An Automated Microfluidic System for the Generation of Droplet Interface Bilayer Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Microchip Fabrication
2.3. Electrical Recordings
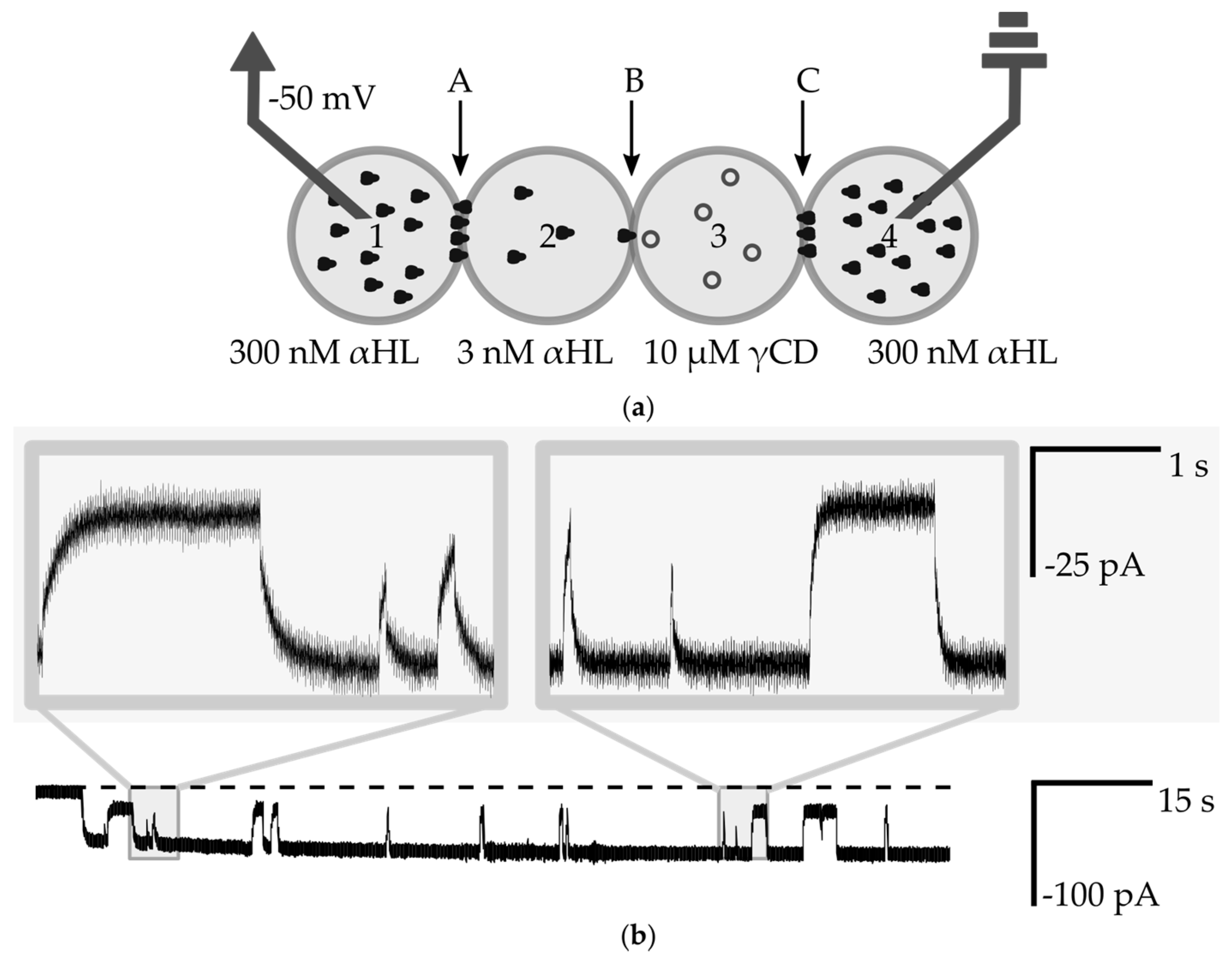
3. Results and Discussion
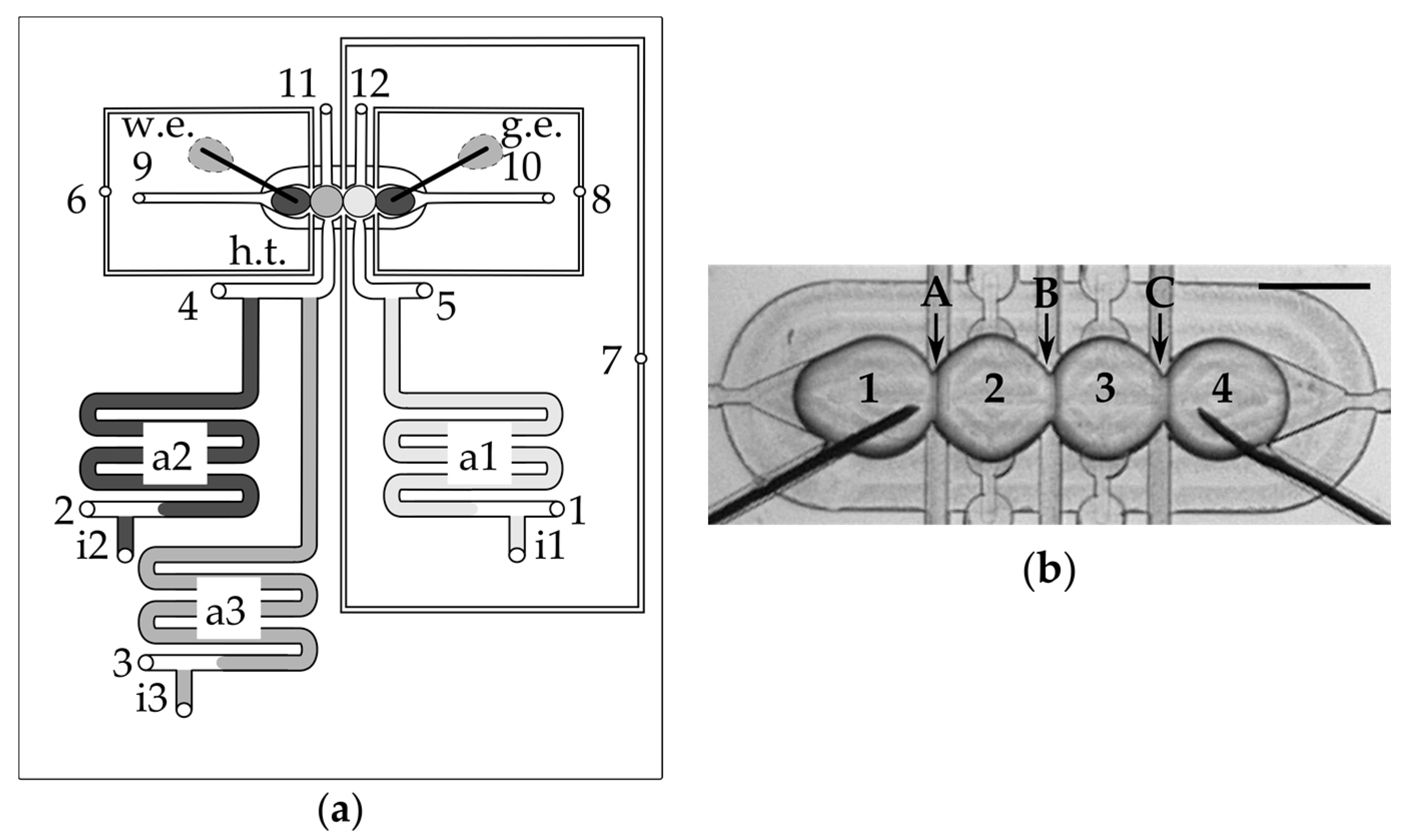
3.1. Layout and Operation of the Device
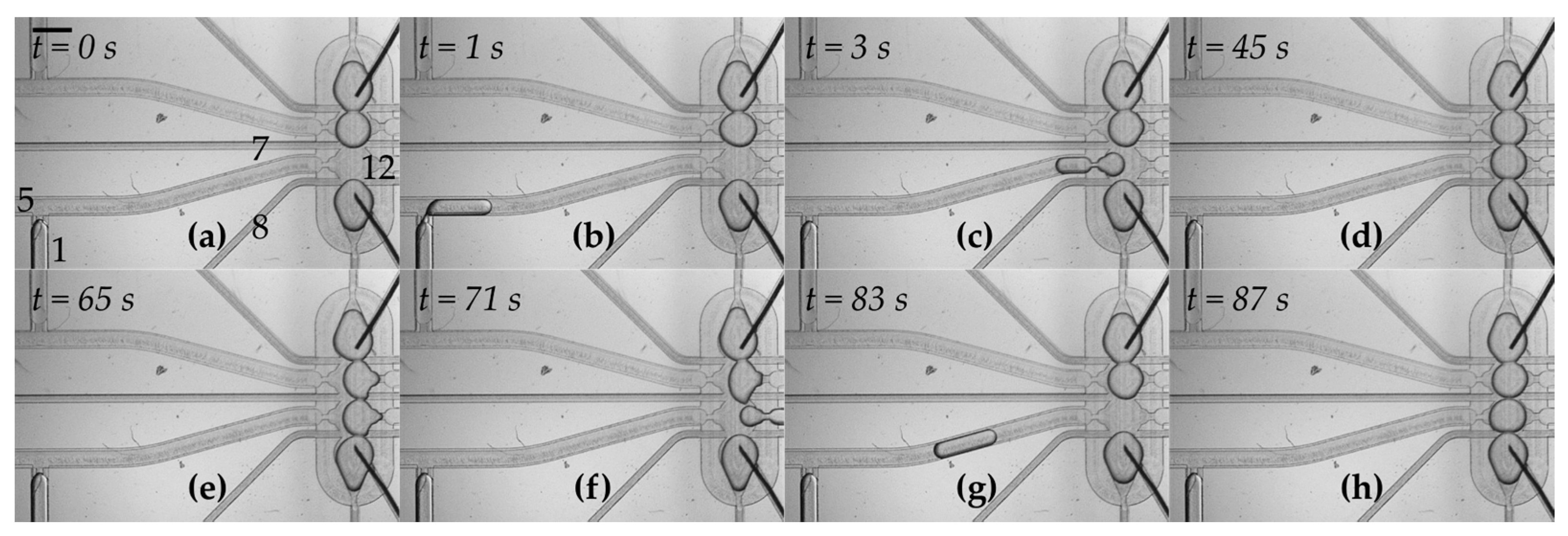
3.2. Droplets Form a Network in a Trap
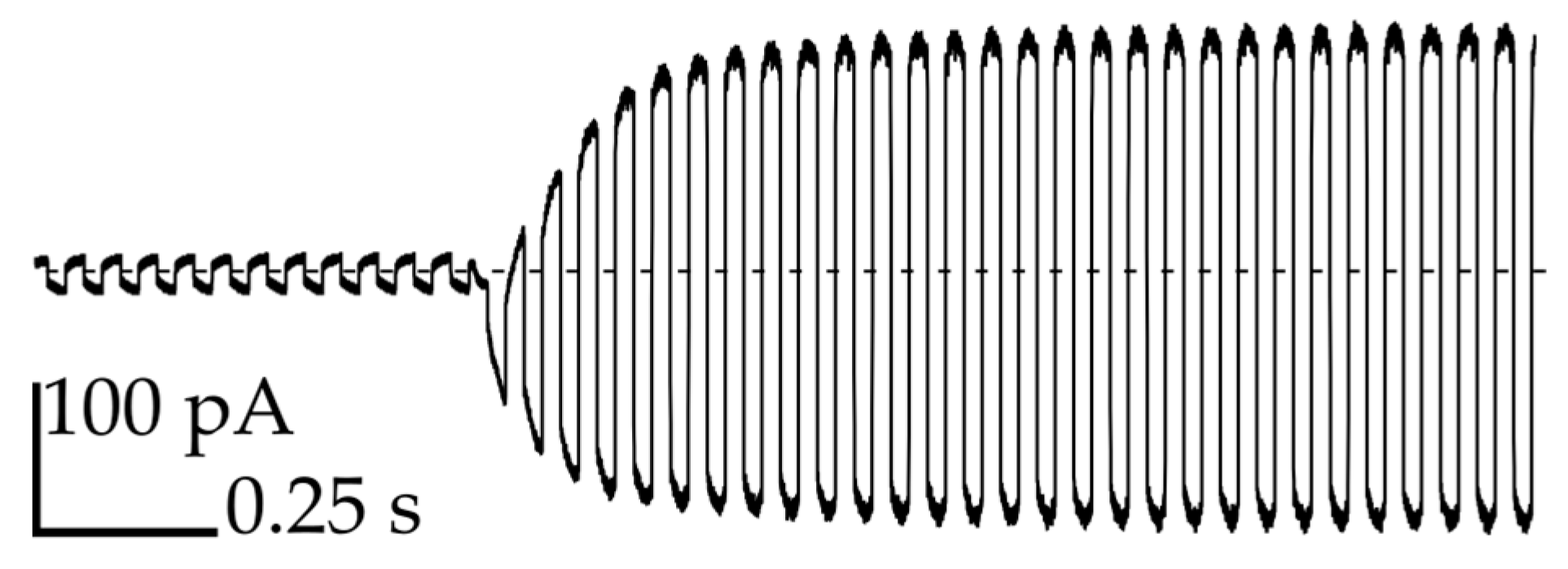
3.3. Transmission of Signal through the Network
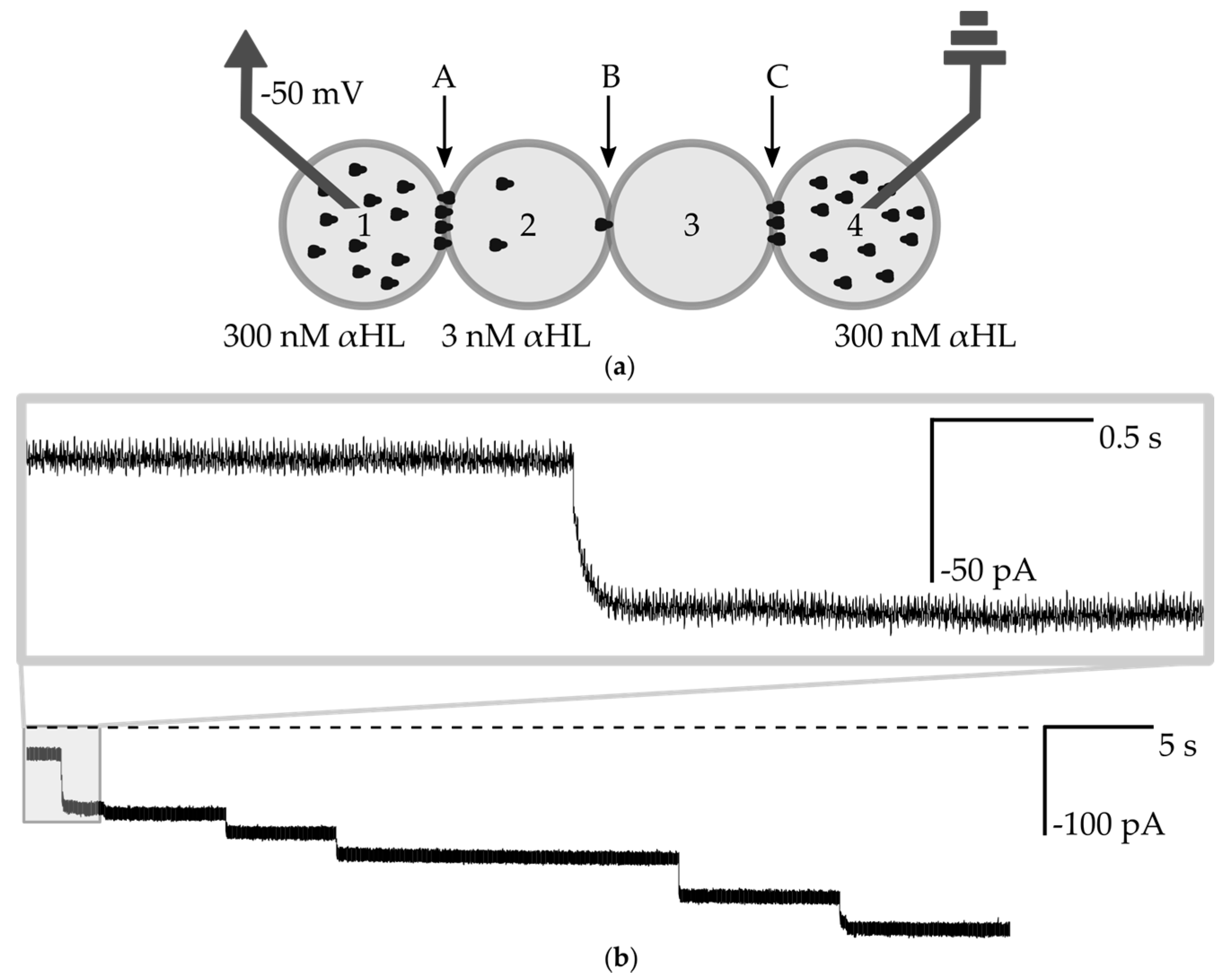
3.4. Measurements of the Interaction of a Nanopore with Small Molecules
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bayley, H.; Cronin, B.; Heron, A.; Holden, M.A.; Hwang, W.L.; Syeda, R.; Thompson, J.; Wallace, M. Droplet interface bilayers. Mol. Biosyst. 2008, 4, 1191–1208. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Takeuchi, S. Artificial Cell Membrane Systems for Biosensing Applications. Anal. Chem. 2017, 89, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.A.; Needham, D.; Bayley, H. Functional Bionetworks from Nanoliter Water Droplets. J. Am. Chem. Soc. 2007, 129, 8650–8655. [Google Scholar] [CrossRef] [PubMed]
- Maglia, G.; Heron, A.J.; Hwang, W.L.; Holden, M.A.; Mikhailova, E.; Li, Q.; Cheley, S.; Bayley, H. Droplet networks with incorporated protein diodes show collective properties. Nat. Nano 2009, 4, 437–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar, G.; Heron, A.J.; Bayley, H. Formation of droplet networks that function in aqueous environments. Nat. Nano 2011, 6, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Guzowski, J.; Jakiela, S.; Korczyk, P.M.; Garstecki, P. Custom tailoring multiple droplets one-by-one. Lab Chip 2013, 13, 4308–4311. [Google Scholar] [CrossRef] [PubMed]
- Friddin, M.S.; Bolognesi, G.; Elani, Y.; Brooks, N.J.; Law, R.V.; Seddon, J.M.; Neil, M.A.A.; Ces, O. Optically assembled droplet interface bilayer (OptiDIB) networks from cell-sized microdroplets. Soft Matter 2016, 12, 7731–7734. [Google Scholar] [CrossRef] [PubMed]
- Yasuga, H.; Kawano, R.; Takinoue, M.; Tsuji, Y.; Osaki, T.; Kamiya, K.; Miki, N.; Takeuchi, S. Logic Gate Operation by DNA Translocation through Biological Nanopores. PLoS ONE 2016, 11, e0149667. [Google Scholar] [CrossRef] [PubMed]
- Carreras, P.; Elani, Y.; Law, R.V.; Brooks, N.J.; Seddon, J.M.; Ces, O. A microfluidic platform for size-dependent generation of droplet interface bilayer networks on rails. Biomicrofluidics 2015, 9, 064121. [Google Scholar] [CrossRef] [PubMed]
- Zagnoni, M.; Cooper, J.M. A microdroplet-based shift register. Lab Chip 2010, 10, 3069–3073. [Google Scholar] [CrossRef] [PubMed]
- King, P.H.; Jones, G.; Morgan, H.; de Planque, M.R.R.; Zauner, K.-P. Interdroplet bilayer arrays in millifluidic droplet traps from 3D-printed moulds. Lab Chip 2014, 14, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Schlicht, B.; Zagnoni, M. Droplet-interface-bilayer assays in microfluidic passive networks. Sci. Rep. 2015, 5, 9951. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-A.; Srijanto, B.; Collier, C.P.; Retterer, S.T.; Sarles, S.A. Hydrodynamic trapping for rapid assembly and in situ electrical characterization of droplet interface bilayer arrays. Lab Chip 2016, 16, 3576–3588. [Google Scholar] [CrossRef] [PubMed]
- Czekalska, M.A.; Kaminski, T.S.; Jakiela, S.; Tanuj Sapra, K.; Bayley, H.; Garstecki, P. A droplet microfluidic system for sequential generation of lipid bilayers and transmembrane electrical recordings. Lab Chip 2015, 15, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Villar, G.; Graham, A.D.; Bayley, H. A Tissue-Like Printed Material. Science 2013, 340, 48. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.A. Chapter 11—Building interconnected membrane networks. In Methods in Cell Biology; Ross, J., Marshall, W.F., Eds.; Academic Press: New York, NY, USA, 2015; Volume 128, pp. 201–222. [Google Scholar]
- Elani, Y.; Gee, A.; Law, R.V.; Ces, O. Engineering multi-compartment vesicle networks. Chem. Sci. 2013, 4, 3332–3338. [Google Scholar] [CrossRef]
- Stanley, C.E.; Elvira, K.S.; Niu, X.Z.; Gee, A.D.; Ces, O.; Edel, J.B.; deMello, A.J. A microfluidic approach for high-throughput droplet interface bilayer (DIB) formation. Chem. Commun. 2010, 46, 1620–1622. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Kawano, R.; Osaki, T.; Kamiya, K.; Miki, N.; Takeuchi, S. Droplet Split-and-Contact Method for High-Throughput Transmembrane Electrical Recording. Anal. Chem. 2013, 85, 10913–10919. [Google Scholar] [CrossRef] [PubMed]
- Tomoike, F.; Tonooka, T.; Osaki, T.; Takeuchi, S. Repetitive formation of optically-observable planar lipid bilayers by rotating chambers on a microaperture. Lab Chip 2016, 16, 2423–2426. [Google Scholar] [CrossRef] [PubMed]
- Baaken, G.; Ankri, N.; Schuler, A.-K.; Rühe, J.; Behrends, J.C. Nanopore-Based Single-Molecule Mass Spectrometry on a Lipid Membrane Microarray. ACS Nano 2011, 5, 8080–8088. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Tsuji, Y.; Sato, K.; Osaki, T.; Kamiya, K.; Hirano, M.; Ide, T.; Miki, N.; Takeuchi, S. Automated Parallel Recordings of Topologically Identified Single Ion Channels. Sci. Rep. 2013, 3, 1995. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Holden, M.A.; Hwang, W.L.; Bayley, H. Screening Blockers against a Potassium Channel with a Droplet Interface Bilayer Array. J. Am. Chem. Soc. 2008, 130, 15543–15548. [Google Scholar] [CrossRef] [PubMed]
- Churski, K.; Kaminski, T.S.; Jakiela, S.; Kamysz, W.; Baranska-Rybak, W.; Weibel, D.B.; Garstecki, P. Rapid screening of antibiotic toxicity in an automated microdroplet system. Lab Chip 2012, 12, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Korczyk, P.M.; Derzsi, L.; Jakiela, S.; Garstecki, P. Microfluidic traps for hard-wired operations on droplets. Lab Chip 2013, 13, 4096–4102. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Holden, M.A.; White, S.; Bayley, H. Electrical Behavior of Droplet Interface Bilayer Networks: Experimental Analysis and Modeling. J. Am. Chem. Soc. 2007, 129, 11854–11864. [Google Scholar] [CrossRef]
- Gross, L.C.M.; Heron, A.J.; Baca, S.C.; Wallace, M.I. Determining Membrane Capacitance by Dynamic Control of Droplet Interface Bilayer Area. Langmuir 2011, 27, 14335–14342. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of Staphylococcal α-Hemolysin, a Heptameric Transmembrane Pore. Science 1996, 274, 1859. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, T.S.; Jakiela, S.; Czekalska, M.A.; Postek, W.; Garstecki, P. Automated generation of libraries of nL droplets. Lab. Chip 2012, 12, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Gielen, F.; Edel, J.B.; deMello, A.J. A microdroplet dilutor for high-throughput screening. Nat. Chem. 2011, 3, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Holden, M.A.; Pentelute, B.L.; Collier, R.J. Ultrasensitive detection of protein translocated through toxin pores in droplet-interface bilayers. Proc. Natl. Acad. Sci. USA 2011, 108, 16577–16581. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lein, M.; Gunderson, C.; Holden, M.A. Direct Quantitation of Peptide-Mediated Protein Transport across a Droplet–Interface Bilayer. J. Am. Chem. Soc. 2011, 133, 15818–15821. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Ziegler, D.; Arnaut, V.; Martin, T.G.; Kapsner, K.; Henneberg, K.; Bausch, A.R.; Dietz, H.; Simmel, F.C. Molecular transport through large-diameter DNA nanopores. Nat. Commun. 2016, 7, 12787. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.R.; Göpfrich, K.; Wood, J.W.; Thacker, V.V.; Stulz, E.; Keyser, U.F.; Howorka, S. Lipid-Bilayer-Spanning DNA Nanopores with a Bifunctional Porphyrin Anchor. Angew. Chem. Int. Ed Engl. 2013, 52, 12069–12072. [Google Scholar] [CrossRef] [PubMed]
- Guzowski, J.; Korczyk, P.M.; Jakiela, S.; Garstecki, P. Automated high-throughput generation of droplets. Lab Chip 2011, 11, 3593–3595. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czekalska, M.A.; Kaminski, T.S.; Horka, M.; Jakiela, S.; Garstecki, P. An Automated Microfluidic System for the Generation of Droplet Interface Bilayer Networks. Micromachines 2017, 8, 93. https://doi.org/10.3390/mi8030093
Czekalska MA, Kaminski TS, Horka M, Jakiela S, Garstecki P. An Automated Microfluidic System for the Generation of Droplet Interface Bilayer Networks. Micromachines. 2017; 8(3):93. https://doi.org/10.3390/mi8030093
Chicago/Turabian StyleCzekalska, Magdalena A., Tomasz S. Kaminski, Michal Horka, Slawomir Jakiela, and Piotr Garstecki. 2017. "An Automated Microfluidic System for the Generation of Droplet Interface Bilayer Networks" Micromachines 8, no. 3: 93. https://doi.org/10.3390/mi8030093






