Fabrication of Graphene Aerogels with Heavily Loaded Metallic Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of Graphene Oxide (GO) Using Modified Hummers’ Method
2.3. Preparation of GO Aerogels Loaded with Metal Salts
2.4. Preparation of Reduced Graphene Oxide (rGO) Aerogels Decorated with Metal Nanoparticles
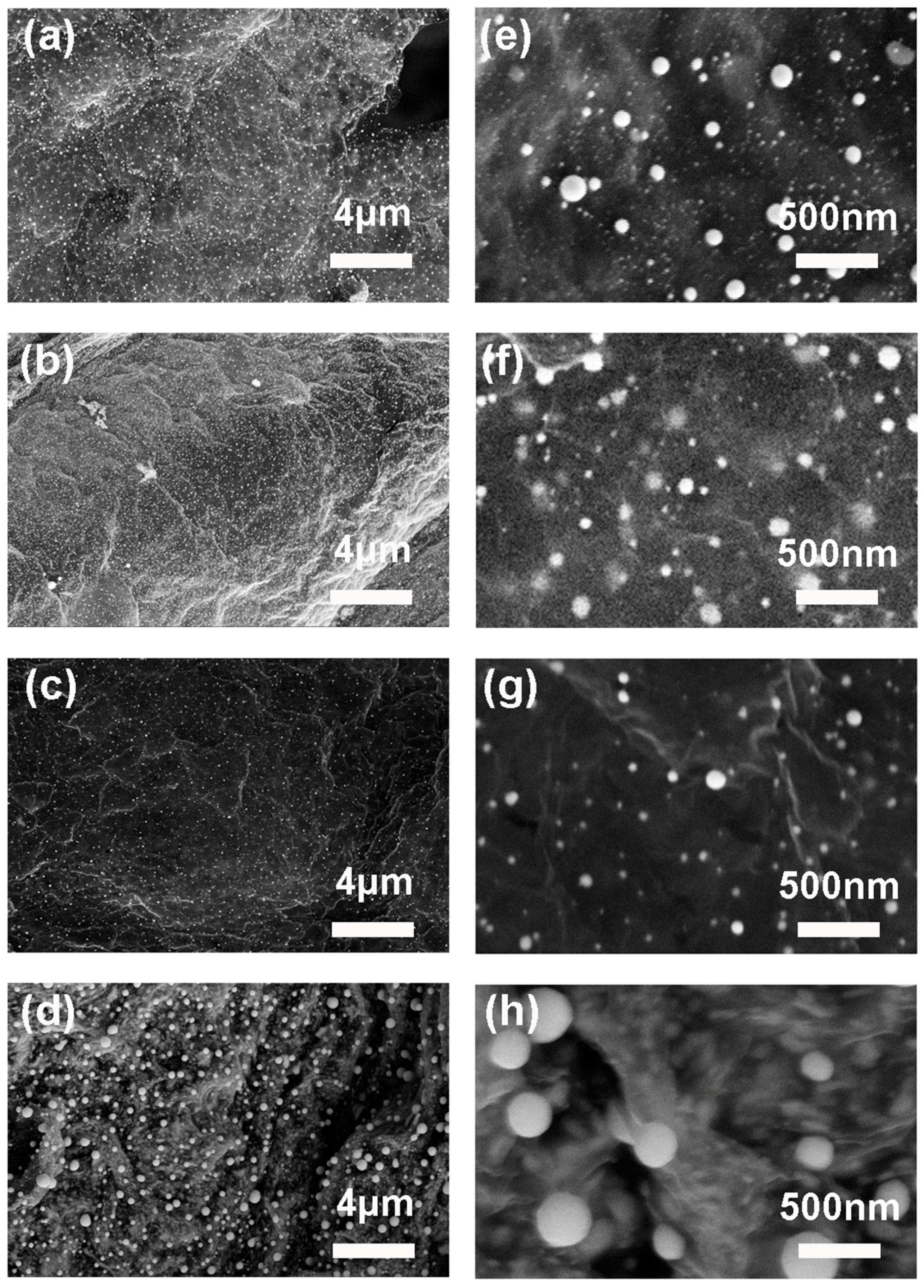
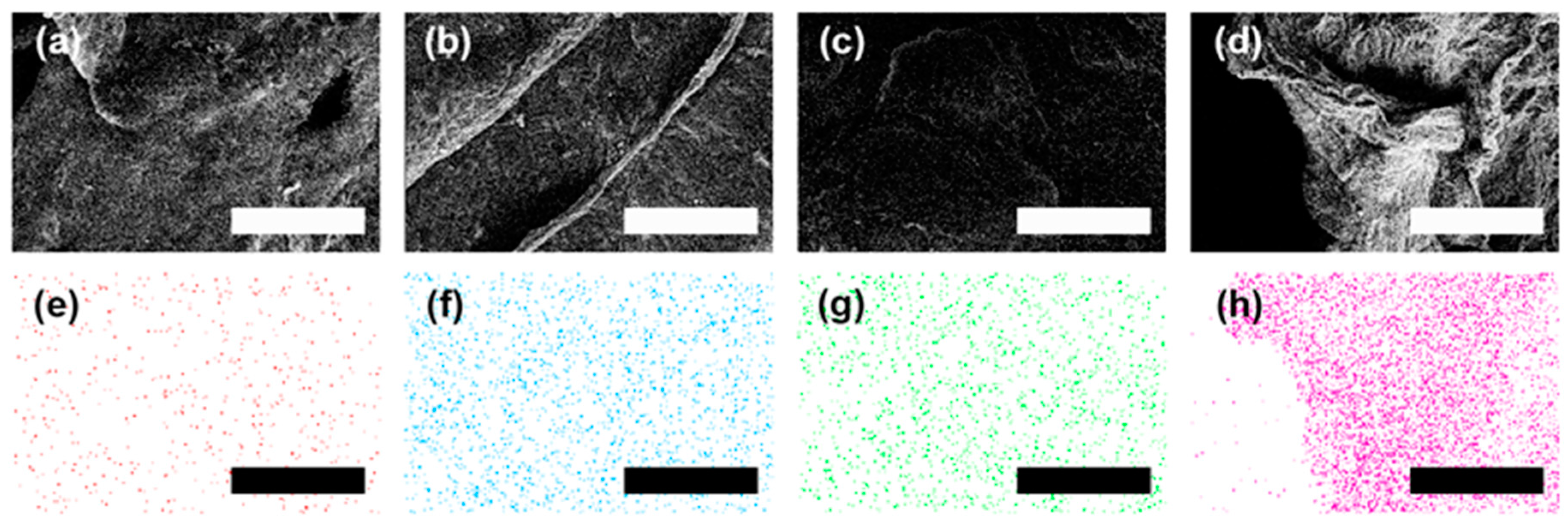
2.5. Characterization
2.6. Sensing Hydrogen Gas Using rGO Aerogels Decorated with Pd Nanoparticles
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
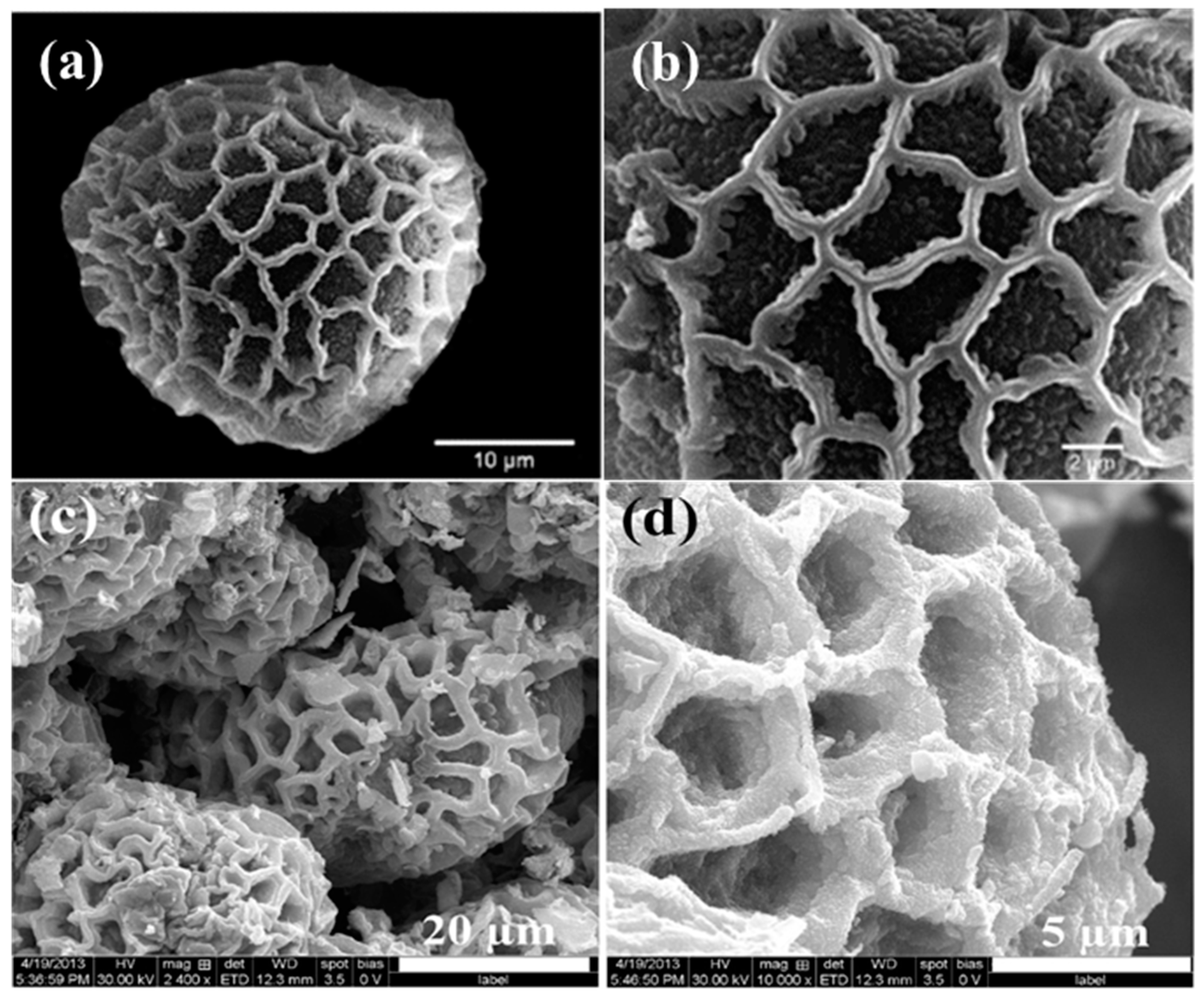
- Song, F.; Su, H.; Chen, J.; Moon, W.-J.; Lau, W.M.; Zhang, D. Impatiens msisimwanensis (Balsaminaceae): Description, pollen morphology and phylogenetic position of a new East African species. S. Afr. J. Bot. 2009, 75, 104–109. [Google Scholar]
- Fazil, A.A.; Bhanu, J.U.; Amutha, A.; Joicy, S.; Ponpandian, N.; Amirthapandian, S.; Panigrahi, B.K.; Thangadurai, P. A facile bio-replicated synthesis of SnO2 motifs with porous surface by using pollen grains of Peltophorum pterocarpum as a template. Microporous Mesoporous Mater. 2015, 212, 91–99. [Google Scholar] [CrossRef]
- Edlund, A.F.; Swanson, R.; Preuss, D. Pollen and stigma structure and function: The role of diversity in pollination. Plant Cell 2004, 16, S84–S97. [Google Scholar] [CrossRef] [PubMed]
- Benton, R. Sensitivity and specificity in drosophila pheromone perception. Trends Neurosci. 2007, 30, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wang, Z.; Cai, C.; Shen, H.; Liang, F.; Wang, D.; Wang, C.; Zhu, T.; Guo, J.; Wang, Y.; et al. Bioinspired materials: From low to high dimensional structure. Adv. Mater. 2014, 26, 6994–7017. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, J.; Qiu, L.; Li, D. Bioinspired effective prevention of restacking in multilayered graphene films: Towards the next generation of high-performance supercapacitors. Adv. Mater. 2011, 23, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.M.; Gibson, M.; Monagle, S.; Patterson, Z.; Elisseeff, J.H. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc. Natl. Acad. Sci. USA 2012, 109, 10012–10017. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Su, H.; Han, J.; Lau, W.M.; Moon, W.; Zhang, D. Bioinspired hierarchical tin oxide scaffolds for enhanced gas sensing properties. J. Phys. Chem. C 2012, 116, 10274–10281. [Google Scholar] [CrossRef]
- Spitzer, D.; Cottineau, T.; Piazzon, N.; Josset, S.; Schnell, F.; Pronkin, S.N.; Savinova, E.R.; Keller, V. Bio-inspired nanostructured sensor for the detection of ultralow concentrations of explosives. Angew. Chem. Int. Ed. 2012, 51, 5334–5338. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Li, T.; Ma, J.; Li, K.; Zuo, X. Silver nanoparticles selectively deposited on graphene-colloidal carbon sphere composites and their application for hydrogen peroxide sensing. Sens. Actuators B 2017, 239, 1205–1212. [Google Scholar] [CrossRef]
- Amanulla, B.; Palanisamy, S.; Chen, S.; Velusamy, V.; Chiu, T.; Chen, T.; Ramaraj, S.K. A non-enzymatic amperometric hydrogen peroxide sensor based on iron nanoparticles decorated reduced graphene oxide nanocomposite. J. Colloid Interface Sci. 2017, 487, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Thirumalraj, B.; Rajkumar, C.; Chen, S.; Barathi, P. Highly stable biomolecule supported by gold nanoparticles/graphene nanocomposite as a sensing platform for H2O2 biosensor application. J. Mater. Chem. B 2016, 4, 6335–6343. [Google Scholar] [CrossRef]
- Cadore, A.R.; Mania, E.; de Morais, E.A.; Watanabe, K.; Taniguchi, T.; Lacerda, R.G.; Campos, L.C. Metal-graphene heterojunction modulation via H2 interaction. Appl. Phys. Lett. 2016, 109, 033109–033114. [Google Scholar] [CrossRef]
- Wu, J.; Feng, S.; Wei, X.; Shen, J.; Lu, W.; Shi, H.; Tao, K.; Lu, S.; Sun, T.; Yu, L.; et al. Facile synthesis of 3D graphene flowers for ultrasensitive and highly reversible gas sensing. Adv. Funct. Mater. 2016, 26, 7462–7469. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Zhang, X.; Zhang, H. 3D porous and redox-active prussian blue-in-graphene aerogels for highly efficient electrochemical detection of H2O2. J. Mater. Chem. 2012, 22, 22090–22096. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Y.; Guo, L.; Li, L. Facile one-step synthesis of a 3D macroscopic SnO2-graphene aerogel and its application as a superior anode material for Li-ion batteries. RSC Adv. 2013, 3, 11489–11492. [Google Scholar] [CrossRef]
- Wu, T.; Chen, M.; Zhang, L.; Xu, X.; Liu, Y.; Yan, J.; Wang, W.; Gao, J. Three-dimensional graphene-based aerogels prepared by a self-assembly process and its excellent catalytic and absorbing performance. J. Mater. Chem. A 2013, 1, 7612–7621. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, D.; Han, S.; Huang, Y.; Li, S.; He, M.; Zhang, F.; Feng, X. Self-assembled Fe2O3/graphene aerogel with high lithium storage performance. ACS Appl. Mater. Interfaces 2013, 5, 3764–3769. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, C.; Li, X.; Zhang, L.; Ma, Y.; Zhang, L.; Xu, X.; Xia, F.; Wang, W.; Gao, J. A one-step method for reduction and self-assembling of graphene oxide into reduced graphene oxide aerogels. J. Mater. Chem. A 2013, 1, 2869–2877. [Google Scholar] [CrossRef]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutierrez, M.C.; del Monte, F. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Irin, F.; Ma, L.; Bhattacharia, S.K.; Hedden, R.C.; Green, M.J. Rheology and morphology of pristine graphene/polyacrylamide gels. ACS Appl. Mater. Interfaces 2013, 5, 8633–8640. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Xu, M.; Bao, S.; Cai, C.; Lu, Z.; Chai, H.; Yang, F.; Wei, H. Self-assembly of three-dimensional interconnected graphene-based aerogels and its application in supercapacitors. J. Colloid Interface Sci. 2013, 407, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Feng, J.; Wu, P. Deposition of three-dimensional graphene aerogel on nickel foam as a binder-free supercapacitor electrode. ACS Appl. Mater. Interfaces 2013, 5, 7122–7129. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; McInnis, M.; Calderon, J.; Seal, S.; Zhai, L.; Thomas, J. Functionalized graphene aerogel composites for high-performance asymmetric supercapacitors. Nano Energy 2015, 11, 611–620. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Z.; Li, J.; Zhang, J.; Sui, X.; Zhang, L. One-pot synthesis of a three-dimensional graphene aerogel supported pt catalyst for methanol electrooxidation. RSC Adv. 2015, 5, 98160–98165. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, P.; Bhatnagar, A.; Prakash Patel, C.R.; Singh, A.P.; Dhawan, S.K.; Gupta, B.K.; Srivastava, O.N. A highly porous, light weight 3D sponge like graphene aerogel for electromagnetic interference shielding applications. RSC Adv. 2015, 5, 107083–107087. [Google Scholar] [CrossRef]
- Ren, L.; Hui, K.N.; Hui, S.K.; Liu, Y.; Qi, X.; Zhong, J.; Du, Y.; Yang, J. 3D hierarchical porous graphene aerogel with tunable meso-pores on graphene nanosheets for high-performance energy storage. Sci. Rep. 2015, 5, 14229. [Google Scholar] [CrossRef] [PubMed]
- Harley-Trochimczyk, A.; Chang, J.; Zhou, Q.; Dong, J.; Pham, T.; Worsley, M.A.; Maboudian, R.; Zettl, A.; Mickelson, W. Catalytic hydrogen sensing using microheated platinum nanoparticle-loaded graphene aerogel. Sens. Actuators B Chem. 2015, 206, 399–406. [Google Scholar] [CrossRef]
- Peng, L.; Zheng, Y.; Li, J.; Jin, Y.; Gao, C. Monolithic neat graphene oxide aerogel for efficient catalysis of S → O acetyl migration. ACS Catal. 2015, 5, 3387–3392. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Gao, L.; Li, C.M. Au nanoparticles–3D graphene hydrogel nanocomposite to boost synergistically in situ detection sensitivity toward cell-released nitric oxide. ACS Appl. Mater. Interfaces 2015, 7, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Y.; Li, P.; Gao, C. Strong, conductive, lightweight, neat graphene aerogel fibers with aligned pores. ACS Nano 2012, 6, 7103–7113. [Google Scholar] [CrossRef] [PubMed]
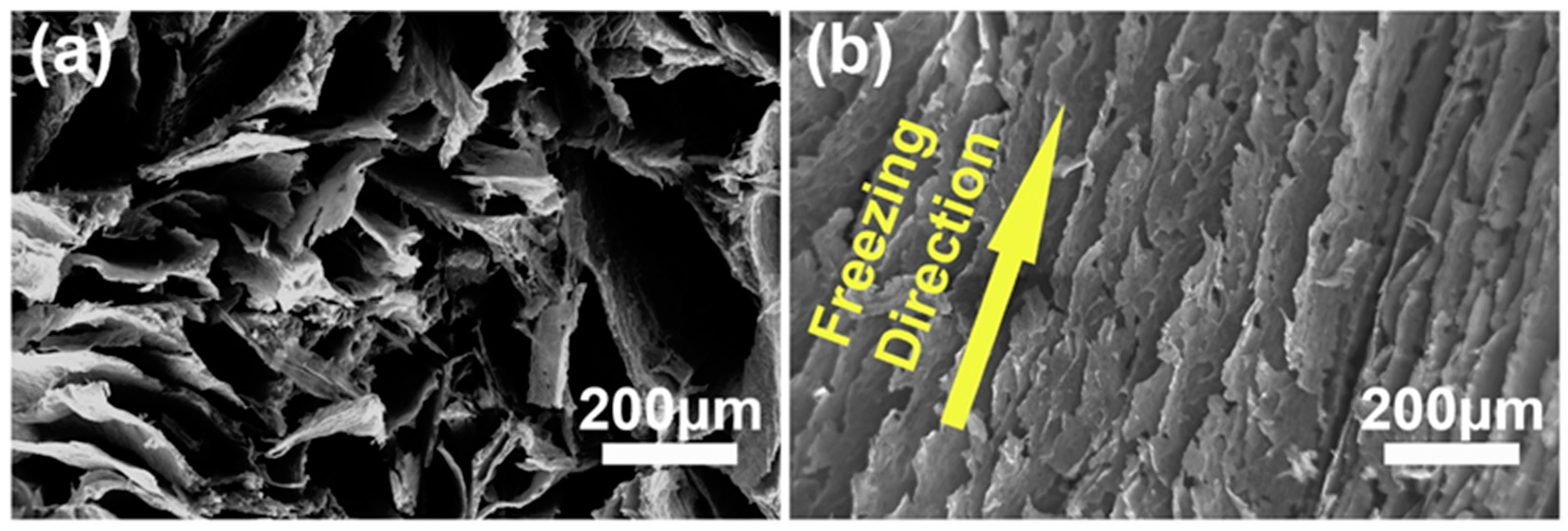
- Schiffres, S.N.; Harish, S.; Maruyama, S.; Shiomi, J.; Malen, J.A. Tunable electrical and thermal transport in ice-templated multilayer graphene nanocomposites through freezing rate control. ACS Nano 2013, 7, 11183–11189. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, J.; Wang, Z.; Li, Z.; Li, J.; Gu, Z.; Wang, G. Novel graphene-gold nanohybrid with excellent electrocatalytic performance for the electrochemical detection of glucose. Sens. Actuators B Chem. 2015, 208, 421–428. [Google Scholar]
- Yin, P.T.; Kim, T.H.; Choi, J.W.; Lee, K.B. Prospects for graphene-nanoparticle-based hybrid sensors. Phys. Chem. Chem. Phys 2013, 15, 12785–12799. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Lee, S.; Shin, C.; Park, S.; Kwon, S.J.; Park, H.S. One-pot self-assembled, reduced graphene oxide/palladium nanoparticle hybrid aerogels for electrocatalytic applications. Electrochim. Acta 2015, 180, 902–908. [Google Scholar] [CrossRef]
- Cong, H.; Ren, X.; Wang, P.; Yu, S. Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, H.; Tang, Y.; Luo, S. Controllable growth of graphene/Cu composite and its nanoarchitecture-dependent electrocatalytic activity to hydrazine oxidation. J. Mater. Chem. A 2014, 2, 4580–4587. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Zhu, Y.; Stoller, M.D.; Cai, W.; Velamakanni, A.; Piner, R.D.; Chen, D.; Ruoff, R.S. Exfoliation of graphite oxide in propylene carbonate and thermal reduction of the resulting graphene oxide platelets. ACS Nano 2010, 4, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, G. Critical Survey of Stability Constants of Edta Complexes: Critical Evaluation of Equilibrium Constants in Solution: Stability Constants of Metal Complexes; Pergamon Press: New York, NY, USA, 1977; pp. 5–7. [Google Scholar]
- Chisolm, J.J. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J. Pediatr. 1968, 73, 1–38. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Peng, C.; Yang, W.; Guo, J.; Zheng, Y.; Chen, P.; Huang, T.; Xu, J. Pd nanoparticles supported on three-dimensional graphene aerogels as highly efficient catalysts for methanol electrooxidation. Electrochim. Acta 2015, 178, 838–846. [Google Scholar] [CrossRef]
- Kumar, R.; Varandani, D.; Mehta, B.; Singh, V.; Wen, Z.; Feng, X.; Müllen, K. Fast response and recovery of hydrogen sensing in Pd–Pt nanoparticle–graphene composite layers. Nanotechnology 2011, 22, 275719. [Google Scholar] [CrossRef] [PubMed]
- Militello, M.C.; Simko, S.J. Elemental palladium by XPS. Surf. Sci. Spectra 1994, 3, 387–394. [Google Scholar] [CrossRef]
- Militello, M.C.; Simko, S.J. Palladium chloride (PdCl2) by XPS. Surf. Sci. Spectra 1994, 3, 402–409. [Google Scholar] [CrossRef]
- Militello, M.C.; Simko, S.J. Palladium oxide (PdO) by XPS. Surf. Sci. Spectra 1994, 3, 395–401. [Google Scholar] [CrossRef]
- Wojnicki, M.; PacŁAwski, K.; Socha, R.P.; Fitzner, K. Adsorption and reduction of platinum(IV) chloride complex ions on activated carbon. Trans. Nonferr. Met. Soc. China 2013, 23, 1147–1156. [Google Scholar] [CrossRef]
- Bancroft, G.M.; Adams, I.; Coatsworth, L.L.; Bennewitz, C.D.; Brown, J.D.; Westwood, W.D. Esca study of sputtered platinum films. Anal. Chem. 1975, 47, 586–588. [Google Scholar] [CrossRef]
- Luo, S.; Shen, P.K. Concave platinum–copper octopod nanoframes bounded with multiple high-index facets for efficient electrooxidation catalysis. ACS Nano 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xie, J.; Feng, X.; Li, A. Edta-directed self-assembly and enhanced catalytic properties of sphere-constructed platinum nanochains. J. Phys. D Appl. Phys. 2010, 43, 115403. [Google Scholar] [CrossRef]
- Lian, K.; Thorpe, S.J.; Kirk, D.W. Electrochemical and surface characterization of electrocatalytically active amorphous Ni Co alloys. Electrochim. Acta 1992, 37, 2029–2041. [Google Scholar] [CrossRef]
- Venezia, A.M.; Bertoncello, R.; Deganello, G. X-ray photoelectron spectroscopy investigation of pumice-supported nickel catalysts. Surf. Interface Anal. 1995, 23, 239–247. [Google Scholar] [CrossRef]
- Mansour, A.N.; Melendres, C.A. Characterization of electrochemically prepared γ-niooh by XPS. Surf. Sci. Spectra 1994, 3, 271–278. [Google Scholar] [CrossRef]
- Cao, X.; Cao, L.; Yao, W.; Ye, X. Influences of dopants on the electronic structure of SnO2 thin films. Thin Solid Films 1998, 317, 443–445. [Google Scholar] [CrossRef]
- Willemen, H.; Van De Vondel, D.F.; Van Der Kelen, G.P. An esca study of tin compounds. Inorg. Chim. Acta 1979, 34, 175–180. [Google Scholar] [CrossRef]
- Shuttleworth, D. Preparation of metal-polymer dispersions by plasma techniques. An esca investigation. J. Phys. Chem. 1980, 84, 1629–1634. [Google Scholar] [CrossRef]
- Paparazzo, E.; Moretto, L.; D’Amato, C.; Palmieri, A. X-ray photoemission spectroscopy and scanning auger microscopy studies of a roman lead pipe ‘fistula’. Surf. Interface Anal. 1995, 23, 69–76. [Google Scholar] [CrossRef]
- Wu, X.; Wen, T.; Guo, H.; Yang, S.; Wang, X.; Xu, A. Biomass-derived sponge-like carbonaceous hydrogels and aerogels for supercapacitors. ACS Nano 2013, 7, 3589–3597. [Google Scholar] [CrossRef] [PubMed]
- Tortello, M.; Colonna, S.; Bernal, M.; Gomez, J.; Pavese, M.; Novara, C.; Giorgis, F.; Maggio, M.; Guerra, G.; Saracco, G.; et al. Effect of thermal annealing on the heat transfer properties of reduced graphite oxide flakes: A nanoscale characterization via scanning thermal microscopy. Carbon 2016, 109, 390–401. [Google Scholar] [CrossRef]
- Gerenser, L.J. Photoemission investigation of silver/poly(ethylene terephthalate) interfacial chemistry: The effect of oxygen-plasma treatment. J. Vac. Sci. Technol. A 1990, 8, 3682–3691. [Google Scholar] [CrossRef]
- Lange, U.; Hirsch, T.; Mirsky, V.M.; Wolfbeis, O.S. Hydrogen sensor based on a graphene—Palladium nanocomposite. Electrochim. Acta 2011, 56, 3707–3712. [Google Scholar] [CrossRef]
- Mubeen, S.; Zhang, T.; Yoo, B.; Deshusses, M.A.; Myung, N.V. Palladium nanoparticles decorated single-walled carbon nanotube hydrogen sensor. J. Phys. Chem. C 2007, 111, 6321–6327. [Google Scholar] [CrossRef]
- Czerwosz, E.; Dłużewski, P.; Kozłowski, M.; Krawczyk, S.; Rymarczyk, J. Preparation and properties of carbon-palladium multilayer for hydrogen detection. Vacuum 2016, 128, 265–271. [Google Scholar] [CrossRef]
- Yaqoob, U.; Uddin, A.S.M.I.; Chung, G.-S. Foldable hydrogen sensor using Pd nanocubes dispersed into multiwall carbon nanotubes-reduced graphene oxide network assembled on nylon filter membrane. Sens. Actuators B Chem. 2016, 229, 355–361. [Google Scholar] [CrossRef]
- Hong, J.; Lee, S.; Seo, J.; Pyo, S.; Kim, J.; Lee, T. A highly sensitive hydrogen sensor with gas selectivity using a PMMA membrane-coated Pd nanoparticle/single-layer graphene hybrid. ACS Appl. Mater. Interfaces 2015, 7, 3554–3561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xue, Q.; Du, Y.; Ling, C.; Xing, W. Highly enhanced sensitivity of hydrogen sensors using novel palladium-decorated graphene nanoribbon film/SiO2/Si structures. J. Mater. Chem. A 2014, 2, 15931–15937. [Google Scholar] [CrossRef]
- Johnson, J.L.; Behnam, A.; Pearton, S.J.; Ural, A. Hydrogen sensing using Pd-functionalized multi-layer graphene nanoribbon networks. Adv. Mater. 2010, 22, 4877–4880. [Google Scholar] [CrossRef] [PubMed]

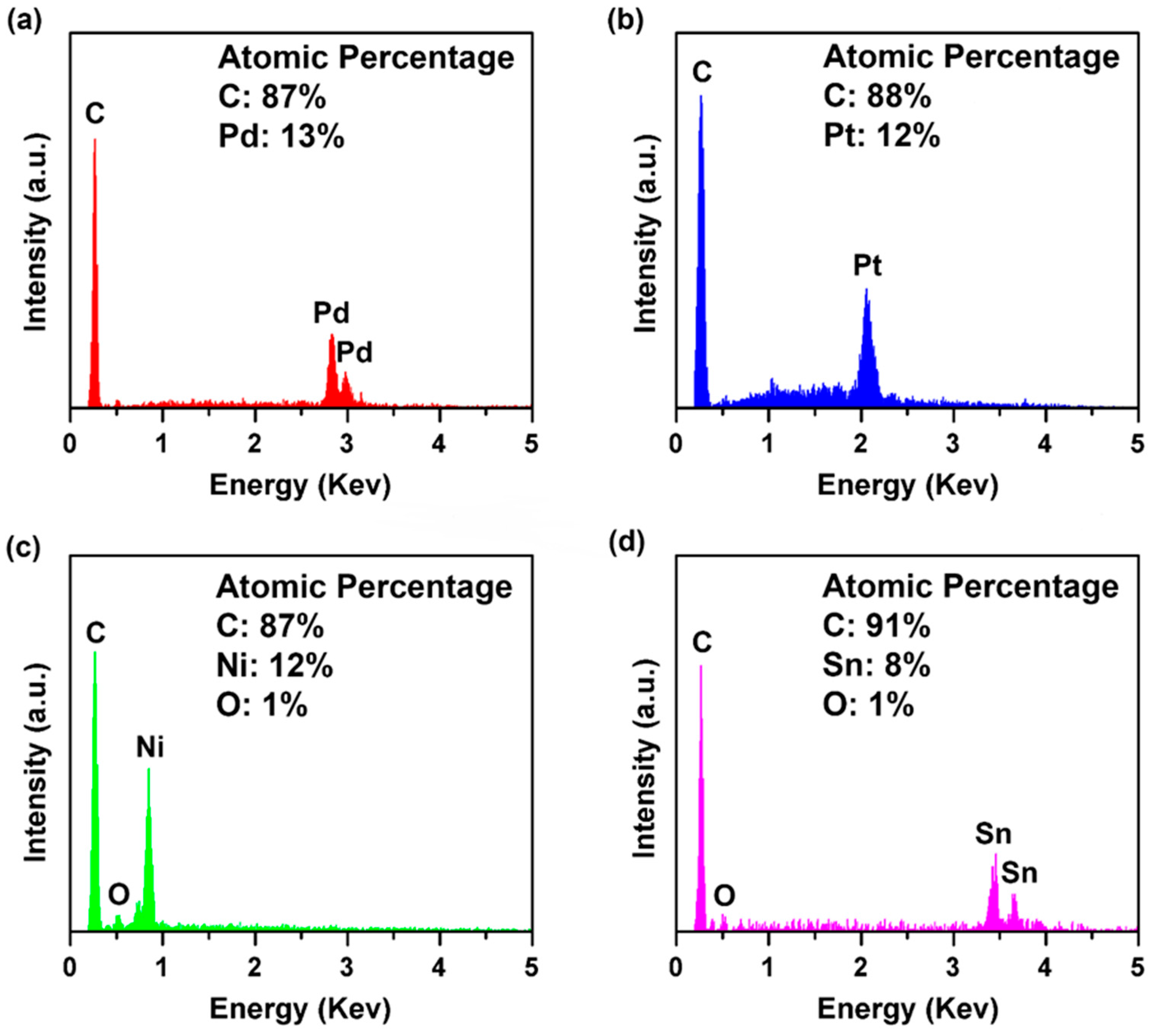
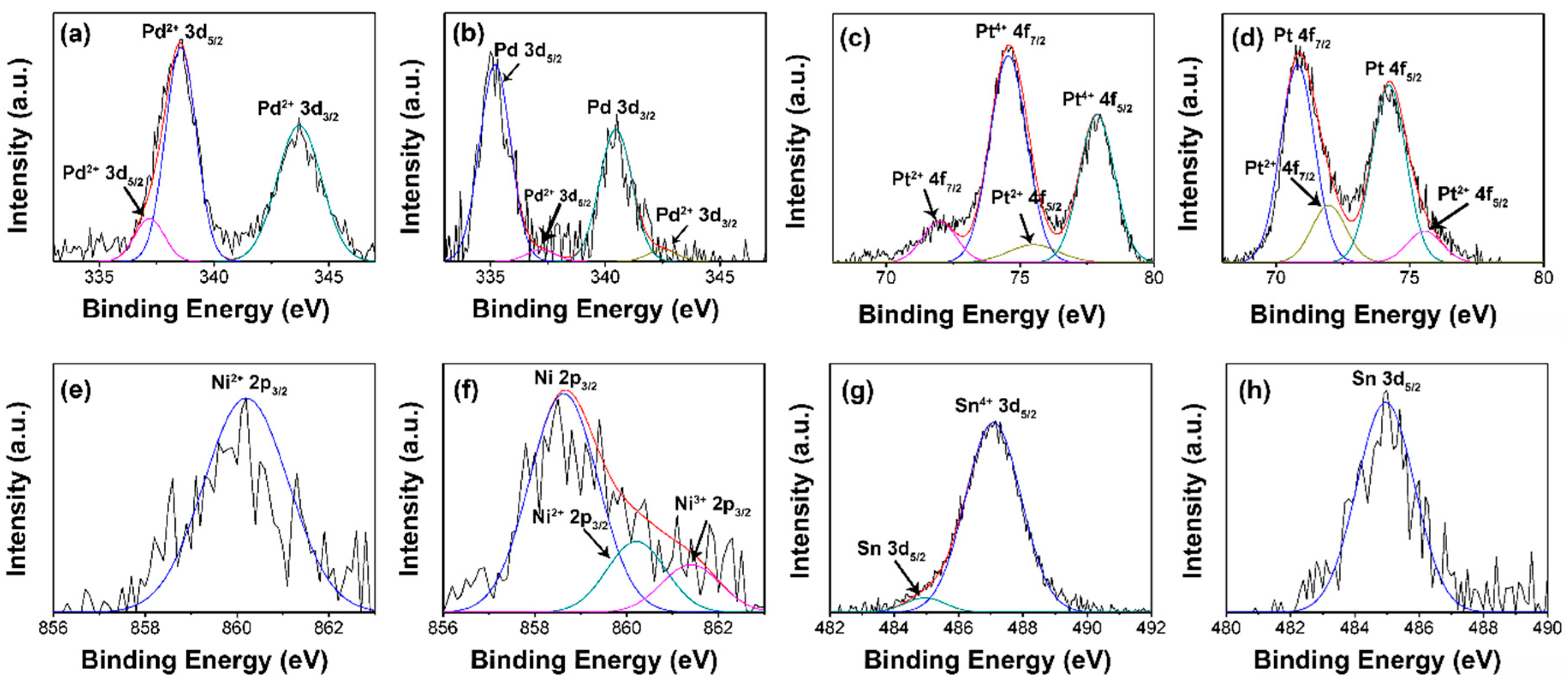
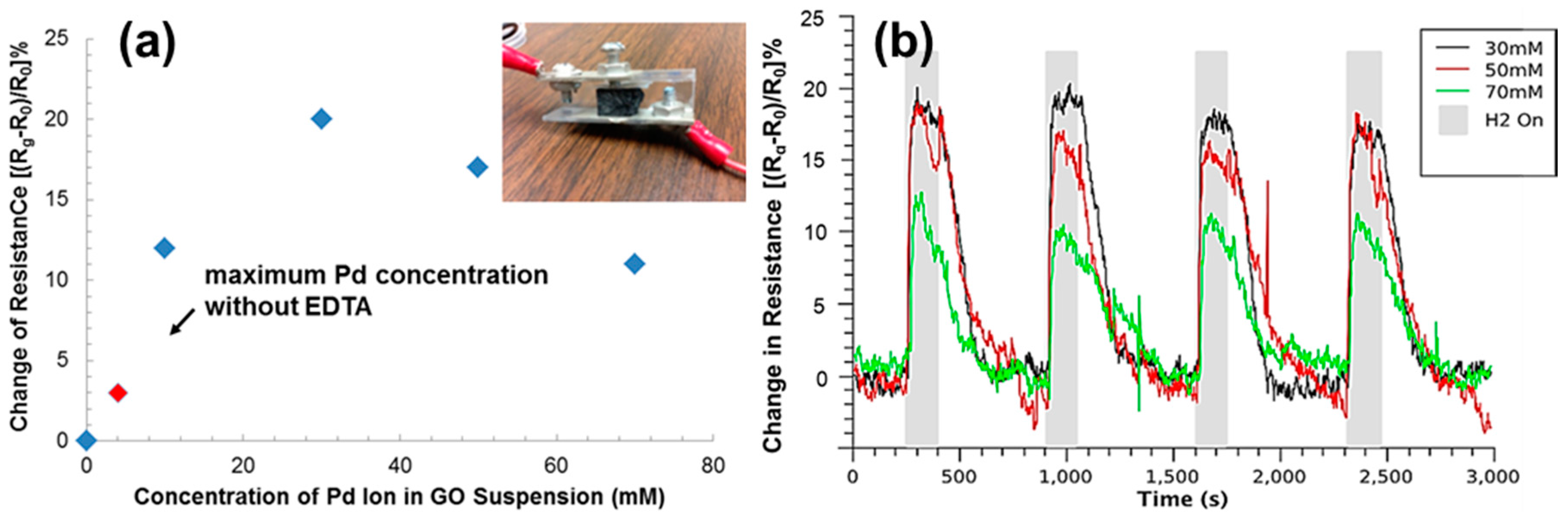
| Material | Method | Response Time | Recovery Time | Detection Range | Reference |
|---|---|---|---|---|---|
| Pd nanoparticles on CNTs | chemical reduction | 18 min. (300 ppm) and 7 min (3000 ppm) | 20 min (100 ppm) and 55 min (1000 ppm) | 100–1000 ppm | [66] |
| Pd and C60 | physical vapor deposition | 500 s | 500 s | 1% | [67] |
| Pd nanocubes on CNTs | chemical reduction | 15 min | 5 min. | 10 ppm–1% | [68] |
| Pd nanoparticles on graphene | galvanic displacement reaction | 2 min | 5 min | 25 ppm–2% | [69] |
| Pd nanoparticles on graphene nanoribbon films | chemical reduction | 750 s | tens of min | 100 ppm–1% | [70] |
| Pd nanoparticles on graphene nanoribbon films | e-beam evaporation | 15 min | 10 min | 40–8000 ppm | [71] |
| Pd nanoparticles on graphene aerogels | chemical reduction | 25 s | 175 s | 100–1000 ppm | current work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Barrios, E.; McInnis, M.; Zuyus, J.; Zhai, L. Fabrication of Graphene Aerogels with Heavily Loaded Metallic Nanoparticles. Micromachines 2017, 8, 47. https://doi.org/10.3390/mi8020047
Shen C, Barrios E, McInnis M, Zuyus J, Zhai L. Fabrication of Graphene Aerogels with Heavily Loaded Metallic Nanoparticles. Micromachines. 2017; 8(2):47. https://doi.org/10.3390/mi8020047
Chicago/Turabian StyleShen, Chen, Elizabeth Barrios, Matthew McInnis, Joseph Zuyus, and Lei Zhai. 2017. "Fabrication of Graphene Aerogels with Heavily Loaded Metallic Nanoparticles" Micromachines 8, no. 2: 47. https://doi.org/10.3390/mi8020047






