New Endoscopic Imaging Technology Based on MEMS Sensors and Actuators
Abstract
:1. Introduction
2. MEMS Based Endoscopic Ultrasound Imaging
2.1. MEMS Based Acoustic Transducers
2.1.1. CMUT (Capacitive Micromachined Ultrasonic Transducers)
2.1.2. PMUT (Piezoelectric Micromachined Ultrasonic Transducer)
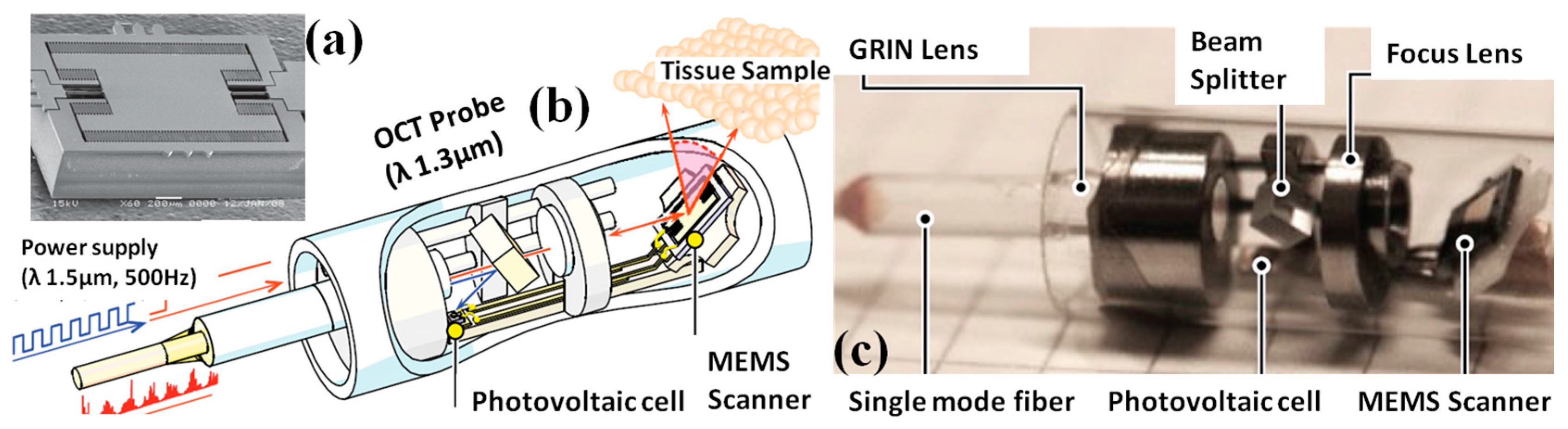
3. MEMS Based Optical Coherence Tomography (OCT) Endomicroscopes
3.1. MEMS Based Laser Source for OCT Endomicroscope
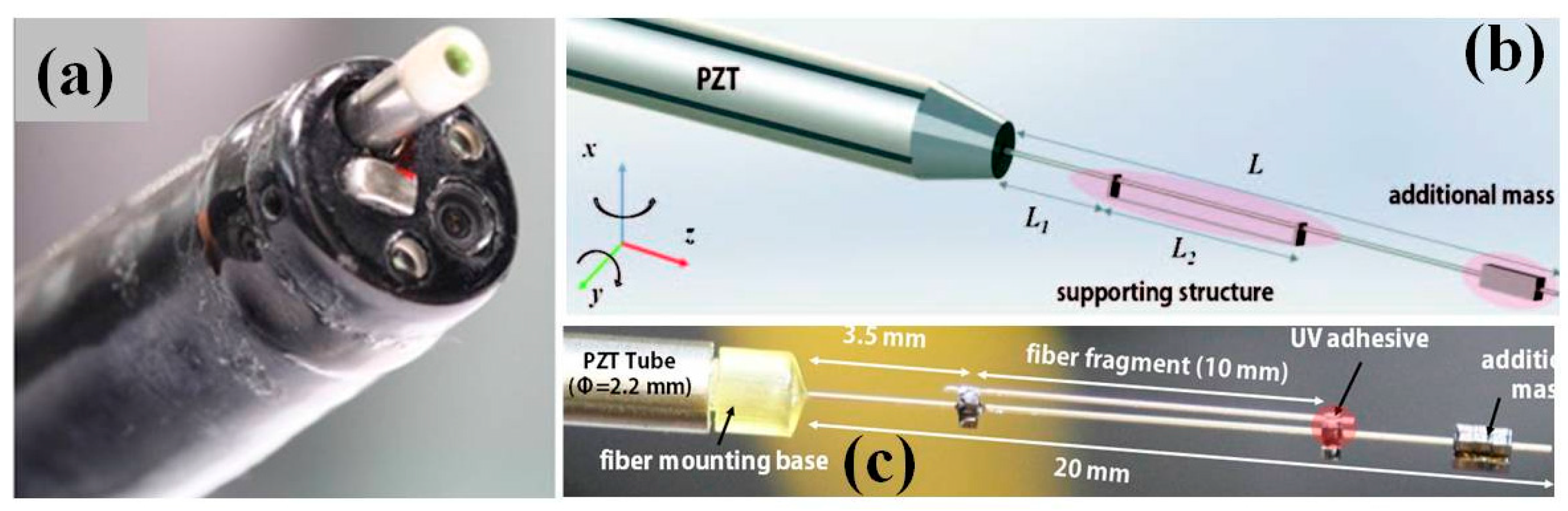
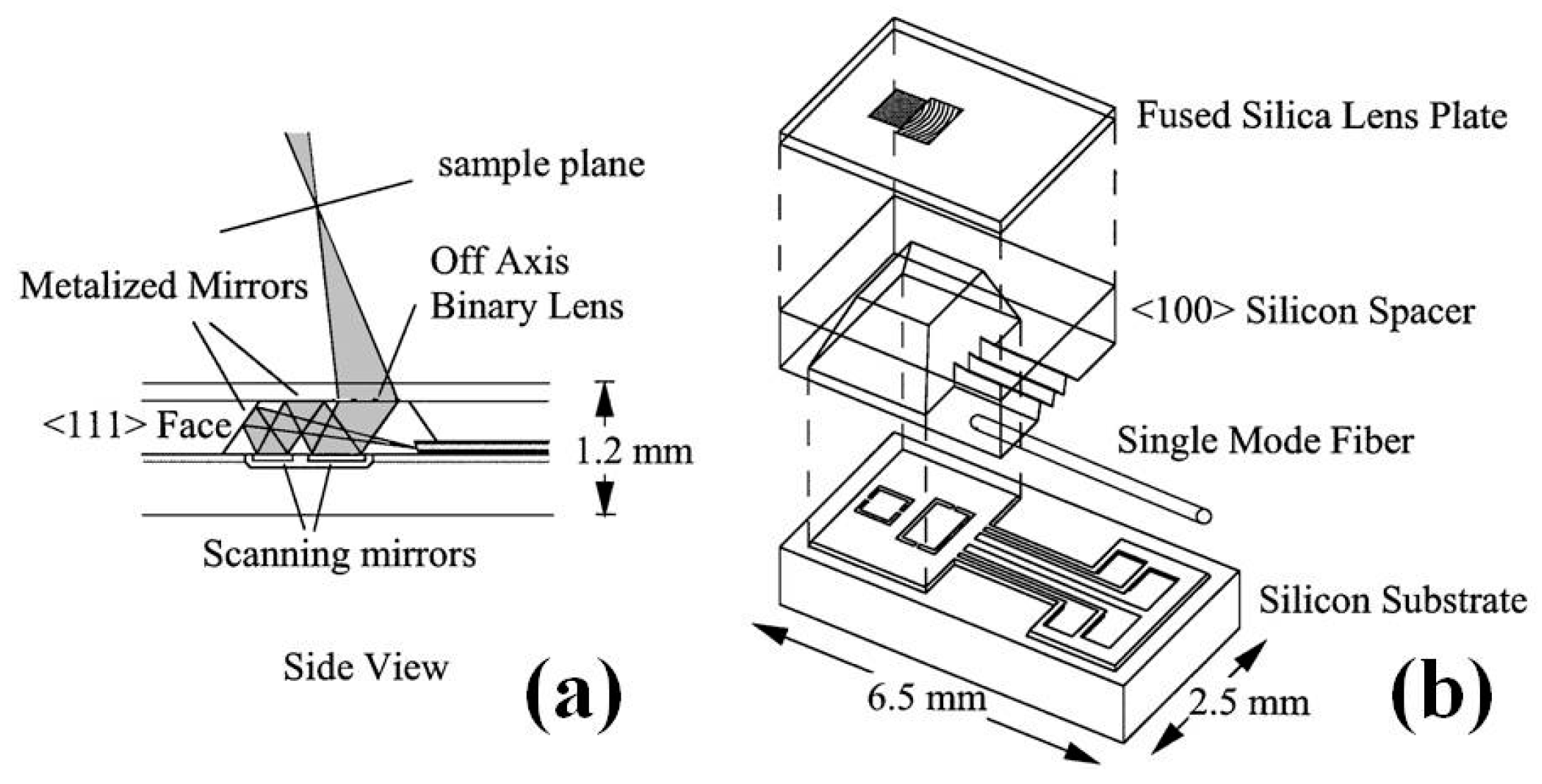
3.2. MEMS Scanners and Actuators for OCT Endomicroscope
4. Confocal Endomicroscope
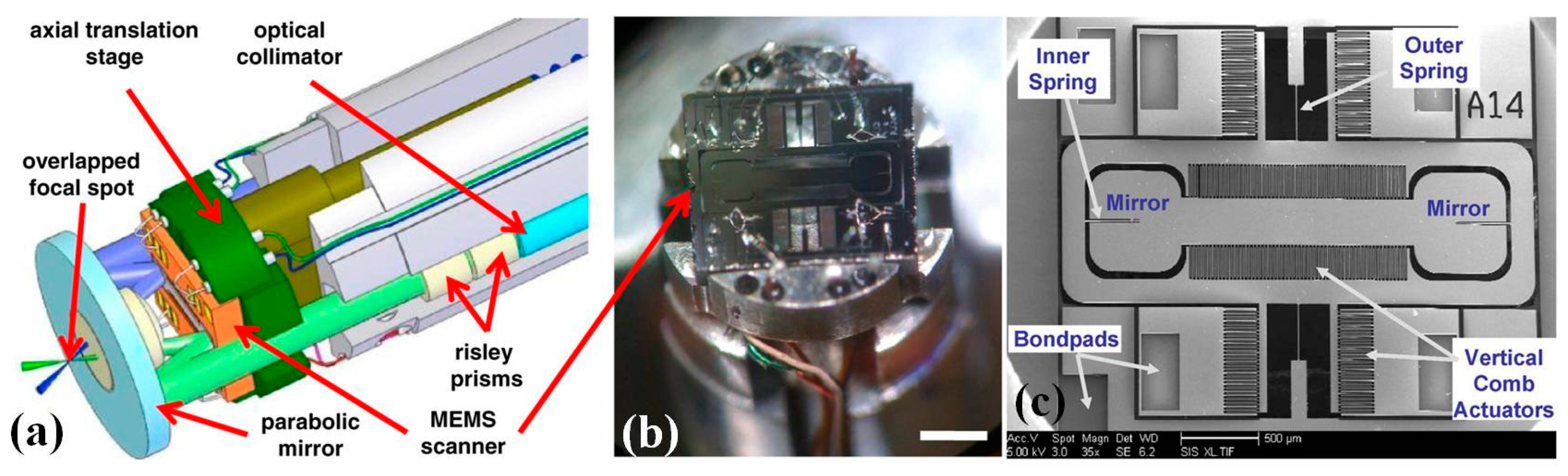
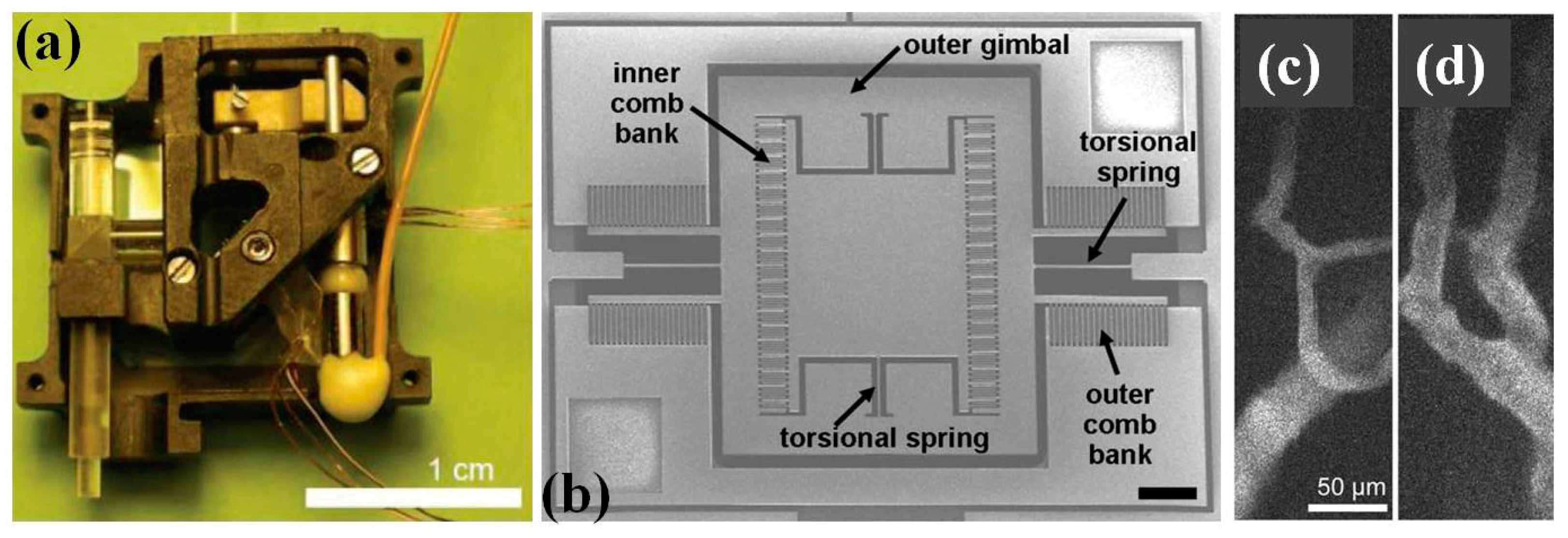
4.1. MEMS Scanner and Actuator for Confocal Endomicroscope
4.2. MEMS Deformable Reflective Mirror
4.3. MEMS Tunable Lens
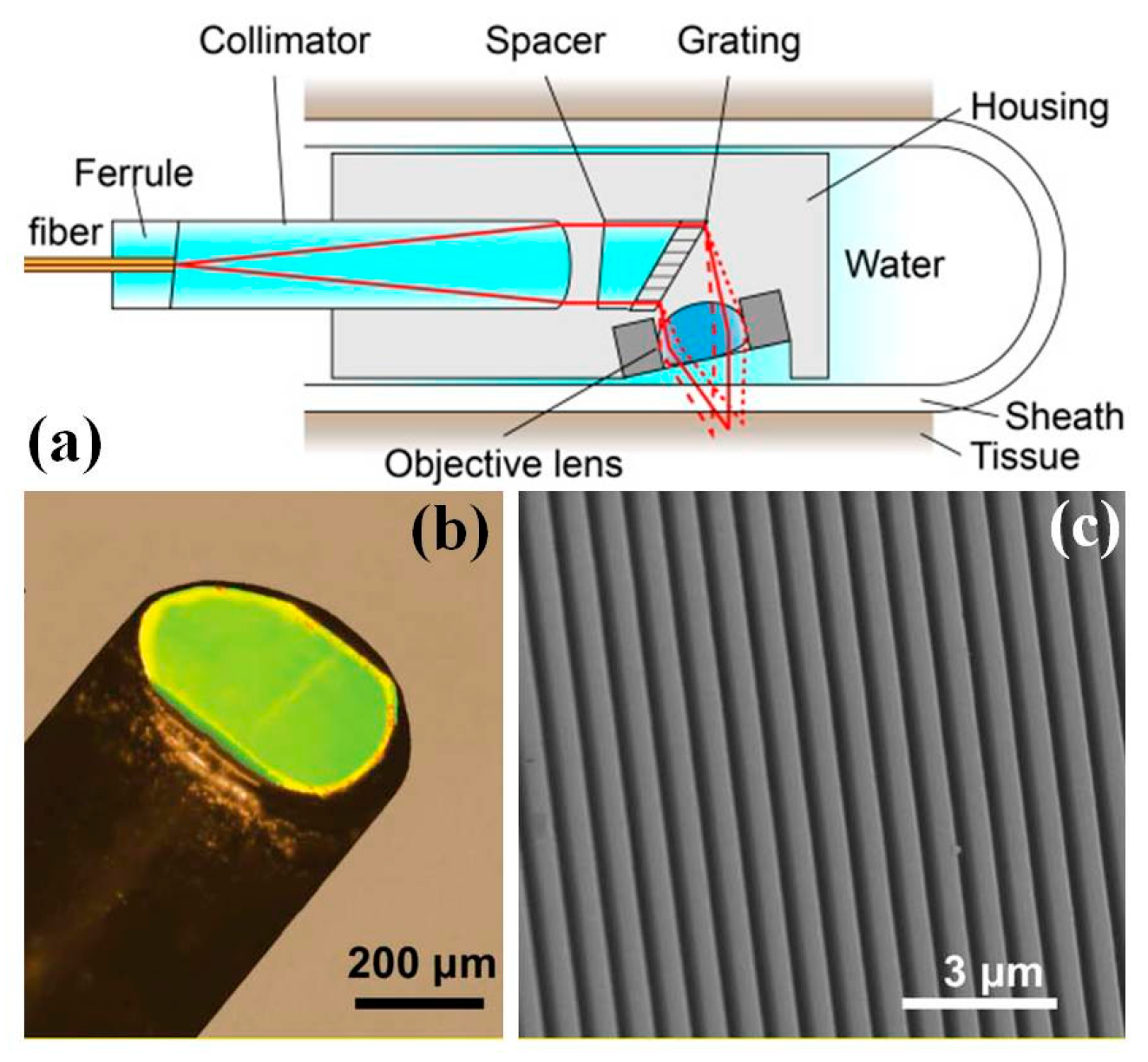
4.4. MEMS Grating for Spectral Encoded Confocal Endomicroscope
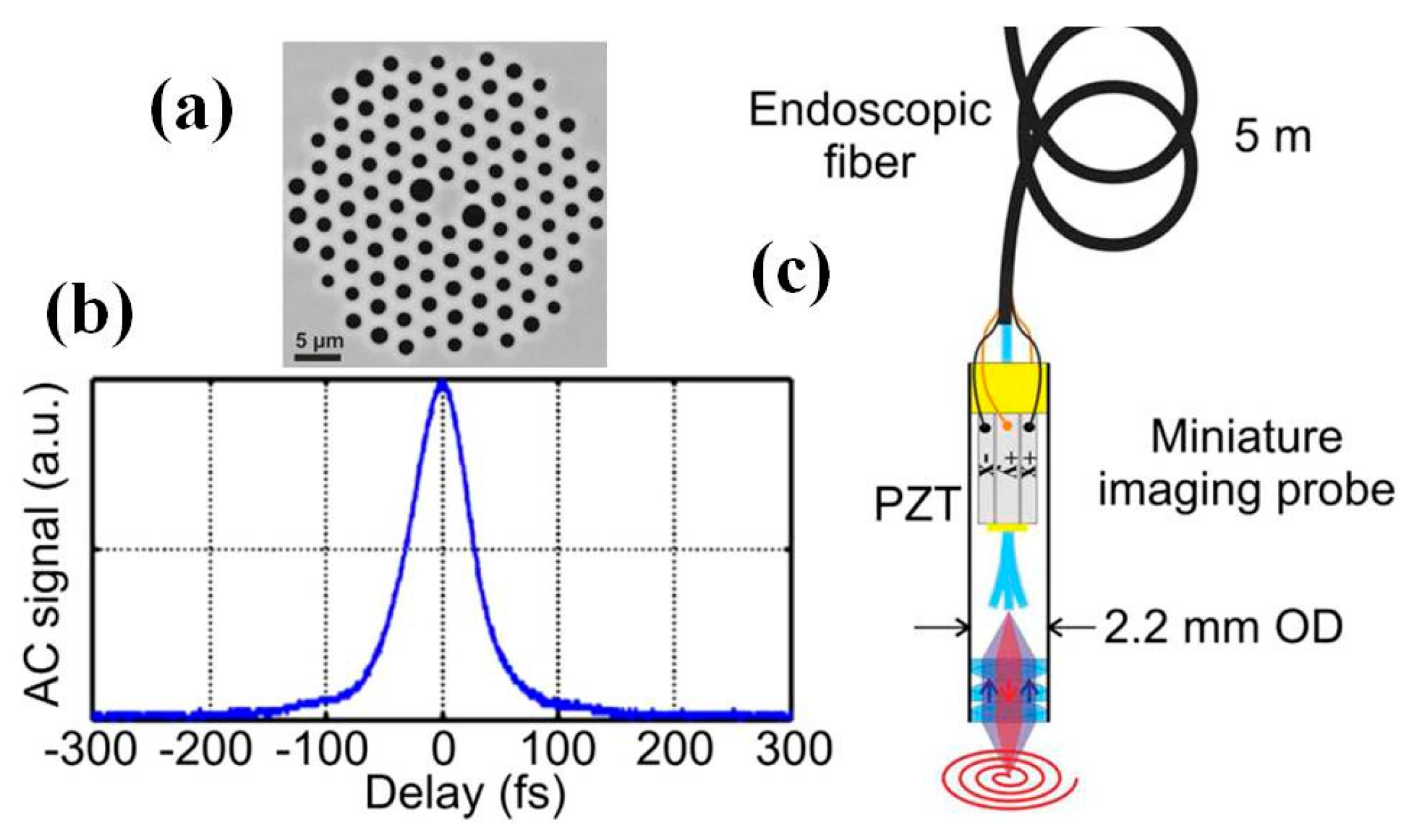
5. Multiphoton Endomicroscope
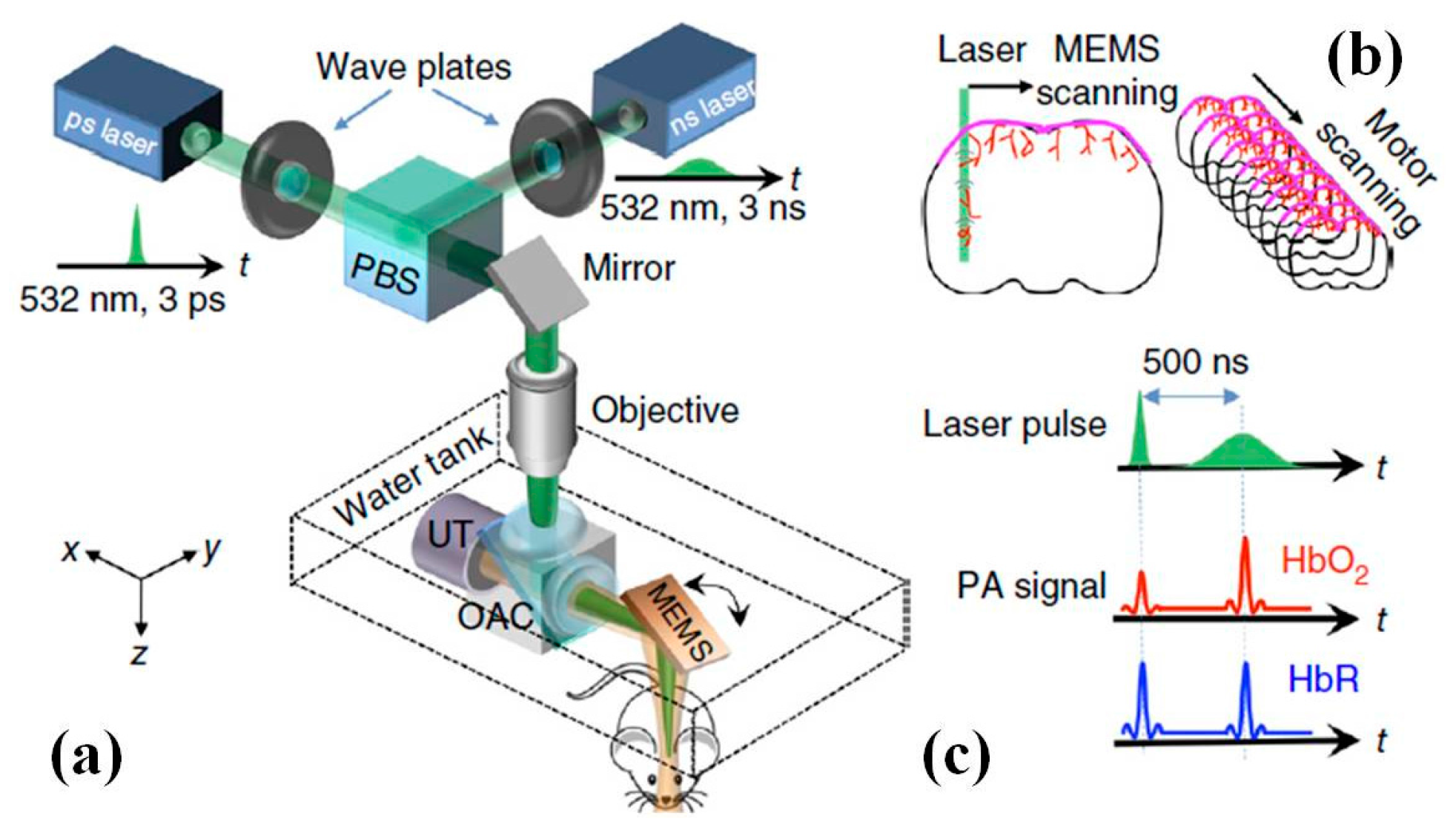
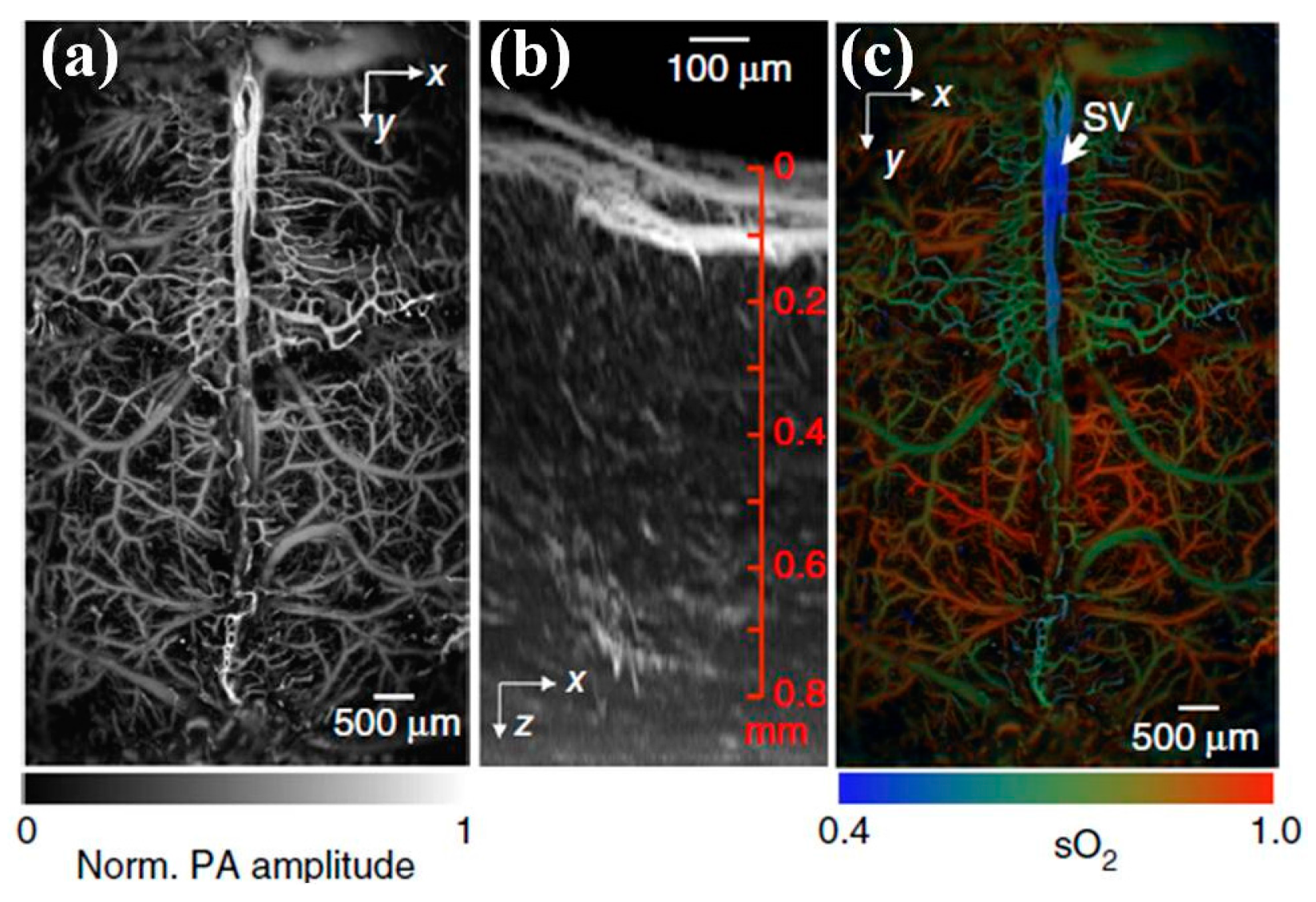
6. Photoacoustic Imaging
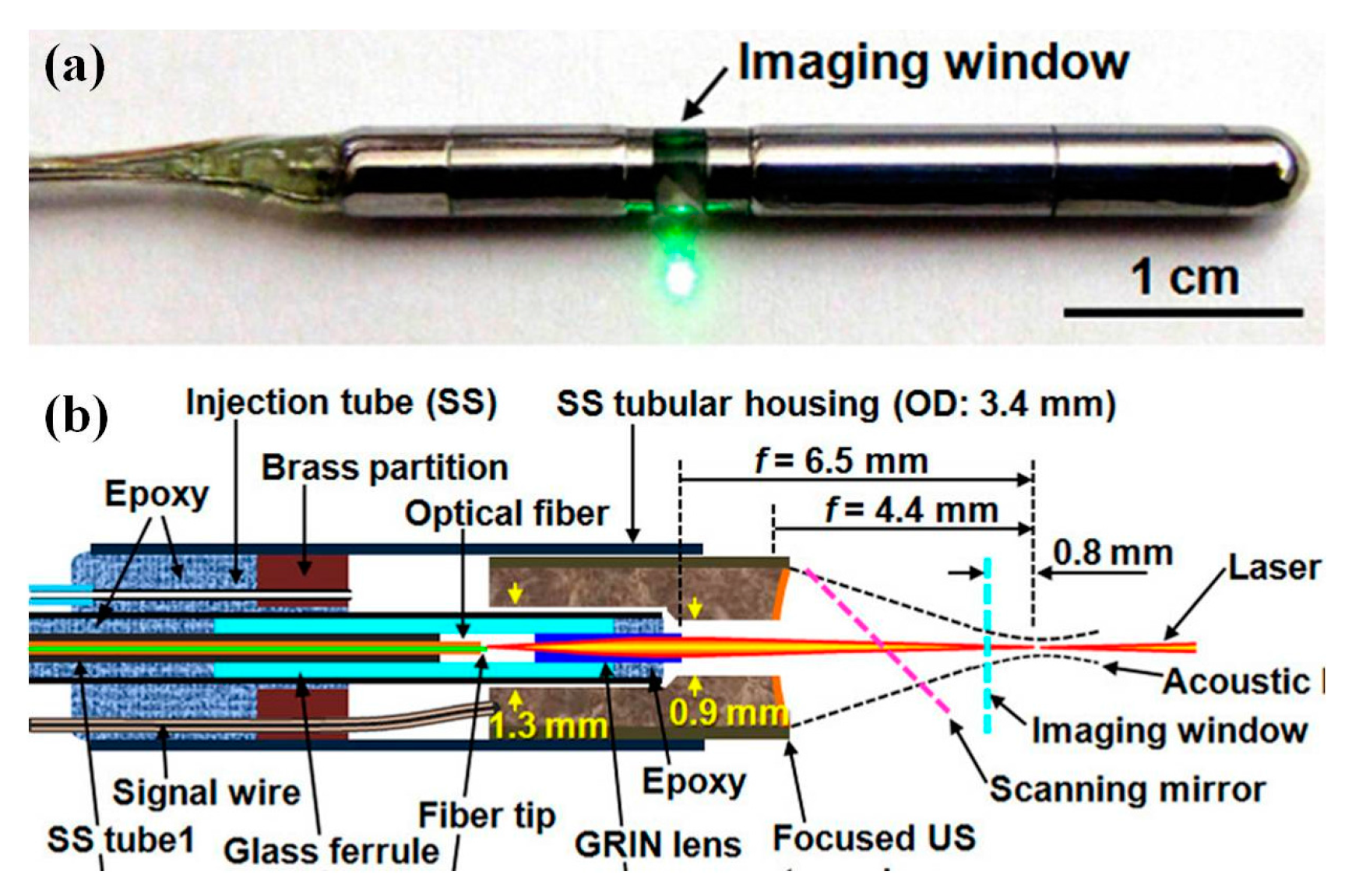
6.1. MEMS Scanner and Actuator Based Photoacoustic Imaging System
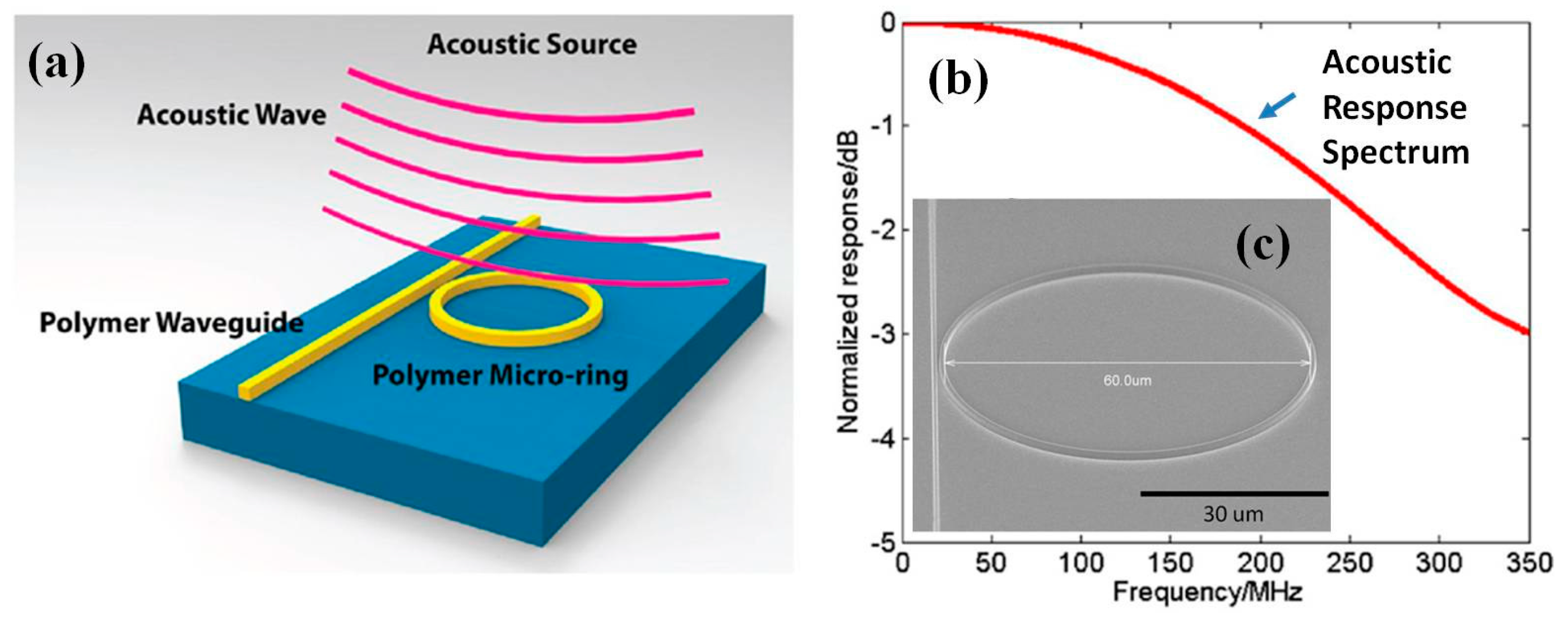
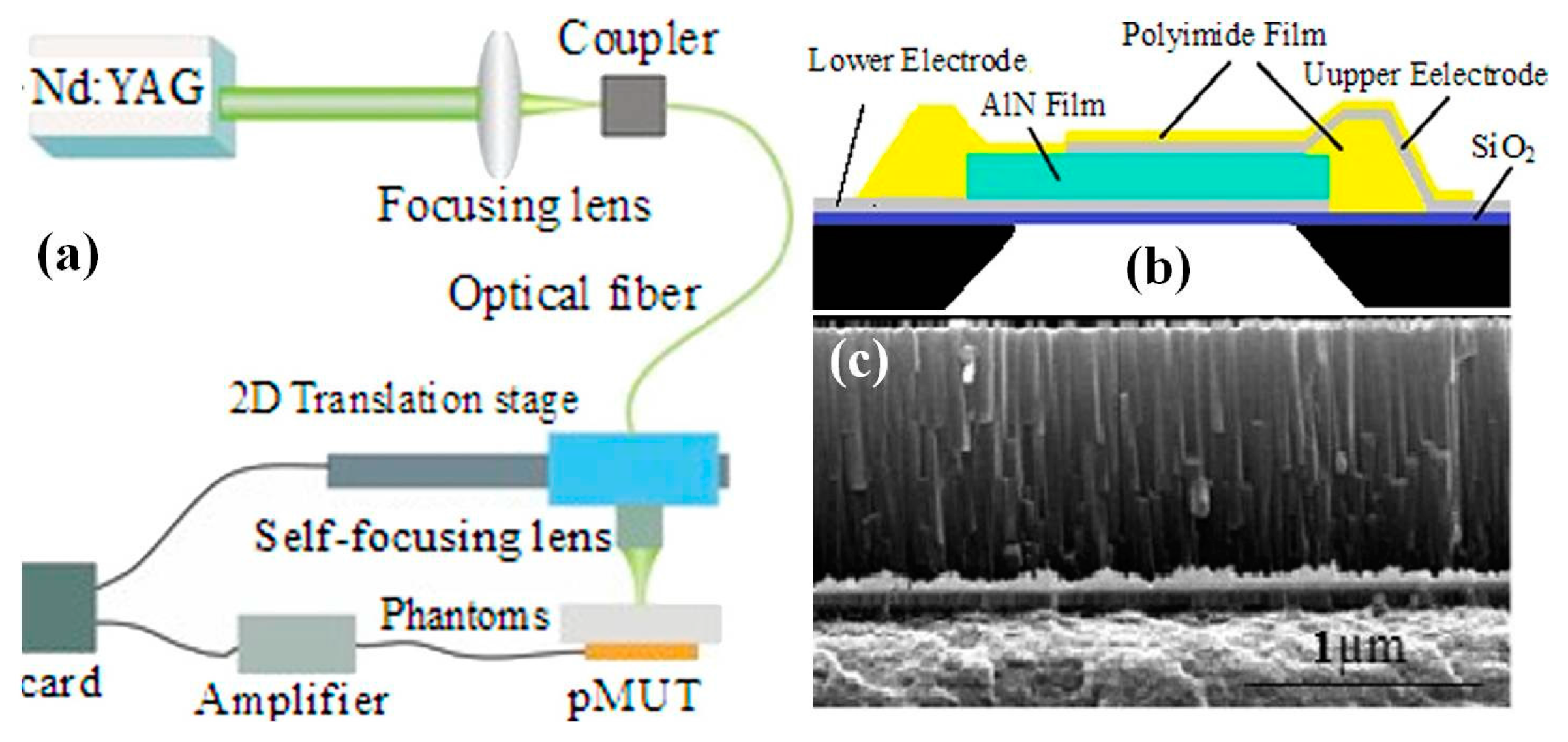
6.2. MEMS Acoustic Sensor for Photoacoustic Endomicroscope
6.2.1. Microring for Photoacoustic Endomicroscope
6.2.2. PMUT for Photoacoustic Endomicroscope
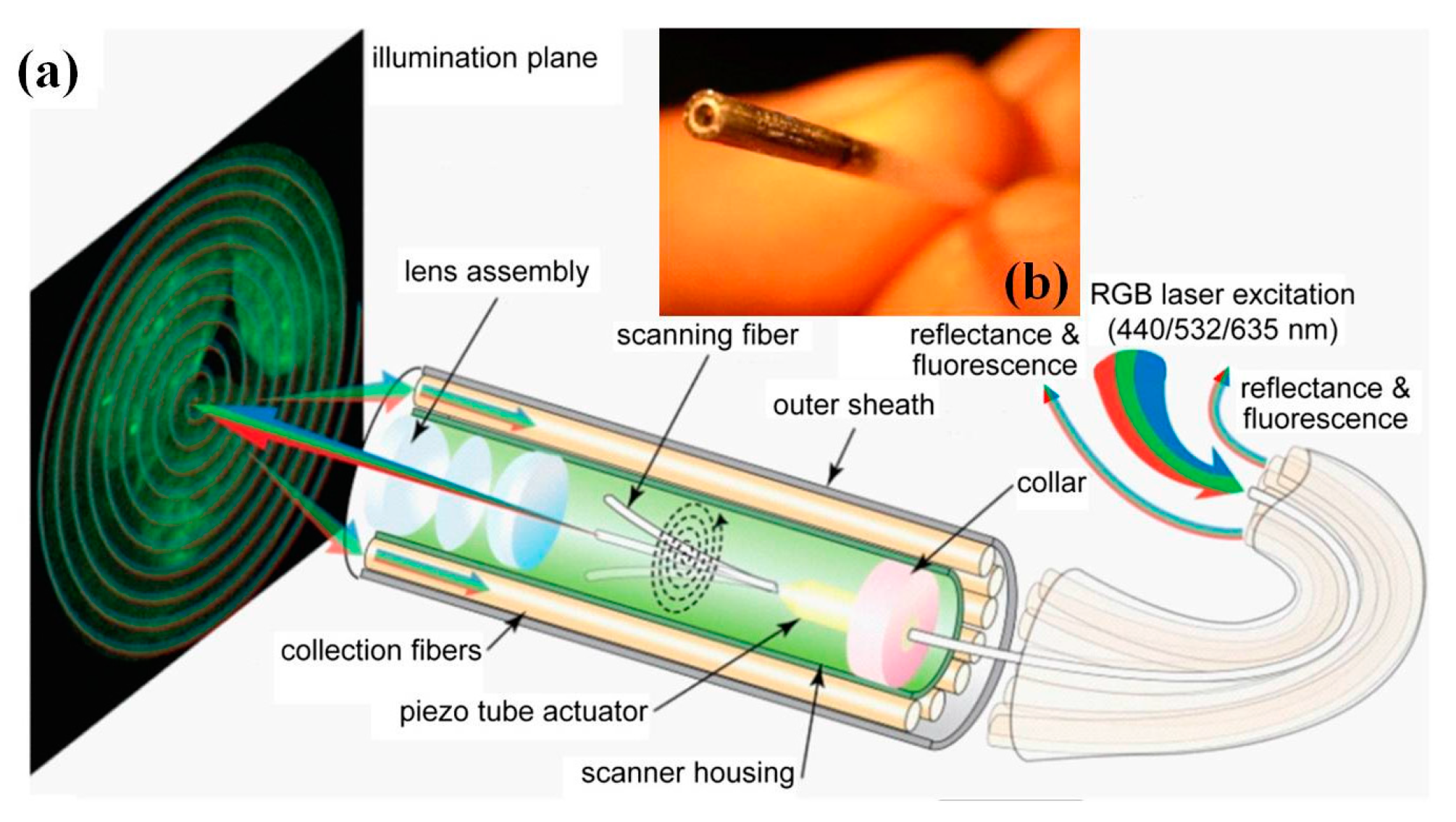
7. Wide-Field Fluorescent Endoscope
8. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PZT | Lead zirconate titanate |
| PVDF | Polyvinylidene difluoride |
| CMUT | Capacitive micromachined ultrasonic transducer |
| PMUT | Piezoelectric micromachined ultrasonic transducer |
| SS-OCT | Sweep source optical coherent tomography |
| VCSEL | Vertical cavity surface emitting laser |
| FWHM | Full width half maximum |
| DRIE | Deep reactive ion etching |
| SOI | Silicon-on-insulator |
| DAC | Dual-axis confocal |
| SVC | Staggered vertical combdrive |
| FOV | Field-of-view |
| PMT | Photomultiplier tubes |
| SECM | Spectrally encoded confocal microscope |
| NA | Numerical aperture |
| PAEM | Photoacoustic endomicroscope |
| MRR | Microring resonator |
References
- American Cancer Society. Cancer Facts and Figures 2015; American Cancer Society: Atlanta, GA, USA, 2015. [Google Scholar]
- Rubin, R.; Strayer, D.S. (Eds.) Rubin’s Pathology: Clinicopathologic Foundations of Medicine, 6th ed.; Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Pomper, M.G. Molecular imaging: An overview. Acad. Radiol. 2011, 8, 1141–1153. [Google Scholar] [CrossRef]
- Öincr, O.; Johnson, J.; Karaman, M.; Demirci, U.; Kaviani, K.; Lee, T.T.H.; Khuri-Yakub, B.T. Capacitive micromachined ultrasonic transducers: Next-generation arrays for acoustic imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 1596–1610. [Google Scholar]
- Solgaard, O.; Godil, A.A.; Howe, R.T.; Lee, L.P.; Peter, Y.A.; Zappe, H. Optical MEMS: From micromirrors to complex systems. J. Microelectromechan. Syst. 2014, 23, 517–538. [Google Scholar] [CrossRef]
- Flusberg, B.A.; Cocker, E.D.; Piyawattanametha, W.; Jung, J.C.; Cheung, E.L.M.; Schnitzer, M.J. Fiber-optic fluorescence imaging. Nat. Methods 2005, 2, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Fujimoto, J.G. (Eds.) Optical Coherence Tomography: Technology and Applications, 2nd ed.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Pawley, J.B. (Ed.) Handbook of Biological Confocal Microscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Fritjof, H.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar]
- Ling, F.; Gu, M. Fibre-optic nonlinear optical microscopy and endoscopy. J. Microsc. 2007, 226, 195–206. [Google Scholar]
- Zhou, Y.; Wang, L.V. Translational Photoacoustic Microscopy. In Frontiers in Biophotonics for Translational Medicine; Springer: Singapore, 2016; pp. 47–73. [Google Scholar]
- Elahi, S.F.; Wang, T.D. Future and advances in endoscopy. J. Biophoton. 2011, 4, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Khemthongchareooen, N.; Jolivot, R.; Rattanavarin, S.; Piyawattanametha, W. Advances in imaging probes and optical microendoscopic imaging techniques for early in vivo cancer assessment. Adv. Drug Del. Rev. 2014, 74, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Tadigadapa, S.; Mateti, K. Piezoelectric MEMS sensors: State-of-the-art and perspectives. Meas. Sci. Technol. 2009, 20, 092001. [Google Scholar] [CrossRef]
- Qiu, Y.; Gigliotti, J.V.; Wallace, M.; Griggio, F.; Demore, C.E.M.; Cochran, S.; Trolier-McKinstry, S. Piezoelectric Micromachined Ultrasound Transducer (PMUT) arrays for integrated sensing, actuation and imaging. Sensors 2015, 15, 8020–8041. [Google Scholar] [CrossRef] [PubMed]
- Cannat, J.M.; Williams, J.; Zhou, Q.; Ritter, T.; Shung, K.K. Development of a 35-MHz piezo-composite ultrasound array for medical imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 224–236. [Google Scholar] [CrossRef]
- Ling, T.; Chen, S.L.; Guo, L.J. Fabrication and characterization of high Q polymer micro-ring resonator and its application as a sensitive ultrasonic detector. Opt. Express 2011, 19, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Chen, S.L.; Guo, L.J. High-sensitivity and wide-directivity ultrasound detection using high Q polymer microring resonators. Appl. Phys. Lett. 2011, 98, 204103. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.; Hurrell, A.; Shaw, A.; Zhang, E.; Beard, P. A Fabry–Pérot fiber-optic ultrasonic hydrophone for the simultaneous measurement of temperature and acoustic pressure. J. Acoust. Soc. Am. 2009, 125, 3611–3622. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Solgaard, O. Monolithic Silicon Photonic Crystal Fiber Tip Sensors. In Lab-on-Fiber Technology; Springer International Publishing: New York, NY, USA, 2015; pp. 69–90. [Google Scholar]
- Ergun, A.S.; Huang, Y.; Zhuang, X.; Oralkan, O.; Yarahoglu, G.G.; Khuri-Yakub, B.T. Capacitive micromachined ultrasonic transducers: Fabrication technology. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 2242–2258. [Google Scholar] [PubMed]
- Degertekin, F.L.; Guldiken, R.O.; Karaman, M. Annular-ring CMUT arrays for forward-looking IVUS: Transducer characterization and imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Kupnik, M.; Watkins, R.D.; Pauly, K.B.; Khuri-Yakub, B.T. Capacitive micromachined ultrasonic transducers for therapeutic ultrasound applications. IEEE Trans. Biomed. Eng. 2010, 57, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.N.; Truong, U.T.; Nikoozadeh, A.; Oralkan, O.; Seo, C.H.; Cannata, J.; Dentinger, A.; Thomenius, K.; de la Rama, A.; Nguyen, T.; et al. First in vivo use of a capacitive micromachined ultrasound transducer array-based imaging and ablation catheter. J. Ultrasound Med. 2012, 31, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Kamaya, A.; Machtaler, S.; Sanjani, S.S.; Nikoozadeh, A.F.; Sommer, G.; Khuri-Yakub, B.T.; Willman, J.K.; Desser, T.S. New technologies in clinical ultrasound. Semin. Roentgenol. 2013, 48, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Dausch, D.E.; Castellucci, J.B.; Chou, D.R.; Von Ramm, O.T. Theory and operation of 2-D array piezoelectric micromachined ultrasound transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- Mastronardi, V.M.; Guido, F.; Amato, M.; De Vittorio, M.; Petroni, S. Piezoelectric ultrasonic transducer based on flexible AlN. Microelectron. Eng. 2014, 121, 59–63. [Google Scholar] [CrossRef]
- Akhbari, S.; Sammoura, F.; Yang, C.; Mahmoud, M.; Aqab, N.; Li, L. Bimorph pMUT with dual electrodes. In Proceedings of the 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; pp. 928–931. [Google Scholar]
- Lu, Y.; Rozen, O.; Tang, H.Y.; Smith, G.L.; Fung, S.; Boser, B.E.; Polcawich, R.G.; Horsley, D. Broadband piezoelectric micromachined ultrasonic transducers based on dual resonance modes. In Proceedings of the 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; pp. 146–149. [Google Scholar]
- Lu, Y.; Horsley, D. Modeling, fabrication, and characterization of piezoelectric micromachined ultrasonic transducer arrays based on cavity SOI wafers. IEEE J. Microelectromech. Syst. 2015, 24, 1142–1149. [Google Scholar] [CrossRef]
- Lu, Y.; Tang, H.Y.; Fung, S.; Boser, B.E.; Horsley, D. Short-range and high-resolution ultrasound imaging using an 8 MHz Aluminum Nitride PMUT array. In Proceedings of the 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; pp. 140–143. [Google Scholar]
- Lu, Y.; Heidari, A.; Horsley, D. A high fill-factor annular array of high frequency piezoelectric micromachined ultrasonic transducers. IEEE J. Microelectromech. Syst. 2015, 24, 904–913. [Google Scholar] [CrossRef]
- Sun, J.; Xie, H. MEMS-based endoscopic optical coherence tomography. Int. J. Opt. 2011, 2011, 825629. [Google Scholar] [CrossRef]
- Sakai, T. A high speed MEMS scanner for 140-kHz SS-OCT. In Proceedings of the 16th International Conference on Optical MEMS and Nanophotonics, Istanbul, Turkey, 8–11 August 2011; pp. 73–74. [Google Scholar]
- Potsaid, B.; Jayaraman, V.; Fujimoto, J.G.; Jiang, J.; Heim, P.; Cable, A.E. MEMS tunable VCSEL light source for ultrahigh speed 60 kHz-1 MHz axial scan rate and long range centimeter class OCT imaging. Proc. SPIE 2012, 8213, 82130M. [Google Scholar] [CrossRef]
- Ahsen, O.; Tao, Y.; Potsaid, B.M.; Sheikine, Y.; Jiang, J.; Grulkowski, I.; Tsai, T.H.; et al. Swept source optical coherence microscopy using a 1310 nm VCSEL light source. Opt. Express 2013, 21, 18021–18033. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Potsaid, B.; Tao, Y.; Jayaraman, V.; Jiang, J.; Heim, P.; Kraus, M.F.; Zhou, C.; Hornegger, J.; Mashimo, H.; et al. Ultrahigh speed endoscopic optical coherence tomography using micromotor imaging catheter and VCSEL technology. Biomed. Opt. Express 2013, 4, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Demis, J.; Burgner, C.; Potsaid, B.; Robertson, M.; Lee, B.W.; Choi, W.; Cable, A.; Fujimoto, J.; Jayaraman, V. Wideband electrically-pumped 1050 nm MEMS-tunable VCSEL for ophthalmic imaging. J. Lightwave Technol. 2015, 33, 3461–3468. [Google Scholar]
- Choi, W.J.; Wang, R.K. Swept-source optical coherence tomography powered by a 1.3-μm vertical cavity surface emitting laser enables 2.3-mm-deep brain imaging in mice in vivo. J. Biomed. Opt. 2015, 20, 106004. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xie, H.; Fedder, G.K. Endoscopic optical coherence tomography based on a microelectromechanical mirror. Opt. Lett. 2001, 26, 1966–1968. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Mukai, D.S.; Brenner, M.; Chen, Z. In vivo endoscopic optical coherence tomography by use of a rotational microelectromechanical system probe. Opt. Lett. 2004, 29, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.M.; Yazdanfar, S.; Rao, K.D.; Izatt, J.A.; Smith, S.W. Electrostatic micromachine scanning mirror for optical coherence tomography. Opt. Lett. 2003, 28, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Piyawattanametha, W.; Patterson, P.R.; Hah, D.; Toshiyoshi, H.; Wu, M.C. Surface- and bulk- micromachined two-dimensional scanner driven by angular vertical comb actuators. J. Microelectromech. Syst. 2005, 14, 1329–1338. [Google Scholar] [CrossRef]
- Aguirre, A.D.; Hertz, P.R.; Chen, Y.; Fujimoto, J.G.; Piyawattanametha, W.; Fan, L.; Wu, M.C. Two-axis MEMS scanning catheter for ultrahigh resolution three-dimensional and en face imaging. Opt. Exp. 2007, 15, 2445–2453. [Google Scholar] [CrossRef]
- Milanovic, V.; Matus, G.A.; McCormick, D.T. Gimbal-less monolithic silicon actuators for tip-tilt-piston micromirror applications. IEEE J. Sel. Top. Quantum Electron. 2004, 10, 462–471. [Google Scholar] [CrossRef]
- Jung, W.; McCormick, D.; Zhang, J.; Wang, L.; Tien, N.C.; Chen, Z. Three-dimensional endoscopic optical coherence tomography by use of a two-axis microelectromechanical scanning mirror. Appl. Phys. Lett. 2006, 88, 163901–163903. [Google Scholar] [CrossRef]
- Lu, C.D.; Kraus, M.F.; Potsaid, B.; Liu, J.J.; Choi, W.; Jayaraman, V.; Cable, A.E.; Hornegger, J.; Duker, J.S.; Fujimoto, J.G. Handheld ultrahigh speed swept source optical coherence tomography instrument using a MEMS scanning mirror. Biomed. Opt. Exp. 2014, 5, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Nakada, M.; Chong, C.; Morosawa, A.; Isamoto, K.; Suzuki, T.; Fujita, H.; Toshiyoshi, H. Optical coherence tomography by all-optical MEMS fiber endoscope. IEICE Electron. Exp. 2010, 7, 428–433. [Google Scholar] [CrossRef]
- Nakada, M.; Chong, C.; Morosawa, A.; Isamoto, K.; Suzuki, T.; Fujita, H.; Toshiyoshi, H. A behavioral model for optically powered OCT endoscope with a micro electrostatic vertical-comb optical scanner. IEEJ Trans. Electr. Electron. Eng. 2014, 9, 448–458. [Google Scholar] [CrossRef]
- Liu, X.; Cobb, M.J.; Chen, Y.; Kimmey, M.B.; Li, X. Rapid-scanning forward-imaging miniature endoscope for real-time optical coherence tomography. Opt. Lett. 2004, 29, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, N.; Huo, T.; Wang, C.; Zheng, J.; Zhou, T.; Xue, P. Tiny endoscopic optical coherence tomography probe driven by a miniaturized hollow ultrasonic motor. J. Biomed. Opt. 2013, 18, 086011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Tsai, T.-H.; Ahsen, O.; Liang, K.; Lee, H.C.; Xue, P.; Li, X.; Fujimoto, J.G. Compact piezoelectric transducer fiber scanning probe for optical coherence tomography. Opt. Lett. 2014, 39, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Song, C.; Kang, M.; Jeong, Y.; Jeong, K.H. Forward imaging OCT endoscopic catheter based on MEMS lens scanning. Opt. Lett. 2012, 37, 2673–2675. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Song, C.; Kang, M.; Jeong, Y.; Jeong, K.H. Lissajous fiber scanning for forward viewing optical endomicroscopy using asymmetric stiffness modulation. Opt. Express 2014, 22, 5818–5825. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Seo, Y.H.; Hwang, K.; Lim, J.K.; Yoon, S.Z.; Jeong, K.H. Micromachined tethered silicon oscillator for an endomicroscopic Lissajous fiber scanner. Opt. Lett. 2014, 39, 6675–6678. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kopa, A.; Pan, Y.; Fedder, G.K.; Xie, H. A two-axis electrothermal micromirror for endoscopic optical coherence tomography. IEEE J. Sel. Top. Quantum Electron. 2004, 10, 636–642. [Google Scholar] [CrossRef]
- Sun, J.; Guo, S.; Wu, L.; Liu, L.; Choe, S.W.; Sorg, B.S.; Xie, H. 3D in vivo optical coherence tomography based on a lowvoltage, large-scan-range 2D MEMS mirror. Opt. Exp. 2010, 18, 12065–12075. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, S.R.; Wu, L.; Sun, J.; Choe, S.; Sorg, B.S.; Xie, H. A 2.8-mm imaging probe based on a high-fill factor MEMS mirror and wire-bonding-free packaging for endoscopic optical coherence tomography. J. Microelectromech. Syst. 2012, 21, 1291–1302. [Google Scholar] [CrossRef]
- Wang, D.; Fu, L.; Wang, X.; Gong, Z.; Samuelson, S.; Duan, C.; Jia, H.; Ma, J.S.; Xie, H. Endoscopic swept-source optical coherence tomography based on a two-axis microelectromechanical system mirror. J. Biomed. Opt. 2013, 18, 086005–086009. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Wang, D.; Zhou, Z.; Liang, P.; Samuelson, S.; Pozzi, A.; Xie, H. Swept-source common-path optical coherence tomography with a MEMS endoscopic imaging probe. Proc. SPIE 2014, 8934, 89342N. [Google Scholar] [CrossRef]
- Xu, Y.; Singh, J.; Premachandran, C.S.; Khairyanto, A.; Chen, K.W.S.; Chen, N.; Sheppard, C.J.R.; Olivo, M. Design and development of a 3D scanning MEMS OCT probe using a novel SiOB package assembly. J. Micromech. Microeng. 2008, 18, 125005–125012. [Google Scholar] [CrossRef]
- Xu, Y.; Singh, J.; Selvaratnam, T.; Chen, N. Two-axis gimbal-less electrothermal micromirror for large-angle circumferential scanning. IEEE J. Sel. Top. Quantum Electron. 2009, 15, 1432–1438. [Google Scholar]
- Kim, K.H.; Park, B.H.; Maguluri, G.N.; Lee, T.W.; Rogomentich, F.J.; Bancu, M.G.; Bouma, B.E.; de Boer, J.F.; Bernstein, J.J. Two-axis magnetically-driven MEMS scanning catheter for endoscopic high-speed optical coherence tomography. Opt. Exp. 2007, 15, 18130–18140. [Google Scholar] [CrossRef]
- Gilchrist, K.H.; McNabb, R.P.; Izatt, J.A.; Grego, S. Piezoelectric scanning mirrors for endoscopic optical coherence tomography. J. Micromech. Microeng. 2009, 19, 095012–095022. [Google Scholar] [CrossRef]
- Naono, T.; Fujii, T.; Esashi, M.; Tanaka, S. A large-scan-angle piezoelectric MEMS optical scanner actuated by a Nb-doped PZT thin film. J. Micromech. Microeng. 2014, 24, 015010–015021. [Google Scholar] [CrossRef]
- Aljasem, K.; Froehly, L.; Seifert, A.; Zappe, H. Scanning and tunable micro-optics for endoscopic optical coherence tomography. J. Microelectromech. Syst. 2011, 20, 1462–1472. [Google Scholar] [CrossRef]
- Dickensheets, D.L.; Kino, G.S. A micromachined scanning confocal optical microscope. Opt. Lett. 1996, 21, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Maitland, K.C.; Shin, H.J.; Ra, H.; Lee, D.; Solgaard, O.; Richards-Kortum, R. Single fiber confocal microscope with a two axis gimbaled MEMS scanner for cellular imaging. Opt. Exp. 2006, 14, 8604–8612. [Google Scholar] [CrossRef]
- Shin, H.J.; Pierce, M.C.; Lee, D.; Ra, H.; Solgaard, O.; Richards-Kortum, R. Fiber-optic confocal microscope using a MEMS scanner and miniature objective lens. Opt. Exp. 2007, 15, 9113–9122. [Google Scholar] [CrossRef]
- Wang, Y.; Raj, M.; McGuff, H.S.; Bhave, G.; Yang, B.; Shen, T.; Zhang, X. Portable oral cancer detection using aminiature confocal imaging probe with a large field of view. J. Micromech. Microeng. 2012, 22, 065001–065010. [Google Scholar] [CrossRef]
- Liu, J.T.C.; Mandella, M.J.; Ra, H.; Wong, L.K.; Solgaard, O.; Kino, G.S.; Piyawattanametha, W.; Contag, C.H.; Wang, T.D. Miniature near-infrared dual-axes confocal microscope utilizing a two-dimensional microelectromechanical systems scanner. Opt. Lett. 2007, 32, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Piyawattanametha, W.; Wang, T.D. MEMS-based dual-axes confocal microendoscopy. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 804–814. [Google Scholar] [CrossRef] [PubMed]
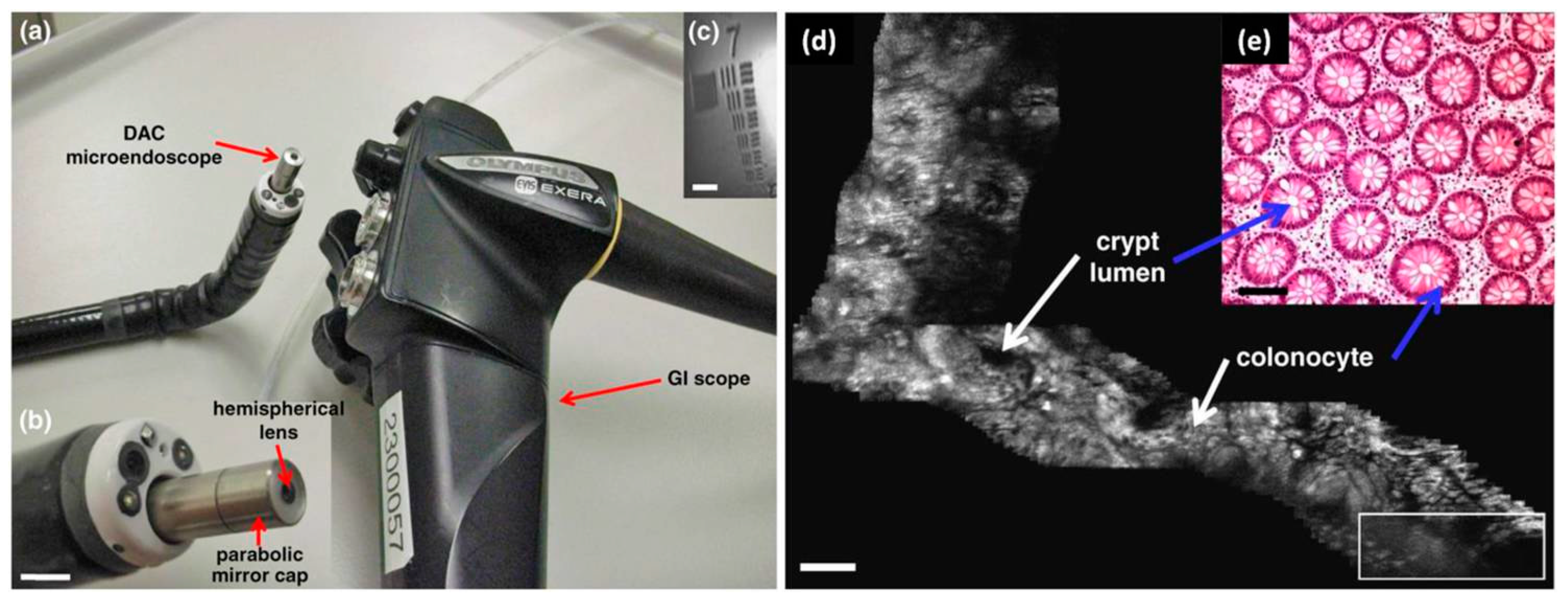
- Piyawattanametha, W.; Ra, H.; Qiu, Z.; Friedland, S.; Liu, J.T.C.; Loewke, K.; Kino, G.S.; Solgaard, O.; Wang, T.D.; Mandella, M.J.; et al. In vivo near-infrared dual-axis confocal microendoscopy in the human lower gastrointestinal tract. J. Biomed. Opt. 2012, 17, 0211021–0211024. [Google Scholar] [CrossRef] [PubMed]
- Ra, H.; Piyawattanametha, W.; Mandella, M.J.; Hsiung, P.L.; Hardy, J.; Wang, T.D.; Contag, C.H.; Kino, G.S.; Solgaard, O. Three-dimensional in vivo imaging by a handheld dual-axes confocal microscope. Opt. Exp. 2008, 16, 7224–7232. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, Z.; Duan, X.; Khondee, S.; Joshi, B.; Mandella, M.J.; Oldham, K.; Kurabayashi, K.; Wang, T.D. Targeted vertical cross-sectional imaging with handheld near-infrared dual axes confocal fluorescence endomicroscope. Biomed. Opt. Exp. 2013, 4, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Khondee, S.; Duan, X.; Li, H.; Mandella, M.J.; Joshi, B.P.; Zhou, Q.; Owens, S.R.; Kurabayashi, K.; Oldham, K.R.; et al. Vertical cross-sectional imaging of colonic dysplasia in vivo with multi-spectral dual axes confocal endomicroscopy. Gastroenterology 2014, 146, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Shahid, W.; Qiu, Z.; Duan, X.; Li, H.; Wang, T.D.; Oldham, K.R. Modeling and simulation of a parametrically resonant micromirror with duty-cycled excitation. J. Microelectromech. Syst. 2014, 23, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Mandella, M.J.; Kino, G.S.; Contag, C.H.; Solgaard, O. 3-D MEMS scanning system for dual-axis confocal microendoscopy. In Proceedings of the International Conference on Optical MEMS and Nanophotonics, Istanbul, Turkey, 8–11 August 2011; pp. 71–72. [Google Scholar]
- Jeong, J.W.; Kim, S.; Solgaard, O. Split-frame gimbaled 2-D MEMS scanner for miniature dual-axis confocal microendoscopes fabricated by front-side processing. J. Microelectromech. Syst. 2012, 21, 308–315. [Google Scholar] [CrossRef]
- Liu, L.; Xie, H. 3-D confocal laser scanning microscopy based on a full-MEMS scanning system. IEEE Photon. Technol. Lett. 2013, 25, 1478–1480. [Google Scholar] [CrossRef]
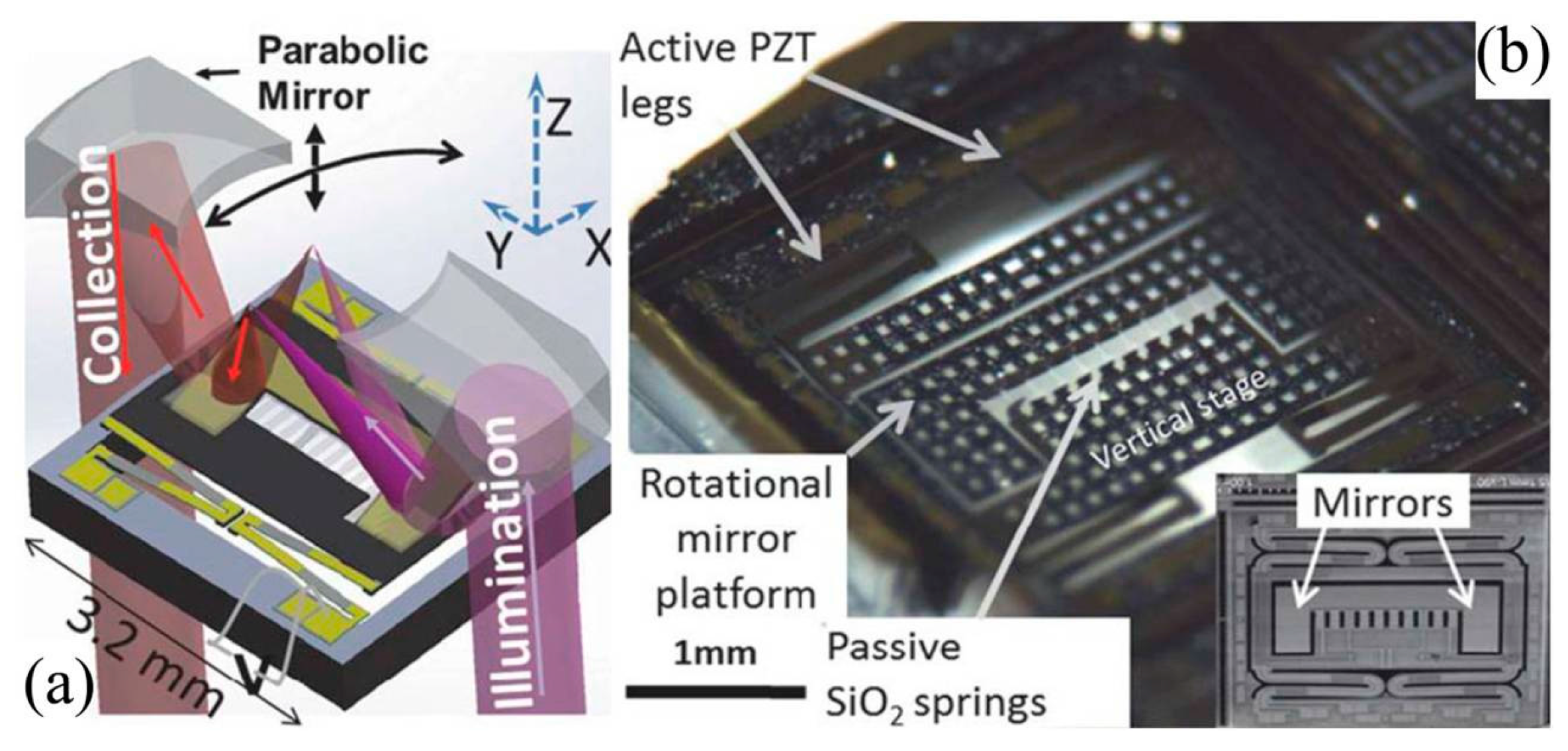
- Qiu, Z.; Pulskamp, J.S.; Lin, X.; Rhee, C.H.; Wang, T.D.; Polcawich, R.G.; Oldham, K. Large displacement vertical translational actuator based on piezoelectric thin films. J. Micromech. Microeng. 2010, 20, 075016. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Rhee, C.H.; Choi, J.; Wang, T.D.; Oldham, K.R. Large stroke vertical PZT microactuator with high-speed rotational scanning. J. Microelectromech. Syst. 2014, 23, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Qiu, Z.; Rhee, C.H.; Wang, T.D.; Oldham, K. A three-degree-of-freedom thin-film PZT-actuated microactuator with large out-of-plane displacement. J. Micromech. Microeng. 2014, 24, 075017. [Google Scholar] [CrossRef] [PubMed]
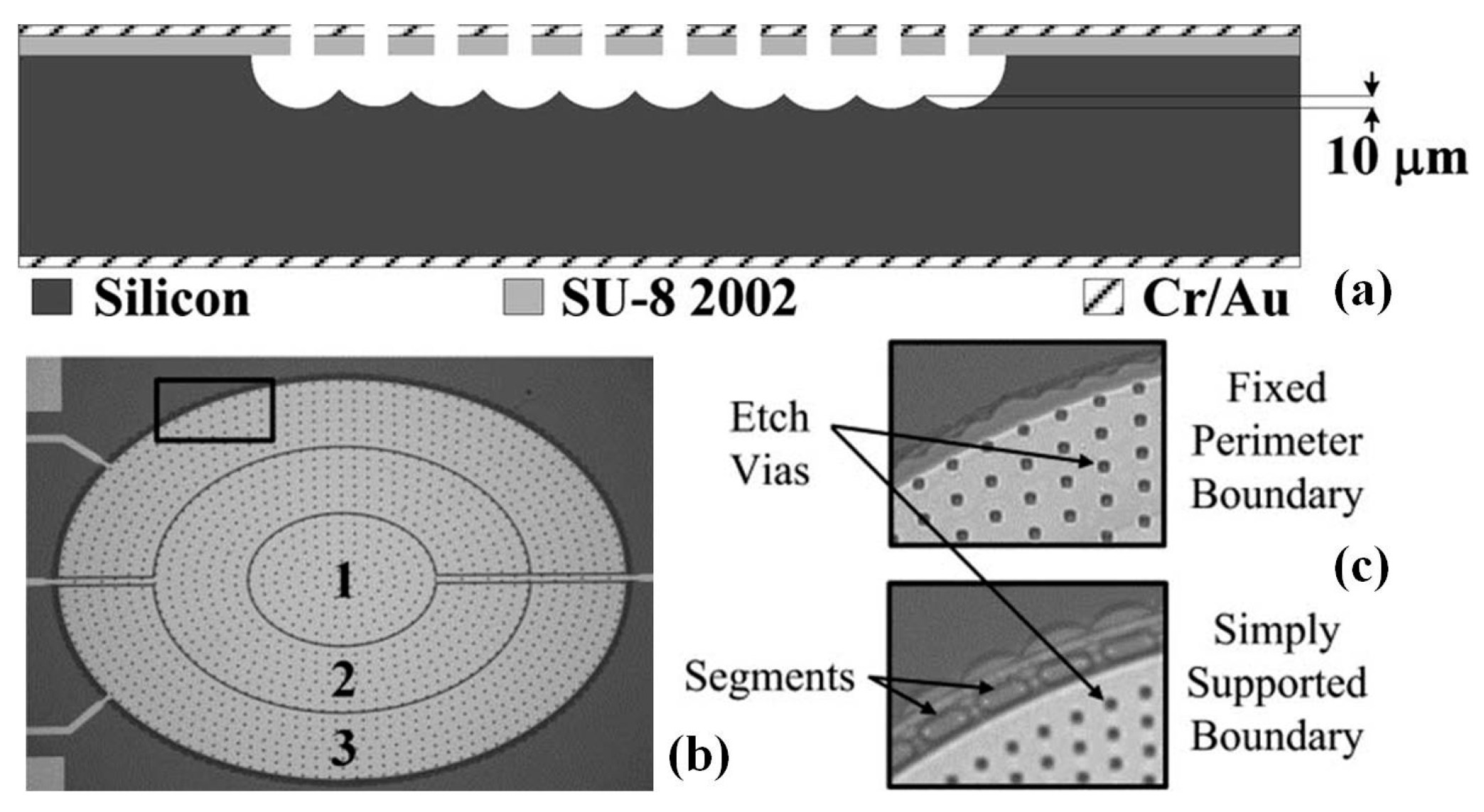
- Moghimi, M.J.; Wilson, C.R.; Dickensheets, D.L. Improved micro-optoelectromechanical systems deformable mirror for in vivo optical microscopy. J. Micro/Nanolithogr. MEMS MOEMS 2012, 11, 043006. [Google Scholar] [CrossRef]
- Lukes, S.J.; Dickensheets, D.L. SU-8 2002 surface micromachined deformable membrane mirrors. J. Microelectromech. Syst. 2013, 22, 94–106. [Google Scholar] [CrossRef]
- Moghimi, M.J.; Chattergoon, K.N.; Wilson, C.R.; Dickensheets, D.L. High speed focus control MEMS mirror with controlled air damping for vital microscopy. J. Microelectromech. Syst. 2013, 22, 938–948. [Google Scholar] [CrossRef]
- Cu-Nguyen, P.H.; Grewe, A.; Hillenbrand, M.; Sinzinger, S.; Seifert, A.; Zappe, H. Tunable hyperchromatic lens system for confocal hyperspectral sensing. Opt. Express 2013, 21, 27611–27621. [Google Scholar] [CrossRef] [PubMed]
- Niklas, W.; Meinert, T.; Zappe, H.; Seifert, A. Tunable MEMS fiber scanner for confocal microscopy. In Proceedings of the 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 881–884. [Google Scholar]
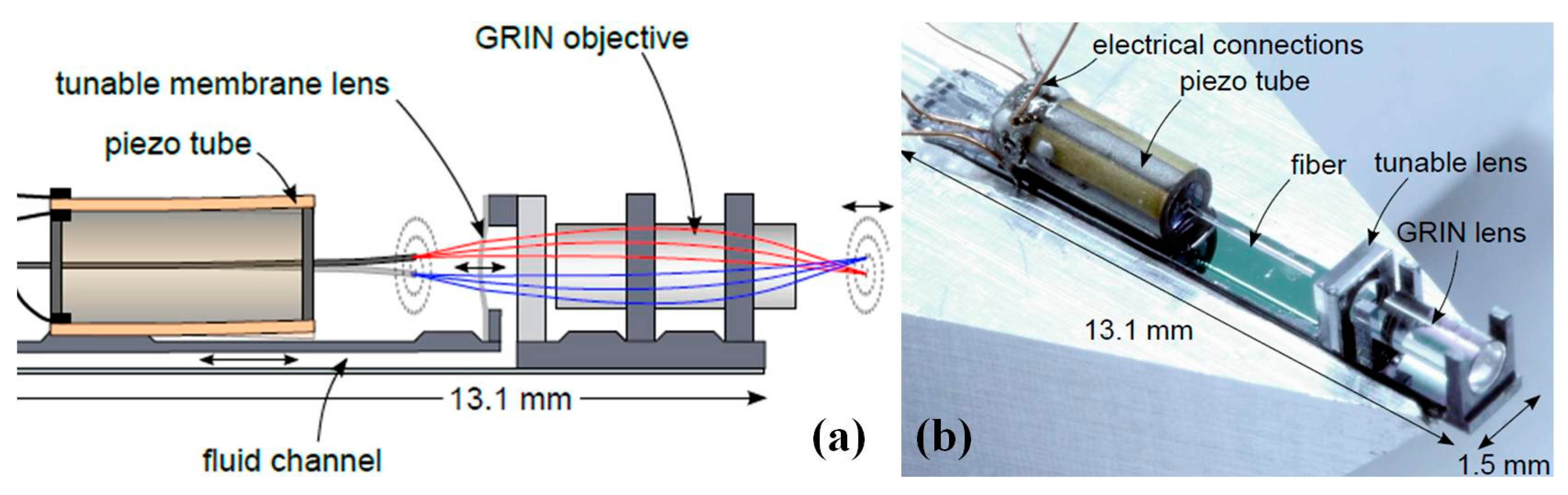
- Kim, M.; Kang, D.; Wu, T.; Tabatabaei, N.; Carruth, R.W.; Martinez, R.V.; Whitesides, G.M.; Nakajima, Y.; Tearney, G.J. Miniature objective lens with variable focus for confocal endomicroscopy. Biomed. Opt. Express 2014, 5, 4350–4361. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, B.N.; Losacco, J.T.; Cormack, R.; Weir, R.; Bright, V.M.; Gopinath, J.T.; Restrepo, D.; Gibson, E.A. Miniaturized fiber-coupled confocal fluorescence microscope with an electrowetting variable focus lens using no moving parts. Opt. Lett. 2015, 40, 2553–2556. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Martinez, R.V.; Whitesides, G.M.; Tearney, G.J. Miniature grating for spectrally-encoded endoscopy. Lab Chip 2013, 13, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Carruth, R.W.; Kim, M.; Schlachter, S.C.; Shishkov, M.; Woods, K.; Tabatabaei, N.; Wu, T.; Tearney, G.J. Endoscopic probe optics for spectrally encoded confocal microscopy. Biomed. Opt. Express 2013, 4, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Schlachter, S.C.; Kang, D.; Gora, M.J.; Vacas-Jacques, P.; Wu, T.; Carruth, R.W.; Wilsterman, E.J.; Bouma, B.E.; Woods, K.; Tearney, G.J. Spectrally encoded confocal microscopy of esophageal tissues at 100 kHz line rate. Biomed. Opt. Express 2013, 4, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, F.; Fee, M.S.; Tank, D.W.; Denk, W. A miniature head-mounted two-photon microscope: High-resolution brain imaging in freely moving animals. Neuron 2001, 31, 903–912. [Google Scholar] [CrossRef]
- Piyawattanametha, W.; Barretto, R.P.J.; Ko, T.H.; Flusberg, B.A.; Cocker, E.D.; Ra, H.; Lee, D.; Solgaard, O.; Schnitzer, M.J. Fast-scanning two-photon fluorescence imaging based on a microelectromechanical systems two-dimensional scanning mirror. Opt. Lett. 2006, 31, 2018–2020. [Google Scholar] [CrossRef] [PubMed]
- Piyawattanametha, W.; Cocker, E.D.; Burns, L.D.; Barretto, R.P.J.; Jung, J.C.; Ra, H.; Solgaard, O.; Schnitzer, M.J. In vivo brain imaging using a portable 2.9 g two-photon microscope based on a microelectromechanical systems scanning mirror. Opt. Lett. 2009, 34, 2309–2311. [Google Scholar] [CrossRef] [PubMed]
- Hoy, C.L.; Durr, N.J.; Chen, P.; Piyawattanametha, W.; Ra, H.; Solgaard, O.; Ben-Yakar, A. Miniaturized probe for femtosecond laser microsurgery and two-photon imaging. Opt. Exp. 2008, 16, 9996–10005. [Google Scholar] [CrossRef]
- Hoy, C.L.; Ferhanoğlu, O.; Yildirim, M.; Piyawattanametha, W.; Ra, H.; Solgaard, O.; Ben-Yakar, A. Optical design and imaging performance testing of a 9.6-mm diameter femtosecond laser microsurgery probe. Opt. Exp. 2011, 19, 10536–10552. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Tang, S.; McCormic, D.T.; Xie, T.; Ahn, Y.C.; Su, J.; Tomov, I.V.; Krasieva, T.B.; Tromberg, B.J.; Chen, Z. Miniaturized probe based on a microelectromechanical system mirror for multiphoton microscopy. Opt. Lett. 2008, 33, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Jung, W.; McCormick, D.; Xie, T.; Su, J.; Ahn, Y.C.; Tromberg, B.J.; Chen, Z. Design and implementation of fiber-based multiphoton endoscopy with microelectromechanical systems scanning. J. Biomed. Opt. 2009, 14, 034005–034011. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, C.J.; Johnston, R.S.; Seibel, E.J.; Helmchen, F. Ultra-compact fiber-optic two-photon microscope for functional fluorescence imaging in vivo. Opt. Express 2008, 16, 5556–5564. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.R.; Brown, C.M.; Ouzounov, D.G.; Pavlova, I.; Kobat, D.; Webb, W.W.; Xu, C. Compact and flexible raster scanning multiphoton endoscope capable of imaging unstained tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 17598–17603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Akins, M.L.; Muraria, K.; Xi, J.; Li, M.J.; Luby-Phelps, K.; Mahendroo, M.; Li, X. A compact fiber-optic SHG scanning endomicroscope and its application to visualize cervical remodeling during pregnancy. Proc. Natl. Acad. Sci. USA 2012, 109, 12878–12883. [Google Scholar] [CrossRef] [PubMed]
- Ducourthial, G.; Leclerc, P.; Mansuryan, T.; Fabert, M.; Brevier, J.; Habert, R.; Braud, F.; Batrin, R.; Vever-Bizet, C.; Bourg-Heckly, G.; et al. Development of a real-time flexible multiphoton microendoscope for label-free imaging in a live animal. Sci. Rep. 2015, 5, 18303. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Choi, H.; So, P.T.C.; Culpepper, M.L. Thermomechanical actuator-based three-axis optical scanner for high-speed two-photon endomicroscope imaging. J. Microelectromech. Syst. 2014, 23, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Duan, C.; Liu, L.; Li, X.; Xie, H. A non-resonant fiber scanner based on an electrothermally-actuated MEMS stage. Sens. Actuators A: Phys. 2015, 233, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Li, C.; Chen, R.; Rao, B.; Yao, J.; Yeh, C.H.; Danielli, A.; et al. Optical-resolution photoacoustic endomicroscopy in vivo. Biomed. Opt. Express 2015, 6, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Huang, C.H.; Zou, J. Microfabricated water-immersible scanning mirror with a small form factor for handheld ultrasound and photoacoustic microscopy. J. Micro/Nanolithogr. MEMS MOEMS 2015, 14, 035004. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.; Yang, J.M.; Maslov, K.I.; Wong, T.T.W.; Li, L.; Huang, C.H.; Zou, J.; Wang, L.V. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods 2015, 12, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, B.Y.; Chen, S.L.; Ling, T.; Guo, L.J.; Li, P.C. All-optical scanhead for ultrasound and photoacoustic dual-modality imaging. Opt. Express 2012, 20, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, S.L.; Ling, T.; Guo, L.J.; Carson, P.L.; Wang, X. Pure optical photoacoustic microscopy. Opt. Express 2011, 19, 9027–9034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, S.L.; Ling, T.; Guo, L. Review of imprinted polymer microring as ultrasound detector: design, fabrication, and characterization. IEEE Sens. J. 2015, 15, 3241–3248. [Google Scholar] [CrossRef]
- Zhang, C.; Ling, T.; Chen, S.L.; Guo, L.J. Ultrabroad bandwidth and highly sensitive optical ultrasonic detector for photoacoustic imaging. ACS Photonics 2014, 1, 1093–1098. [Google Scholar] [CrossRef]
- Dong, B.; Chen, S.; Zhang, Z.; Sun, C.; Zhang, H.F. Photoacoustic probe using a micro-ring resonator ultrasonic sensor for endoscopic applicatons. Opt. Lett. 2014, 39, 4372–4375. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, B.; Zhang, Z.; Zhang, H.F.; Sun, C. A transparent broadband ultrasonic detector based on an optical micro-ring resonator for photoacoustic microscopy. Sci. Rep. 2014, 4, 4496. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, S.; Ma, T.J.; Furukawa, Y.; Wygant, I.O.; Xuefeng, Z.; De La Zerda, A.; Oralkan, O.; Kamaya, A.; Jeffrey, R.B.; Khuri-Yakub, B.T. Three-dimensional photoacoustic imaging using a two-dimensional CMUT array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, S.R.; Ma, T.J.; Vaithilingam, S.; Oralkan, Ö.; Khuri-Yakub, B.T.; Gambhir, S.S. Deep tissue photoacoustic imaging using a miniaturized 2-D capacitive micromachined ultrasonic transducer array. IEEE Trans. Biomed. Eng. 2012, 59, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chu, F.; Liu, X.; Li, Y.; Rong, J.; Jiang, H. AlN-based piezoelectric micromachined ultrasonic transducer for photoacoustic imaging. Appl. Phys. Lett. 2013, 103, 031118. [Google Scholar] [CrossRef]
- Xi, L.; Sun, J.; Zhu, Y.; Wu, L.; Xie, H.; Jiang, H. Photoacoustic imaging based on MEMS mirror scanning. Biomed. Opt. Express 2010, 1, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Engelbrecht, C.J.; Soper, T.D.; Helmchen, F.; Seibel, E.J. Scanning fiber endoscopy with highly flexible, 1 mm catheterscopes for wide-field, full-color imaging. J. Biophotonics 2010, 3, 385–407. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.J.; Cameron, M.L.; Joshi, B.P.; Gaustad, A.; Seibel, E.J.; Wang, T.D. Targeted detection of murine colonic dysplasia in vivo with flexible multispectral scanning fiber endoscopy. J. Biomed. Opt. 2012, 17, 0211031–02110311. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hou, V.; Nelson, L.Y.; Seibel, E.J. Mitigating fluorescence spectral overlap in wide-field endoscopic imaging. J. Biomed. Opt. 2013, 18, 086012. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Xi, L.; Samuelson, S.R.; Xie, H.; Yang, L.; Jiang, H. Microelectromechanical systems scanning-mirror-based handheld probe for fluorescence molecular tomography. Appl. Opt. 2012, 51, 4678–4683. [Google Scholar] [CrossRef] [PubMed]

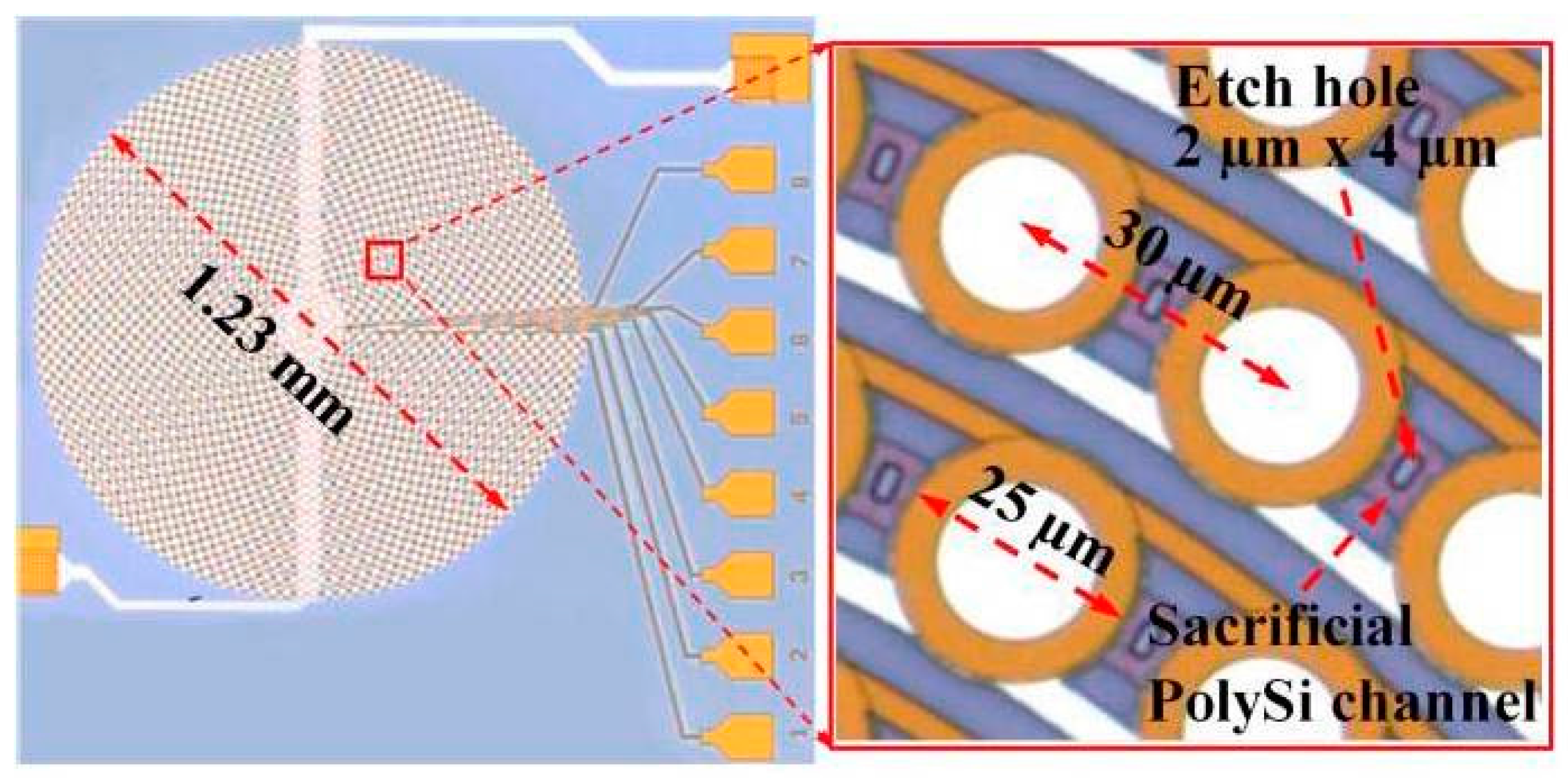
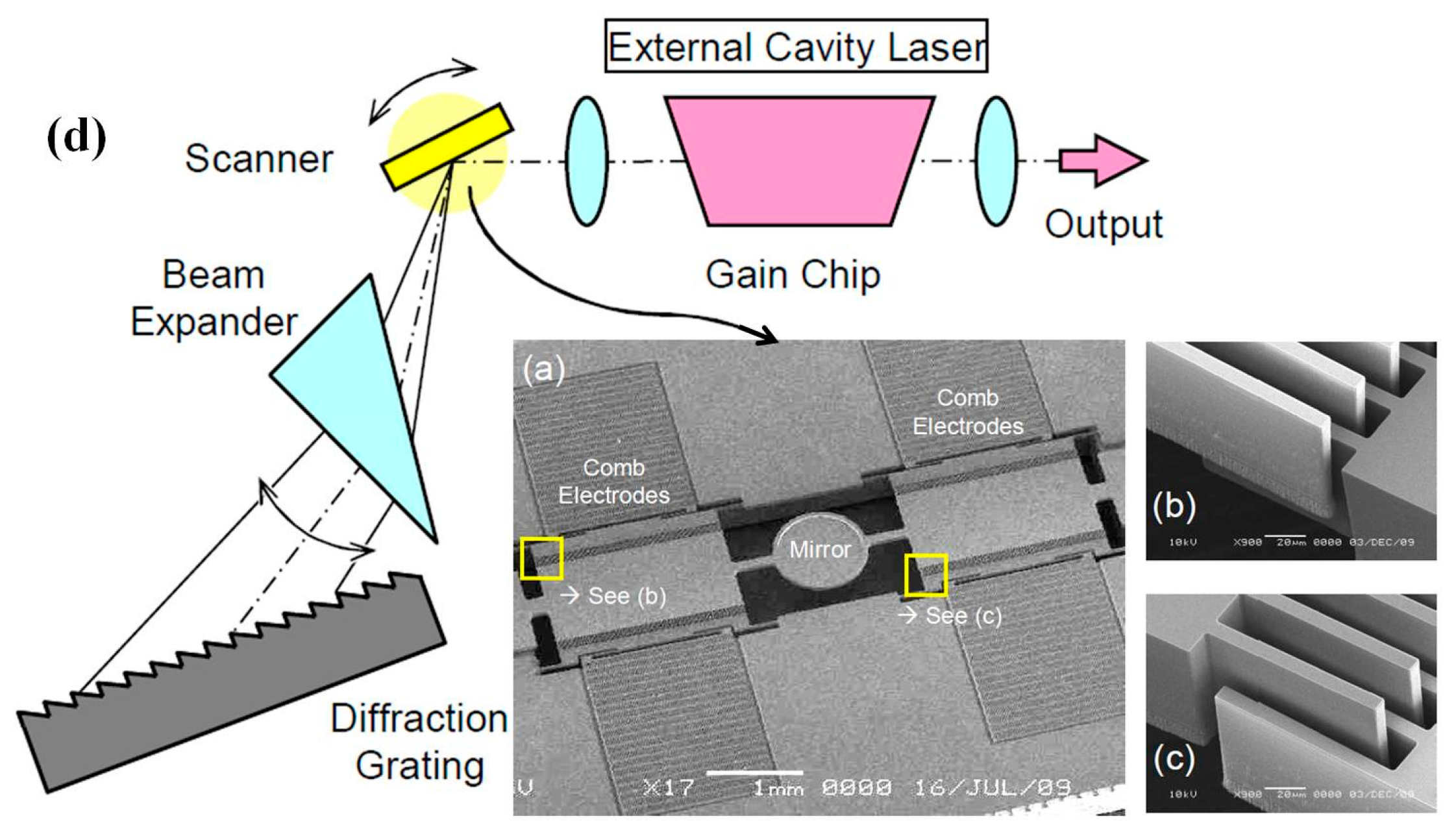
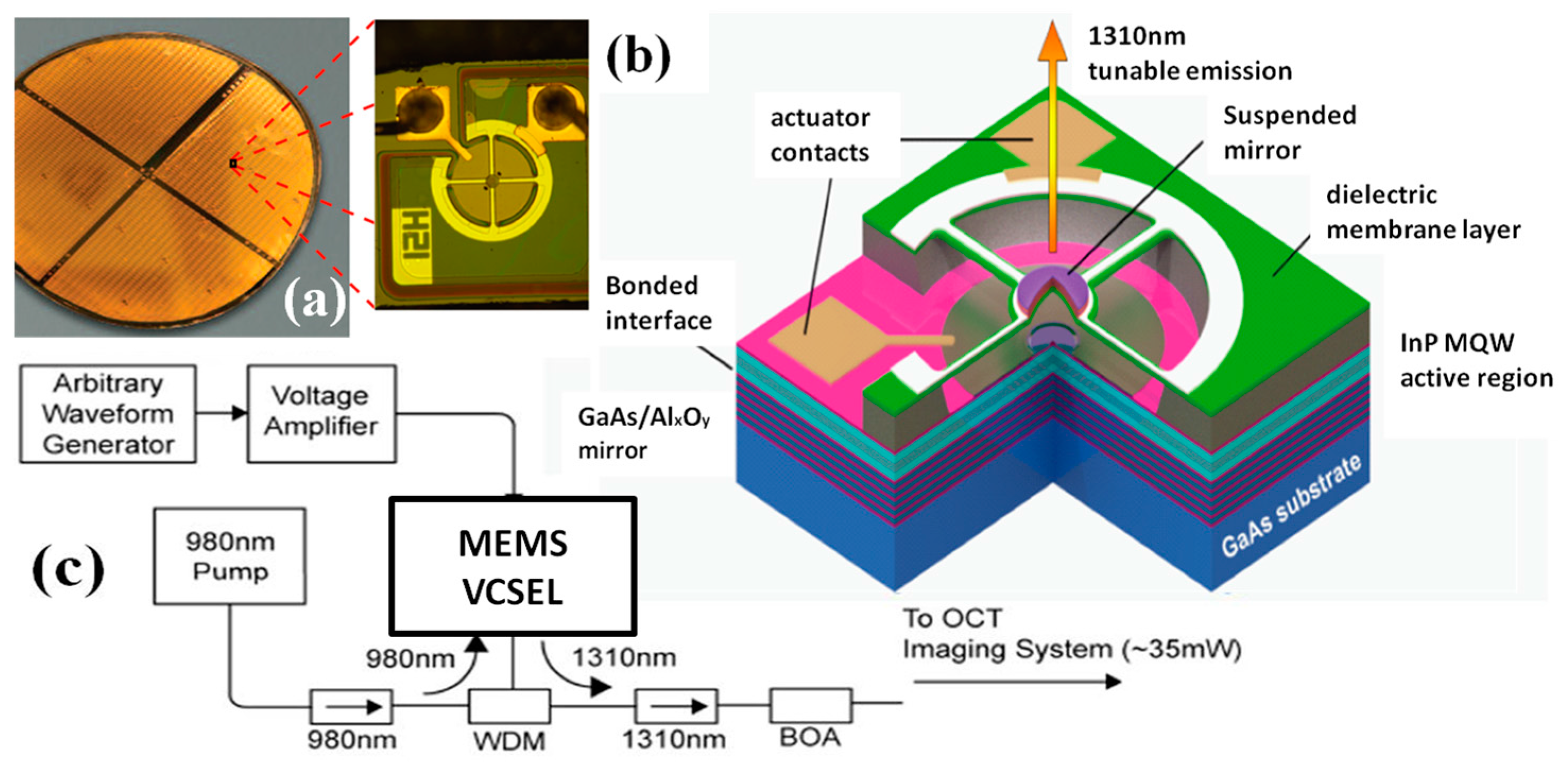
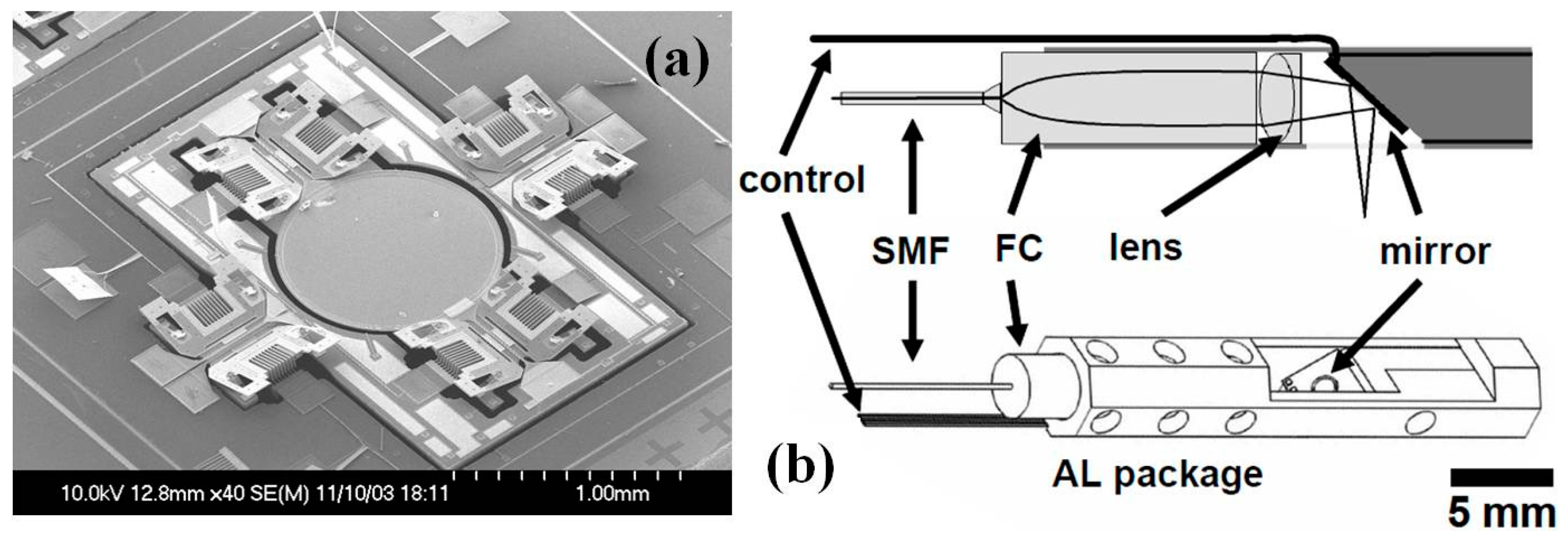


| Characteristics | Conventional | CMUT | PMUT | Fiber Optical | Acoustic Sensor |
|---|---|---|---|---|---|
| Sensors | PZT or PVDF | Capacitive | Thin film AlN/PZT | Microring | Fabry–Pérot Cavity |
| Array | Yes | Yes | Yes | challenging | challenging |
| Footprint | Bulky | OD < 3 mm | OD < 3 mm | <1 mm | <1 mm |
| Sensitivity | Medium | Medium | Medium | ultrahigh | High |
| Modality | Spatial Resolution (µm) | Field-Of-View (FOV) | Imaging Rate (Hz) | Medical Applications | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Fluorescent Wide-Field | 100–300 | ~70°–90° | ~30 | GI, respiratory, ear, urinary, reproductive tracts | High imaging speed, inexpensive laser source, minimal moving parts, commercial devices exist | Relatively low resolution and contrast, no depth sectioning |
| Single-axis confocal | 0.5–5 | 0°–150° | >2 | GI, respiratory, ear, urinary, reproductive tracts | High sensitivity provide functional information miniaturization through proximal or distal ends commercial devices exist | Limited contrast and wavelength, limited tissue penetration (<100 µm), limited working distance, increased aberration due to high NA optics |
| Dual-axis confocal | 3–6 | 250–1000 µm | >15 | Skin, GI tract, liver, head and neck, pancreas | Effective out-of-focus rejection of scattered light for high contrast, deep tissue penetration (~400 µm), relatively isotropic resolution | Low NA optics limits sensitivity, challenging alignment of a dual-beam configuration |
| OCT | 1–15 | 2000–3000 µm | >60 | GI, respiratory, ear, urinary, reproductive tracts | Impressive miniaturization, high sensitivity, dynamic range, high imaging speed, deep tissue penetration (a few mm) | Label-free imaging, expensive detector array, Short dynamic range along depth |
| Two-photon | 0.5–2 | 200–500 µm | >5 | GI, respiratory, tracts | High resolution and contrast, deep tissue penetration (~500 µm ~1 mm) less photobleaching and phototoxicity, Commercial devices exist | Relatively expensive laser source and optics, need dispersion compensation or special fibers to maintain pulse shape |
| Optical resolution photoacoustic microscope (OR-PAM) | ~5 | 1000 µm | 10 | Breast, brain | High spatial resolution and contrast high imaging speed, deep tissue penetration (a few mm) | Relatively expensive laser source progress on miniaturization is still ongoing |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Z.; Piyawattanamatha, W. New Endoscopic Imaging Technology Based on MEMS Sensors and Actuators. Micromachines 2017, 8, 210. https://doi.org/10.3390/mi8070210
Qiu Z, Piyawattanamatha W. New Endoscopic Imaging Technology Based on MEMS Sensors and Actuators. Micromachines. 2017; 8(7):210. https://doi.org/10.3390/mi8070210
Chicago/Turabian StyleQiu, Zhen, and Wibool Piyawattanamatha. 2017. "New Endoscopic Imaging Technology Based on MEMS Sensors and Actuators" Micromachines 8, no. 7: 210. https://doi.org/10.3390/mi8070210






