Thermoplastic Micromodel Investigation of Two-Phase Flows in a Fractured Porous Medium
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagent
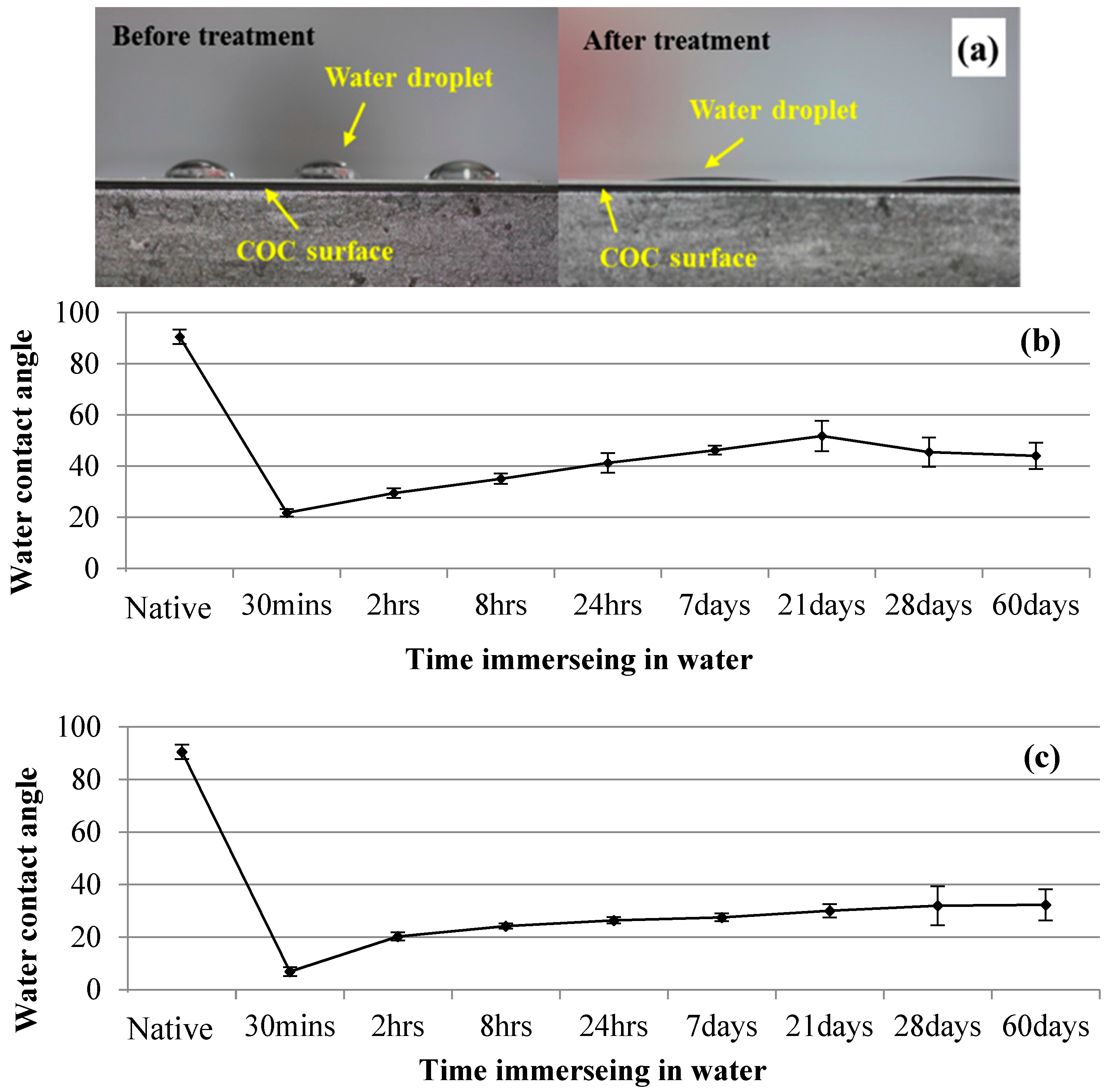
2.2. Contact Angle Measurement
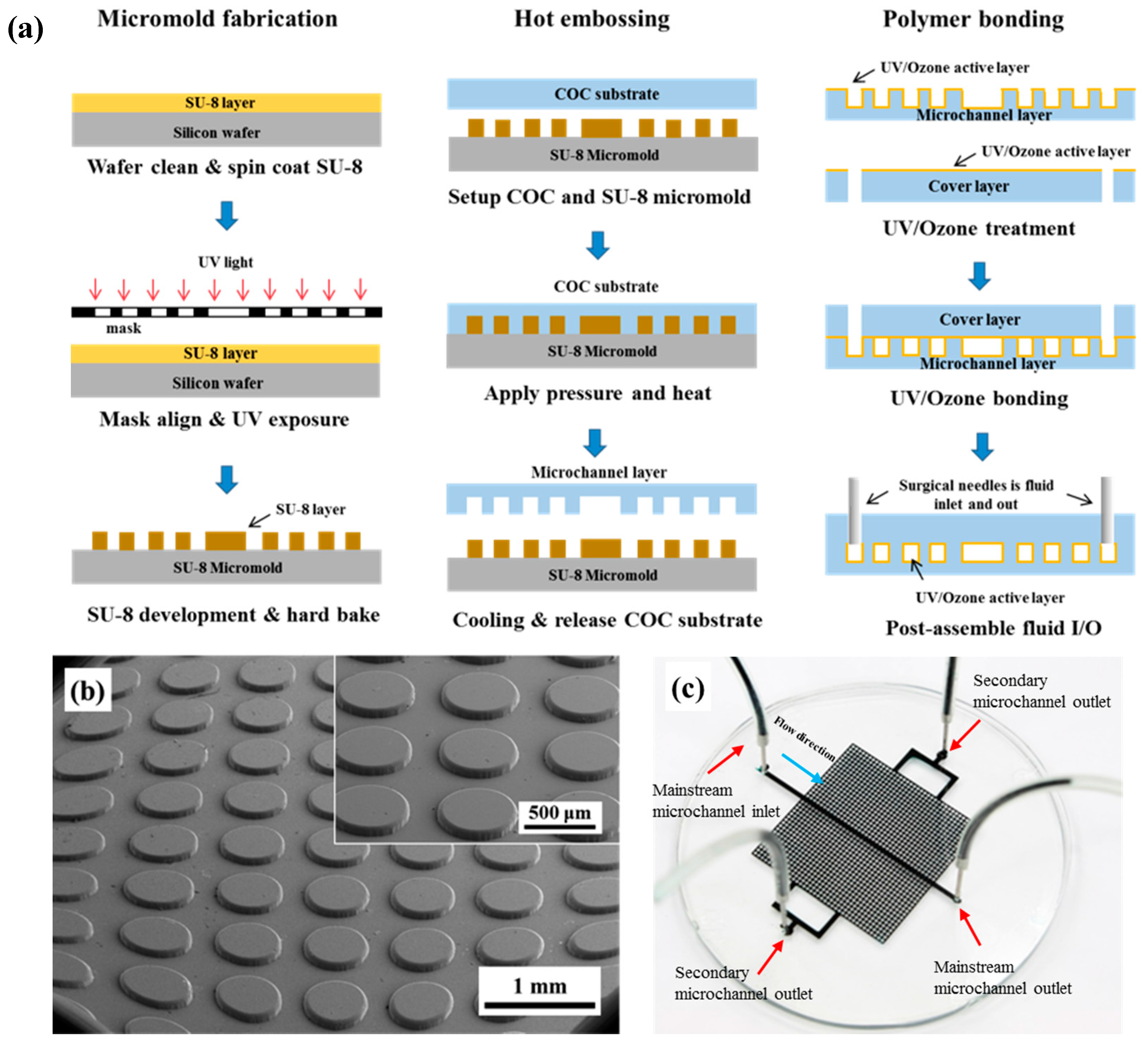
2.3. Micromold Fabrication
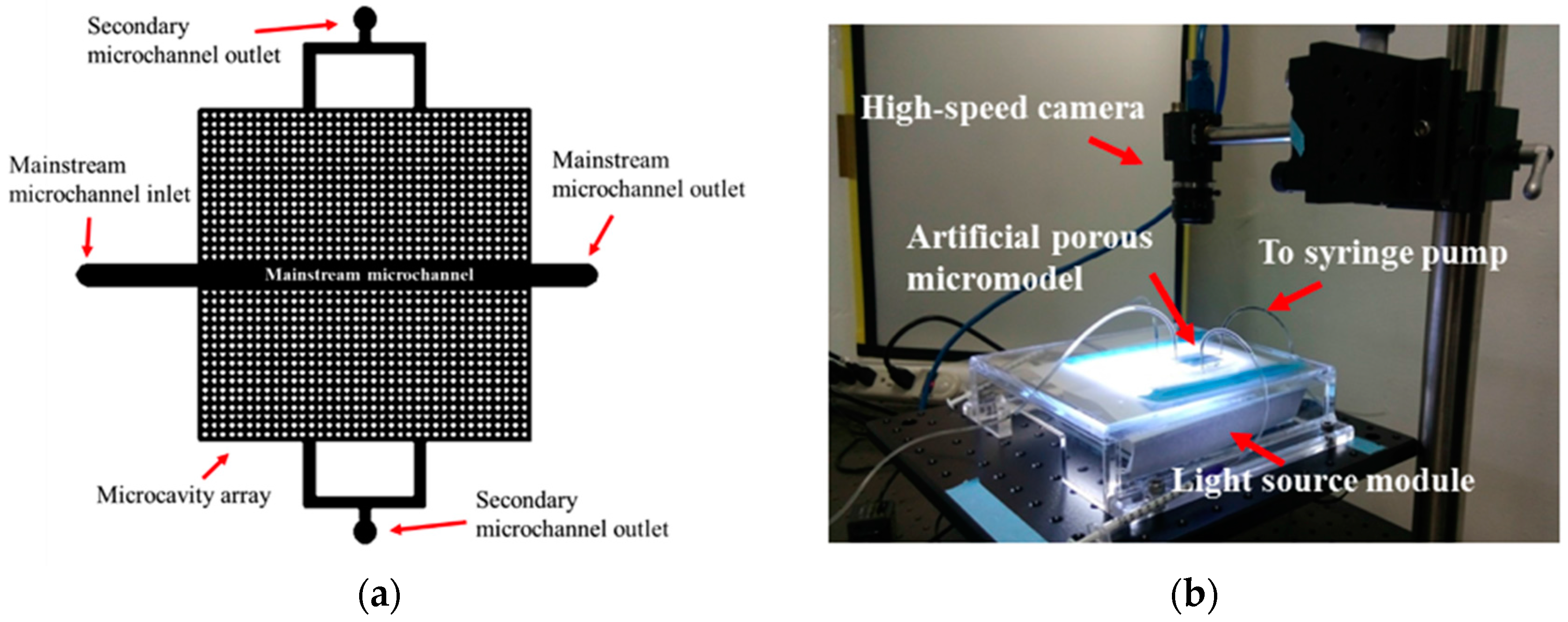
2.4. Micromodel Design and Experimental Setup
3. Results and Discussion
3.1. Polymer Micromodel Fabrication
3.2. Microchannel Surface Wettability Characterization
3.3. Results of the Two-Phase-Flow Micromodel Experiments
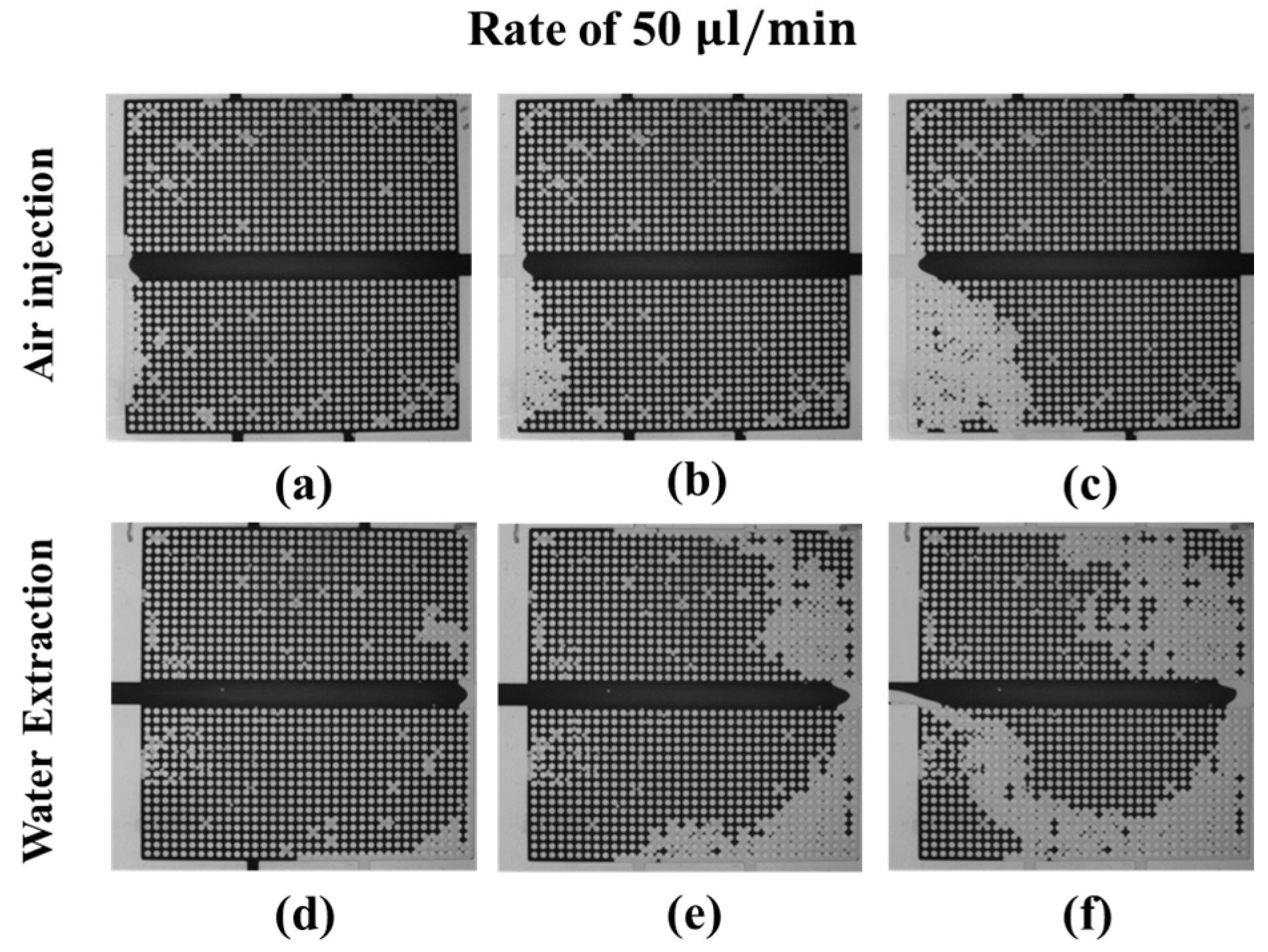
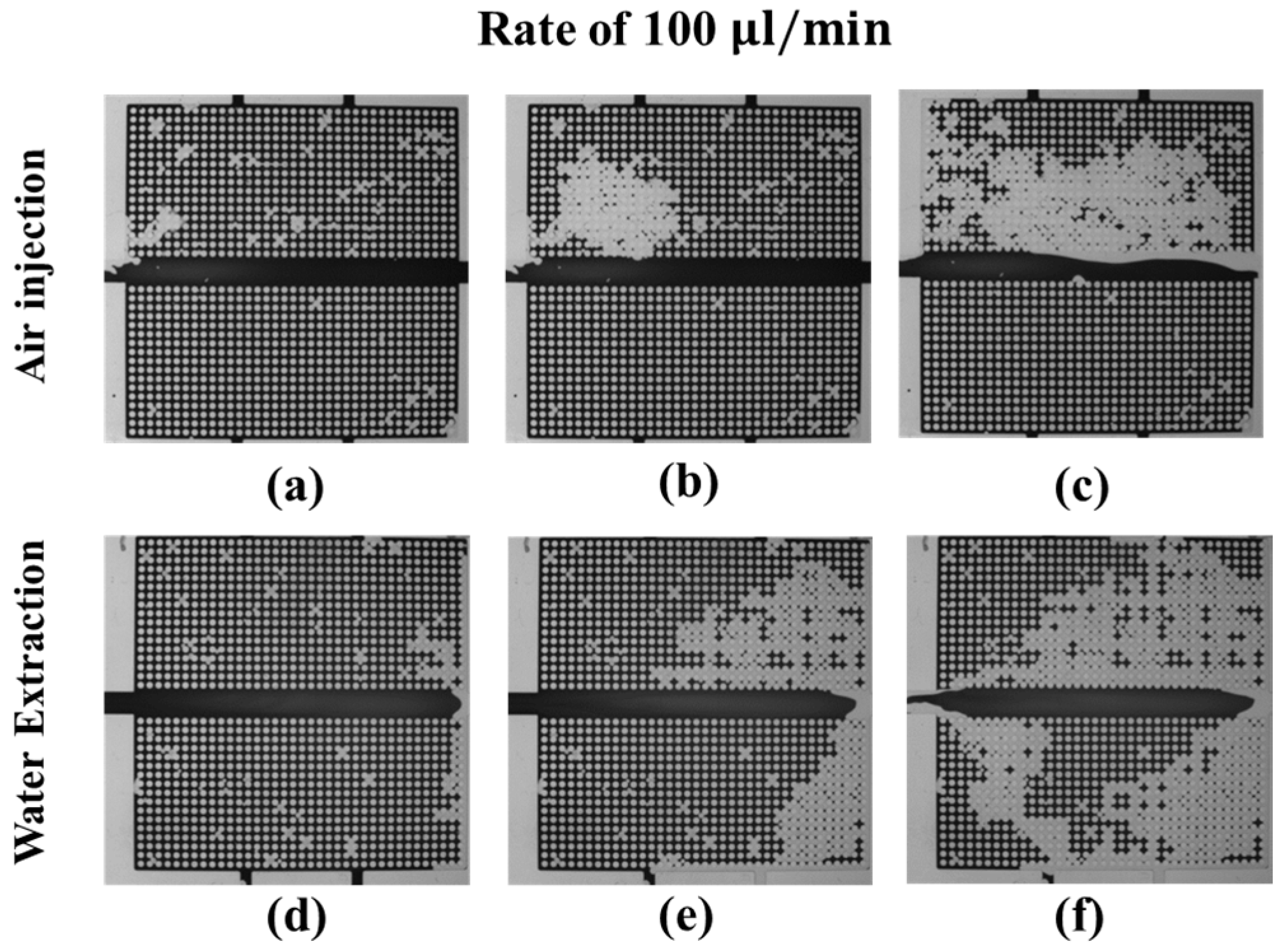
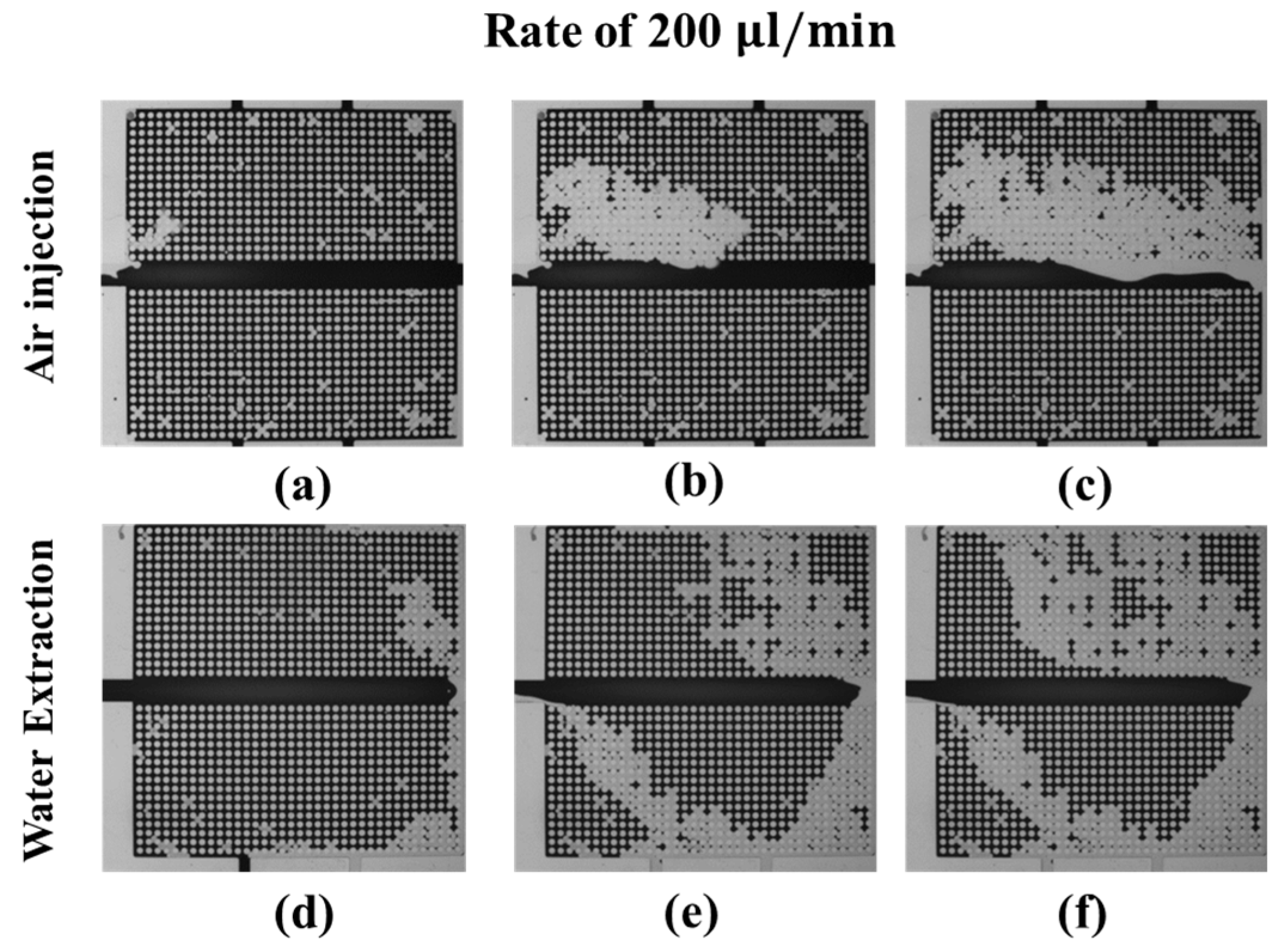
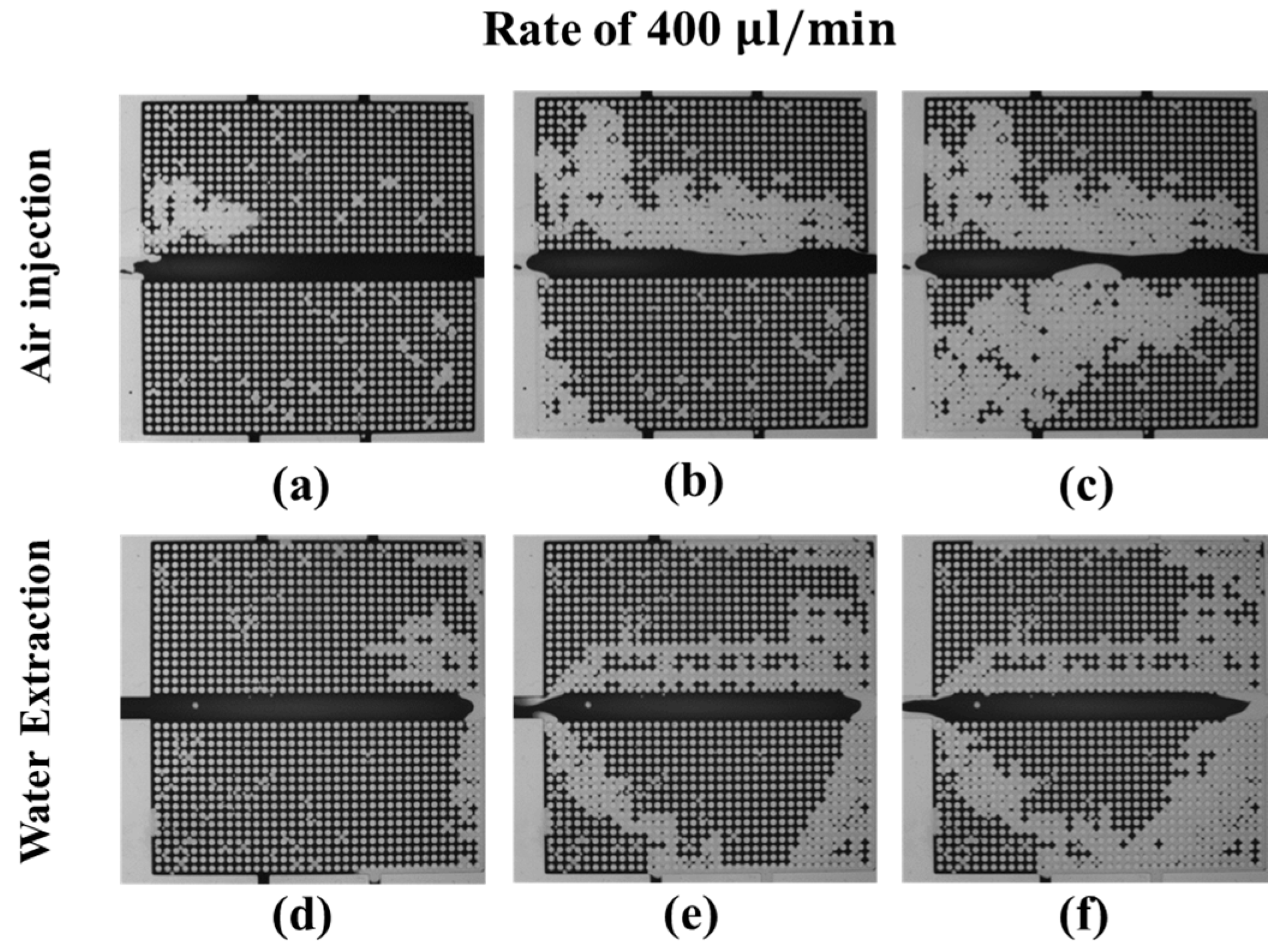
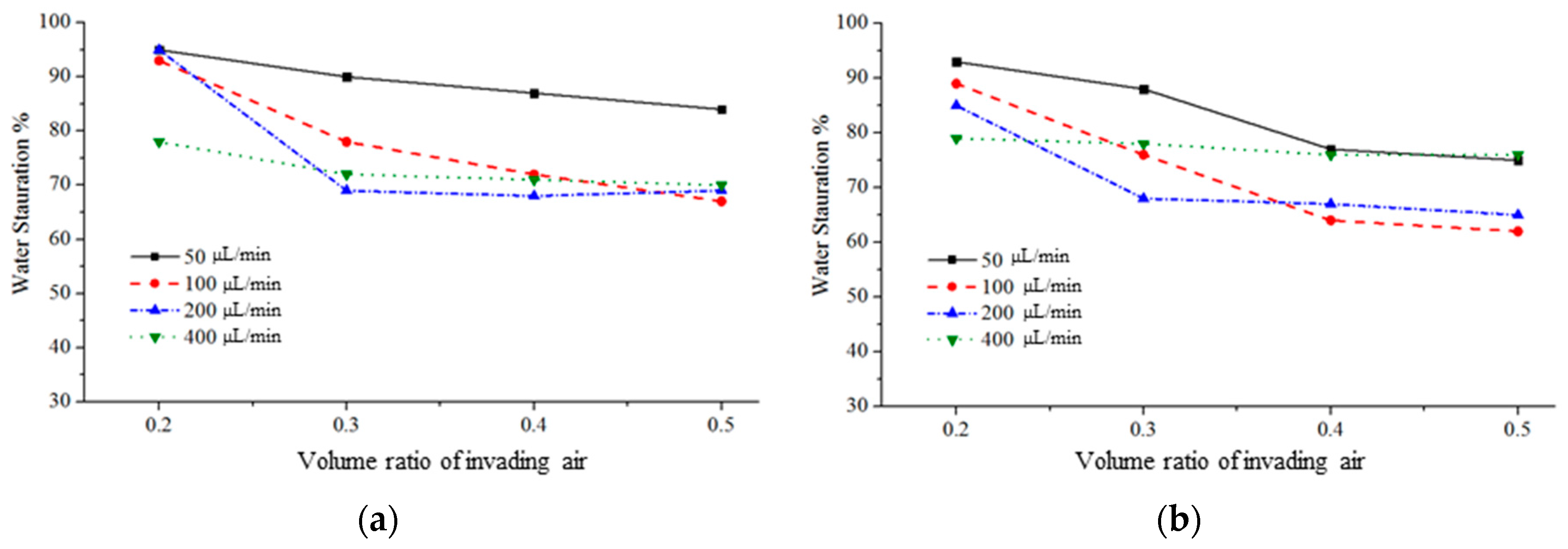
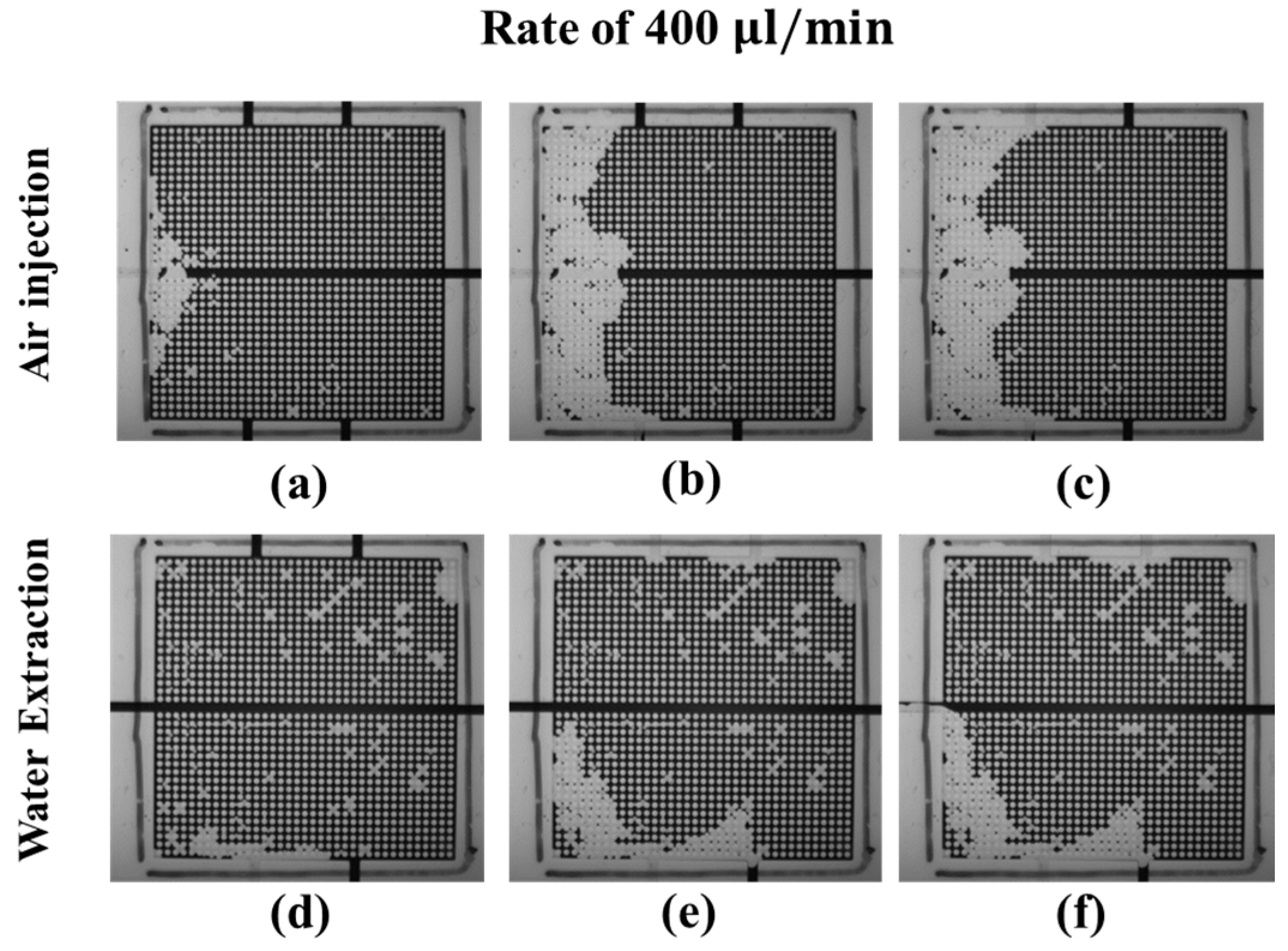
3.3.1. Water–Air Displacement: Air Injection and Water Extraction
3.3.2. Oil-Water Displacement: Water Injection
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rangel-German, E.; Kovscek, A. A micromodel investigation of two-phase matrix-fracture transfer mechanisms. Water Resour. Res. 2006, 42. [Google Scholar] [CrossRef]
- Conn, C.A.; Ma, K.; Hirasaki, G.J.; Biswal, S.L. Visualizing oil displacement with foam in a microfluidic device with permeability contrast. Lab Chip 2014, 14, 3968–3977. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Tokunaga, T.K.; Tsang, C.-F.; Bodvarsson, G.S. Improved glass micromodel methods for studies of flow and transport in fractured porous media. Water Resour. Res. 1996, 32, 1955–1964. [Google Scholar] [CrossRef]
- Long, J.; Witherspoon, P.A. The relationship of the degree of interconnection to permeability in fracture networks. J. Geophys. Res. Solid Earth 1985, 90, 3087–3098. [Google Scholar] [CrossRef]
- Karadimitriou, N.K.; Hassanizadeh, S.M. A review of micromodels and their use in two-phase flow studies. Vadose Zone J. 2012, 11. [Google Scholar] [CrossRef]
- Chatenever, A.; Calhoun, J.C., Jr. Visual examinations of fluid behavior in porous media-part I. J. Pet. Technol. 1952, 4, 149–156. [Google Scholar] [CrossRef]
- Nuss, W.; Whiting, R. Technique for reproducing rock pore space: Geological notes. AAPG Bull. 1947, 31, 2044–2049. [Google Scholar]
- Tsakiroglou, C.; Avraam, D. Fabrication of a new class of porous media models for visualization studies of multiphase flow processes. J. Mater. Sci. 2002, 37, 353–363. [Google Scholar] [CrossRef]
- Cheng, J.T.; Pyrak-Nolte, L.J.; Nolte, D.D.; Giordano, N.J. Linking pressure and saturation through interfacial areas in porous media. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Blunt, M.; King, P. Relative permeabilities from two-and three-dimensional pore-scale network modelling. Transp. Porous Media 1991, 6, 407–433. [Google Scholar] [CrossRef]
- Chatzis, I.; Dullien, F. Dynamic immiscible displacement mechanisms in pore doublets: Theory versus experiment. J. Colloid Interface Sci. 1983, 91, 199–222. [Google Scholar] [CrossRef]
- Cuenca, A.; Chabert, M.; Morvan, M.; Bodiguel, H. Axisymmetric drainage in hydrophobic porous media micromodels. Oil Gas Sci. Technol. 2012, 67, 953–962. [Google Scholar] [CrossRef]
- Corapcioglu, M.Y.; Fedirchuk, P. Glass Bead Micromodel Study of Solute Transport; FAO: Rome, Italy, 1999. [Google Scholar]
- Mohammadi, S.; Maghzi, A.; Ghazanfari, M.H.; Masihi, M.; Mohebbi, A.; Kharrat, R. On the control of glass micro-model characteristics developed by laser technology. Energy Sources Part A 2013, 35, 193–201. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, C.Y.; Hilpert, M.; Kuhlenschmidt, M.S.; Kuhlenschmidt, T.B.; Nguyen, T.H. Transport of cryptosporidium parvum oocysts in a silicon micromodel. Environ. Sci. Technol. 2012, 46, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.T.; Giordano, N. Fluid flow through nanometer-scale channels. Phys. Rev. E 2002, 65. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Gartner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Roh, C.; Lee, J.; Kang, C. The deformation of polydimethylsiloxane (PDMS) microfluidic channels filled with embedded circular obstacles under certain circumstances. Molecules 2016, 21, 798. [Google Scholar] [CrossRef] [PubMed]
- Attia, U.M.; Marson, S.; Alcock, J.R. Micro-injection moulding of polymer microfluidic devices. Microfluid. Nanofluid. 2009, 7, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Attia, U.M.; Marson, S.; Alcock, J.R. Design and fabrication of a three-dimensional microfluidic device for blood separation using micro-injection moulding. J. Eng. Manuf. 2014, 228, 941–949. [Google Scholar] [CrossRef]
- Jeon, J.S.; Chung, S.; Kamm, R.D.; Charest, J.L. Hot embossing for fabrication of a microfluidic 3D cell culture platform. Biomed. Microdevices 2011, 13, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.S.; Chiang, Y.C.; Lin, S.C.; Cheng, H.C.; Huang, C.F.; Shen, Y.K.; Lin, Y. Fabrication of microfluidic chip using micro-hot embossing with micro electrical discharge machining mold. Polym. Adv. Technol. 2012, 23, 57–64. [Google Scholar] [CrossRef]
- Li, Y.; Buch, J.S.; Rosenberger, F.; DeVoe, D.L.; Lee, C.S. Integration of isoelectric focusing with parallel sodium dodecyl sulfate gel electrophoresis for multidimensional protein separations in a plastic microfludic network. Anal. Chem. 2004, 76, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Chen, T.Y.; Woon, W.Y.; Lo, C.J. Rapid polymer microchannel fabrication by hot roller embossing process. Microsyst. Technol. 2012, 18, 713–722. [Google Scholar] [CrossRef]
- Yeo, L.P.; Ng, S.H.; Wang, Z.F.; Xia, H.M.; Wang, Z.P.; Thang, V.S.; Zhong, Z.W.; de Rooij, N.F. Investigation of hot roller embossing for microfluidic devices. J. Micromech. Microeng. 2010, 20, 015017. [Google Scholar] [CrossRef]
- Tsao, C.-W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluid. 2008, 6, 1–16. [Google Scholar] [CrossRef]
- Koesdjojo, M.T.; Koch, C.R.; Remcho, V.T. Technique for microfabrication of polymeric-based microchips from an SU-8 master with temperature-assisted vaporized organic solvent bonding. Anal. Chem. 2009, 81, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Riegger, L.; Strohmeier, O.; Faltin, B.; Zengerle, R.; Koltay, P. Adhesive bonding of microfluidic chips: Influence of process parameters. J. Micromech. Microeng. 2010, 20, 087003. [Google Scholar] [CrossRef]
- Tsao, C.W.; Hromada, L.; Liu, J.; Kumar, P.; DeVoe, D.L. Low temperature bonding of PMMA and COC microfluidic substrates using UV/Ozone surface treatment. Lab Chip 2007, 7, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, V.; Park, D.K.; Hwang, H.; Chantiwas, R.; Soper, S.A.; Cho, Y.K. Simple room temperature bonding of thermoplastics and poly(dimethylsiloxane). Lab Chip 2011, 11, 962–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truckenmuller, R.; Ahrens, R.; Cheng, Y.; Fischer, G.; Saile, V. An ultrasonic welding based process for building up a new class of inert fluidic microsensors and -actuators from polymers. Sens. Actuators A Phys. 2006, 132, 385–392. [Google Scholar] [CrossRef]
- Hirasaki, G.; Zhang, D.L. Surface chemistry of oil recovery from fractured, oil-wet, carbonate formations. SPE J. 2004, 9, 151–162. [Google Scholar] [CrossRef]
- Carrillo, M.; Yates, S.; Letey, J. Measurement of initial soil-water contact angle of water repellent soils. Soil Sci. Soc. Am. J. 1999, 63, 433–436. [Google Scholar] [CrossRef]
- Letey, J.; Osborn, J.; Pelishek, R. Measurement of liquid-solid contact angles in soil and sand. Soil Sci. 1962, 93, 149–153. [Google Scholar] [CrossRef]
- Technical Datasheet. Available online: http://www.topas.com/tech-center/datasheets (accessed on 19 Feburary 2017).
- Peters, F.; Arabali, D. Interfacial tension between oil and water measured with a modified contour method. Colloids Surf. A Physicochem. Eng. Asp. 2013, 426, 1–5. [Google Scholar] [CrossRef]
- McDowell, C.J.; Powers, S.E. Mechanisms affecting the infiltration and distribution of ethanol-blended gasoline in the vadose zone. Environ. Sci. Technol. 2003, 37, 1803–1810. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, S.-Y.; Zhang, Z.-Y.; Tsao, C.-W. Thermoplastic Micromodel Investigation of Two-Phase Flows in a Fractured Porous Medium. Micromachines 2017, 8, 38. https://doi.org/10.3390/mi8020038
Hsu S-Y, Zhang Z-Y, Tsao C-W. Thermoplastic Micromodel Investigation of Two-Phase Flows in a Fractured Porous Medium. Micromachines. 2017; 8(2):38. https://doi.org/10.3390/mi8020038
Chicago/Turabian StyleHsu, Shao-Yiu, Zhong-Yao Zhang, and Chia-Wen Tsao. 2017. "Thermoplastic Micromodel Investigation of Two-Phase Flows in a Fractured Porous Medium" Micromachines 8, no. 2: 38. https://doi.org/10.3390/mi8020038





