Optical Flow Cell for Measuring Size, Velocity and Composition of Flowing Droplets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fabrication of Microchip
2.3. Droplet Generation
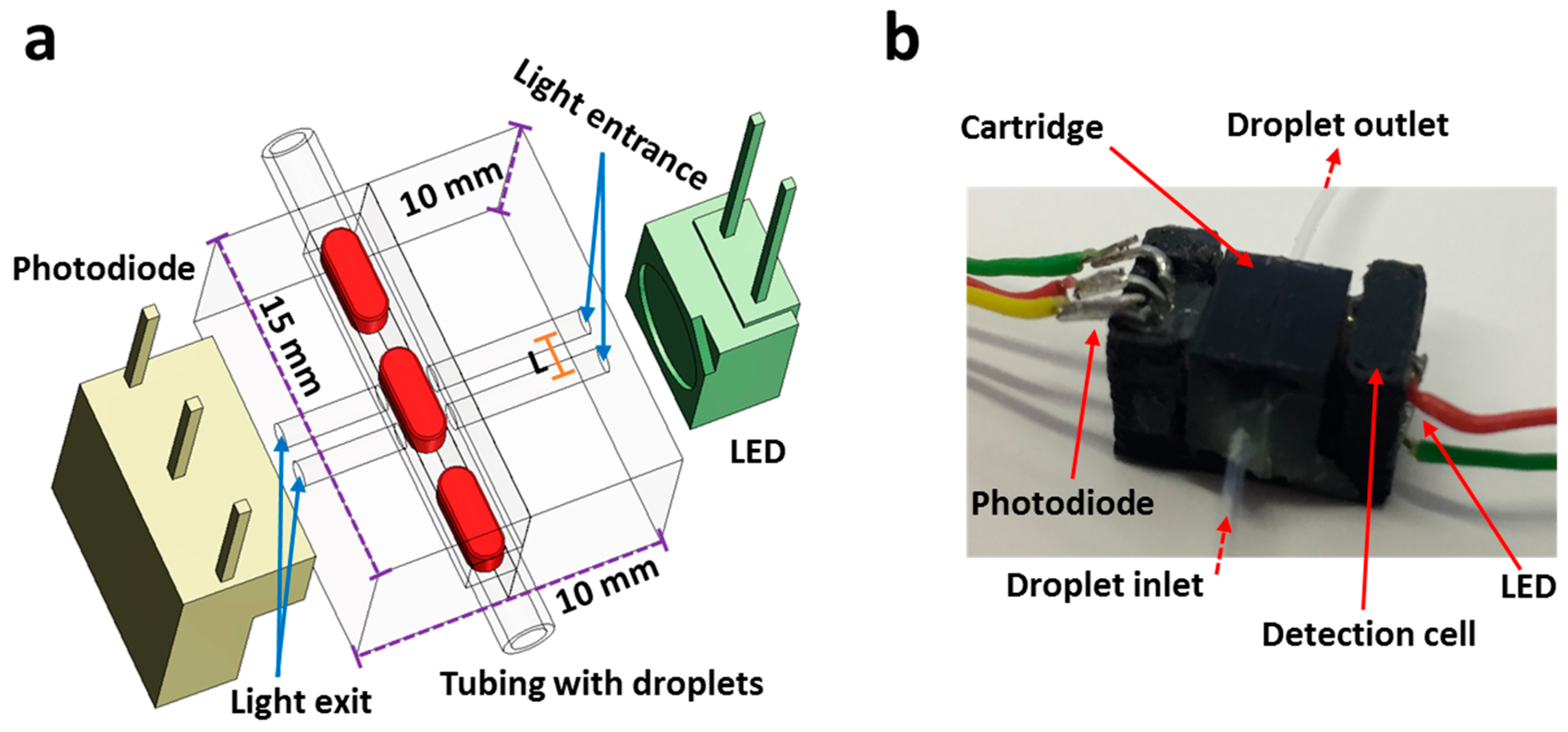
2.4. Fabrication of the Flow Cell
2.5. Data Processing
3. Results and Discussion
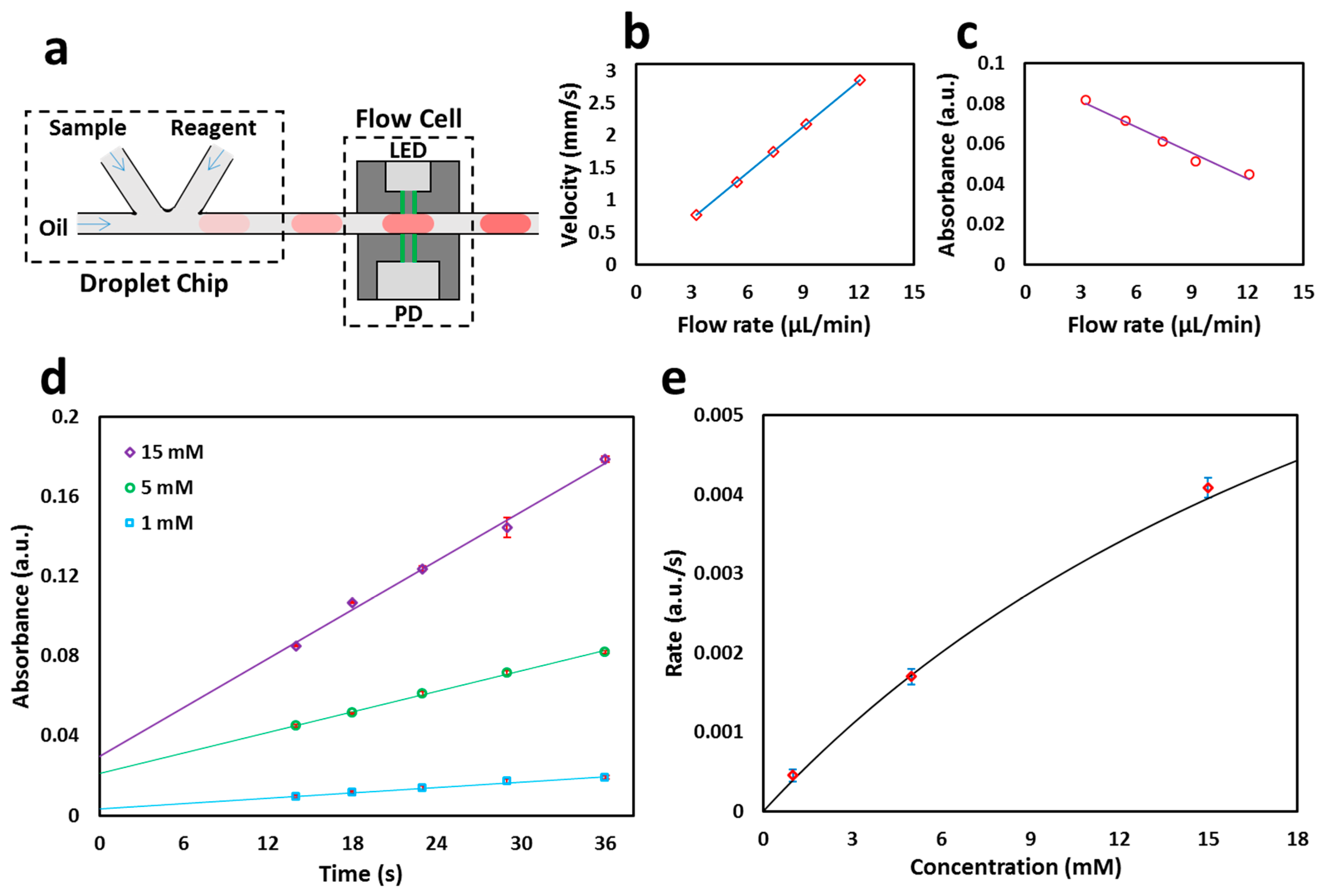
3.1. Operation of the Flow Cell
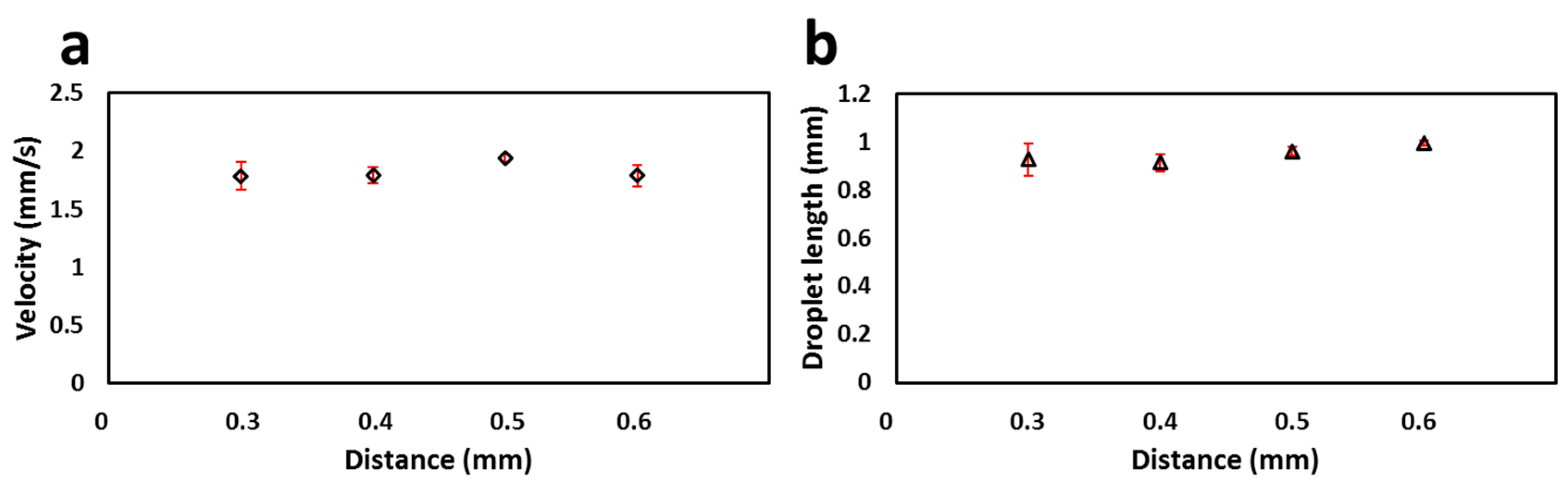
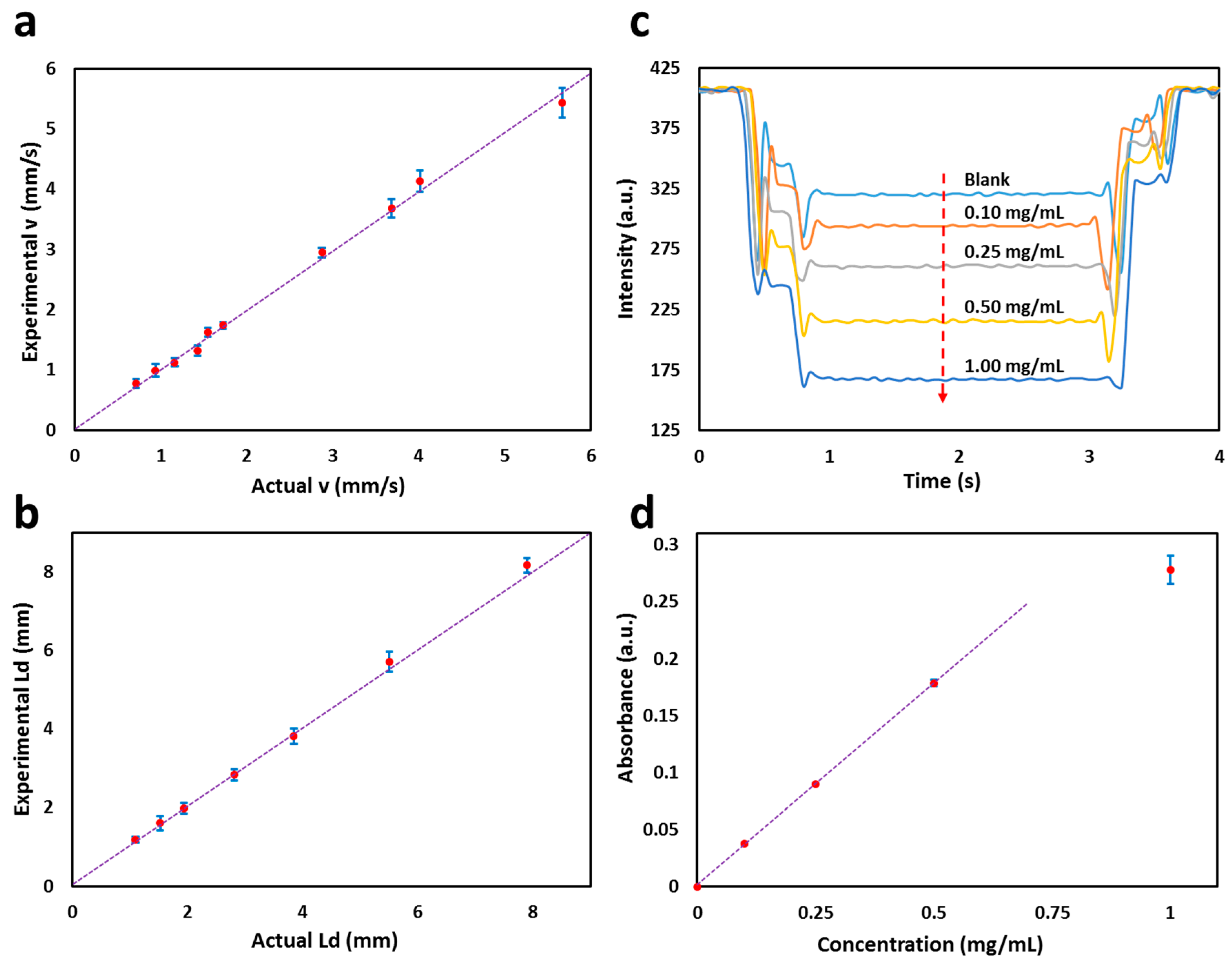
3.2. Calibration of the Flow Cell
3.3. Glucose Colorimetric Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, H.; Tice, J.D.; Ismagilov, R.F. A Microfluidic system for controlling reaction networks in time doppler-based flow rate sensing in microfluidic channels. Angew. Chem. Int. Ed. 2003, 42, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.; Bakal, A.; Tzur, M.; Veinguer, M.; Mazurski, N.; Cohen, N.; Levy, U. Doppler-based flow rate sensing in microfluidic channels. Sensors 2014, 14, 16799–16807. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, S.L.; Joensson, H.N.; Svahn, H.A. Multiplex analysis of enzyme kinetics and inhibition by droplet microfluidics using picoinjectors. Lab Chip 2013, 13, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Nightingale, A.M.; Niu, X. Continuous measurement of enzymatic kinetics in droplet flow for point-of-care monitoring. Analyst 2016, 141, 3266–3273. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.M.N.; Dong, T.; Hank, U.; Hoivik, N. Recent developments in optical detection technologies in lab-on-a-chip devices for biosensing applications. Sensors 2014, 14, 15458. [Google Scholar] [CrossRef] [PubMed]
- Wanh, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar]
- Mitton, K.P.; Trevithick, J.R. High-performance liquid chromatography-electrochemical detection of antioxidants in vertebrate lens: Glutathione, tocopherol, and ascorbate. Methods Enzymol. 1994, 233, 523–539. [Google Scholar] [PubMed]
- Wongkaew, N.; He, P.; Kurth, V.; Surareungchai, W.; Baeumner, A.J. Multi-channel PMMA microfluidic biosensor with integrated IDUAs for electrochemical detection. Anal. Bioanal. Chem. 2013, 18, 5965–5974. [Google Scholar] [CrossRef] [PubMed]
- Elbuken, C.; Glawdel, T.; Chan, D.; Ren, C.L. Detection of microdroplet size and speed using capacitive sensors. Sens. Actuators A Phys. 2011, 171, 55–62. [Google Scholar] [CrossRef]
- Dong, T.; Barbosa, C. Capacitance variation induced by microfluidic two-phase flow across insulated interdigital electrodes in lab-on-chip devices. Sensors 2015, 15, 2694–2708. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, M.; Peng, S.; Wen, W.; Sheng, P. Real-time detection, control, and sorting of microfluidic droplets. Biomicrofluidics 2007, 1, 044101. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Darhuber, A.A.; Troian, S.M.; Wagner, S. Capacitive sensing of droplets for microfluidic devices based on thermocapillary actuation. Lab Chip 2004, 5, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Demori, M.; Ferraria, V.; Strazzab, D.; Poesio, P. A capacitive sensor system for the analysis of two-phase flows of oil and conductive water. Sens. Actuators A Phys. 2010, 163, 172–179. [Google Scholar] [CrossRef]
- Ernst, A.; Streule, W.; Schmitt, N.; Zengerle, R.; Koltay, P. A capacitive sensor for non-contact nanoliter droplet detection. Sens. Actuators A Phys. 2009, 153, 57–63. [Google Scholar] [CrossRef]
- De Saint Vincent, M.R.; Cassagnre, S.; Plantard, J.; Delville, J.P. Real-time droplet caliper for digital microfluidics. Microfluid. Nanofluid. 2012, 13, 261–271. [Google Scholar] [CrossRef]
- Ferraro, D.; Champ, J.; Teste, B.; Serra, M.; Malaquin, L.; Viovy, J.-L.; de Cremoux, P.; Descroix, S. Microfluidic platform combining droplets and magnetic tweezers: Application to HER2 expression in cancer diagnosis. Sci. Rep. 2016, 6, 25540. [Google Scholar] [CrossRef] [PubMed]
- Jakiela, S.; Makulska, S.; Korczyk, P.M.; Garstecki, P. Speed of flow of individual droplets in microfluidic channels as a function of the capillary number, volume of droplets and contrast of viscosities. Lab Chip 2011, 21, 3603–3608. [Google Scholar] [CrossRef] [PubMed]
- Revellina, R.; Dupontb, V.; Ursenbachera, T.; Thomea, J.R.; Zunc, I. Characterization of diabatic two-phase flows in microchannels: Flow parameter results for R-134a in a 0.5 mm channel. Int. J. Multiph. Flow 2006, 32, 755–774. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Morgan, H.; Zhang, X.; Niu, X. Droplet interfaced parallel and quantitative microfluidic-based separations. Anal. Chem. 2015, 87, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Karmali, K.; Karmali, A.; Teixeira, A.; Curto, M.J.M. Assay for glucose oxidase from Aspergillus niger and Penicillium amagasakiense by Fourier transform infrared spectroscopy. Anal. Biochem. 2004, 333, 320–327. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.-u.; Nightingale, A.M.; Niu, X. Optical Flow Cell for Measuring Size, Velocity and Composition of Flowing Droplets. Micromachines 2017, 8, 58. https://doi.org/10.3390/mi8020058
Hassan S-u, Nightingale AM, Niu X. Optical Flow Cell for Measuring Size, Velocity and Composition of Flowing Droplets. Micromachines. 2017; 8(2):58. https://doi.org/10.3390/mi8020058
Chicago/Turabian StyleHassan, Sammer-ul, Adrian M. Nightingale, and Xize Niu. 2017. "Optical Flow Cell for Measuring Size, Velocity and Composition of Flowing Droplets" Micromachines 8, no. 2: 58. https://doi.org/10.3390/mi8020058





