Electrical Tweezer for Droplet Transportation, Extraction, Merging and DNA Analysis
Abstract
:1. Introduction
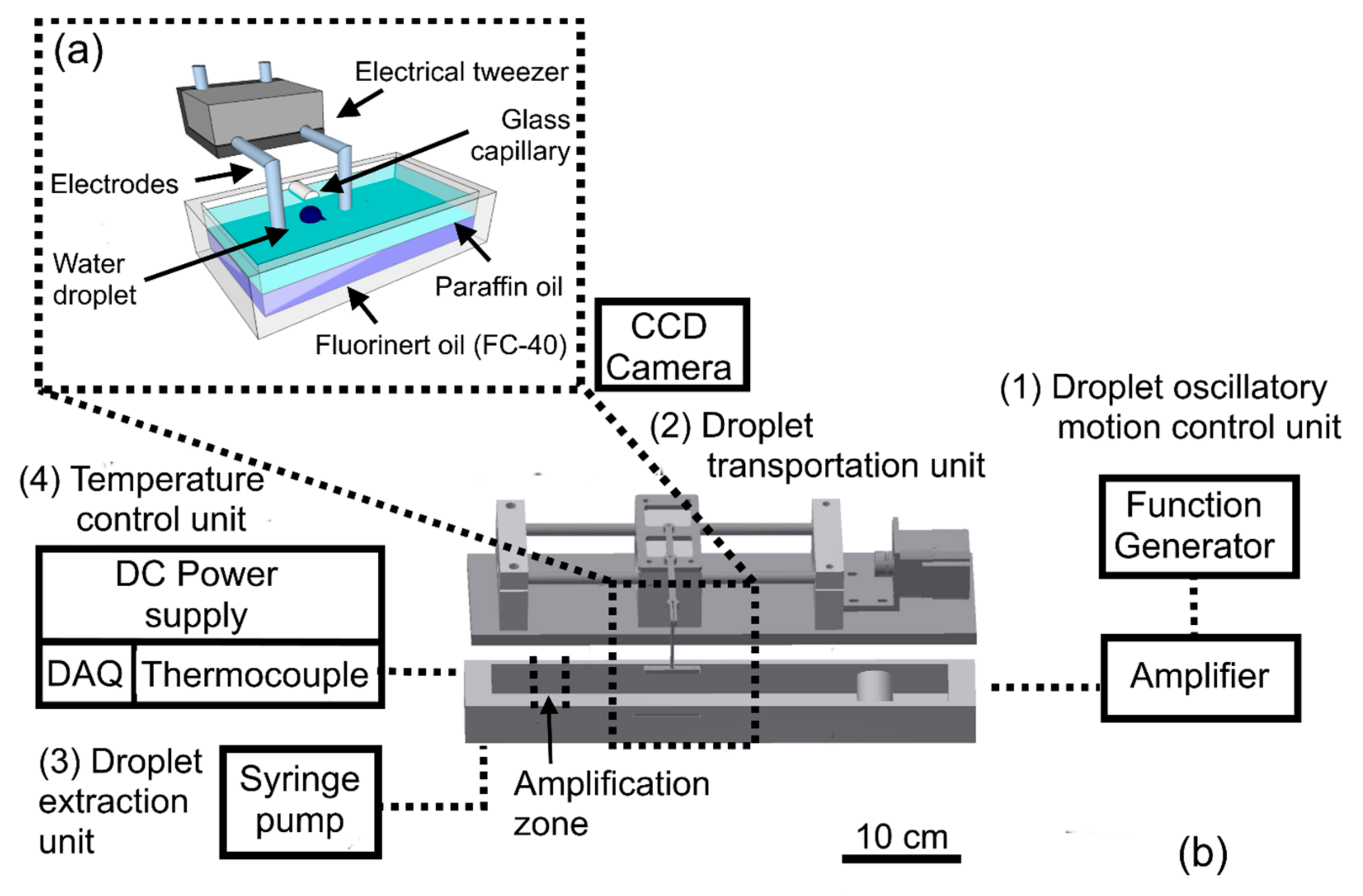
2. Design of the Electrical Tweezer
3. Experimental Setup
3.1. Image Analysis
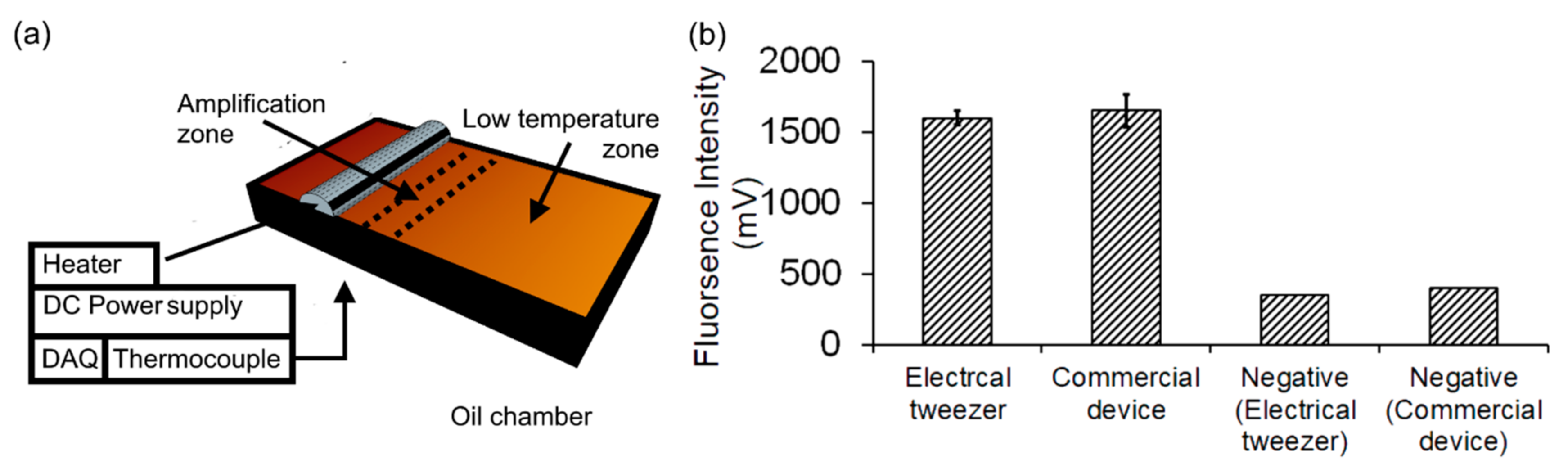
3.2. Loop-Mediated Isothermal Amplification (LAMP) Assays
4. Results and Discussions
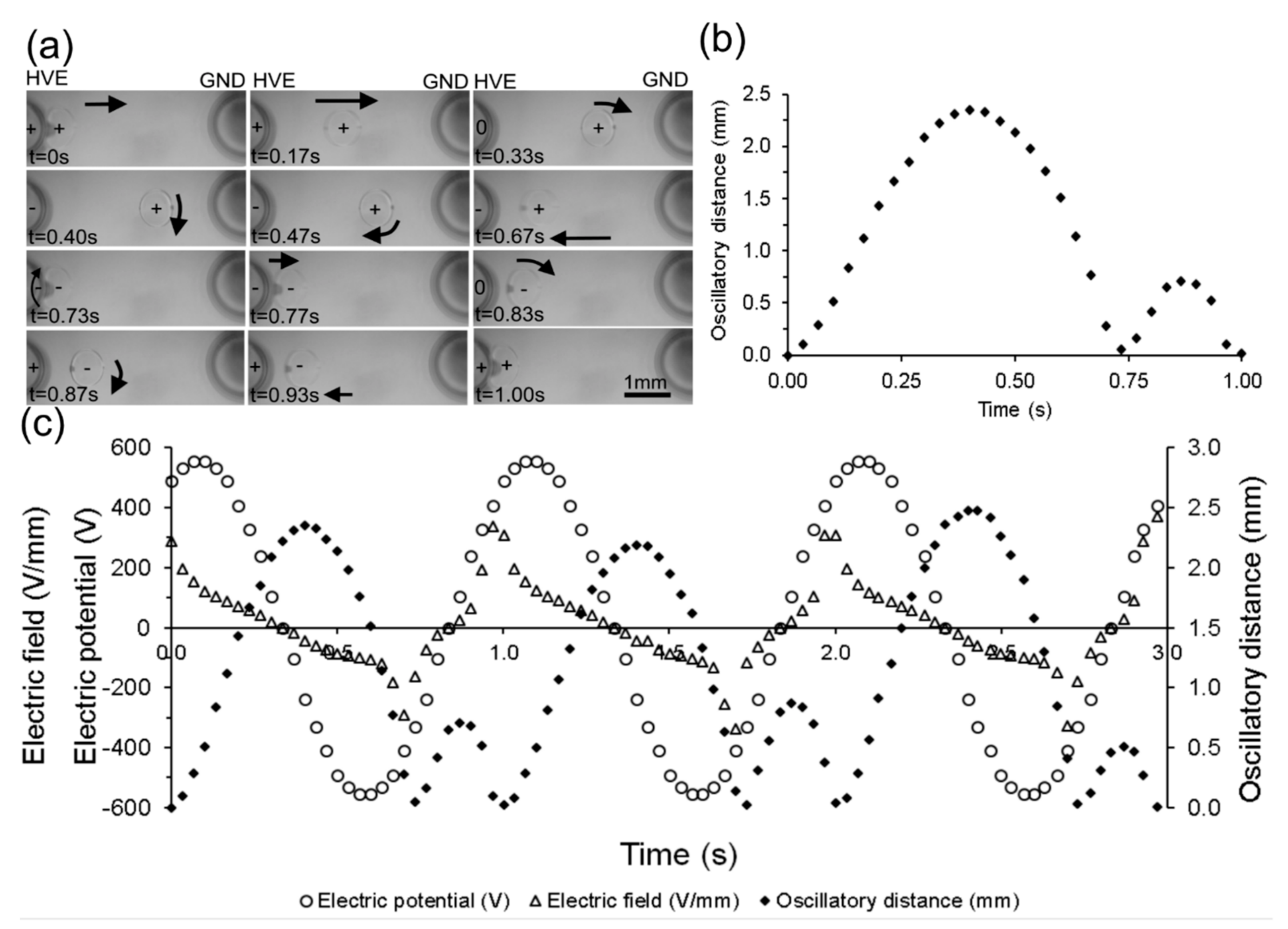
4.1. Droplet Motion under Alternating Current (AC) Electric Field
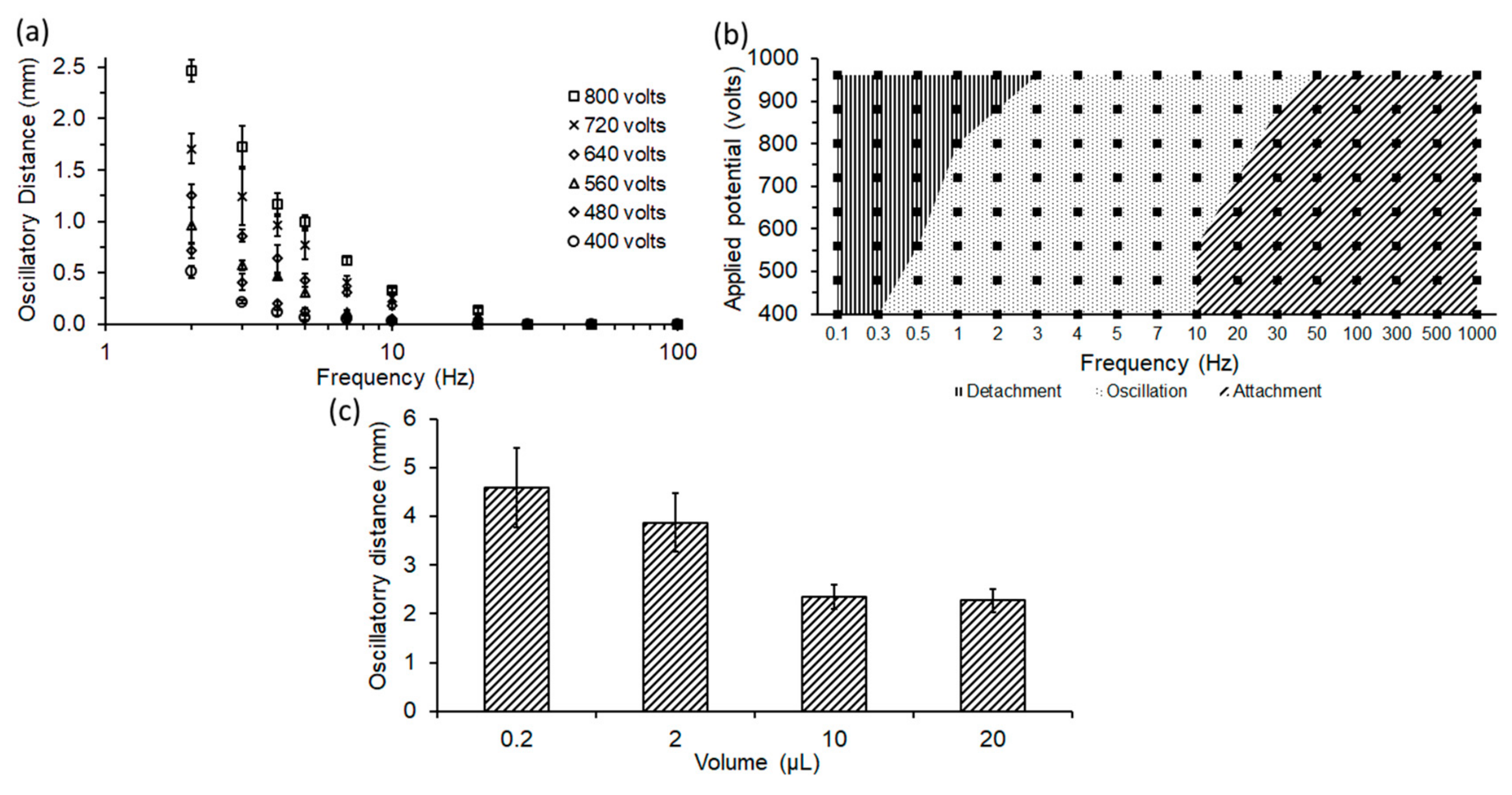
4.2. Effect of Electric Field and Frequency
4.3. Effect of Volume
5. Electrical Tweezers for Microfluidic Unit Operations
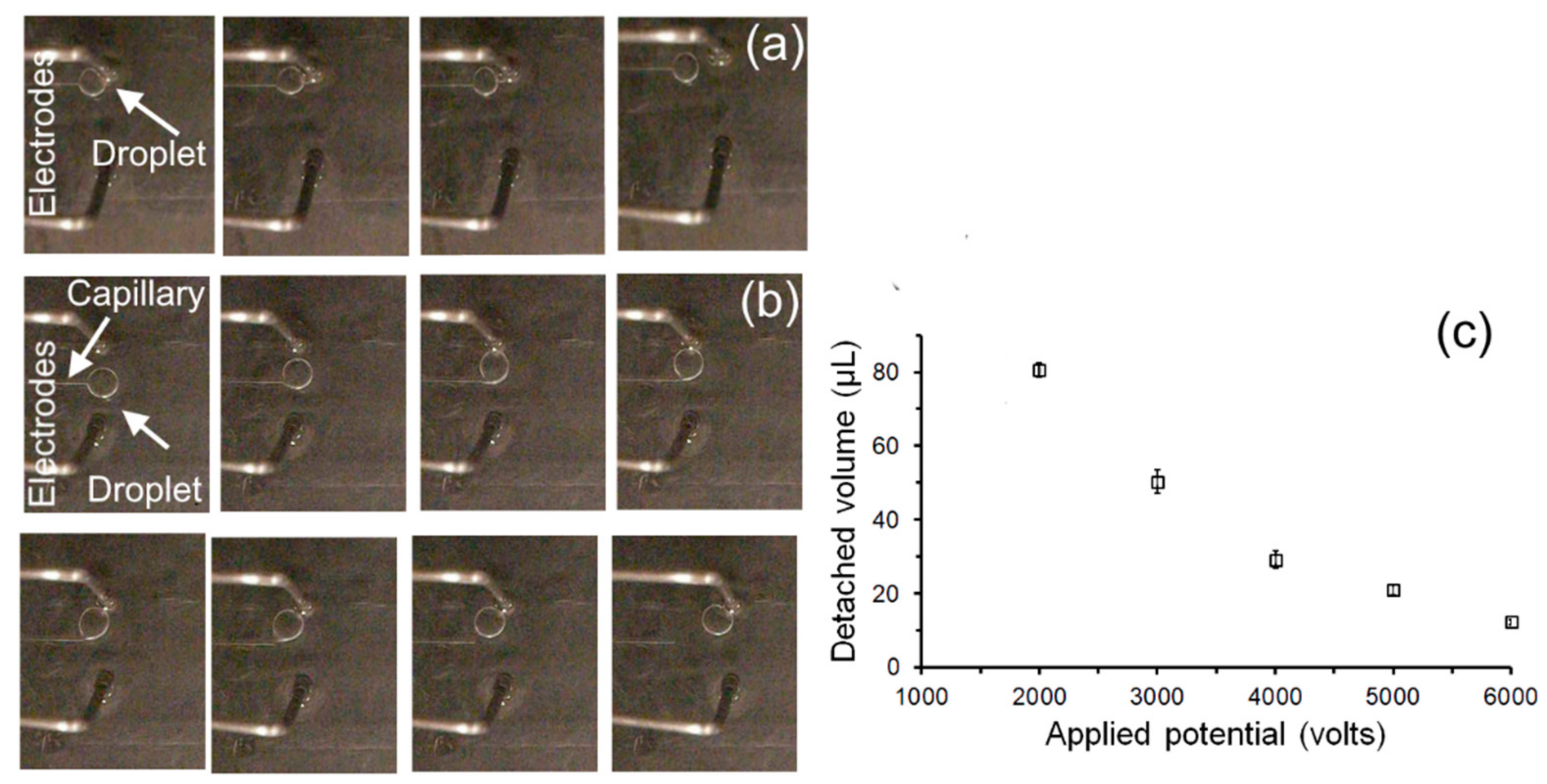
5.1. Droplet Transportation
5.2. Droplet Extraction
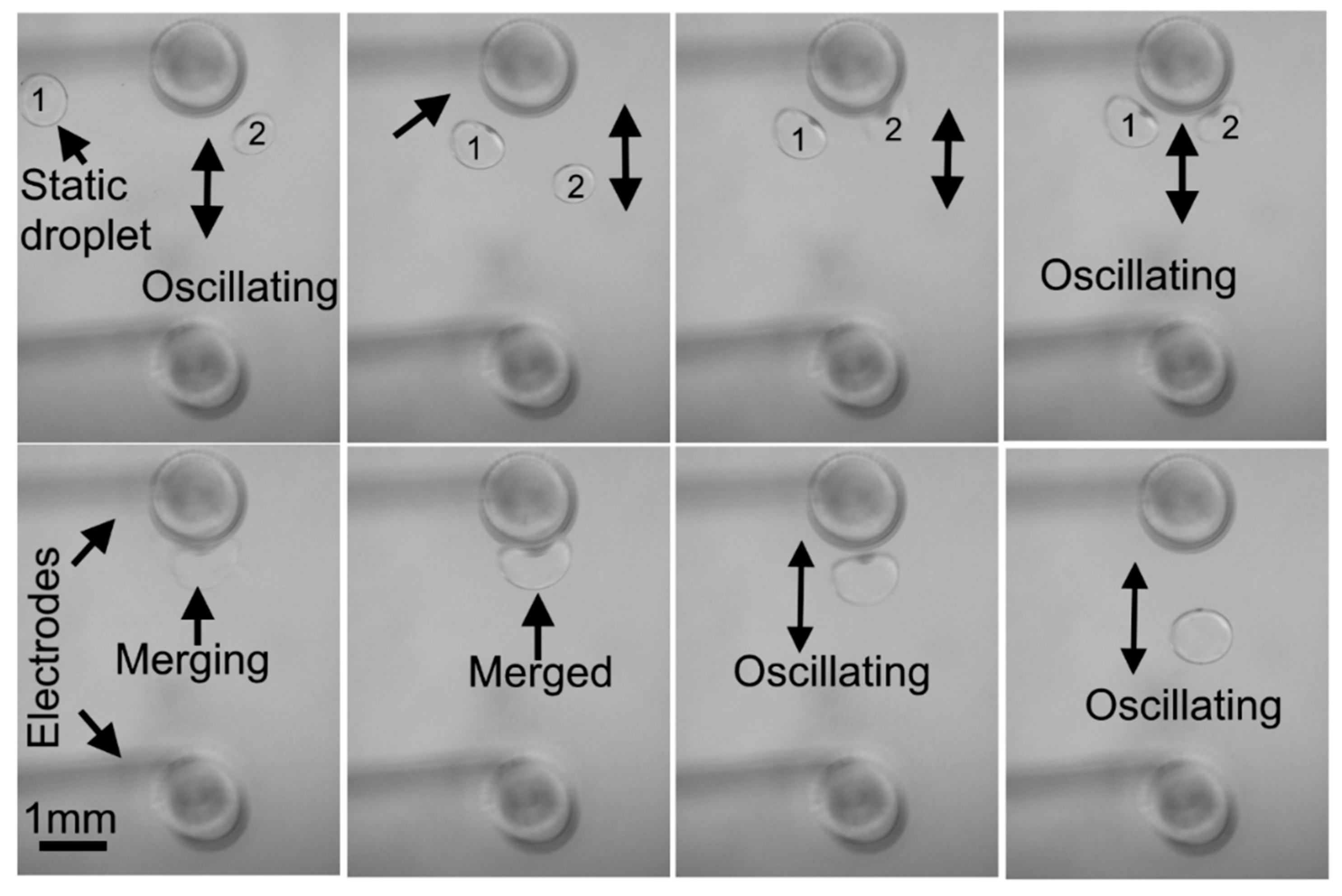
5.3. Droplet Merging
6. DNA Analysis through Amplification
7. Conclusions
Author Contributions
Conflicts of Interest
References
- You, D.J.; Tran, P.L.; Kwon, H.-J.; Patel, D.; Yoon, J.-Y. Very quick reverse transcription polymerase chain reaction for detecting 2009 H1N1 influenza A using wire-guide droplet manipulations. Faraday Discuss. 2011, 149, 159. [Google Scholar] [CrossRef] [PubMed]
- Im, D.J.; Noh, J.; Yi, N.W.; Park, J.; Kang, I.S. Influences of electric field on living cells in a charged water-in-oil droplet under electrophoretic actuation. Biomicrofluidics 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W.T.S. Microdroplets in microfluidics: An evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed. 2010, 49, 5846–5868. [Google Scholar] [CrossRef] [PubMed]
- Köster, S.; Angilè, F.E.; Duan, H.; Agresti, J.J.; Wintner, A.; Schmitz, C.; Rowat, A.C.; Merten, C.A.; Pisignano, D.; Griffiths, A.D.; et al. Drop-based microfluidic devices for encapsulation of single cells. Lab Chip 2008, 8, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Taly, V.; Kelly, B.T.; Griffiths, A.D. Droplets as microreactors for high-throughput biology. ChemBioChem 2007, 8, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Lee, G.B.; Huang, F.C.; Chen, Y.Y.; Lin, J.L. Integrated polymerase chain reaction chips utilizing digital microfluidics. Biomed. Microdevices 2006, 8, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Im, D.J.; Yoo, B.S.; Ahn, M.M.; Moon, D.; Kang, I.S. Digital electrophoresis of charged droplets. Anal. Chem. 2013, 85, 4038–4044. [Google Scholar] [CrossRef] [PubMed]
- Im, D.J.; Noh, J.; Moon, D.; Kang, I.S. Electrophoresis of a charged droplet in a dielectric liquid for droplet actuation. Anal. Chem. 2011, 83, 5168–5174. [Google Scholar] [CrossRef] [PubMed]
- Marre, S.; Jensen, K.F. Synthesis of micro and nanostructures in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1183–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, W.-Y.; Liu, K.; Lin, R.J.; Selke, M.; Kolb, H.C.; Zhang, N.; Zhao, X.-Z.; Phelps, M.E.; Shen, C.K.F.; et al. An integrated microfluidic device for large-scale in situ click chemistry screening. Lab Chip 2009, 9, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Cottam, B.F.; Krishnadasan, S.; Demello, A.J.; Demello, J.C.; Shaffer, M.S.P. Accelerated synthesis of titanium oxide nanostructures using microfluidic chips. Lab Chip 2007, 7, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.-H.; Choi, K.M.; Tseng, W.-Y.; Tan, Y.-C.; Shea, K.J.; Lee, A.P. Alternating droplet generation and controlled dynamic droplet fusion in microfluidic device for CdS nanoparticle synthesis. Lab Chip 2006, 6, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Yobas, L.; Martens, S.; Ong, W.-L.; Ranganathan, N. High-performance flow-focusing geometry for spontaneous generation of monodispersed droplets. Lab Chip 2006, 6, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Utada, A.; Lorenceau, E.; Link, D.; Kaplan, P. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-C.; Fisher, J.S.; Lee, A.I.; Cristini, V.; Lee, A.P. Design of microfluidic channel geometries for the control of droplet volume, chemical concentration, and sorting. Lab Chip 2004, 4, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, T.; Roberts, R.W.; Arnold, F.H.; Quake, S.R. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett. 2001, 86, 4163–4166. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.G.; Shenderov, A.D.; Fair, R.B. Electrowetting-based actuation of droplets for integrated microfluidicsElectronic supplementary information (ESI) available: Six videos showing droplet flow, droplet dispensing and electrowetting. Lab Chip 2002, 2, 96. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.R.; Moon, H.; Kim, C.-J.; Loo, J.A.; Garrell, R.L. Electrowetting-based microfluidics for analysis of peptides and proteins by matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2004, 76, 4833–4838. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-Y.; You, D.J. Backscattering particle immunoassays in wire-guide droplet manipulations. J. Biol. Eng. 2008, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.; Wheeler, A.R. The digital revolution: A new paradigm for microfluidics. Adv. Mater. 2009, 21, 920–925. [Google Scholar] [CrossRef]
- Washizu, M.; Member, S. Electrostatic Actuation of Liquid Droplets for Microreactor Applications. Water 1998, 34, 732–737. [Google Scholar]
- Cho, S.K.; Moon, H.; Kim, C.J. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar]
- Paik, P.; Pamula, V.K.; Pollack, M.G.; Fair, R.B. Electrowetting-based droplet mixers for microfluidic systems. Lab Chip 2003, 3, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.M.; Oh, H.C.; Kang, I.S. Electrical charging of a conducting water droplet in a dielectric fluid on the electrode surface. J. Colloid Interface Sci. 2008, 322, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-M.; Kang, I.S. A novel actuation method of transporting droplets by using electrical charging of droplet in a dielectric fluid. Biomicrofluidics 2009, 3, 22402. [Google Scholar] [PubMed]
- Droplet, C.W.; Mumm, F.; van Helvoort, A.T.J.; Sikorski, P. Easy Route to Superhydrophobic. 2009, 3, 2647–2652. [Google Scholar]
- Harshman, D.K.; Reyes, R.; Park, T.S.; You, D.J.; Song, J.Y.; Yoon, J.Y. Enhanced nucleic acid amplification with blood in situ by wire-guided droplet manipulation (WDM). Biosens. Bioelectron. 2014, 53, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Angus, S.V.; Cho, S.; Harshman, D.K.; Song, J.-Y.; Yoon, J.-Y. A portable, shock-proof, surface-heated droplet PCR system for Escherichia coli detection. Biosens. Bioelectron. 2015, 74, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, A.; Chong, S.; Mahony, J.; Deen, M.J.; Selvaganapathy, P.R. Electrical Tweezer for Droplet Transportation, Extraction, Merging and DNA Analysis. Micromachines 2017, 8, 353. https://doi.org/10.3390/mi8120353
Shahid A, Chong S, Mahony J, Deen MJ, Selvaganapathy PR. Electrical Tweezer for Droplet Transportation, Extraction, Merging and DNA Analysis. Micromachines. 2017; 8(12):353. https://doi.org/10.3390/mi8120353
Chicago/Turabian StyleShahid, Ali, Sylvia Chong, James Mahony, M. Jamal Deen, and P. Ravi Selvaganapathy. 2017. "Electrical Tweezer for Droplet Transportation, Extraction, Merging and DNA Analysis" Micromachines 8, no. 12: 353. https://doi.org/10.3390/mi8120353




