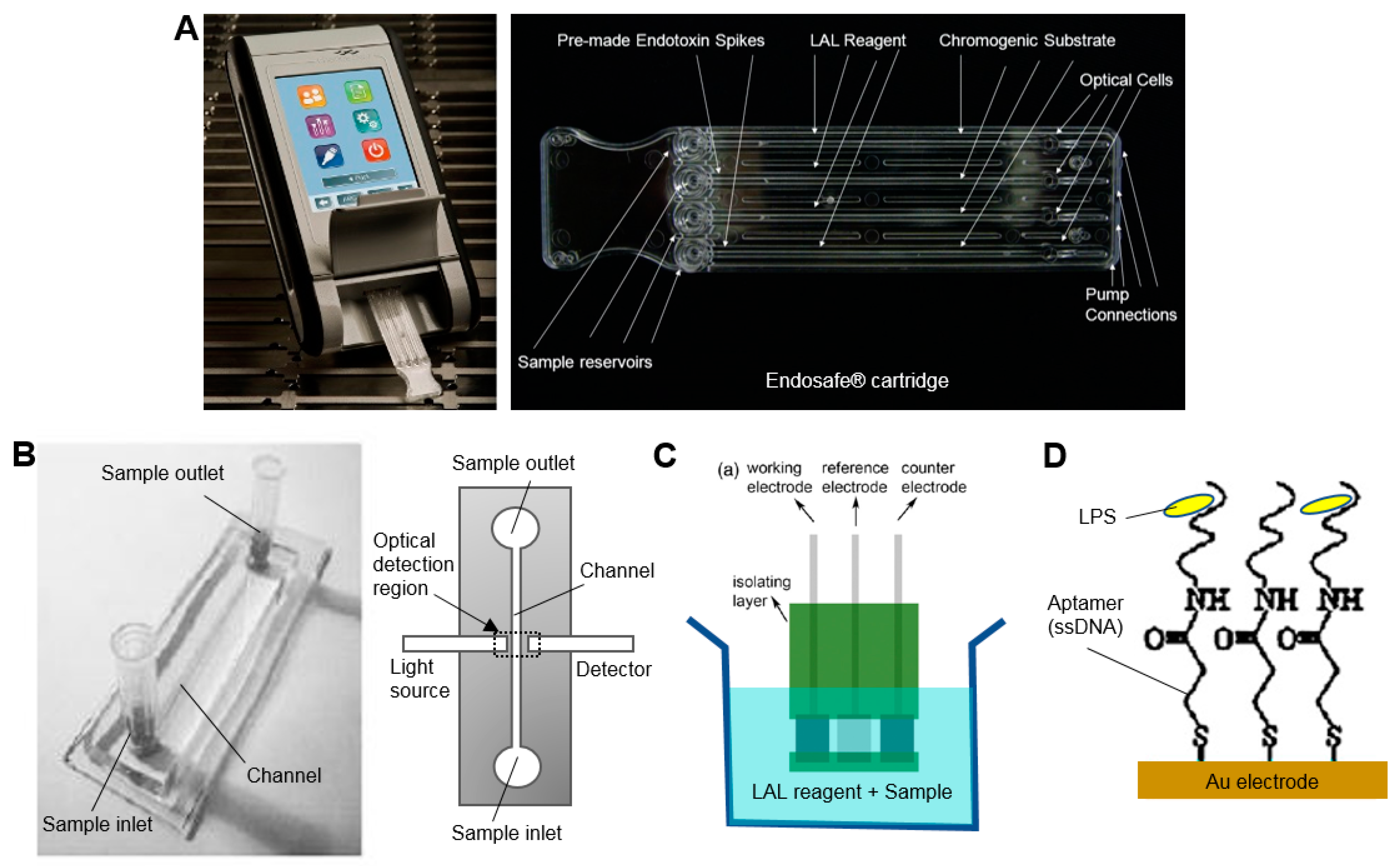
2.4. Bacterial Endotoxin Test
2.5. Chemical Purity and Identity
2.6. Kryptofix 2.2.2 (K222)
2.7. Residual Organic Solvents
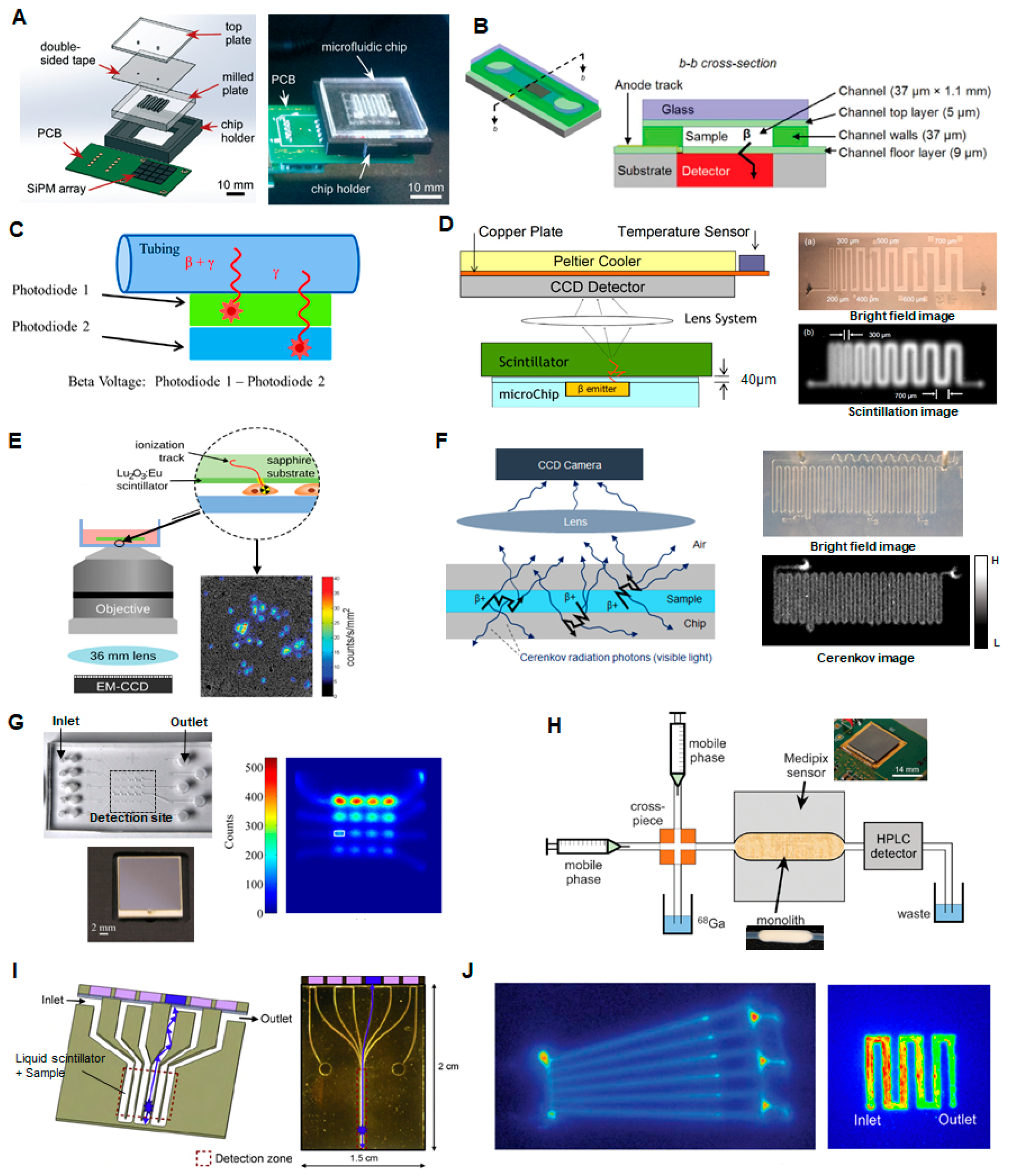
2.8. Radioactivity Measurement (Radioactivity Concentration)
2.9. Radionuclidic Purity and Identity
2.10. Radiochemical Purity and Identity
2.11. Molar Activity
3. Outlook
Acknowledgments
Conflicts of Interest
References
- Mariani, G.; Bruselli, L.; Kuwert, T.; Kim, E.E.; Flotats, A.; Israel, O.; Dondi, M.; Watanabe, N. A review on the clinical uses of SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1959–1985. [Google Scholar] [CrossRef] [PubMed]
- Phelps, M.E. PET: Molecular Imaging and Its Biological Applications, 1st ed.; Springer Science & Business Media: Berlin, Germany, 2004; ISBN 0-387-40359-0. [Google Scholar]
- Phelps, M.E. PET: The Merging of Biology and Imaging into Molecular Imaging. J. Nucl. Med. 2000, 41, 661–681. [Google Scholar] [PubMed]
- Bansal, A.; Pandey, M.K.; Demirhan, Y.E.; Nesbitt, J.J.; Crespo-Diaz, R.J.; Terzic, A.; Behfar, A.; DeGrado, T.R. Novel 89Zr cell labeling approach for PET-based cell trafficking studies. EJNMMI Res. 2015, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Glaudemans, A.W.J.M.; de Vries, E.F.J.; Galli, F.; Dierckx, R.A.J.O.; Slart, R.H.J.A.; Signore, A. The Use of 18F-FDG-PET/CT for Diagnosis and Treatment Monitoring of Inflammatory and Infectious Diseases. Clin. Dev. Immunol. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, F.; Kirjavainen, A.K.; Forsback, S.; Krzyczmonik, A.; Keller, T.; Newington, I.M.; Glaser, M.; Luthra, S.K.; Solin, O.; Gouverneur, V. Organomediated Enantioselective 18F Fluorination for PET Applications. Angew. Chem. Int. Ed. 2015, 54, 13366–13369. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.L.; Willowson, K.P. An Evidence-Based Review of Quantitative SPECT Imaging and Potential Clinical Applications. J. Nucl. Med. 2013, 54, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.M.; Scott, P.J.H.; Thompson, S. Clinical Applications of Radiolabeled Peptides for PET. Semin. Nucl. Med. 2017, 47, 493–523. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.M.; Tremoleda, J.L.; Bayomy, T.B.; Gsell, W. Molecular SPECT Imaging: An Overview. Available online: https://www.hindawi.com/journals/ijmi/2011/796025/abs/ (accessed on 19 August 2017).
- Fermi, E. Quality Control of PET Radiopharmaceuticals. In Molecular Imaging: Radiopharmaceuticals for PET and SPECT; Springer: Berlin/Heidelberg, Germany, 2009; pp. 197–204. ISBN 978-3-540-76734-3. [Google Scholar]
- Scott, P.J.H.; Hockley, B.G. Radiochemical Syntheses, Radiopharmaceuticals for Positron Emission Tomography; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-118-14033-8. [Google Scholar]
- U.S. Pharmacopeia (USP). General Chapter <823>: Positron Emission Tomography Drugs for Compounding, Investigation, and Research Uses; USP: Rockville, MD, USA, 2011. [Google Scholar]
- CFR-Code of Federal Regulations Title 21. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm (accessed on 30 June 2013).
- European Directorate for the Quality of Medicines & Healthcare. Technical Guide for the Elaboration of Monographs on Radiopharmaceutical Preparations; European Directorate for the Quality of Medicines & Healthcare: Strasbourg, France, 2010. [Google Scholar]
- Hung, J.C. Comparison of Various Requirements of the Quality Assurance Procedures for 18F-FDG Injection. J. Nucl. Med. 2002, 43, 1495–1506. [Google Scholar] [PubMed]
- Yu, S. Review of 18F-FDG synthesis and quality control. Biomed. Imaging Interv. J. 2006, 2, e57. [Google Scholar] [CrossRef] [PubMed]
- Lodi, F.; Boschi, S. Quality Control of PET Radiopharmaceuticals. In Basic Science of PET Imaging; Springer: Cham, Switzerland, 2017; pp. 105–126. ISBN 978-3-319-40068-6. [Google Scholar]
- Ferguson, D.; McGrath, S.; O’Hara, G.; Marshall, C. Investigation of staff finger doses during quality control of FDG production. Health Phys. 2011, 100, 523–529. [Google Scholar] [CrossRef] [PubMed]
- QC1. Automatic Quality Control for PET and MI Tracers. Available online: http://www.qc1.com/ (accessed on 1 December 2012).
- Trace-Ability Inc. SBIR Source. Available online: http://sbirsource.com/sbir/firms/26162-trace-ability-inc (accessed on 26 December 2015).
- Anzellotti, A.I.; McFarland, A.R.; Ferguson, D.; Olson, K.F. Towards the Full Automation of QC Release Tests for [18F] fluoride-labeled Radiotracers. Curr. Org. Chem. 2013, 17, 2153–2158. [Google Scholar] [CrossRef]
- Awasthi, V.; Watson, J.; Gali, H.; Matlock, G.; McFarland, A.; Bailey, J.; Anzellotti, A. A “dose on demand” Biomarker Generator for automated production of [18F] F- and [18F]FDG. Appl. Radiat. Isot. 2014, 89, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Elizarov, A.M.; van Dam, R.M.; Shin, Y.S.; Kolb, H.C.; Padgett, H.C.; Stout, D.; Shu, J.; Huang, J.; Daridon, A.; Heath, J.R. Design and Optimization of Coin-Shaped Microreactor Chips for PET Radiopharmaceutical Synthesis. J. Nucl. Med. 2010, 51, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Rensch, C.; Jackson, A.; Lindner, S.; Salvamoser, R.; Samper, V.; Riese, S.; Bartenstein, P.; Wängler, C.; Wängler, B. Microfluidics: A Groundbreaking Technology for PET Tracer Production? Molecules 2013, 18, 7930–7956. [Google Scholar] [CrossRef] [PubMed]
- Pascali, G.; Watts, P.; Salvadori, P. Microfluidics in radiopharmaceutical chemistry. Nucl. Med. Biol. 2013, 40, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Keng, P.Y.; van Dam, R.M. Digital Microfluidics: A New Paradigm for Radiochemistry. Mol. Imaging 2015, 14, 579–594. [Google Scholar]
- Chao, P.H.; Collins, J.; Argus, J.P.; Tseng, W.-Y.; Lee, J.T.; van Dam, R.M. Automatic concentration and reformulation of PET tracers via microfluidic membrane distillation. Lab Chip 2017, 17, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Pascali, G.; Nannavecchia, G.; Pitzianti, S.; Salvadori, P.A. Dose-on-demand of diverse 18F-fluorocholine derivatives through a two-step microfluidic approach. Nucl. Med. Biol. 2011, 38, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Hansteen, O.H.; Jakobsen, J.B.; Lindgaard, S.-E.; Franci, X.; Roed, L.; Andersen, A.; Johansen, G.; Knuttel, T.; Langseth, K.M. Quality Control Devices and Methods for Radiopharmaceuticals. U.S. Patent 9291606, 22 March 2016. [Google Scholar]
- Tarn, M.D.; Isu, A.; Archibald, S.J.; Pamme, N. On-chip absorbance spectroscopy for the determination of optical clarity and pH for the quality control testing of [18F] FDG radiotracer. In Proceedings of the 18th International Conference on Miniaturized Systems for Chemistry and Life Sciences, San Antonio, TX, 26–30 October 2014; pp. 1077–1079. [Google Scholar]
- Rushworth, C.M.; Davies, J.; Cabral, J.T.; Dolan, P.R.; Smith, J.M.; Vallance, C. Cavity-enhanced optical methods for online microfluidic analysis. Chem. Phys. Lett. 2012, 554, 1–14. [Google Scholar] [CrossRef]
- Khan, M.I.; Mukherjee, K.; Shoukat, R.; Dong, H. A review on pH sensitive materials for sensors and detection methods. Microsyst. Technol. 2017, 23, 4391–4404. [Google Scholar] [CrossRef]
- Maruyama, H.; Arai, F.; Fukuda, T. On-chip pH measurement using functionalized gel-microbeads positioned by optical tweezers. Lab Chip 2008, 8, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Maclin, A.Q.; Kim, M.D.; Dergunov, S.A.; Pinkhassik, E.; Lindner, E. Small-Volume pH Sensing with a Capillary Optode Utilizing Dye-Loaded Porous Nanocapsules in a Hydrogel Matrix. Electroanalysis 2015, 27, 733–744. [Google Scholar] [CrossRef]
- Mela, P.; Onclin, S.; Goedbloed, M.H.; Levi, S.; García-Parajó, M.F.; van Hulst, N.F.; Ravoo, B.J.; Reinhoudt, D.N.; van den Berg, A. Monolayer-functionalized microfluidics devices for optical sensing of acidity. Lab Chip 2005, 5, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Florea, L.; Fay, C.; Lahiff, E.; Phelan, T.; O’Connor, N.E.; Corcoran, B.; Diamond, D.; Benito-Lopez, F. Dynamic pH mapping in microfluidic devices by integrating adaptive coatings based on polyaniline with colorimetric imaging techniques. Lab Chip 2013, 13, 1079–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on Hydrogel-based pH Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Thong Trinh, Q.; Gerlach, G.; Sorber, J.; Arndt, K.-F. Hydrogel-based piezoresistive pH sensors: Design, simulation and output characteristics. Sens. Actuators B Chem. 2006, 117, 17–26. [Google Scholar] [CrossRef]
- Hilt, J.Z.; Gupta, A.K.; Bashir, R.; Peppas, N.A. Ultrasensitive BioMEMS Sensors Based on Microcantilevers Patterned with Environmentally Responsive Hydrogels. Biomed. Microdevices 2003, 5, 177–184. [Google Scholar] [CrossRef]
- Lin, C.-F.; Lee, G.-B.; Wang, C.-H.; Lee, H.-H.; Liao, W.-Y.; Chou, T.-C. Microfluidic pH-sensing chips integrated with pneumatic fluid-control devices. Biosens. Bioelectron. 2006, 21, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Suzuki, M. A Microfluidic pH Measurement Device with a Flowing Liquid Junction. Sensors 2017, 17, 1563. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, HHS. Current good manufacturing practice for positron emission tomography drugs. Fed. Regist. 2009, 74, 65409–65436. [Google Scholar]
- U.S. Pharmacopeia (USP). General Chapters: <71> STERILITY TESTS. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_c71.html (accessed on 9 September 2017).
- Foudeh, A.M.; Didar, T.F.; Veres, T.; Tabrizian, M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip 2012, 12, 3249–3266. [Google Scholar] [CrossRef] [PubMed]
- Bridle, H.; Miller, B.; Desmulliez, M.P.Y. Application of microfluidics in waterborne pathogen monitoring: A review. Water Res. 2014, 55, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Brandão, D.; Liébana, S.; Pividori, M.I. Multiplexed detection of foodborne pathogens based on magnetic particles. New Biotechnol. 2015, 32, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Mairhofer, J.; Roppert, K.; Ertl, P. Microfluidic Systems for Pathogen Sensing: A Review. Sensors 2009, 9, 4804–4823. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, G.-Y.; Seok Seo, T. An integrated passive micromixer—Magnetic separation—Capillary electrophoresis microdevice for rapid and multiplex pathogen detection at the single-cell level. Lab Chip 2011, 11, 3465–3470. [Google Scholar] [CrossRef] [PubMed]
- Lantz, A.W.; Bao, Y.; Armstrong, D.W. Single-Cell Detection: Test of Microbial Contamination Using Capillary Electrophoresis. Anal. Chem. 2007, 79, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Morgan, H. Single-cell microfluidic impedance cytometry: A review. Microfluid. Nanofluid. 2010, 8, 423–443. [Google Scholar] [CrossRef]
- Varshney, M.; Li, Y. Interdigitated array microelectrodes based impedance biosensors for detection of bacterial cells. Biosens. Bioelectron. 2009, 24, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Haandbæk, N.; With, O.; Bürgel, S.C.; Heer, F.; Hierlemann, A. Resonance-enhanced microfluidic impedance cytometer for detection of single bacteria. Lab Chip 2014, 14, 3313–3324. [Google Scholar] [CrossRef] [PubMed]
- U.S. Pharmacopeia (USP). General Chapters: <85> BACTERIAL ENDOTOXINS TEST. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_c85.html (accessed on 12 August 2017).
- Das, A.P.; Kumar, P.S.; Swain, S. Recent advances in biosensor based endotoxin detection. Biosens. Bioelectron. 2014, 51, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Charles River Laboratories, Inc. Endotoxin Testing Systems. Available online: http://www.criver.com/products-services/rapid-micro/endosafe/endotoxin-rapid-testing-systems (accessed on 16 September 2017).
- Suh, C.W.; Hwang, S.Y.; Choi, H.J.; Seong, G.H.; Ahn, Y.M.; Kim, Y.S.; Lee, E.K. Feasibility of on-chip detection of endotoxin by LAL test. Biotechnol. Bioprocess Eng. 2004, 9, 132–136. [Google Scholar] [CrossRef]
- Miao, P.; Han, K.; Qi, J.; Zhang, C.; Liu, T. Electrochemical investigation of endotoxin induced limulus amebocyte lysate gel-clot process. Electrochem. Commun. 2013, 26, 29–32. [Google Scholar] [CrossRef]
- Noda, K.; Goto, H.; Murakami, Y.; Ahmed, A.B.F.; Kuroda, A. Endotoxin assay by bioluminescence using mutant firefly luciferase. Anal. Biochem. 2010, 397, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Lin, M.; Lee, H.; Cho, M.; Choe, W.-S.; Lee, Y. Determination of endotoxin through an aptamer-based impedance biosensor. Biosens. Bioelectron. 2012, 32, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Makszin, L.; Kilár, A.; Felső, P.; Péterfi, Z.; Kocsis, B.; Kilár, F. Quantitative microfluidic analysis of S- and R-type endotoxin components with chip capillary electrophoresis. Electrophoresis 2012, 33, 3351–3360. [Google Scholar] [CrossRef] [PubMed]
- Franck, D.; Nann, H.; Davi, P.; Schubiger, P.A.; Ametamey, S.M. Faster analysis of radiopharmaceuticals using ultra performance liquid chromatography (UPLC®) in combination with low volume radio flow cell. Appl. Radiat. Isot. 2009, 67, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Kryza, D.; Janier, M. Radio-UHPLC: A tool for rapidly determining the radiochemical purity of technetium-99m radiopharmaceuticals? Appl. Radiat. Isot. 2013, 78, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, D.S.; Shepodd, T.J.; Kirby, B.J. Microchip HPLC of Peptides and Proteins. Anal. Chem. 2005, 77, 2997–3000. [Google Scholar] [CrossRef] [PubMed]
- Agilent|HPLC-Chip/MS System Solutions. Available online: http://www.agilent.com/en-us/products/liquid-chromatography/low-flow-lc-systems/1260-infinity-hplc-chip-ms-system/gp42776 (accessed on 18 October 2017).
- Šesták, J.; Moravcová, D.; Kahle, V. Instrument platforms for nano liquid chromatography. J. Chromatogr. A 2015, 1421, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Killeen, K. The fundamental aspects and applications of Agilent HPLC-Chip. J. Sep. Sci. 2007, 30, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.R.; Manz, A. Present state of microchip electrophoresis: State of the art and routine applications. J. Chromatogr. A 2015, 1382, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.P.; Cranny, A.; Harris, N.R.; Green, N.G.; Wharton, J.A.; Wood, R.J.K.; Stokes, K.R. Review on the development of truly portable and in-situ capillary electrophoresis systems. Meas. Sci. Technol. 2013, 24, 042001. [Google Scholar] [CrossRef]
- Janasek, D.; Franzke, J.; Manz, A. Scaling and the design of miniaturized chemical-analysis systems. Nature 2006, 442, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Jimidar, M. Capillary Electrophoresis Methods for Pharmaceutical Analysis; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 978-0-08-055961-2. [Google Scholar]
- Mironov, G.G.; Clouthier, C.M.; Akbar, A.; Keillor, J.W.; Berezovski, M.V. Simultaneous analysis of enzyme structure and activity by kinetic capillary electrophoresis-MS. Nat. Chem. Biol. 2016, 12, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Morbioli, G.G.; Mazzu-Nascimento, T.; Aquino, A.; Cervantes, C.; Carrilho, E. Recombinant drugs-on-a-chip: The usage of capillary electrophoresis and trends in miniaturized systems—A review. Anal. Chim. Acta 2016, 935, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.S.; Ly, J.; Jones, J.; Cheung, S.; van Dam, R.M. Novel volumetric method for highly repeatable injection in microchip electrophoresis. Anal. Chim. Acta 2017, 985, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Breadmore, M.C.; Shallan, A.I.; Rabanes, H.R.; Gstoettenmayr, D.; Abdul Keyon, A.S.; Gaspar, A.; Dawod, M.; Quirino, J.P. Recent advances in enhancing the sensitivity of electrophoresis and electrochromatography in capillaries and microchips (2010–2012). Electrophoresis 2013, 34, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Holzgrabe, U.; Brinz, D.; Kopec, S.; Weber, C.; Bitar, Y. Why not using capillary electrophoresis in drug analysis? Electrophoresis 2006, 27, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Kaniansky, D.; Rajec, P.; Švec, A.; Marák, J.; Kovaľ, M.; Lúčka, M.; Franko, Š.; Sabanoš, G. On-column radiometric detector for capillary isotachophoresis. J. Radioanal. Nucl. Chem. 1989, 129, 305–325. [Google Scholar] [CrossRef]
- Kaniansky, D.; Rajec, P.; Švec, A.; Havaši, P.; Macášek, F. On-line radiometric detection in capillary isotachophoresis. J. Chromatogr. A 1983, 258, 238–243. [Google Scholar] [CrossRef]
- Pentoney, S.L.; Zare, R.N.; Quint, J.F. On-line radioisotope detection for capillary electrophoresis. Anal. Chem. 1989, 61, 1642–1647. [Google Scholar] [CrossRef]
- Altria, K.D.; Simpson, C.F.; Bharij, A.K.; Theobald, A.E. A gamma-ray detector for capillary zone electrophoresis and its use in the analysis of some radiopharmaceuticals. Electrophoresis 1990, 11, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, R.; Noll, B.; Johannsen, B. Capillary electrophoresis of 99mtechnetium radiopharmaceuticals. J. Chromatogr. B Biomed. Sci. Appl. 1999, 724, 365–371. [Google Scholar] [CrossRef]
- Cheung, S.; Ly, J.; Lazari, M.; Sadeghi, S.; Keng, P.Y.; van Dam, R.M. The separation and detection of PET tracers via capillary electrophoresis for chemical identity and purity analysis. J. Pharm. Biomed. Anal. 2014, 94, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.L.; Martin, R.S. Integration of on-chip peristaltic pumps and injection valves with microchip electrophoresis and electrochemical detection. Electrophoresis 2010, 31, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Kubáň, P.; Hauser, P.C. Effects of the cell geometry and operating parameters on the performance of an external contactless conductivity detector for microchip electrophoresis. Lab Chip 2005, 5, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Kubáň, P.; Hauser, P.C. A review of the recent achievements in capacitively coupled contactless conductivity detection. Anal. Chim. Acta 2008, 607, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Kubáň, P.; Hauser, P.C. Ten years of axial capacitively coupled contactless conductivity detection for CZE—A review. Electrophoresis 2009, 30, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.E.; Krattiger, B.; Maystre, F.; Widmer, H.M. On-column laser-based refractive index detector for capillary electrophoresis. Anal. Chem. 1991, 63, 2689–2697. [Google Scholar] [CrossRef]
- Redman, E.A.; Mellors, J.S.; Starkey, J.A.; Ramsey, J.M. Characterization of Intact Antibody Drug Conjugate Variants Using Microfluidic Capillary Electrophoresis—Mass Spectrometry. Anal. Chem. 2016, 88, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, K.; Lee, M.; Flynn, G.C. Microchip assays for screening monoclonal antibody product quality. Electrophoresis 2008, 29, 4993–5002. [Google Scholar] [CrossRef] [PubMed]
- Chaly, T.; Dahl, J.R. Thin layer chromatographic detection of Kryptofix 2.2.2 in the routine synthesis of [18F] 2-fluoro-2-deoxy-d-glucose. Int. J. Radiat. Appl. Instrum. B 1989, 16, 385–387. [Google Scholar] [CrossRef]
- Mock, B.H.; Winkle, W.; Vavrek, M.T. A color spot test for the detection of Kryptofix 2.2.2 in [18F] FDG preparations. Nucl. Med. Biol. 1997, 24, 193–195. [Google Scholar] [CrossRef]
- Ferrieri, R.A.; Schlyer, D.J.; Alexoff, D.L.; Fowler, J.S.; Wolf, A.P. Direct analysis of Kryptofix 2.2.2 in 18FDG by gas chromatography using a nitrogen-selective detector. Nucl. Med. Biol. 1993, 20, 367–369. [Google Scholar] [CrossRef]
- Lao, Y.; Yang, C.; Zou, W.; Gan, M.; Chen, P.; Su, W. Quantification of Kryptofix 2.2.2 in [18F] fluorine-labelled radiopharmaceuticals by rapid-resolution liquid chromatography. Nucl. Med. Commun. 2012, 33, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, B.X.; Channing, M.A.; Eckelman, W.C. Quantification of Kryptofix 2.2.2 in 2-[18F] FDG and other radiopharmaceuticals by LC/MS/MS. Nucl. Med. Biol. 2002, 29, 125–129. [Google Scholar] [CrossRef]
- Sun, X.; Gan, H.; Qiao, J.; Zhu, L.; Liu, Y.; Zhong, J. Rapid Detection of the Residual Kryptofix 2.2.2 Levels in [18F]-Labeled Radiopharmaceuticals by Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry. Anal. Lett. 2011, 44, 1197–1205. [Google Scholar] [CrossRef]
- Nakao, R.; Ito, T.; Yamaguchi, M.; Suzuki, K. Simultaneous analysis of FDG, ClDG and Kryptofix 2.2.2 in [18F] DG preparation by high-performance liquid chromatography with UV detection. Nucl. Med. Biol. 2008, 35, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Charles, O.C. Radioanalysis for PET imaging pharmaceuticals: On-chip Detection of Kryptofix 2.2.2. Int. J. Sci. Eng. Res. 2016, 7, 478–494. [Google Scholar]
- Tarn, M.D.; Esfahani, M.M.N.; Patinglag, L.; Chan, Y.C.; Buch, J.X.; Onyije, C.C.; Gawne, P.J.; Gambin, D.J.B.; Brown, N.J.; Archibald, S.J.; et al. Microanalytical devices towards integrated quality control testing of [18F]FDG radiotracer. In Proceedings of the 21st International Conference on Miniaturized Systems for Chemistry and Life Sciences, Savannah, GA, USA, 22–26 October 2017; pp. 559–560. [Google Scholar]
- Anzellotti, A.I.; McFarland, A.R.; Olson, K.F. A rapid and simple colorimetric test for 2,2,2-cryptand (Kryptofix 2.2.2.) in solution. Anal. Methods 2013, 5, 4317–4320. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). FDA Guidance for Industry Q3C Impurities: Residual Solvents; FDA: Silver Springs, MD, USA, 1997.
- Koziorowski, J. A simple method for the quality control of [18F] FDG. Appl. Radiat. Isot. 2010, 68, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Archibald, S.; Pamme, N.; Brown, N.; Tarn, M. Integrated microfluidic lab-on-a-chip systems for 18F radiotracer synthesis, purification and quality control. J. Nucl. Med. 2015, 56, 167. [Google Scholar]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef] [PubMed]
- Nutt, R.; Giamis, A.M.; Mcfarland, A. Quality Control Module for Biomarker Generator System. U.S. Patent 20110070158 A1, 24 March 2011. [Google Scholar]
- Bhushan, A.; Yemane, D.; Trudell, D.; Overton, E.B.; Goettert, J. Fabrication of micro-gas chromatograph columns for fast chromatography. Microsyst. Technol. 2007, 13, 361–368. [Google Scholar] [CrossRef]
- Tzeng, T.H.; Kuo, C.Y.; Wang, S.Y.; Huang, P.K.; Huang, Y.M.; Hsieh, W.C.; Huang, Y.J.; Kuo, P.H.; Yu, S.A.; Lee, S.C.; et al. A Portable Micro Gas Chromatography System for Lung Cancer Associated Volatile Organic Compound Detection. IEEE J. Solid State Circuits 2016, 51, 259–272. [Google Scholar] [CrossRef]
- Taggart, M.P.; Tarn, M.D.; Esfahani, M.M.N.; Schofield, D.M.; Brown, N.J.; Archibald, S.J.; Deakin, T.; Pamme, N.; Thompson, L.F. Development of radiodetection systems towards miniaturised quality control of PET and SPECT radiopharmaceuticals. Lab Chip 2016, 16, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Convert, L.; Girard Baril, F.; Boisselle, V.; Pratte, J.-F.; Fontaine, R.; Lecomte, R.; Charette, P.G.; Aimez, V. Blood compatible microfluidic system for pharmacokinetic studies in small animals. Lab Chip 2012, 12, 4683–4692. [Google Scholar] [CrossRef] [PubMed]
- Dooraghi, A.A.; Carroll, L.; Collins, J.; van Dam, R.M.; Chatziioannou, A.F. ARAS: An automated radioactivity aliquoting system for dispensing solutions containing positron-emitting radioisotopes. EJNMMI Res. 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Vu, N.T.; Yu, Z.T.; Silverman, R.W.; Tseng, H.R.; Chatziioannou, A.F. Optimization of design parameters of a prototype CCD-based lens-coupled imaging system for the detection of beta particles in a microfluidic chip. In Proceedings of the 2007 IEEE Nuclear Science Symposium Conference Record, Honolulu, HI, USA, 26 October–3 November 2007; Volume 6, pp. 4615–4619. [Google Scholar]
- Sengupta, D.; Miller, S.; Marton, Z.; Chin, F.; Nagarkar, V.; Pratx, G. Bright Lu2O3: Eu Thin-Film Scintillators for High-Resolution Radioluminescence Microscopy. Adv. Healthc. Mater. 2015, 4, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Taschereau, R.; Olma, S.; Liu, K.; Chen, Y.-C.; Shen, C.K.-F.; van Dam, R.M.; Chatziioannou, A.F. Cerenkov radiation imaging as a method for quantitative measurements of beta particles in a microfluidic chip. Phys. Med. Biol. 2009, 54, 6757–6771. [Google Scholar] [CrossRef] [PubMed]
- Dooraghi, A.A.; Vu, N.T.; Silverman, R.W.; Farrell, R.; Shah, K.S.; Wang, J.; Heath, J.R.; Chatziioannou, A.F. Betabox: A beta particle imaging system based on a position sensitive avalanche photodiode. Phys. Med. Biol. 2013, 58, 3739. [Google Scholar] [CrossRef] [PubMed]
- Tarn, M.D.; Maneuski, D.; Alexander, R.; Brown, N.J.; O’Shea, V.; Pimlott, S.L.; Pamme, N.; Archibald, S.J. Positron detection in silica monoliths for miniaturised quality control of PET radiotracers. Chem. Commun. 2016, 52, 7221–7224. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, A.; Gorini, B.; Haguenauer, M.; Jiguet, S.; Miotto, G.L.; Vandelli, W.; Triviño, N.V.; Renaud, P. Scintillation particle detection based on microfluidics. Sens. Actuators Phys. 2010, 162, 272–275. [Google Scholar] [CrossRef]
- Lavén, M.; Velikyan, I.; Djodjic, M.; Ljung, J.; Berglund, O.; Markides, K.; Långström, B.; Wallenborg, S. Imaging of peptide adsorption to microfluidic channels in a plastic compact disc using a positron emitting radionuclide. Lab Chip 2005, 5, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Liang, V.; Cheung, S.; Woo, S.; Wu, C.; Ly, J.; Deng, Y.; Eddings, M.; van Dam, R.M. Reusable electrochemical cell for rapid separation of [18F] fluoride from [18O] water for flow-through synthesis of 18F-labeled tracers. Appl. Radiat. Isot. 2013, 75, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Vu, N.T.; Chung, Y.H.; Yu, Z.T.; Silverman, R.W.; Taschereau, R.; Tseng, H.R.; Chatziioannou, A.F. Detection of Beta Particles in a Microfluidic Chip Using a Scintillator and CCD. In Proceedings of the 2006 IEEE Nuclear Science Symposium Conference Record, San Diego, CA, USA, 29 October–1 November 2006; Volume 4, pp. 1977–1981. [Google Scholar]
- Pratx, G.; Chen, K.; Sun, C.; Martin, L.; Carpenter, C.M.; Olcott, P.D.; Xing, L. Radioluminescence Microscopy: Measuring the Heterogeneous Uptake of Radiotracers in Single Living Cells. PLoS ONE 2012, 7, e46285. [Google Scholar] [CrossRef] [PubMed]
- Dooraghi, A.A.; Keng, P.Y.; Chen, S.; Javed, M.R.; Kim, C.-J.; Chatziioannou, A.F.; van Dam, R.M. Optimization of microfluidic PET tracer synthesis with Cerenkov imaging. Analyst 2013, 138, 5654–5664. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Kim, J.; Johnson, D.; Dooraghi, A.A.; Mai, W.X.; Ta, L.; Chatziioannou, A.F.; Phelps, M.E.; Nathanson, D.A.; Heath, J.R. Quantitative assessments of glycolysis from single cells. Technology 2015, 3, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Maneuski, D.; Giacomelli, F.; Lemaire, C.; Pimlott, S.; Plenevaux, A.; Owens, J.; O’Shea, V.; Luxen, A. On the use of positron counting for radio-Assay in nuclear pharmaceutical production. Appl. Radiat. Isot. 2017, 125, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Maoddi, P.; Mapelli, A.; Bagiacchi, P.; Gorini, B.; Haguenauer, M.; Miotto, G.L.; Garcia, R.M.; Tehrani, F.S.; Veneziano, S.; Renaud, P. Scintillation detectors based on silicon microfluidic channels. J. Instrum. 2014, 9, C01019. [Google Scholar] [CrossRef]
- Ory, D.; Van den Brande, J.; de Groot, T.; Serdons, K.; Bex, M.; Declercq, L.; Cleeren, F.; Ooms, M.; Van Laere, K.; Verbruggen, A.; et al. Retention of [18F] fluoride on reversed phase HPLC columns. J. Pharm. Biomed. Anal. 2015, 111, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Archibald, S.J.; He, P.; Haswell, S.J.; Pamme, N.; Brown, N.J.; Tarn, M.D.; Alexander, R.; Esfahani, M.M.N. Method and Apparatus for the Analysis of Compounds. Patent Application WO/2016/063068, 28 April 2016. [Google Scholar]
- Marchand, P.; Bekaert, V.; Ouadi, A.; Laquerriere, P.; Brasse, D.; Curien, H. Forty Years of 18F-Labeled Compound Development in an Open Access Database. J. Nucl. Med. 2013, 54, 15N–17N. [Google Scholar]
- Radiosynthesis Database of PET Probes (RaDaP). Available online: http://www.nirs.qst.go.jp/research/division/mic/db2/ (accessed on 8 May 2017).
- Keng, P.Y.; Esterby, M.; van Dam, R.M. Emerging Technologies for Decentralized Production of PET Tracers. In Positron Emission Tomography—Current Clinical and Research Aspects; Hsieh, C.-H., Ed.; InTech: Rijeka, Croatia, 2012; pp. 153–182. ISBN 978-953-307-824-3. [Google Scholar]
- Keng, P.Y.; Sergeev, M.; van Dam, R.M. Advantages of Radiochemistry in Microliter Volumes. In Perspectives on Nuclear Medicine for Molecular Diagnosis and Integrated Therapy; Kuge, Y., Shiga, T., Tamaki, N., Eds.; Springer: Japan, 2016; pp. 93–111. ISBN 978-4-431-55892-7. [Google Scholar]
- Pascali, G.; Salvadori, P.A. Opportunities and challenges in the utilization of microfluidic technologies to the production of radiopharmaceuticals. Chim. Oggi Chem. Today 2016, 34, 28–32. [Google Scholar]
- Shah, K.S.; Grazioso, R.; Farrell, R.; Glodo, J.; Mcclish, M.A.; Entine, G.; Dokhale, P.A.; Cherry, S.R. Position sensitive APDs for small animal PET imaging. In Proceedings of the 2002 IEEE Nuclear Science Symposium Conference Record, Norfolk, VA, USA, 10–16 November 2002; Volume 3, pp. 1411–1415. [Google Scholar]

| QC Test | Conventional Method (s) | Typical Acceptance Criteria | Examples of Microfluidic Suitable Approaches |
|---|---|---|---|
| pH | pH indicator strips; electronic pH meter | 4.5 < pH < 8.5 | |
| Appearance (color/clarity) | Visual | Clear, colorless, particulate-free |
|
| Sterility | Short term: filter integrity test (e.g., bubble point test); long term: Bacterial culture | Long term: No bacterial growth observed |
|
| Bacterial endotoxin | LAL test | 175 EU/V |
|
| Chemical identity/purity | HPLC/UV | Varies |
|
| Kryptofix 2.2.2 | Color spot test | <50 µg/mL (USP); 2.2 mg/V (EP) |
|
| Residual organic solvents | Gas chromatography; HPLC/RI | Varies (e.g., MeCN 4.1 mg/day, EtOH 50 mg/day, DMSO 50 mg/day, DCM 6 mg/day, DMF8.8 mg/day) |
|
| Radioactivity concentration | Dose calibrator | Varies | |
| Radionuclidic identity | Half-life measurement with dose calibrator | Varies (e.g., 105-115 min for 18F-labled tracers) |
|
| Radionuclidic purity | Gamma spectrometer | Match expected energy spectrum |
|
| Radiochemical identity and purity | Radio-HPLC; radio-TLC | >95%; (>90% for [18F]FDG) | |
| Specific activity | Radio-HPLC and dose calibrator | Varies |
|
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, N.S.; Sadeghi, S.; Van Dam, R.M. Recent Progress toward Microfluidic Quality Control Testing of Radiopharmaceuticals. Micromachines 2017, 8, 337. https://doi.org/10.3390/mi8110337
Ha NS, Sadeghi S, Van Dam RM. Recent Progress toward Microfluidic Quality Control Testing of Radiopharmaceuticals. Micromachines. 2017; 8(11):337. https://doi.org/10.3390/mi8110337
Chicago/Turabian StyleHa, Noel S., Saman Sadeghi, and R. Michael Van Dam. 2017. "Recent Progress toward Microfluidic Quality Control Testing of Radiopharmaceuticals" Micromachines 8, no. 11: 337. https://doi.org/10.3390/mi8110337





