A Review of the State of Dry Adhesives: Biomimetic Structures and the Alternative Designs They Inspire
Abstract
:1. Introduction
2. Design
2.1. Desirable Properties for Dry Adhesive Systems
2.1.1. Strong and Reversible Adhesion
2.1.2. High Adhesion to Preload Ratio
2.1.3. Durability
2.2. Fundamental Concepts for Creating a Dry Adhesive System
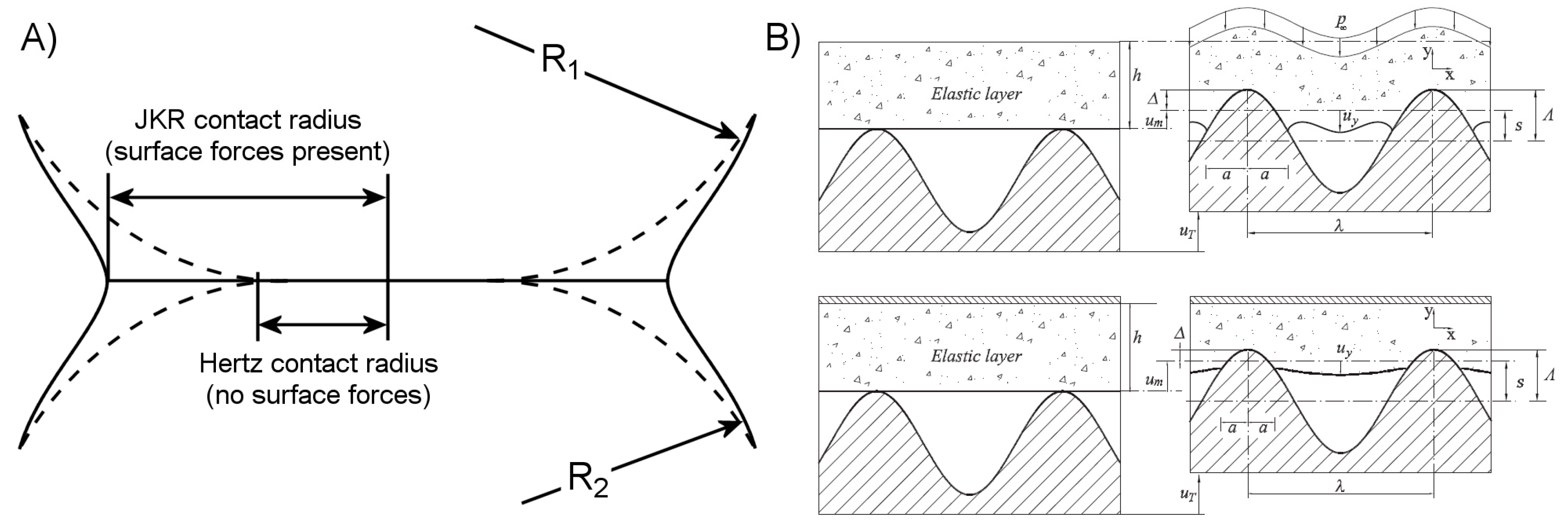
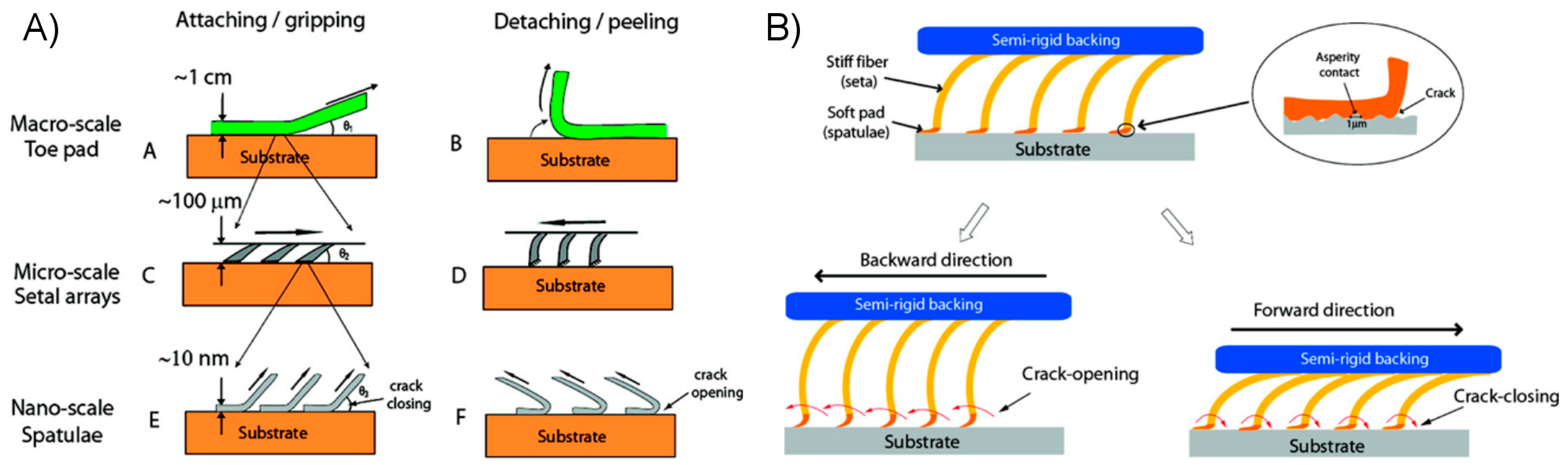
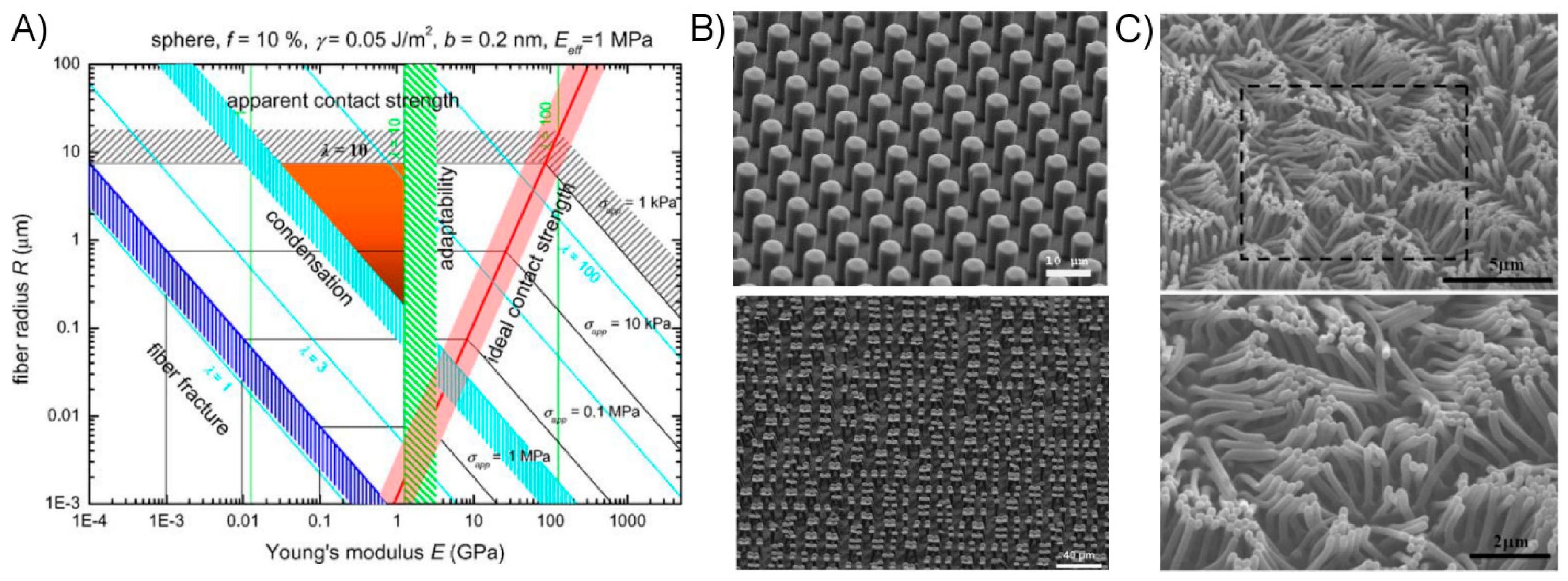
2.2.1. Attractive Forces
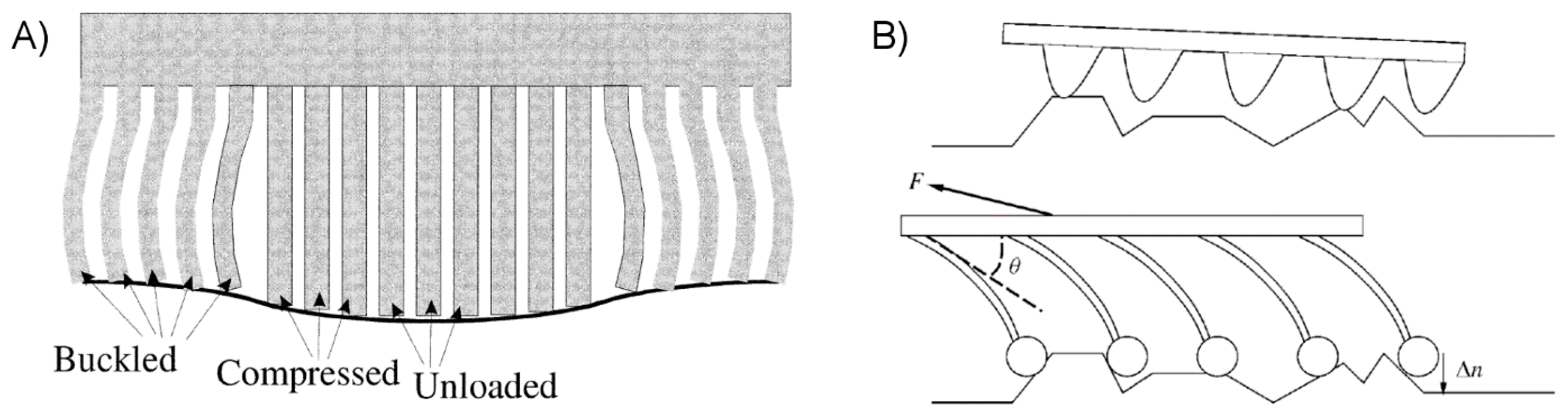
2.2.2. Controlling Elastic Energy
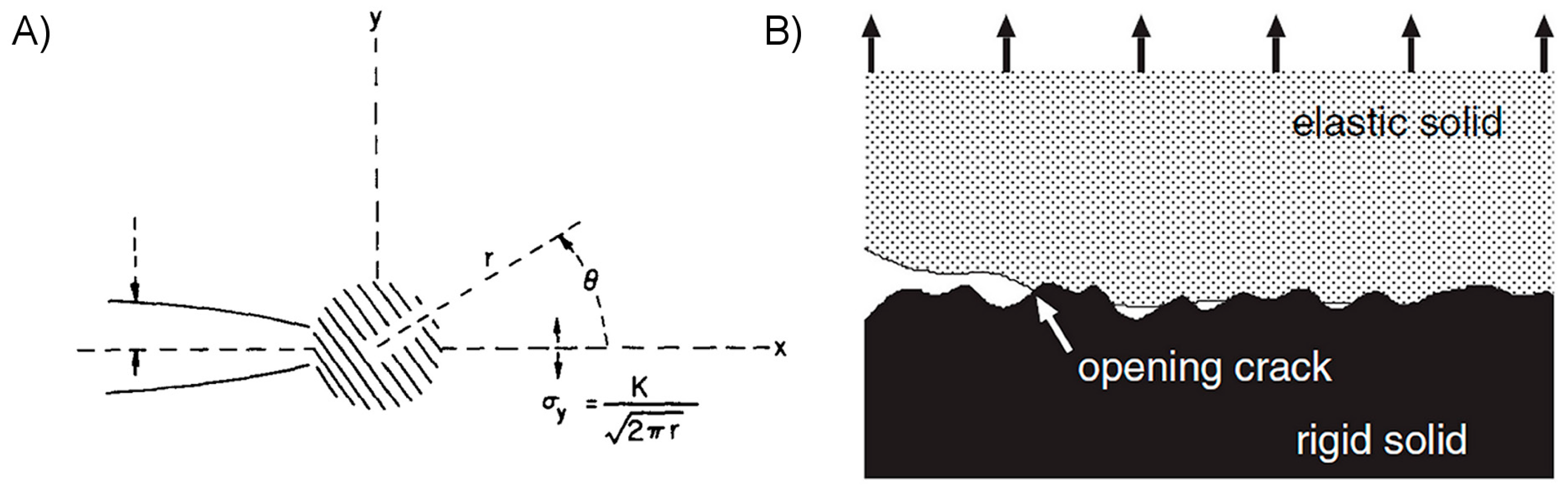
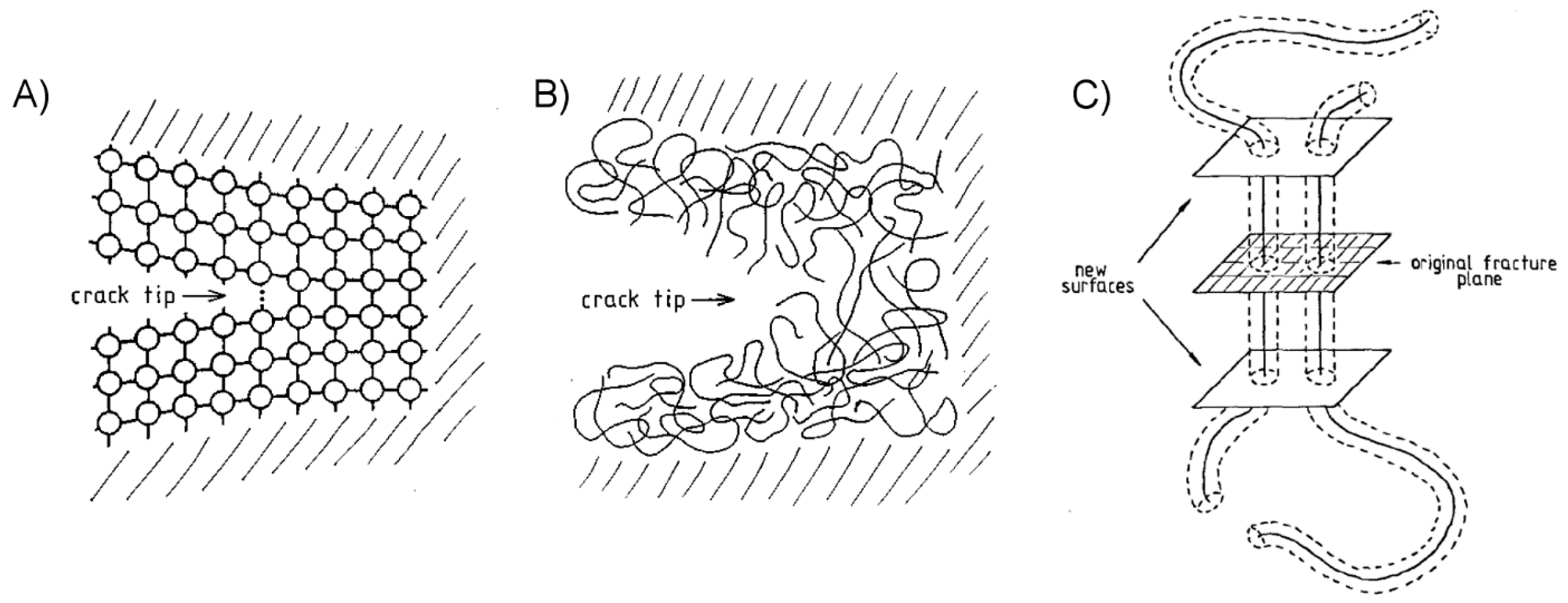
2.2.3. Enhancing Work of Adhesion through Energy Dissipation and Absorption
2.3. Strategies for Creating a Successful Dry Adhesive
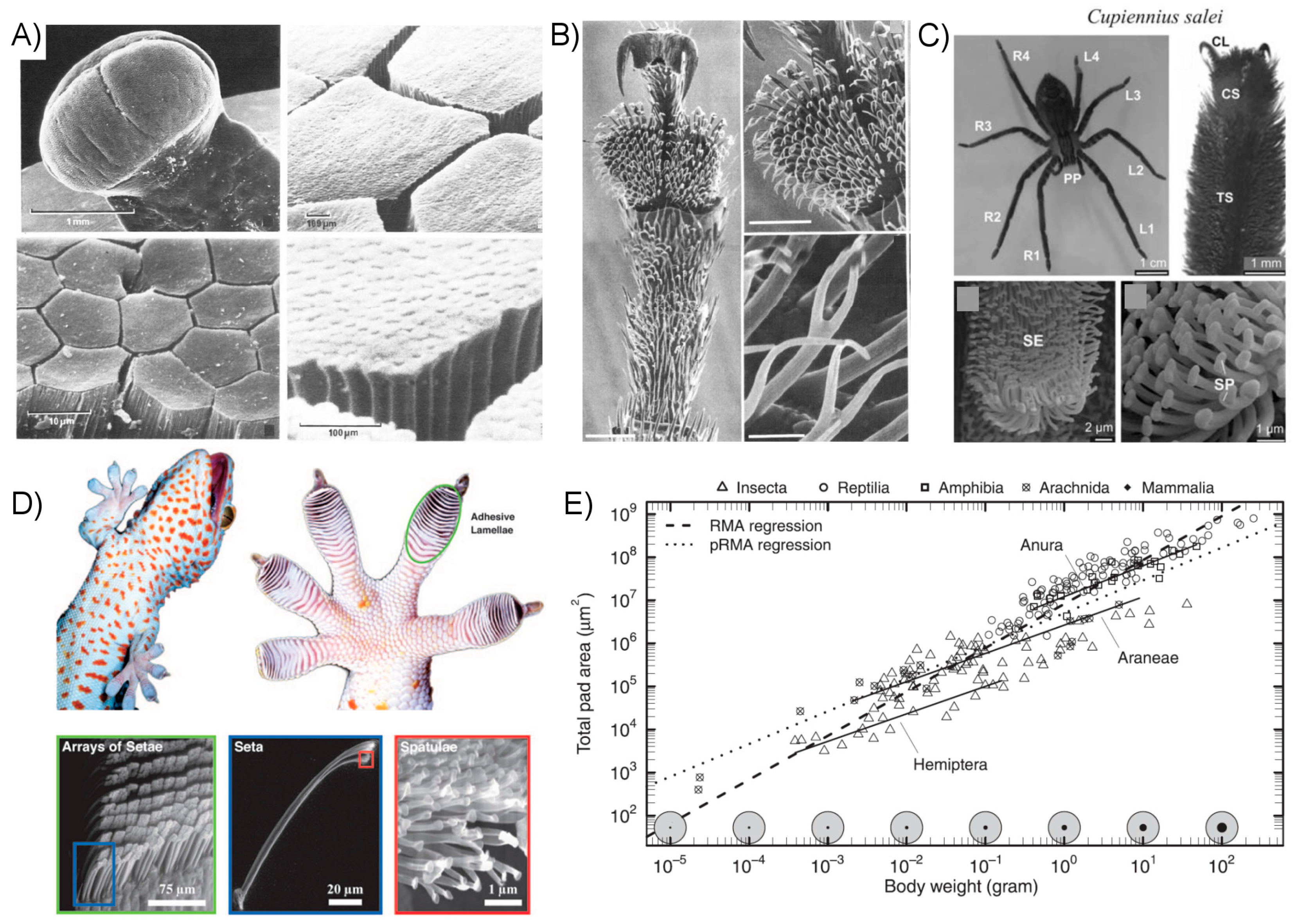
2.3.1. Observations of Natural Systems
2.3.2. Biomimetic Artificial Fibrillar Dry Adhesives
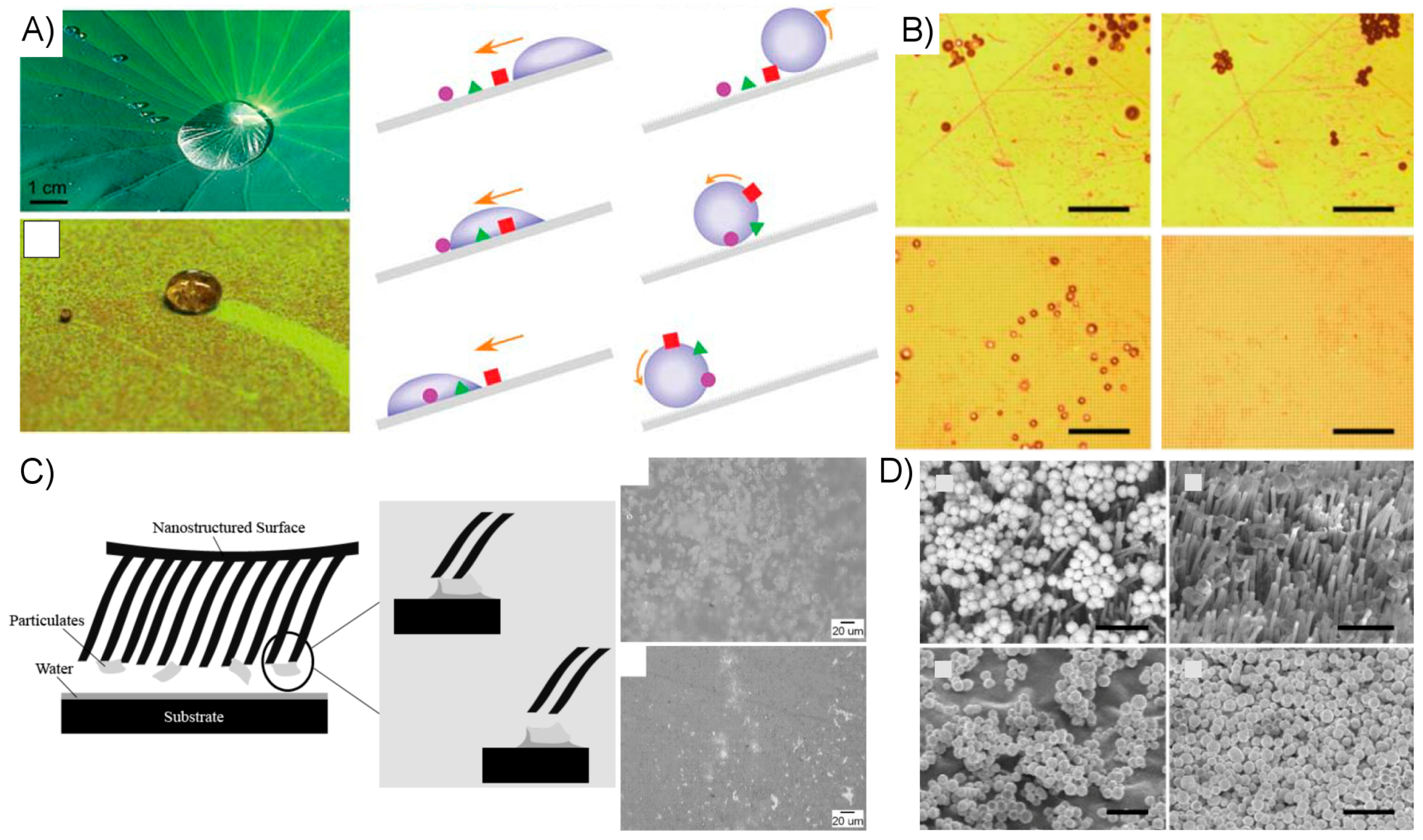
2.3.3. Alternative Strategies for Enhancing Artificial Dry Adhesive Performance
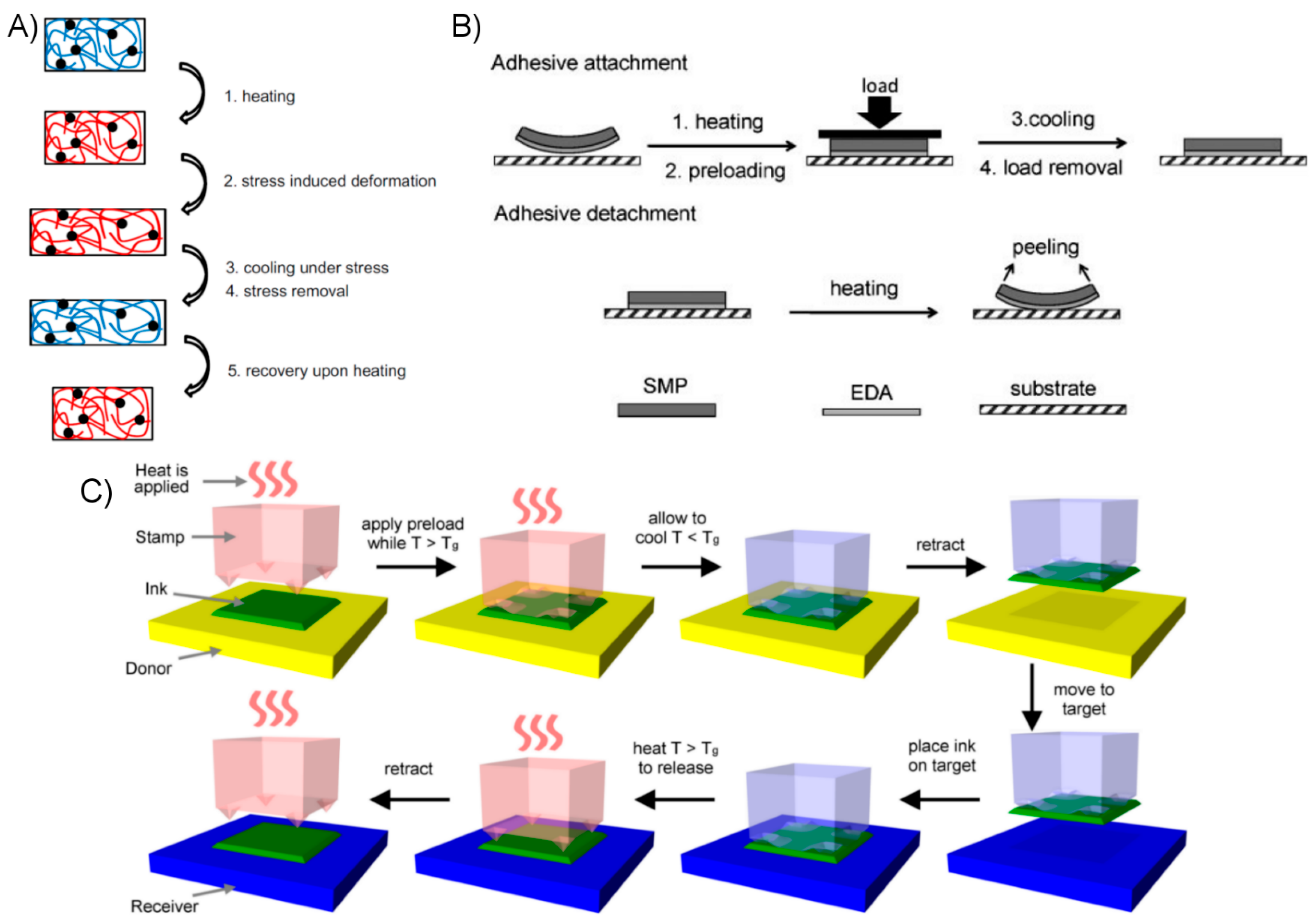
- A bond phase, wherein the SMP is heated above its Tg to increase its compliance, allowing thorough conformation to the opposing substrate,
- A cooling and unloading phase, wherein the SMP is cooled below its Tg to reduce compliance and fix its shape, at which point it has maximized its adhesive bond strength,
- A removal phase, wherein the SMP is re-heated above its Tg, increasing its compliance and releasing stored strains so that it may be removed easily.
3. Fabrication
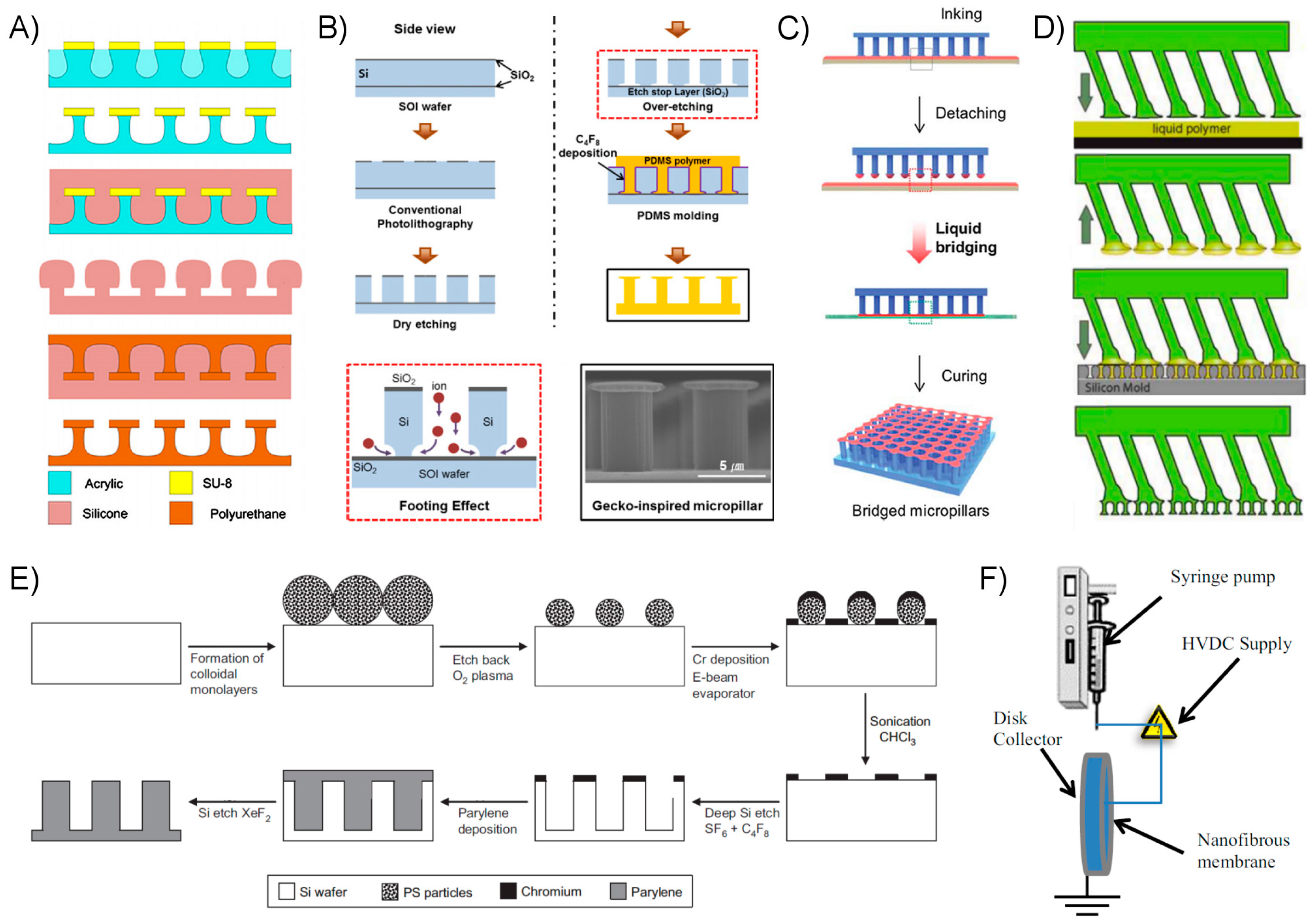
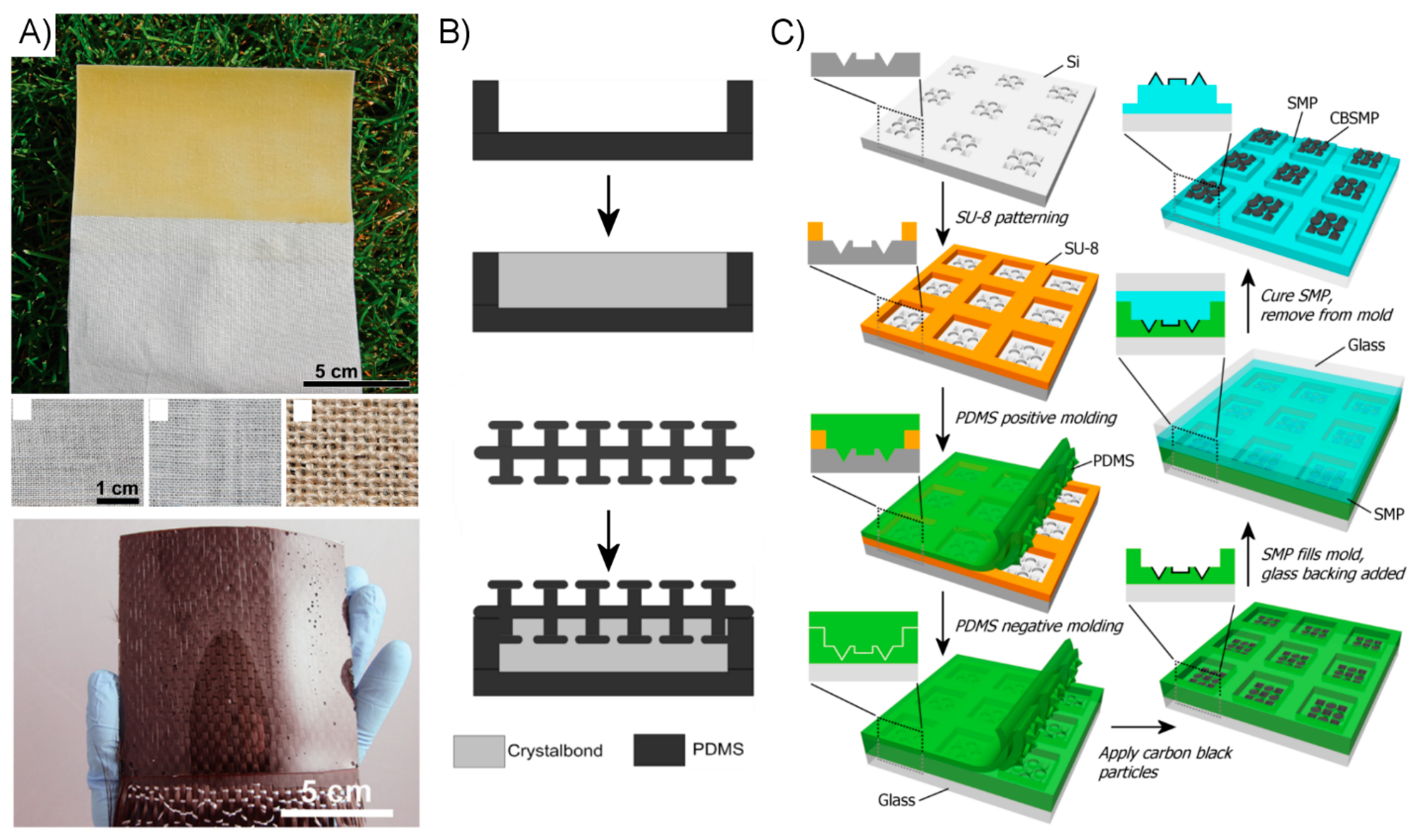
3.1. Fibrillar Fabrication Methods and Examples
3.2. Alternative Approach Fabrication Methods and Examples
4. Performance Metrics and Test Results
4.1. Performance Metrics
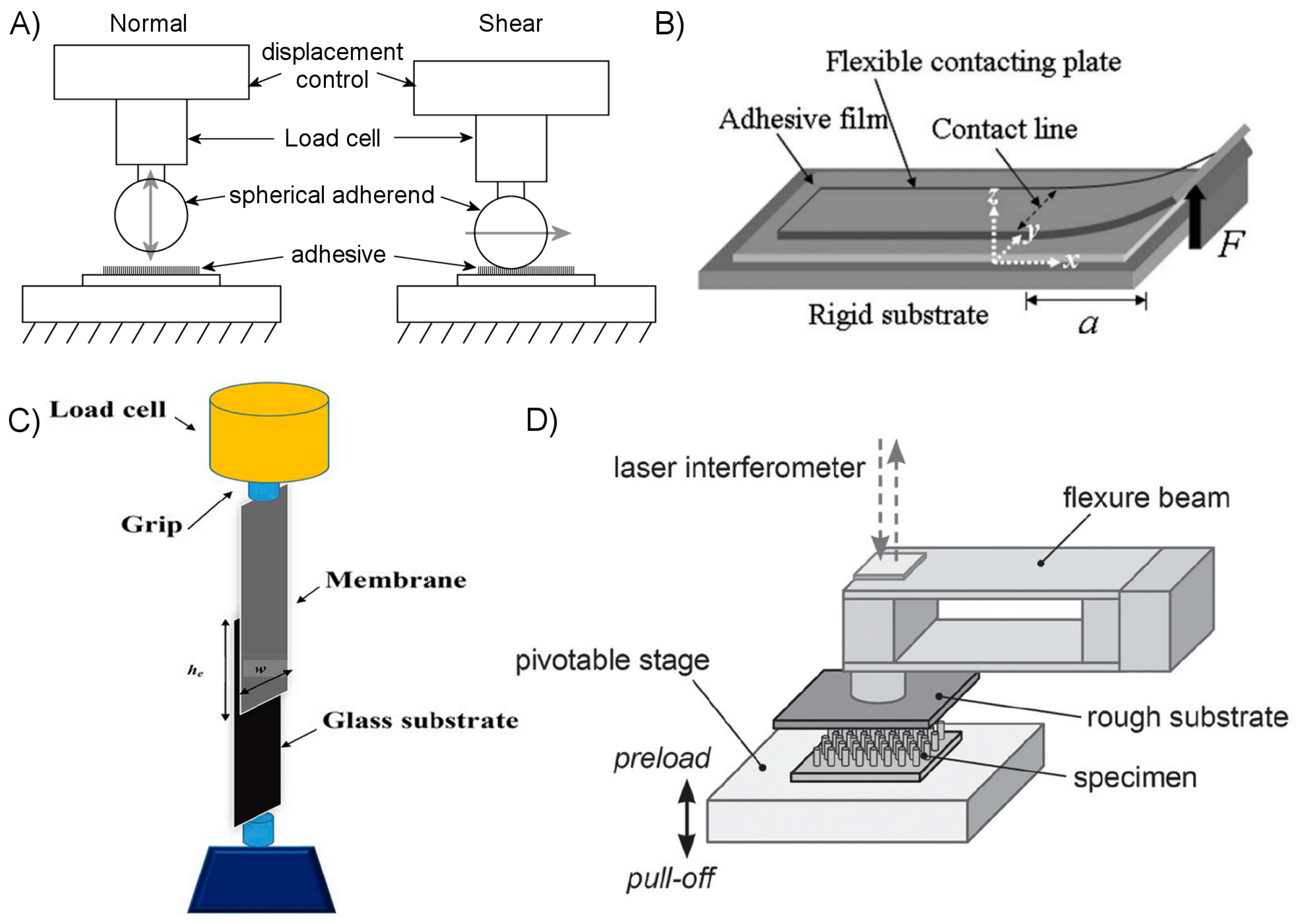
4.2. Test Methods
4.3. Test Results
5. Applications and Outlook
Acknowledgments
Conflicts of Interest
References
- Autumn, K.; Sitti, M.; Liang, Y.A.; Peattie, A.M.; Hansen, W.R.; Sponberg, S.; Kenny, T.W.; Fearing, R.; Israelachvili, J.N.; Full, R.J. Evidence for van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci. USA 2002, 99, 12252–12256. [Google Scholar] [CrossRef] [PubMed]
- Henning, B. Morphologie und histologie der tarsen von Tettigonia viridissima L. (Orthoptera, Ensifera). Z. Für Morphol. Tiere 1974, 79, 323–342. [Google Scholar]
- Federle, W.; Brainerd, E.L.; McMahon, T.A.; Hölldobler, B. Biomechanics of the movable pretarsal adhesive organ in ants and bees. Proc. Natl. Acad. Sci. USA 2001, 98, 6215–6220. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.F.G.; Croghan, P.C.; Gowing, R.P. The mechanism by which aphids adhere to smooth surfaces. J. Exp. Biol. 1990, 152, 243–253. [Google Scholar]
- Gravish, N.; Wilkinson, M.; Autumn, K. Frictional and elastic energy in gecko adhesive detachment. J. R. Soc. Interface 2008, 5, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Federle, W. Why are so many adhesive pads hairy? J. Exp. Biol. 2006, 209, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Autumn, K. Gecko Adhesion: Structure, Function, and Applications. MRS Bull. 2007, 32, 473–478. [Google Scholar] [CrossRef]
- Ruibal, R.; Ernst, V. The structure of the digital setae of lizards. J. Morphol. 1965, 117, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Boesel, L.F.; Greiner, C.; Arzt, E.; del Campo, A. Gecko-Inspired Surfaces: A Path to Strong and Reversible Dry Adhesives. Adv. Mater. 2010, 22, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Pang, C.; Jeong, H.-E.; Kim, H.-N.; Yoon, H.; Jung, H.-S.; Suh, K.-Y. Towards the Next Level of Bioinspired Dry Adhesives: New Designs and Applications. Adv. Funct. Mater. 2011, 21, 3606–3616. [Google Scholar] [CrossRef]
- Hu, S.; Xia, Z. Rational Design and Nanofabrication of Gecko-Inspired Fibrillar Adhesives. Small 2012, 8, 2464–2468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Pesika, N.; Zeng, H.; Tian, Y.; Israelachvili, J. Recent advances in gecko adhesion and friction mechanisms and development of gecko-inspired dry adhesive surfaces. Friction 2013, 1, 114–129. [Google Scholar] [CrossRef]
- Heepe, L.; Kovalev, A.E.; Varenberg, M.; Tuma, J.; Gorb, S.N. First mushroom-shaped adhesive microstructure: A review. Theor. Appl. Mech. Lett. 2012, 2, 14008. [Google Scholar] [CrossRef]
- Pattantyus-Abraham, A.; Krahn, J.; Menon, C. Recent Advances in Nanostructured Biomimetic Dry Adhesives. Front. Bioeng. Biotechnol. 2013, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sahay, R.; Low, H.Y.; Baji, A.; Foong, S.; Wood, K.L. A state-of-the-art review and analysis on the design of dry adhesion materials for applications such as climbing micro-robots. RSC Adv. 2015, 5, 50821–50832. [Google Scholar] [CrossRef]
- Cutkosky, M.R. Climbing with adhesion: From bioinspiration to biounderstanding. Interface Focus 2015, 5, 20150015. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.D. Strength Predictions for Lap Joints, Especially with Composite Adherends. A Review. J. Adhes. 1989, 30, 219–242. [Google Scholar] [CrossRef]
- Hansen, W.R.; Autumn, K. Evidence for self-cleaning in gecko setae. Proc. Natl. Acad. Sci. USA 2005, 102, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Clemente, C.J.; Bullock, J.M.R.; Beale, A.; Federle, W. Evidence for self-cleaning in fluid-based smooth and hairy adhesive systems of insects. J. Exp. Biol. 2010, 213, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Genzer, J.; Marmur, A. Biological and Synthetic Self-Cleaning Surfaces. MRS Bull. 2008, 33, 742–746. [Google Scholar] [CrossRef]
- Kim, S.; Cheung, E.; Sitti, M. Wet Self-Cleaning of Biologically Inspired Elastomer Mushroom Shaped Microfibrillar Adhesives. Langmuir 2009, 25, 7196–7199. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Kendall, K.; Roberts, A.D. Surface Energy and the Contact of Elastic Solids. Proc. R. Soc. Lond. Math. Phys. Eng. Sci. 1971, 324, 301–313. [Google Scholar] [CrossRef]
- Autumn, K.; Peattie, A.M. Mechanisms of Adhesion in Geckos. Integr. Comp. Biol. 2002, 42, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Pastewka, L.; Robbins, M.O. Contact between rough surfaces and a criterion for macroscopic adhesion. Proc. Natl. Acad. Sci. USA 2014, 111, 3298–3303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tong, T.; Delzeit, L.; Kashani, A.; Meyyappan, M.; Majumdar, A. Interfacial energy and strength of multiwalled-carbon-nanotube-based dry adhesive. J. Vac. Sci. Technol. B 2006, 24, 331–335. [Google Scholar] [CrossRef]
- Kawai, S.; Foster, A.S.; Björkman, T.; Nowakowska, S.; Björk, J.; Canova, F.F.; Gade, L.H.; Jung, T.A.; Meyer, E. Van der Waals interactions and the limits of isolated atom models at interfaces. Nat. Commun. 2016, 7, 11559. [Google Scholar] [CrossRef] [PubMed]
- London, F. The general theory of molecular forces. Trans. Faraday Soc. 1937, 33. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Schrader, M.E. Young-Dupre Revisited. Langmuir 1995, 11, 3585–3589. [Google Scholar] [CrossRef]
- Girifalco, L.A.; Good, R.J. A Theory for the Estimation of Surface and Interfacial Energies. I. Derivation and Application to Interfacial Tension. J. Phys. Chem. 1957, 61, 904–909. [Google Scholar] [CrossRef]
- Maugis, D. Adherence of Elastomers: Fracture Mechanics Aspects. J. Adhes. 1987, 23, 61–66. [Google Scholar] [CrossRef]
- Hui, C.-Y.; Jagota, A.; Bennison, S.J.; Londono, J.D. Crack blunting and the strength of soft elastic solids. Proc. R. Soc. Lond. Math. Phys. Eng. Sci. 2003, 459, 1489–1516. [Google Scholar] [CrossRef]
- Dhelika, R.; Sawai, K.; Takahashi, K.; Takarada, W.; Kikutani, T.; Saito, S. Electrostatic chuck consisting of polymeric electrostatic inductive fibers for handling of objects with rough surfaces. Smart Mater. Struct. 2013, 22, 95010. [Google Scholar] [CrossRef]
- Krahn, J.; Menon, C. Electro-Dry-Adhesion. Langmuir 2012, 28, 5438–5443. [Google Scholar] [CrossRef] [PubMed]
- Ruffatto, D.; Parness, A.; Spenko, M. Improving controllable adhesion on both rough and smooth surfaces with a hybrid electrostatic/gecko-like adhesive. J. R. Soc. Interface 2014, 11, 20131089. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.H.; Sreekumar, M.; Ponnambalam, S.G. Hybrid electrostatic and elastomer adhesion mechanism for wall climbing robot. Mechatronics 2016, 35, 122–135. [Google Scholar] [CrossRef]
- Cao, C.; Sun, X.; Fang, Y.; Qin, Q.-H.; Yu, A.; Feng, X.-Q. Theoretical model and design of electroadhesive pad with interdigitated electrodes. Mater. Des. 2016, 89, 485–491. [Google Scholar] [CrossRef]
- Jeong, H.E.; Lee, S.H.; Kim, P.; Suh, K.Y. High aspect-ratio polymer nanostructures by tailored capillarity and adhesive force. Colloids Surf. Physicochem. Eng. Asp. 2008, 313–314, 359–364. [Google Scholar] [CrossRef]
- Hanna, G.; Jon, W.; Barnes, W.P.J. Adhesion and Detachment of the Toe Pads of Tree Frogs. J. Exp. Biol. 1991, 155, 103–125. [Google Scholar]
- Ishii, S. Adhesion of a Leaf Feeding Ladybird Epilachna vigintioctomaculta (Coleoptera: Coccinellidae) on a Virtically Smooth Surface. Appl. Entomol. Zool. 1987, 22, 222–228. [Google Scholar]
- Federle, W.; Riehle, M.; Curtis, A.S.G.; Full, R.J. An Integrative Study of Insect Adhesion: Mechanics and Wet Adhesion of Pretarsal Pads in Ants. Integr. Comp. Biol. 2002, 42, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.; Mantz, H.; Spolenak, R.; Mecke, K.; Jacobs, K.; Gorb, S.N.; Arzt, E. Evidence for capillarity contributions to gecko adhesion from single spatula nanomechanical measurements. Proc. Natl. Acad. Sci. USA 2005, 102, 16293–16296. [Google Scholar] [CrossRef] [PubMed]
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [PubMed]
- Gay, C. Stickiness—Some Fundamentals of Adhesion. Integr. Comp. Biol. 2002, 42, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Bates, R. Studies in the nature of adhesive tack. J. Appl. Polym. Sci. 1976, 20, 2941–2954. [Google Scholar] [CrossRef]
- Menga, N.; Afferrante, L.; Carbone, G. Adhesive and adhesiveless contact mechanics of elastic layers on slightly wavy rigid substrates. Int. J. Solids Struct. 2016, 88–89, 101–109. [Google Scholar] [CrossRef]
- Abbot, S. Dahlquist Criterion|Practical Adhesion Science|Prof Steven Abbott. Available online: http://www.stevenabbott.co.uk/practical-adhesion/dahlquist.php (accessed on 28 December 2016).
- Kim, S.; Sitti, M.; Hui, C.-Y.; Long, R.; Jagota, A. Effect of backing layer thickness on adhesion of single-level elastomer fiber arrays. Appl. Phys. Lett. 2007, 91, 161905. [Google Scholar] [CrossRef]
- Arzt, E.; Gorb, S.; Spolenak, R. From micro to nano contacts in biological attachment devices. Proc. Natl. Acad. Sci. USA 2003, 100, 10603–10606. [Google Scholar] [CrossRef] [PubMed]
- Varenberg, M.; Murarash, B.; Kligerman, Y.; Gorb, S.N. Geometry-controlled adhesion: Revisiting the contact splitting hypothesis. Appl. Phys. A 2011, 103, 933–938. [Google Scholar] [CrossRef]
- Jagota, A.; Bennison, S.J. Mechanics of Adhesion through a Fibrillar Microstructure. Integr. Comp. Biol. 2002, 42, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Sitti, M.; Fearing, R.S. Synthetic gecko foot-hair micro/nano-structures as dry adhesives. J. Adhes. Sci. Technol. 2003, 17, 1055–1073. [Google Scholar] [CrossRef]
- Williams, M.L. The continuum interpretation for fracture and adhesion. J. Appl. Polym. Sci. 1969, 13, 29–40. [Google Scholar] [CrossRef]
- Maugis, D.; Barquins, M. Fracture mechanics and the adherence of viscoelastic bodies. J. Phys. Appl. Phys. 1978, 11, 1989. [Google Scholar] [CrossRef]
- Irwin, G.R. Linear fracture mechanics, fracture transition, and fracture control. Eng. Fract. Mech. 1968, 1, 241–257. [Google Scholar] [CrossRef]
- Mulakaluri, N.; Persson, B.N.J. Adhesion between elastic solids with randomly rough surfaces: Comparison of analytical theory with molecular-dynamics simulations. EPL Europhys. Lett. 2011, 96, 66003. [Google Scholar] [CrossRef]
- Bartlett, M.D.; Croll, A.B.; King, D.R.; Paret, B.M.; Irschick, D.J.; Crosby, A.J. Looking Beyond Fibrillar Features to Scale Gecko-Like Adhesion. Adv. Mater. 2012, 24, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Risan, J.; Croll, A.B.; Azarmi, F. Compliance switching for adhesion control. J. Polym. Sci. B Polym. Phys. 2015, 53, 48–57. [Google Scholar] [CrossRef]
- King, D.R.; Crosby, A.J. Optimizing Adhesive Design by Understanding Compliance. ACS Appl. Mater. Interfaces 2015, 7, 27771–27781. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.D.; Crosby, A.J. High Capacity, Easy Release Adhesives from Renewable Materials. Adv. Mater. 2014, 26, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- King, D.R.; Bartlett, M.D.; Gilman, C.A.; Irschick, D.J.; Crosby, A.J. Creating Gecko-Like Adhesives for “Real World” Surfaces. Adv. Mater. 2014, 26, 4345–4351. [Google Scholar] [CrossRef] [PubMed]
- Lake, G.J.; Thomas, A.G. The Strength of Highly Elastic Materials. Proc. R. Soc. Lond. Math. Phys. Eng. Sci. 1967, 300, 108–119. [Google Scholar] [CrossRef]
- Gent, A.N. Adhesion and Strength of Viscoelastic Solids. Is There a Relationship between Adhesion and Bulk Properties? Langmuir 1996, 12, 4492–4496. [Google Scholar] [CrossRef]
- Maugis, D. Subcritical crack growth, surface energy, fracture toughness, stick-slip and embrittlement. J. Mater. Sci. 1985, 20, 3041–3073. [Google Scholar] [CrossRef]
- Schapery, R.A. A theory of crack initiation and growth in viscoelastic media. Int. J. Fract. 1975, 11, 141–159. [Google Scholar] [CrossRef]
- Gent, A.N.; Schultz, J. Effect of Wetting Liquids on the Strength of Adhesion of Viscoelastic Material. J. Adhes. 1972, 3, 281–294. [Google Scholar] [CrossRef]
- Prentice, P. The influence of molecular weight on the fracture of thermoplastic glassy polymers. J. Mater. Sci. 1985, 20, 1445–1454. [Google Scholar] [CrossRef]
- Lakhera, N.; Graucob, A.; Schneider, A.S.; Kroner, E.; Arzt, E.; Yakacki, C.M.; Frick, C.P. Effect of viscoelasticity on the spherical and flat adhesion characteristics of photopolymerizable acrylate polymer networks. Int. J. Adhes. Adhes. 2013, 44, 184–194. [Google Scholar] [CrossRef]
- Chang, R.-J.; Gent, A.N. Effect of interfacial bonding on the strength of adhesion of elastomers. I. Self-adhesion. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1619–1633. [Google Scholar] [CrossRef]
- Saulnier, F.; Ondarçuhu, T.; Aradian, A.; Raphaël, E. Adhesion between a Viscoelastic Material and a Solid Surface. Macromolecules 2004, 37, 1067–1075. [Google Scholar] [CrossRef]
- Persson, B.N.J. On the mechanism of adhesion in biological systems. J. Chem. Phys. 2003, 118, 7614–7621. [Google Scholar] [CrossRef]
- Persson, B.N.J. Fracture of polymers. J. Chem. Phys. 1999, 110, 9713–9724. [Google Scholar] [CrossRef]
- Chung, J.Y.; Chaudhury, M.K. Roles of discontinuities in bio-inspired adhesive pads. J. R. Soc. Interface 2005, 2, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Rice, J.R. A First-Order Perturbation Analysis of Crack Trapping by Arrays of Obstacles. J. Appl. Mech. 1989, 56, 828–836. [Google Scholar] [CrossRef]
- Glassmaker, N.J.; Jagota, A.; Hui, C.-Y.; Noderer, W.L.; Chaudhury, M.K. Biologically inspired crack trapping for enhanced adhesion. Proc. Natl. Acad. Sci. USA 2007, 104, 10786–10791. [Google Scholar] [CrossRef] [PubMed]
- Noderer, W.L.; Shen, L.; Vajpayee, S.; Glassmaker, N.J.; Jagota, A.; Hui, C.-Y. Enhanced adhesion and compliance of film-terminated fibrillar surfaces. Proc. R. Soc. Lond. Math. Phys. Eng. Sci. 2007, 463, 2631–2654. [Google Scholar] [CrossRef]
- Yan, S.; He, L.; Wang, H. Adhesion hysteresis of a film-terminated fibrillar array. Sci. China Phys. Mech. Astron. 2012, 55, 1026–1031. [Google Scholar] [CrossRef]
- Nadermann, N.; Ning, J.; Jagota, A.; Hui, C.-Y. Active Switching of Adhesion in a Film-Terminated Fibrillar Structure. Langmuir 2010, 26, 15464–15471. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Glassmaker, N.J.; Jagota, A.; Hui, C.-Y. Strongly enhanced static friction using a film-terminated fibrillar interface. Soft Matter 2008, 4, 618–625. [Google Scholar] [CrossRef]
- Bae, W.-G.; Kim, D.; Suh, K.-Y. Instantly switchable adhesion of bridged fibrillar adhesive via gecko-inspired detachment mechanism and its application to a transportation system. Nanoscale 2013, 5, 11876–11884. [Google Scholar] [CrossRef] [PubMed]
- Gorb, S.; Jiao, Y.; Scherge, M. Ultrastructural architecture and mechanical properties of attachment pads in Tettigonia viridissima (Orthoptera Tettigoniidae). J. Comp. Physiol. A 2000, 186, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.E. Anatomy of the Honey Bee; Cornell University Press: Ithaca, NY, USA, 1984. [Google Scholar]
- Zhou, Y.; Robinson, A.; Viney, C.; Federle, W. Effect of shear forces and ageing on the compliance of adhesive pads in adult cockroaches. J. Exp. Biol. 2015, 218, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Clemente, C.J.; Dirks, J.-H.; Barbero, D.R.; Steiner, U.; Federle, W. Friction ridges in cockroach climbing pads: Anisotropy of shear stress measured on transparent, microstructured substrates. J. Comp. Physiol. A 2009, 195, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.W. Adaptive features on the tarsi of cockroaches (Insecta: Dictyoptera). Int. J. Insect Morphol. Embryol. 1974, 3, 317–334. [Google Scholar] [CrossRef]
- Dirks, J.-H.; Clemente, C.J.; Federle, W. Insect tricks: Two-phasic foot pad secretion prevents slipping. J. R. Soc. Interface 2010, 7, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.D.; Hardie, J. The organs of adhesion in the aphid Megoura Viciae. J. Exp. Biol. 1988, 136, 209–228. [Google Scholar]
- Federle, W.; Barnes, W.J.P.; Baumgartner, W.; Drechsler, P.; Smith, J.M. Wet but not slippery: Boundary friction in tree frog adhesive toe pads. J. R. Soc. Interface 2006, 3, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Drotlef, D.-M.; Stepien, L.; Kappl, M.; Barnes, W.J.P.; Butt, H.-J.; del Campo, A. Insights into the Adhesive Mechanisms of Tree Frogs using Artificial Mimics. Adv. Funct. Mater. 2013, 23, 1137–1146. [Google Scholar] [CrossRef]
- Kappl, M.; Kaveh, F.; Barnes, W.J.P. Nanoscale friction and adhesion of tree frog toe pads. Bioinspir. Biomim. 2016, 11, 35003. [Google Scholar] [CrossRef] [PubMed]
- Iturri, J.; Xue, L.; Kappl, M.; García-Fernández, L.; Barnes, W.J.P.; Butt, H.-J.; del Campo, A. Torrent Frog-Inspired Adhesives: Attachment to Flooded Surfaces. Adv. Funct. Mater. 2015, 25, 1499–1505. [Google Scholar] [CrossRef]
- Emerson, S.B.; Diehl, D. Toe pad morphology and mechanisms of sticking in frogs. Biol. J. Linn. Soc. 1980, 13, 199–216. [Google Scholar] [CrossRef]
- Stork, N.E. A scanning electron microscope study of tarsal adhesive setae in the Coleoptera. Zool. J. Linn. Soc. 1980, 68, 173–306. [Google Scholar] [CrossRef]
- Niederegger, S.; Gorb, S.N. Friction and adhesion in the tarsal and metatarsal scopulae of spiders. J. Comp. Physiol. A 2006, 192, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Labonte, D.; Clemente, C.J.; Dittrich, A.; Kuo, C.-Y.; Crosby, A.J.; Irschick, D.J.; Federle, W. Extreme positive allometry of animal adhesive pads and the size limits of adhesion-based climbing. Proc. Natl. Acad. Sci. USA 2016, 113, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Bauchnenß, E.; Renner, M. Pulvillus of Calliphora erythrocephala Meig. (Diptera: Calliphoridae). Int. J. Insect Morphol. Embryol. 1977, 6, 225–227. [Google Scholar] [CrossRef]
- Chen, Y.; Shih, M.-C.; Wu, M.-H.; Yang, E.-C.; Chi, K.-J. Underwater attachment using hairs: The functioning of spatula and sucker setae from male diving beetles. J. R. Soc. Interface 2014, 11, 20140273. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, E.; Wolff, J.O.; Arzt, E.; Gorb, S.N. The whole is more than the sum of all its parts: Collective effect of spider attachment organs. J. Exp. Biol. 2014, 217, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.O.; Gorb, S.N. Radial arrangement of Janus-like setae permits friction control in spiders. Sci. Rep. 2013, 3, 1101. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.E.; Peterson, J.A. Convergent and Alternative Designs in the Digital Adhesive Pads of Scincid Lizards. Science 1982, 215, 1509–1511. [Google Scholar] [CrossRef] [PubMed]
- Prowse, M.S.; Wilkinson, M.; Puthoff, J.B.; Mayer, G.; Autumn, K. Effects of humidity on the mechanical properties of gecko setae. Acta Biomater. 2011, 7, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Gravish, N.; Wilkinson, M.; Sponberg, S.; Parness, A.; Esparza, N.; Soto, D.; Yamaguchi, T.; Broide, M.; Cutkosky, M.; Creton, C.; et al. Rate-dependent frictional adhesion in natural and synthetic gecko setae. J. R. Soc. Interface 2010, 7, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.-Y.; Glassmaker, N.J.; Jagota, A. How Compliance Compensates for Surface Roughness in Fibrillar Adhesion. J. Adhes. 2005, 81, 699–721. [Google Scholar] [CrossRef]
- Murphy, M.P.; Kim, S.; Sitti, M. Enhanced Adhesion by Gecko-Inspired Hierarchical Fibrillar Adhesives. ACS Appl. Mater. Interfaces 2009, 1, 849–855. [Google Scholar] [CrossRef] [PubMed]
- McKittrick, J.; Chen, P.-Y.; Bodde, S.G.; Yang, W.; Novitskaya, E.E.; Meyers, M.A. The Structure, Functions, and Mechanical Properties of Keratin. JOM 2012, 64, 449–468. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef]
- Autumn, K.; Dittmore, A.; Santos, D.; Spenko, M.; Cutkosky, M. Frictional adhesion: A new angle on gecko attachment. J. Exp. Biol. 2006, 209, 3569–3579. [Google Scholar] [CrossRef] [PubMed]
- Clemente, C.J.; Federle, W. Pushing versus pulling: Division of labour between tarsal attachment pads in cockroaches. Proc. R. Soc. Lond. B Biol. Sci. 2008, 275, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Choi, J.; Zaghi, G.; Zhang, J.; Carraro, C.; Maboudian, R. Frictional characteristics of stiff, high aspect ratio microfiber arrays based on cyclic olefin polymers. J. Adhes. Sci. Technol. 2017, 31, 1017–1027. [Google Scholar] [CrossRef]
- Del Campo, A.; Greiner, C.; Arzt, E. Contact Shape Controls Adhesion of Bioinspired Fibrillar Surfaces. Langmuir 2007, 23, 10235–10243. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Aksak, B.; Sitti, M. Adhesion and anisotropic friction enhancements of angled heterogeneous micro-fiber arrays with spherical and spatula tips. J. Adhes. Sci. Technol. 2007, 21, 1281–1296. [Google Scholar] [CrossRef]
- Kim, S.; Sitti, M. Biologically inspired polymer microfibers with spatulate tips as repeatable fibrillar adhesives. Appl. Phys. Lett. 2006, 89, 261911. [Google Scholar] [CrossRef]
- Kang, S.M. Bioinspired design and fabrication of green-environmental dry adhesive with robust wide-tip shape. Int. J. Precis. Eng. Manuf. Green Technol. 2016, 3, 189–192. [Google Scholar] [CrossRef]
- Aksak, B.; Murphy, M.P.; Sitti, M. Adhesion of Biologically Inspired Vertical and Angled Polymer Microfiber Arrays. Langmuir 2007, 23, 3322–3332. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.E.; Lee, J.-K.; Kim, H.N.; Moon, S.H.; Suh, K.Y. A nontransferring dry adhesive with hierarchical polymer nanohairs. Proc. Natl. Acad. Sci. USA 2009, 106, 5639–5644. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.E.; Lee, J.-K.; Kwak, M.K.; Moon, S.H.; Suh, K.Y. Effect of leaning angle of gecko-inspired slanted polymer nanohairs on dry adhesion. Appl. Phys. Lett. 2010, 96, 43704. [Google Scholar] [CrossRef]
- Parness, A.; Soto, D.; Esparza, N.; Gravish, N.; Wilkinson, M.; Autumn, K.; Cutkosky, M. A microfabricated wedge-shaped adhesive array displaying gecko-like dynamic adhesion, directionality and long lifetime. J. R. Soc. Interface 2009, 6, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Wettels, N.; Parness, A. Advances in fibrillar on-off polymer adhesive: Sensing and engagement speed. In Proceedings of the 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems, Chicago, IL, USA, 14–18 September 2014; pp. 4266–4271. [Google Scholar]
- Hawkes, E.W.; Eason, E.V.; Asbeck, A.T.; Cutkosky, M.R. The Gecko’s Toe: Scaling Directional Adhesives for Climbing Applications. IEEEASME Trans. Mechatron. 2013, 18, 518–526. [Google Scholar] [CrossRef]
- Kim, S.; Sitti, M.; Xie, T.; Xiao, X. Reversible dry micro-fibrillar adhesives with thermally controllable adhesion. Soft Matter 2009, 5, 3689–3693. [Google Scholar] [CrossRef]
- Zhao, B.; Pesika, N.; Zeng, H.; Wei, Z.; Chen, Y.; Autumn, K.; Turner, K.; Israelachvili, J. Role of Tilted Adhesion Fibrils (Setae) in the Adhesion and Locomotion of Gecko-like Systems. J. Phys. Chem. B 2009, 113, 3615–3621. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Fearing, R.S. Contact Self-Cleaning of Synthetic Gecko Adhesive from Polymer Microfibers. Langmuir 2008, 24, 10587–10591. [Google Scholar] [CrossRef] [PubMed]
- Kustandi, T.S.; Samper, V.D.; Yi, D.K.; Ng, W.S.; Neuzil, P.; Sun, W. Self-Assembled Nanoparticles Based Fabrication of Gecko Foot-Hair-Inspired Polymer Nanofibers. Adv. Funct. Mater. 2007, 17, 2211–2218. [Google Scholar] [CrossRef]
- Spolenak, R.; Gorb, S.; Arzt, E. Adhesion design maps for bio-inspired attachment systems. Acta Biomater. 2005, 1, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Greiner, C.; Spolenak, R.; Arzt, E. Adhesion design maps for fibrillar adhesives: The effect of shape. Acta Biomater. 2009, 5, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Greiner, C.; del Campo, A.; Arzt, E. Adhesion of Bioinspired Micropatterned Surfaces: Effects of Pillar Radius, Aspect Ratio, and Preload. Langmuir 2007, 23, 3495–3502. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, H.S.; Lee, J.; Kim, S.; Lee, K.-H.; Moon, W.; Kwon, T.H. Replication of high-aspect-ratio nanopillar array for biomimetic gecko foot-hair prototype by UV nano embossing with anodic aluminum oxide mold. Microsyst. Technol. 2007, 13, 601–606. [Google Scholar] [CrossRef]
- Pendergraph, S.A.; Bartlett, M.D.; Carter, K.R.; Crosby, A.J. Enhancing Adhesion of Elastomeric Composites through Facile Patterning of Surface Discontinuities. ACS Appl. Mater. Interfaces 2014, 6, 6845–6850. [Google Scholar] [CrossRef] [PubMed]
- Krahn, J.; Bovero, E.; Menon, C. Magnetic Field Switchable Dry Adhesives. ACS Appl. Mater. Interfaces 2015, 7, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Xie, T. Recent advances in polymer shape memory. Polymer 2011, 52, 4985–5000. [Google Scholar] [CrossRef]
- Santhosh Kumar, K.S.; Biju, R.; Reghunadhan Nair, C.P. Progress in shape memory epoxy resins. React. Funct. Polym. 2013, 73, 421–430. [Google Scholar] [CrossRef]
- Rousseau, I.A. Challenges of shape memory polymers: A review of the progress toward overcoming SMP’s limitations. Polym. Eng. Sci. 2008, 48, 2075–2089. [Google Scholar] [CrossRef]
- Xie, T.; Xiao, X. Self-Peeling Reversible Dry Adhesive System. Chem. Mater. 2008, 20, 2866–2868. [Google Scholar] [CrossRef]
- Wang, R.; Xiao, X.; Xie, T. Viscoelastic Behavior and Force Nature of Thermo-Reversible Epoxy Dry Adhesives. Macromol. Rapid Commun. 2010, 31, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Eisenhaure, J.D.; Rhee, S.I.; Al-Okaily, A.M.; Carlson, A.; Ferreira, P.M.; Kim, S. The Use of Shape Memory Polymers for MEMS Assembly. J. Microelectromech. Syst. 2016, 25, 69–77. [Google Scholar] [CrossRef]
- Eisenhaure, J.D.; Xie, T.; Varghese, S.; Kim, S. Microstructured Shape Memory Polymer Surfaces with Reversible Dry Adhesion. ACS Appl. Mater. Interfaces 2013, 5, 7714–7717. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.; Bowen, A.M.; Huang, Y.; Nuzzo, R.G.; Rogers, J.A. Transfer printing techniques for materials assembly and micro/nanodevice fabrication. Adv. Mater. 2012, 24, 5284–5318. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Eisenhaure, J.; Kim, S. Micro-wedge array surface of a shape memory polymer as a reversible dry adhesive. Extreme Mech. Lett. 2016, 9, 207–214. [Google Scholar] [CrossRef]
- Day, P.; Eason, E.V.; Esparza, N.; Christensen, D.; Cutkosky, M. Microwedge Machining for the Manufacture of Directional Dry Adhesives. J. Micro Nano-Manuf. 2013, 1, 011001. [Google Scholar] [CrossRef]
- Yi, H.; Kang, M.; Kwak, M.K.; Jeong, H.E. Simple and Reliable Fabrication of Bioinspired Mushroom-Shaped Micropillars with Precisely Controlled Tip Geometries. ACS Appl. Mater. Interfaces 2016, 8, 22671–22678. [Google Scholar] [CrossRef] [PubMed]
- Sameoto, D.; Menon, C. Deep UV patterning of acrylic masters for molding biomimetic dry adhesives. J. Micromech. Microeng. 2010, 20, 115037. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, S.; Cheng, X.; Gao, X.; Guo, X. Fabrication and Characterization of Gecko-inspired Dry Adhesion, Superhydrophobicity and Wet Self-cleaning Surfaces. J. Bionic Eng. 2016, 13, 132–142. [Google Scholar] [CrossRef]
- Yi, H.; Hwang, I.; Lee, J.H.; Lee, D.; Lim, H.; Tahk, D.; Sung, M.; Bae, W.-G.; Choi, S.-J.; Kwak, M.K.; et al. Continuous and Scalable Fabrication of Bioinspired Dry Adhesives via a Roll-to-Roll Process with Modulated Ultraviolet-Curable Resin. ACS Appl. Mater. Interfaces 2014, 6, 14590–14599. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, H.; Li, X.; Shao, J.; Ding, Y.; Liu, H.; An, N. Biomimetic Mushroom-Shaped Microfibers for Dry Adhesives by Electrically Induced Polymer Deformation. ACS Appl. Mater. Interfaces 2014, 6, 14167–14173. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, M.; Thiel, M.; Worgull, M.; Hölscher, H. 3D Direct Laser Writing of Nano- and Microstructured Hierarchical Gecko-Mimicking Surfaces. Small 2012, 8, 3009–3015. [Google Scholar] [CrossRef] [PubMed]
- Sahay, R.; Parveen, H.; Baji, A.; Ganesh, V.A.; Ranganath, A.S. Fabrication of PVDF hierarchical fibrillar structures using electrospinning for dry-adhesive applications. J. Mater. Sci. 2017, 52, 2435–2441. [Google Scholar] [CrossRef]
- Geim, A.K.; Dubonos, S.V.; Grigorieva, I.V.; Novoselov, K.S.; Zhukov, A.A.; Shapoval, S.Y. Microfabricated adhesive mimicking gecko foot-hair. Nat. Mater. 2003, 2, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Hsu, Y.-N.; Chung, Y.-C. Bio-inspired HDPE-based dry adhesives and their layer-by-layer catechol modification on the surface for use in humid environments. RSC Adv. 2014, 4, 22931–22937. [Google Scholar] [CrossRef]
- Sahay, R.; Parveen, H.; Sargur Ranganath, A.; Anand Ganesh, V.; Baji, A. On the adhesion of hierarchical electrospun fibrous structures and prediction of their pull-off strength. RSC Adv. 2016, 6, 47883–47889. [Google Scholar] [CrossRef]
- Sahay, R.; Baji, A.; Ranganath, A.S.; Anand Ganesh, V. Durable adhesives based on electrospun poly(vinylidene fluoride) fibers. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Rong, Z.; Zhou, Y.; Chen, B.; Robertson, J.; Federle, W.; Hofmann, S.; Steiner, U.; Goldberg-Oppenheimer, P. Bio-Inspired Hierarchical Polymer Fiber–Carbon Nanotube Adhesives. Adv. Mater. 2014, 26, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Du, F.; Ganguli, S.; Roy, A.; Dai, L. Carbon nanotube dry adhesives with temperature-enhanced adhesion over a large temperature range. Nat. Commun. 2016, 7, 13450. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ju, Y.; Xu, B.; Wang, P.; Kojima, N.; Ichioka, K.; Hosoi, A. Mimicking a gecko’s foot with strong adhesive strength based on a spinnable vertically aligned carbon nanotube array. RSC Adv. 2014, 4, 9056–9060. [Google Scholar] [CrossRef]
- Krahn, J.; Sameoto, D.; Menon, C. Controllable biomimetic adhesion using embedded phase change material. Smart Mater. Struct. 2011, 20, 15014. [Google Scholar] [CrossRef]
- Eisenhaure, J.; Kim, S. Laser-Driven Shape Memory Effect for Transfer Printing Combining Parallelism with Individual Object Control. Adv. Mater. Technol. 2016, 1. [Google Scholar] [CrossRef]
- Eisenhaure, J.; Kim, S. An Internally Heated Shape Memory Polymer Dry Adhesive. Polymers 2014, 6, 2274–2286. [Google Scholar] [CrossRef]
- Wang, Y.; Lehmann, S.; Shao, J.; Sameoto, D. Adhesion Circle: A New Approach To Better Characterize Directional Gecko-Inspired Dry Adhesives. ACS Appl. Mater. Interfaces 2017, 9, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.A.; Suresh, S.A.; Jiang, H.; Ulmen, J.V.; Hawkes, E.W.; Christensen, D.L.; Cutkosky, M.R. Tactile sensing for gecko-inspired adhesion. In Proceedings of the 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Hamburg, Germany, 28 September–2 October 2015; pp. 1501–1507. [Google Scholar]
- Eason, E.V.; Hawkes, E.W.; Windheim, M.; Christensen, D.L.; Libby, T.; Cutkosky, M.R. Stress distribution and contact area measurements of a gecko toe using a high-resolution tactile sensor. Bioinspir. Biomim. 2015, 10, 16013. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Zhang, Z.; Chueh, Y.-L.; Ho, J.C.; Lee, J.; Fearing, R.S.; Javey, A. Wet and Dry Adhesion Properties of Self-Selective Nanowire Connectors. Adv. Funct. Mater. 2009, 19, 3098–3102. [Google Scholar] [CrossRef]
- Yang, S.Y.; Carlson, A.; Cheng, H.; Yu, Q.; Ahmed, N.; Wu, J.; Kim, S.; Sitti, M.; Ferreira, P.M.; Huang, Y.; et al. Elastomer Surfaces with Directionally Dependent Adhesion Strength and Their Use in Transfer Printing with Continuous Roll-to-Roll Applications. Adv. Mater. 2012, 24, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, A. Peeling off an adhesive layer with spatially varying modulus. Phys. Rev. E 2010, 81, 21603. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Pang, C.; Suh, K.Y. Shape-Tunable Polymer Nanofibrillar Structures by Oblique Electron Beam Irradiation. Langmuir 2009, 25, 8879–8882. [Google Scholar] [CrossRef] [PubMed]
- Michal, B.T.; Spencer, E.J.; Rowan, S.J. Stimuli-Responsive Reversible Two-Level Adhesion from a Structurally Dynamic Shape-Memory Polymer. ACS Appl. Mater. Interfaces 2016, 8, 11041–11049. [Google Scholar] [CrossRef] [PubMed]
- Glassmaker, N.J.; Jagota, A.; Hui, C.-Y.; Kim, J. Design of biomimetic fibrillar interfaces: 1. Making contact. J. R. Soc. Interface 2004, 1, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Barreau, V.; Hensel, R.; Guimard, N.K.; Ghatak, A.; McMeeking, R.M.; Arzt, E. Fibrillar Elastomeric Micropatterns Create Tunable Adhesion Even to Rough Surfaces. Adv. Funct. Mater. 2016, 26, 4687–4694. [Google Scholar] [CrossRef]
- Keum, H.; Carlson, A.; Ning, H.; Mihi, A.; Eisenhaure, J.D.; Braun, P.V.; Rogers, J.A.; Kim, S. Silicon micro-masonry using elastomeric stamps for three-dimensional microfabrication. J. Micromech. Microeng. 2012, 22, 55018. [Google Scholar] [CrossRef]
- Carlson, A.; Kim-Lee, H.-J.; Wu, J.; Elvikis, P.; Cheng, H.; Kovalsky, A.; Elgan, S.; Yu, Q.; Ferreira, P.M.; Huang, Y.; et al. Shear-enhanced adhesiveless transfer printing for use in deterministic materials assembly. Appl. Phys. Lett. 2011, 98, 264104. [Google Scholar] [CrossRef]
- Luo, X.; Lauber, K.E.; Mather, P.T. A thermally responsive, rigid, and reversible adhesive. Polymer 2010, 51, 1169–1175. [Google Scholar] [CrossRef]
- Castellanos, G.; Arzt, E.; Kamperman, M. Effect of viscoelasticity on adhesion of bioinspired micropatterned epoxy surfaces. Langmuir 2011, 27, 7752–7759. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Dai, L.; Stone, M.; Xia, Z.; Wang, Z.L. Carbon Nanotube Arrays with Strong Shear Binding-On and Easy Normal Lifting-Off. Science 2008, 322, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Dai, L. Gecko-Foot-Mimetic Aligned Single-Walled Carbon Nanotube Dry Adhesives with Unique Electrical and Thermal Properties. Adv. Mater. 2007, 19, 3844–3849. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, H.; Shao, J.; Sameoto, D.; Li, X.; Wang, L.; Hu, H.; Ding, Y.; Lu, B. Switchable Dry Adhesion with Step-like Micropillars and Controllable Interfacial Contact. ACS Appl. Mater. Interfaces 2016, 8, 10029–10037. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Sitti, M. Soft Grippers Using Micro-fibrillar Adhesives for Transfer Printing. Adv. Mater. 2014, 26, 4901–4906. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Lum, G.Z.; Song, S.; Rich, S.; Sitti, M. Phase Change of Gallium Enables Highly Reversible and Switchable Adhesion. Adv. Mater. 2016, 28, 5088–5092. [Google Scholar] [CrossRef] [PubMed]
- Gillies, A.G.; Kwak, J.; Fearing, R.S. Controllable Particle Adhesion with a Magnetically Actuated Synthetic Gecko Adhesive. Adv. Funct. Mater. 2013, 23, 3256–3261. [Google Scholar] [CrossRef]
- Lee, J.; Majidi, C.; Schubert, B.; Fearing, R.S. Sliding-induced adhesion of stiff polymer microfibre arrays. I. Macroscale behaviour. J. R. Soc. Interface 2008, 5, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Fearing, R.S. Wet Self-Cleaning of Superhydrophobic Microfiber Adhesives Formed from High Density Polyethylene. Langmuir 2012, 28, 15372–15377. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Aksak, B.; Sitti, M. Enhanced friction of elastomer microfiber adhesives with spatulate tips. Appl. Phys. Lett. 2007, 91, 221913. [Google Scholar] [CrossRef]
- Yi, H.; Hwang, I.; Sung, M.; Lee, D.; Kim, J.-H.; Kang, S.M.; Bae, W.-G.; Jeong, H.E. Bio-inspired adhesive systems for next-generation green manufacturing. Int. J. Precis. Eng. Manuf. Green Technol. 2014, 1, 347–351. [Google Scholar] [CrossRef]
- Shahsavan, H.; Salili, S.M.; Jákli, A.; Zhao, B. Thermally Active Liquid Crystal Network Gripper Mimicking the Self-Peeling of Gecko Toe Pads. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Keum, H.; Seong, M.; Sinha, S.; Kim, S. Electrostatically driven collapsible Au thin films assembled using transfer printing for thermal switching. Appl. Phys. Lett. 2012, 100, 211904. [Google Scholar] [CrossRef]
- Keum, H.; Kim, S. Micro-masonry for 3D Additive Micromanufacturing. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Keum, H.; Park, K.; Bashir, R.; Kim, S. Micro-Masonry of MEMS Sensors and Actuators. J. Microelectromech. Syst. 2014, 23, 308–314. [Google Scholar] [CrossRef]
- Keum, H.; Yang, Z.; Han, K.; Handler, D.E.; Nguyen, T.N.; Schutt-Aine, J.; Bahl, G.; Kim, S. Microassembly of Heterogeneous Materials using Transfer Printing and Thermal Processing. Sci. Rep. 2016, 6, 29925. [Google Scholar] [CrossRef] [PubMed]
- Bhaswara, A.; Keum, H.; Rhee, S.; Legrand, B.; Mathieu, F.; Kim, S.; Nicu, L.; Leichle, T. Fabrication of nanoplate resonating structures via micro-masonry. J. Micromech. Microeng. 2014, 24, 115012. [Google Scholar] [CrossRef]
- Yoo, B.; Cho, S.; Seo, S.; Lee, J. Elastomeric Angled Microflaps with Reversible Adhesion for Transfer-Printing Semiconductor Membranes onto Dry Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 19247–19253. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-W.; Park, B.; Keum, H.; Kim, S.; Rogers, J.A.; Solgaard, O. Two-axis MEMS scanner with transfer-printed high-reflectivity, broadband monolithic silicon photonic crystal mirrors. Opt. Express 2013, 21, 13800–13809. [Google Scholar] [CrossRef] [PubMed]
- Henrey, M.; Díaz Téllez, J.P.; Wormnes, K.; Pambaguian, L.; Menon, C. Towards the use of mushroom-capped dry adhesives in outer space: Effects of low pressure and temperature on adhesion strength. Aerosp. Sci. Technol. 2013, 29, 185–190. [Google Scholar] [CrossRef]
- Purtov, J.; Frensemeier, M.; Kroner, E. Switchable Adhesion in Vacuum Using Bio-Inspired Dry Adhesives. ACS Appl. Mater. Interfaces 2015, 7, 24127–24135. [Google Scholar] [CrossRef] [PubMed]
- Heepe, L.; Varenberg, M.; Itovich, Y.; Gorb, S.N. Suction component in adhesion of mushroom-shaped microstructure. J. R. Soc. Interface 2011, 8, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Sameoto, D.; Sharif, H.; Menon, C. Investigation of low-pressure adhesion performance of mushroom shaped biomimetic dry adhesives. J. Adhes. Sci. Technol. 2012, 26, 2641–2652. [Google Scholar] [CrossRef]
- Henrey, M.; Tellez, J.P.D.; Wormnes, K.; Pambaguian, L.; Menon, C. Sticking in space: Manufacturing dry adhesives and testing their performance in space environments. In Proceedings of the 12th Symposium on Advanced Space Technologies in Robotics and Automation (ASTRA), ESA/ESTEC, Noordwijk, The Netherlands, 15–17 May 2013. [Google Scholar]
- Kalouche, S.; Wiltsie, N.; Su, H.J.; Parness, A. Inchworm style gecko adhesive climbing robot. In Proceedings of the 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems, Chicago, IL, USA, 14–18 September 2014; pp. 2319–2324. [Google Scholar]
- Amjadi, M.; Kyung, K.-U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Zhang, D.; Zhang, P.; Han, Z. Bioinspired Surface for Surgical Graspers Based on the Strong Wet Friction of Tree Frog Toe Pads. ACS Appl. Mater. Interfaces 2015, 7, 13987–13995. [Google Scholar] [CrossRef] [PubMed]
- Henrey, M.; Ahmed, A.; Boscariol, P.; Shannon, L.; Menon, C. Abigaille-III: A Versatile, Bioinspired Hexapod for Scaling Smooth Vertical Surfaces. J. Bionic Eng. 2014, 11, 1–17. [Google Scholar] [CrossRef]
- Li, Y.; Krahn, J.; Menon, C. Bioinspired Dry Adhesive Materials and Their Application in Robotics: A Review. J. Bionic Eng. 2016, 13, 181–199. [Google Scholar] [CrossRef]
- Kim, S.; Spenko, M.; Trujillo, S.; Heyneman, B.; Santos, D.; Cutkosky, M.R. Smooth Vertical Surface Climbing With Directional Adhesion. IEEE Trans. Robot. 2008, 24, 65–74. [Google Scholar]
- Santos, D.; Kim, S.; Spenko, M.; Parness, A.; Cutkosky, M. Directional Adhesive Structures for Controlled Climbing on Smooth Vertical Surfaces. In Proceedings of the 2007 IEEE International Conference on Robotics and Automation, Roma, Italy, 10–14 April 2007; pp. 1262–1267. [Google Scholar]
- Hawkes, E.W.; Ulmen, J.; Esparza, N.; Cutkosky, M.R. Scaling walls: Applying dry adhesives to the real world. In Proceedings of the 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems, San Francisco, CA, USA, 25–30 September 2011; pp. 5100–5106. [Google Scholar]
- Daler, L.; Klaptocz, A.; Briod, A.; Sitti, M.; Floreano, D. A perching mechanism for flying robots using a fibre-based adhesive. In Proceedings of the 2013 IEEE International Conference on Robotics and Automation (ICRA), Karlsruhe, Germany, 6–10 May 2013; pp. 4433–4438. [Google Scholar]
- Kalantari, A.; Mahajan, K.; Ruffatto, D.; Spenko, M. Autonomous perching and take-off on vertical walls for a quadrotor micro air vehicle. In Proceedings of the 2015 IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, 26–30 May 2015; pp. 4669–4674. [Google Scholar]
- Hawkes, E.W.; Jiang, H.; Cutkosky, M.R. Three-dimensional dynamic surface grasping with dry adhesion. Int. J. Robot. Res. 2016, 35, 943–958. [Google Scholar] [CrossRef]
- Hawkes, E.W.; Eason, E.V.; Christensen, D.L.; Cutkosky, M.R. Human climbing with efficiently scaled gecko-inspired dry adhesives. J. R. Soc. Interface 2015, 12, 20140675. [Google Scholar] [CrossRef] [PubMed]

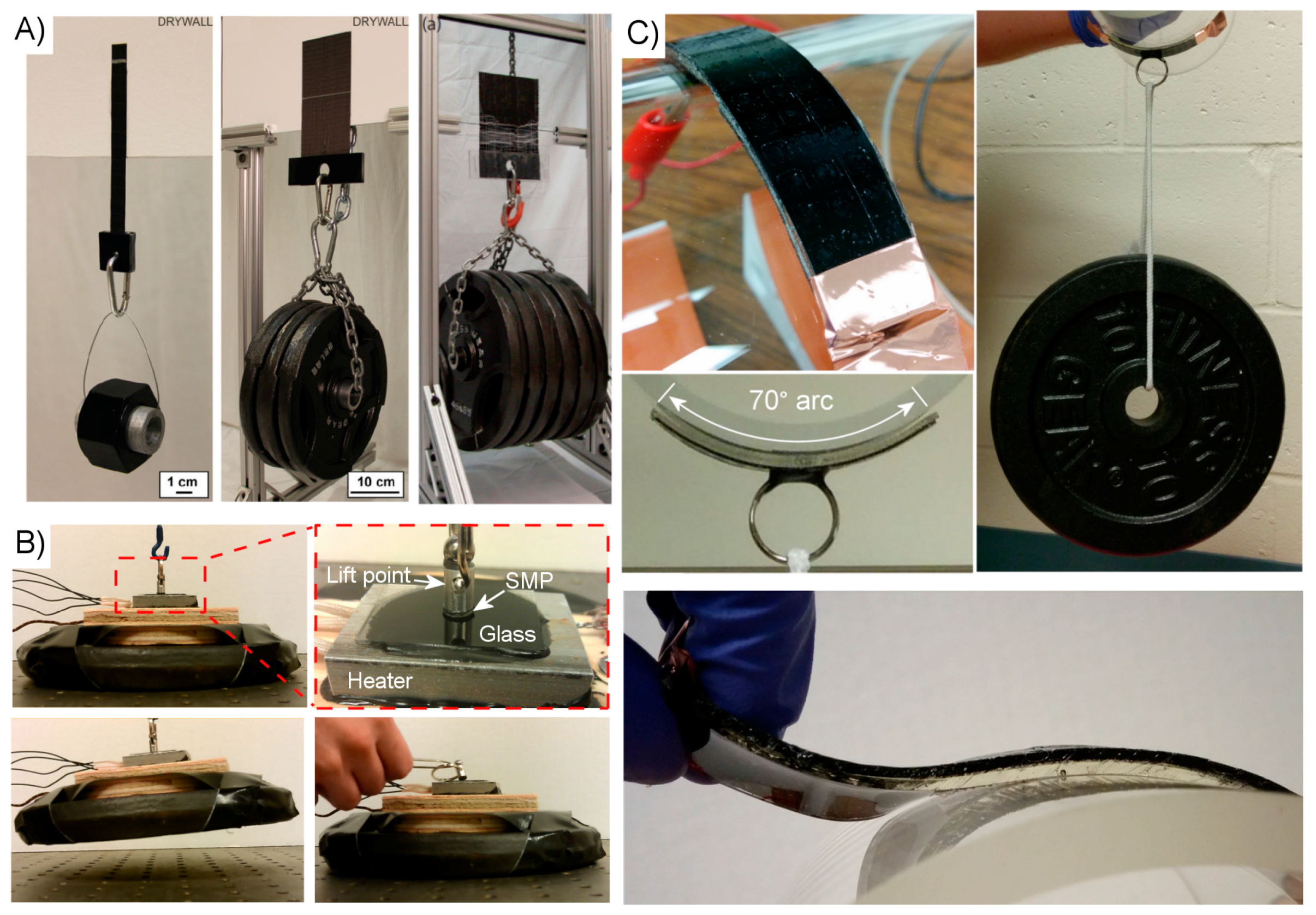
| Surface Structure | Material | Test Method | Test Scale (mm) | Max. Normal Adhesion (N/cm2) | Reversibility | Reference |
|---|---|---|---|---|---|---|
| microtips | epoxy SMP | glass adherend, free-hanging weight | 10 | 200 | ~1000:1 microstructure, rigidity control | [137] |
| flat | epoxy SMP | glass adherend, free-hanging weight | 100 | 5–30 | - | [157] |
| microtips, flat, microspheres | epoxy SMP | Si adherend, load cell w/motor stage | 0.1 | 700 (flat) 560 (microtip) | 2:1 (flat) ~1000:1 (microtip) microstructure, rigidity control | [136] |
| microtips, flat | PDMS | Si adherend, load cell w/motor stage | 0.1 | 3–6 | >100:1 microstructure | [168] |
| flat | PDMS | Si adherend, load cell w/motor stage | 0.1 | 7 | ~100:1 shear displacement | [169] |
| flat/angled | PDMS | Si adherend, load cell w/motor stage | 0.1 | 10 | ~100:1 shear displacement | [162] |
| flat | PU or PDMS/carbon composite | glass adherend, universal mechanical tester, center loading | 100 | 7.5 (a) | 300:1 (b) loading location | [58] |
| spatula microfibrillar, flat | PDMS Crystalbond filler | 6 mm sapphire lens adherend, load cell w/motor stage | 1 | 20 | ~5:1 fibrillar ~20:1 flat rigidity control | [155] |
| flat | PCL and bisphnol-A epoxy | Al and stainless steel adherends, universal mechanical tester | 10 | 80 to 650 ~200 solvent self-bonding | >75:1 heat release | [170] |
| microfibrillar, smooth | epoxy polymer PDMS | 4 mm spherical sapphire adherend, interferometer w/motor stage | 1 | ~1 (c) | 4:1 loading rate | [171] |
| film-terminated fibrillar | PDMS | Si adherend, double-cantilever beam | 1 | 2.6 fibrillar ~4 flat | - | [76] |
| nanofibrillar | CNT | glass adherend, free-hanging weight | 4 | ~10 | ~10:1 loading direction | [172] |
| nanofibrillar | SWCNT, MWCNT | glass adherend, laboratory balance | 4 | 12 MWCNT, 28 SWCNT | - | [173] |
| nanofibrillar | MWCNT | glass adherend, laboratory balance | 2 | 11.7 | - | [26] |
| nanofibrillar | Polyimide | glass adherend, laboratory balance | 10 | 3 | - | [148] |
| microfibrillar | PDMS | Si adherend, displacement sensor w/motor stage | 8 | 0.6 maximum | 20:1 shear displacement | [174] |
| inflatable hemisphere | ST-1060 PU | flat glass adherend, load cell w/motor stage | 10 | ~0.5 | 204:1 inflation displacement | [175] |
| gallium liquid | PDMS with gallium liquid | glass, Au, Si, PDMS adherends, load cell w/motor stage | 1 | 2.9 (smooth glass) 3.74 (silicon) 4.4 (gold) | 178:1 rough glass 113:1 smooth glass 86:1 silicon Ga phase change | [176] |
| thick film-terminated fibrillar | PDMS/Fe-PDMS | spherical glass adherend, load cell w/motor stage | 10 | 2.4 | minimal, magnetic field orientation | [130] |
| micro-ridges | PDMS/Fe-PDMS | <1 mm glass sphere, cantilever deflection measurements | 1 | 0.1 | ~10:1 magnetic field orientation | [177] |
| microfibrillar, various tips | PU ST-1060, ST-1087 | 6 mm glass sphere adherend, load cell w/motor stage | 6 | >0.05 | - | [112] |
| flat | epoxy SMP, elastomer | PC and PP adherends, universal mechanical tester | 10 | 100 | >100:1 shape change | [135] |
| flat, single and dual layer microfibrillar | PU | 12 mm spherical glass adherend, load cell w/motor stage | 1 | 2.7 (flat) 5.9 (single layer) 3.75 (dual layer) | - | [105] |
| Surface Structure | Material | Test Method | Test Scale (mm) | Max. Shear Adhesion (N/cm2) | Reversibility | Reference |
|---|---|---|---|---|---|---|
| nanofibrillar | CNT | Cu adherend, spring scale w/manual force application | 10 | 37 at 25 °C 124 at 1030 °C | - | [153] |
| nanofibrillar | CNT | glass adherend, free-hanging weight | 4 | ~100 | ~10:1 loading direction | [172] |
| nanofibrillar, hierarchical | CNT/SU-8 hierarchical | HMDS-treated 1 mm glass sphere and 1.5 mm roughened steel sphere, load cell w/motor stage | 10 | ~20 | large (a), normal vs. shear loading | [152] |
| nanofibrillar | SWCNT, MWCNT | glass adherend, laboratory balance | 4 | 7 MWCNT, 17 SWCNT | - | [173] |
| nanofibrillar | MWCNT | glass adherend, laboratory balance | 2 | 7.7 | - | [26] |
| spatula microfibrillar | conductive PDMS (carbon black) | PP adherend, spring scale | 100 | 0.4 | - | [35] |
| microfibrillar | PP | glass adherend, load cell w/motor stage | 20 | 2 | ~1000:1 peeling vs. shearing | [178] |
| microfibrillar, various tips | PU ST-1060, ST-1087 | 6 mm diameter glass sphere adherend, load cell w/motor stage | 1 | >0.15 | - | [112] |
| flat | epoxy SMP, elastomer | PC and PP adherends, universal mechanical tester | 10 | 55 | >100:1 shape change | [135] |
| microfibrillar | HDPE | glass adherend, hanging water cup | 10 | 4.7 | - | [179] |
| nanofibrillar | Germanium/Parylene nanowires | self-adhering, wet and dry conditions | 5 | 30 | - | [161] |
| flat | PU or PDMS/carbon composite | glass adherend, universal mechanical tester, center loading | 100 | 29.5 max 26.0 avg. | 300:1 loading location | [58] |
| microfibrillar | PU | 6 mm diameter glass sphere adherend, load cell w/motor stage | 1 | 41 | - | [180] |
| Surface Structure | Material | Test Method | Test Scale (mm) | Work of Adhesion (J/m2) | Reversibility | Reference |
|---|---|---|---|---|---|---|
| film-terminated fibrillar | PDMS | Si adherend, double-cantilever beam | 1 | 0.137 (flat) 1.2 (fibrillar) | - | [76] |
| nanofibrillar | SWCNT, MWCNT | glass adherend, laboratory balance | 4 | 0.07–0.2 | - | [173] |
| nanofibrillar | MWCNT | glass adherend, laboratory balance | 2 | 0.02–0.08 | - | [26] |
| flat, single and dual layer microfibrillar | PU | 12 mm spherical glass adherend, load cell w/motor stage | 1 | 0.002 (flat surface) 0.034 (dual layer) | - | [105] |
| flat, incised | PDMS | silanized glass plate adherend, cantilever actuated by linear motor w/load cell | 10 | ≤0.8 (crosswise incisions) ~0.1 (smooth surface) | - | [74] |
| film-terminated fibrillar | PDMS | 8 mm diameter spherical glass adherend, load cell w/motor stage | 1 | 0.3 (fiber/film) 0.12 (flat control) | - | [77] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisenhaure, J.; Kim, S. A Review of the State of Dry Adhesives: Biomimetic Structures and the Alternative Designs They Inspire. Micromachines 2017, 8, 125. https://doi.org/10.3390/mi8040125
Eisenhaure J, Kim S. A Review of the State of Dry Adhesives: Biomimetic Structures and the Alternative Designs They Inspire. Micromachines. 2017; 8(4):125. https://doi.org/10.3390/mi8040125
Chicago/Turabian StyleEisenhaure, Jeffrey, and Seok Kim. 2017. "A Review of the State of Dry Adhesives: Biomimetic Structures and the Alternative Designs They Inspire" Micromachines 8, no. 4: 125. https://doi.org/10.3390/mi8040125





