Rapid Nucleic Acid Extraction and Purification Using a Miniature Ultrasonic Technique
Abstract
:1. Introduction
2. Materials and Methods
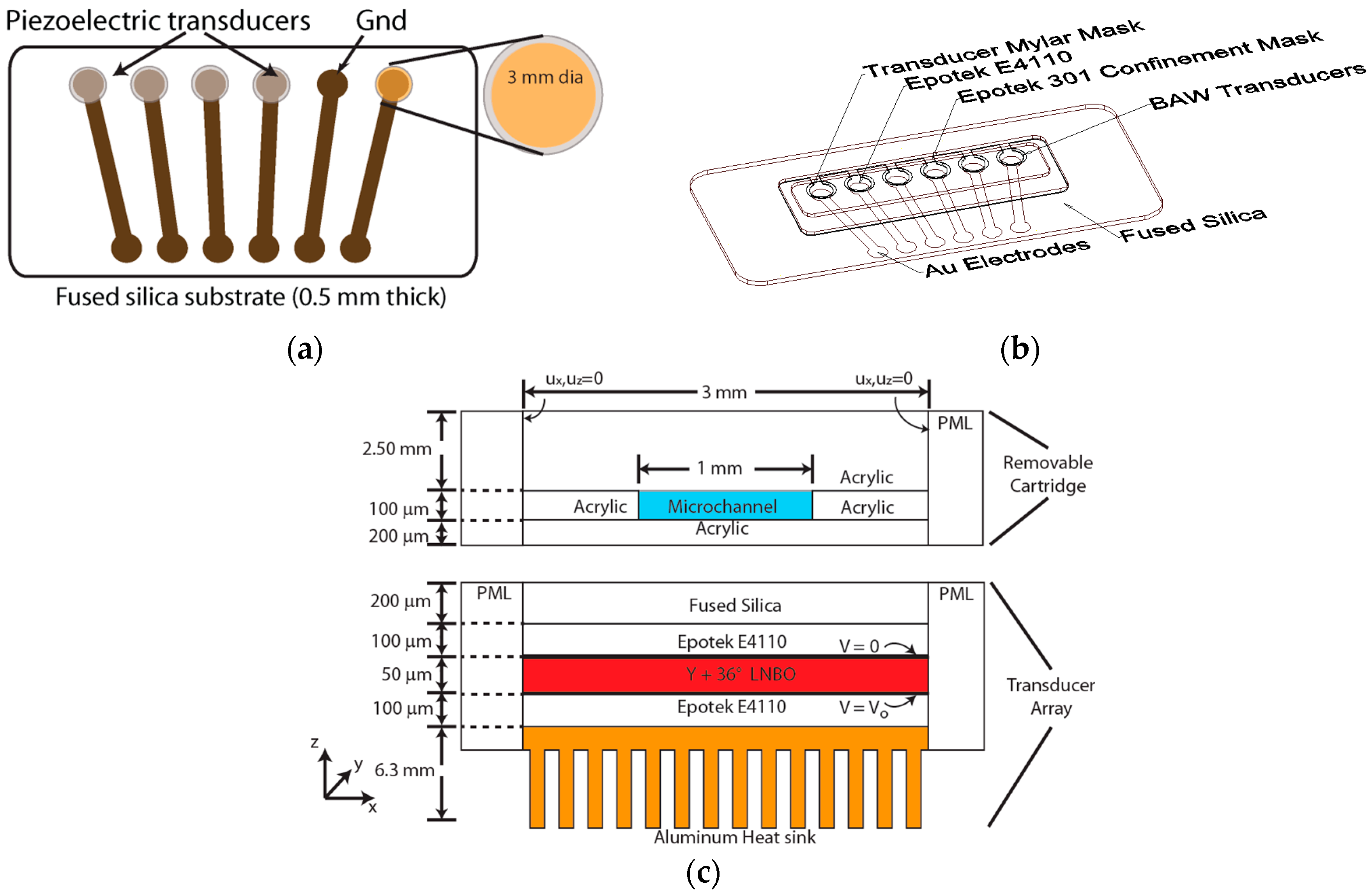
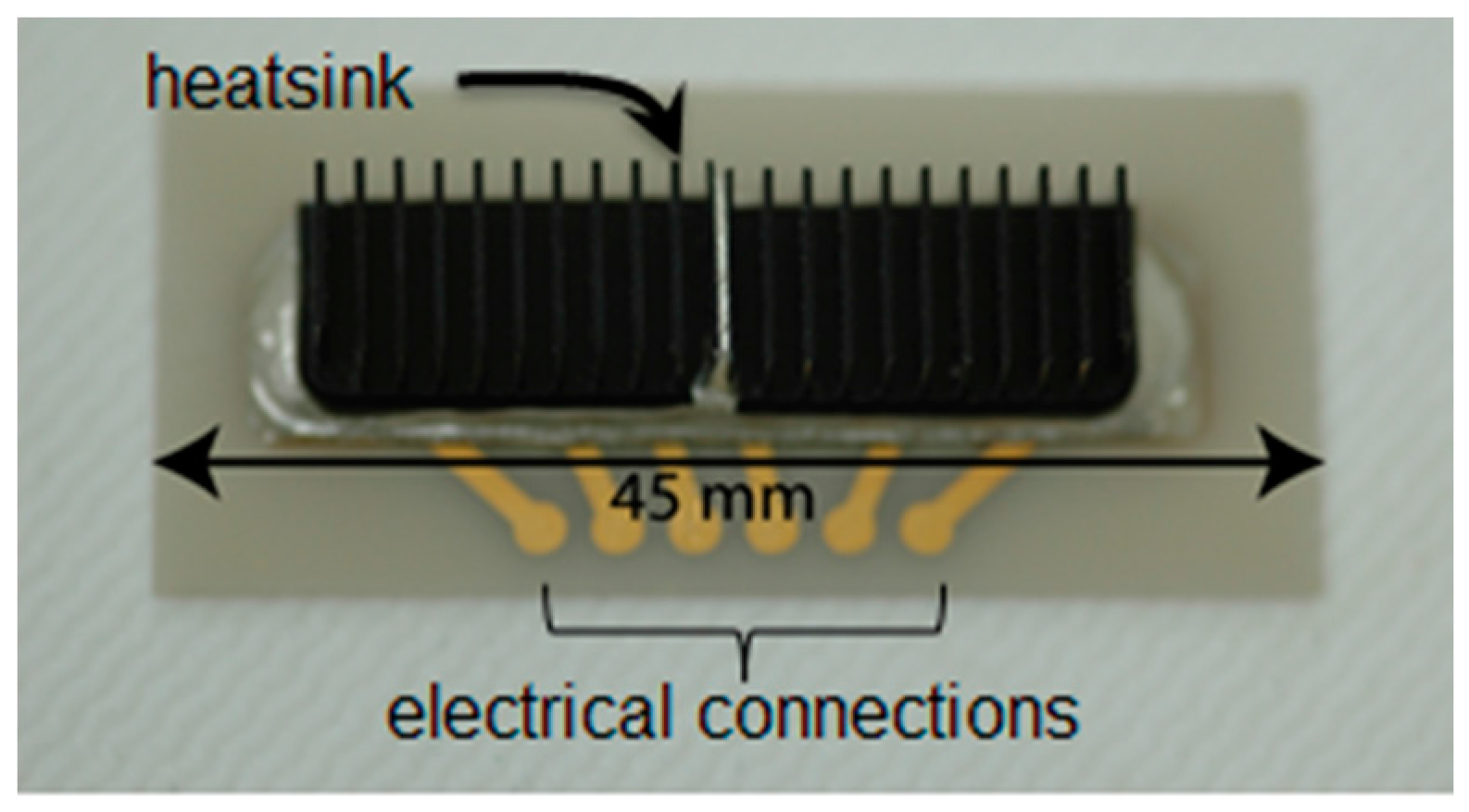
2.1. Transducer Fabrication
2.2. Cell Sample Preparation and Cellular Lysis Quantitation
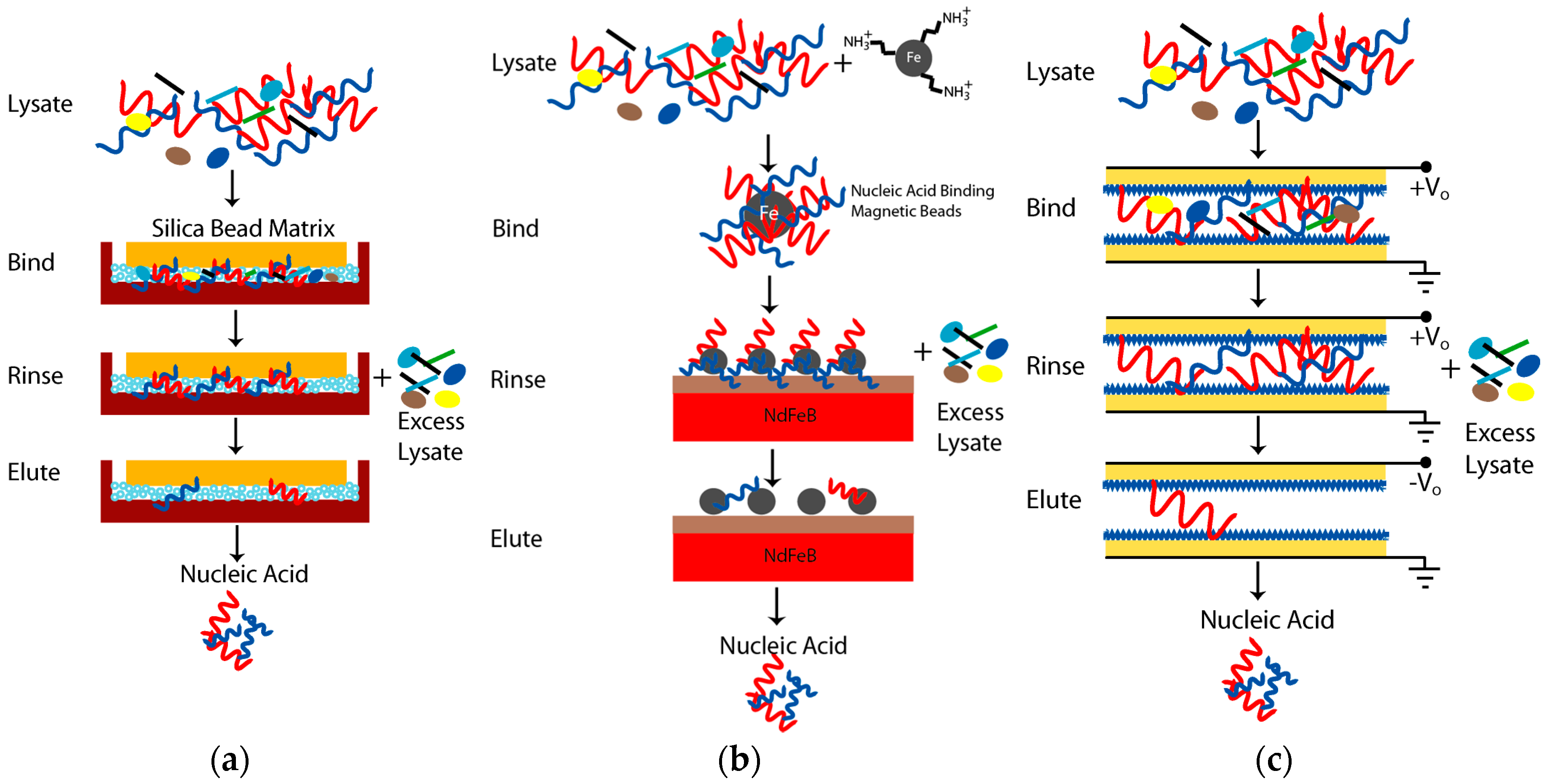
2.3. Nucleic Acid Extraction Approaches with Acoustic Lysis
Sol-Gel Silica Bead Nucleic Acid Extraction
3. Results and Discussion
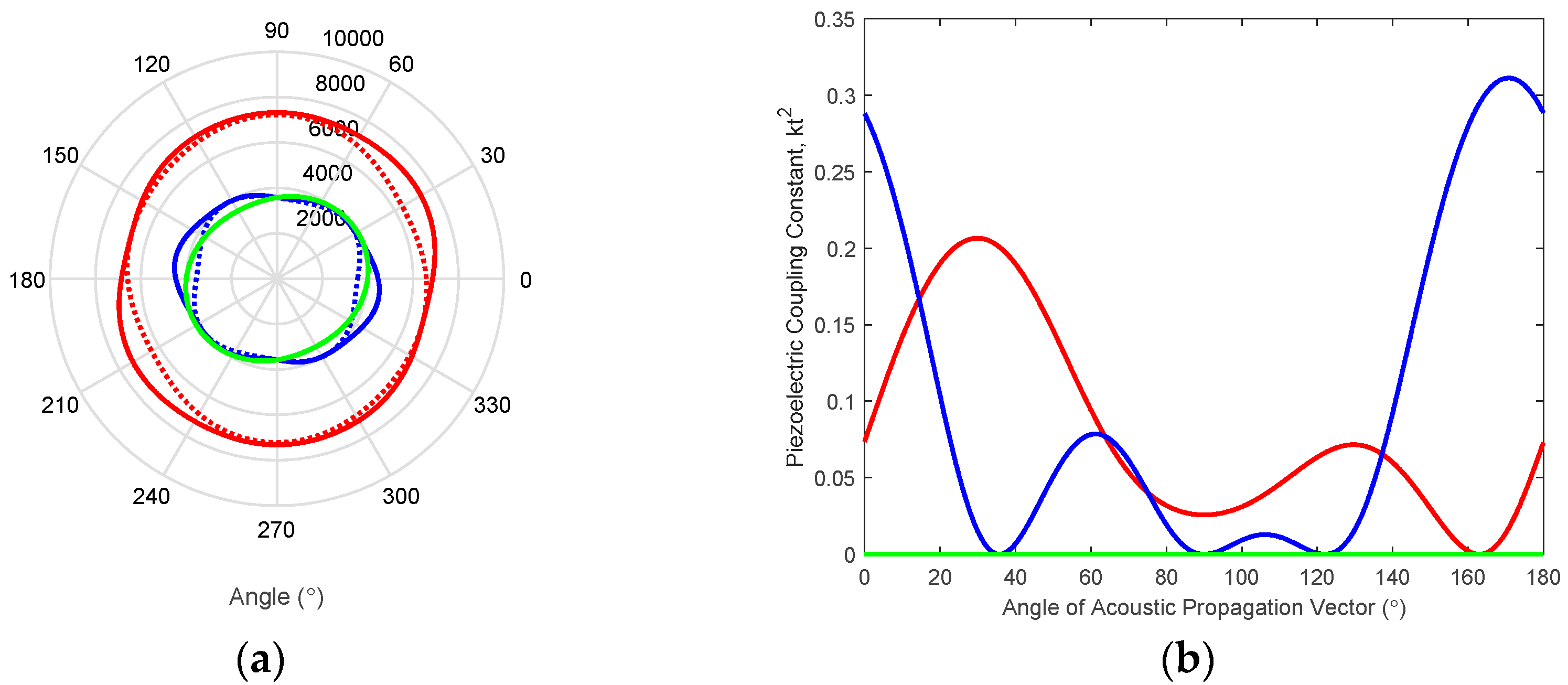
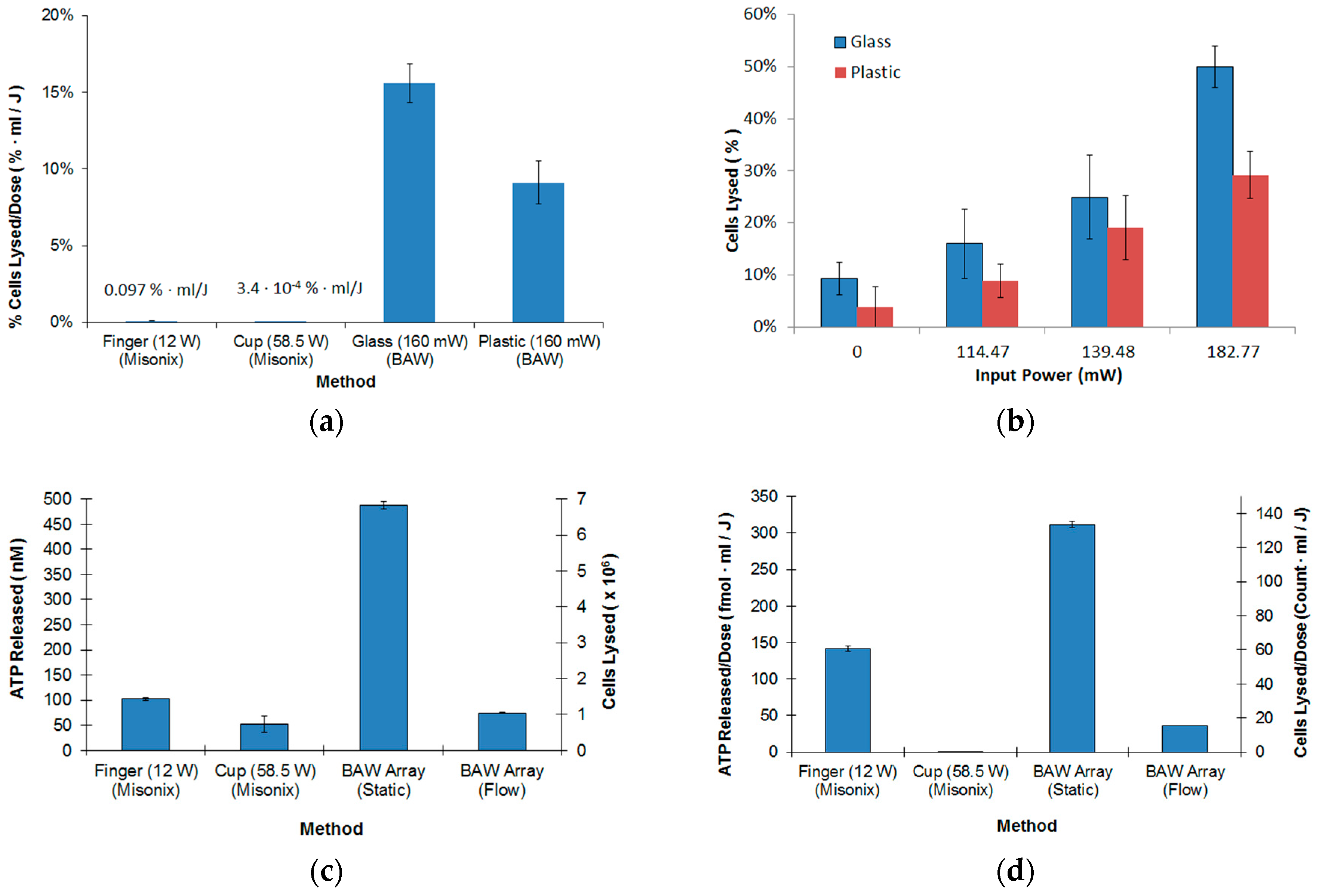
3.1. Miniature BAW Transducer Array and Cellular Lysis
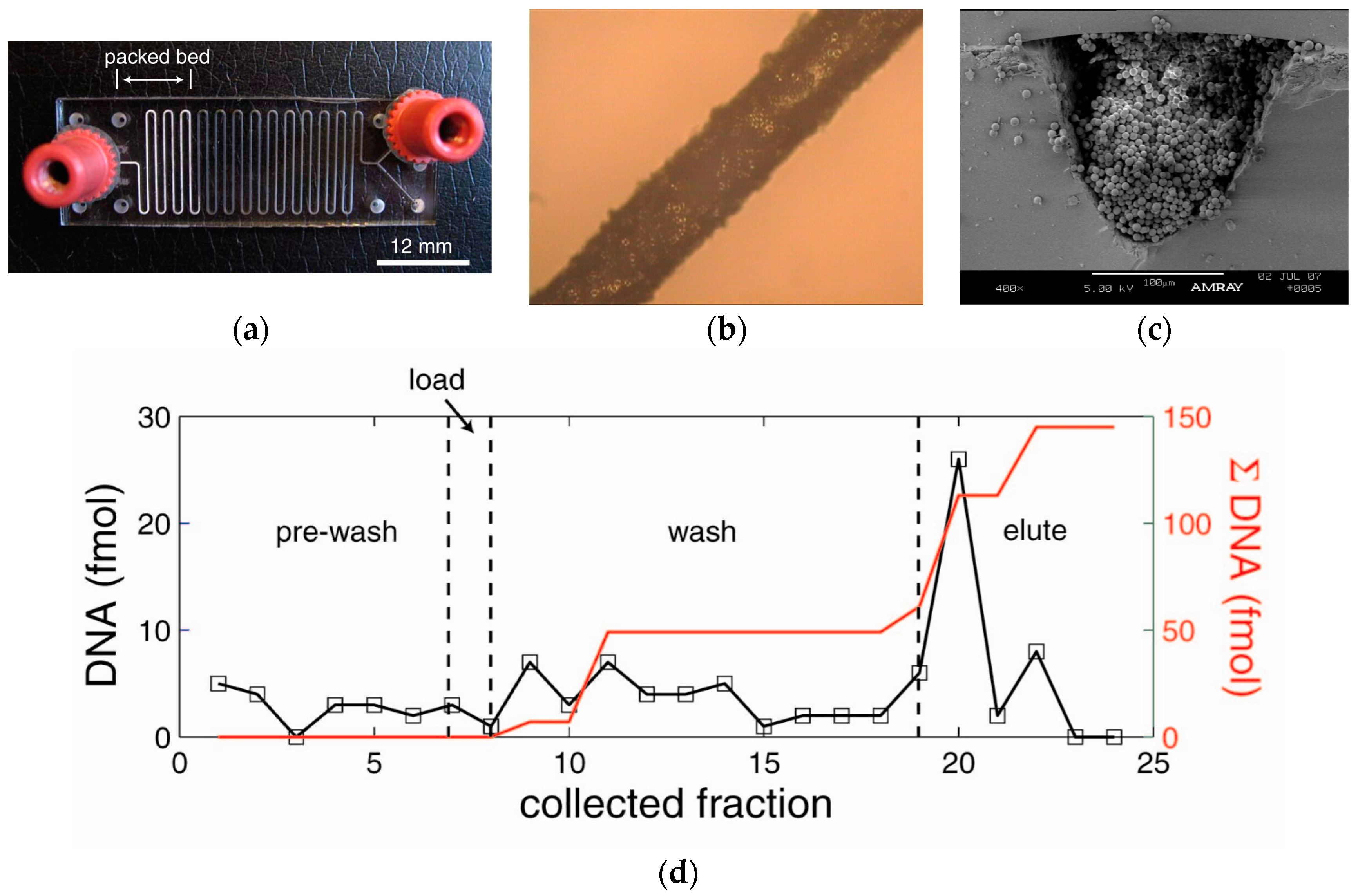
3.2. Sol-Gel Packed Microchannels
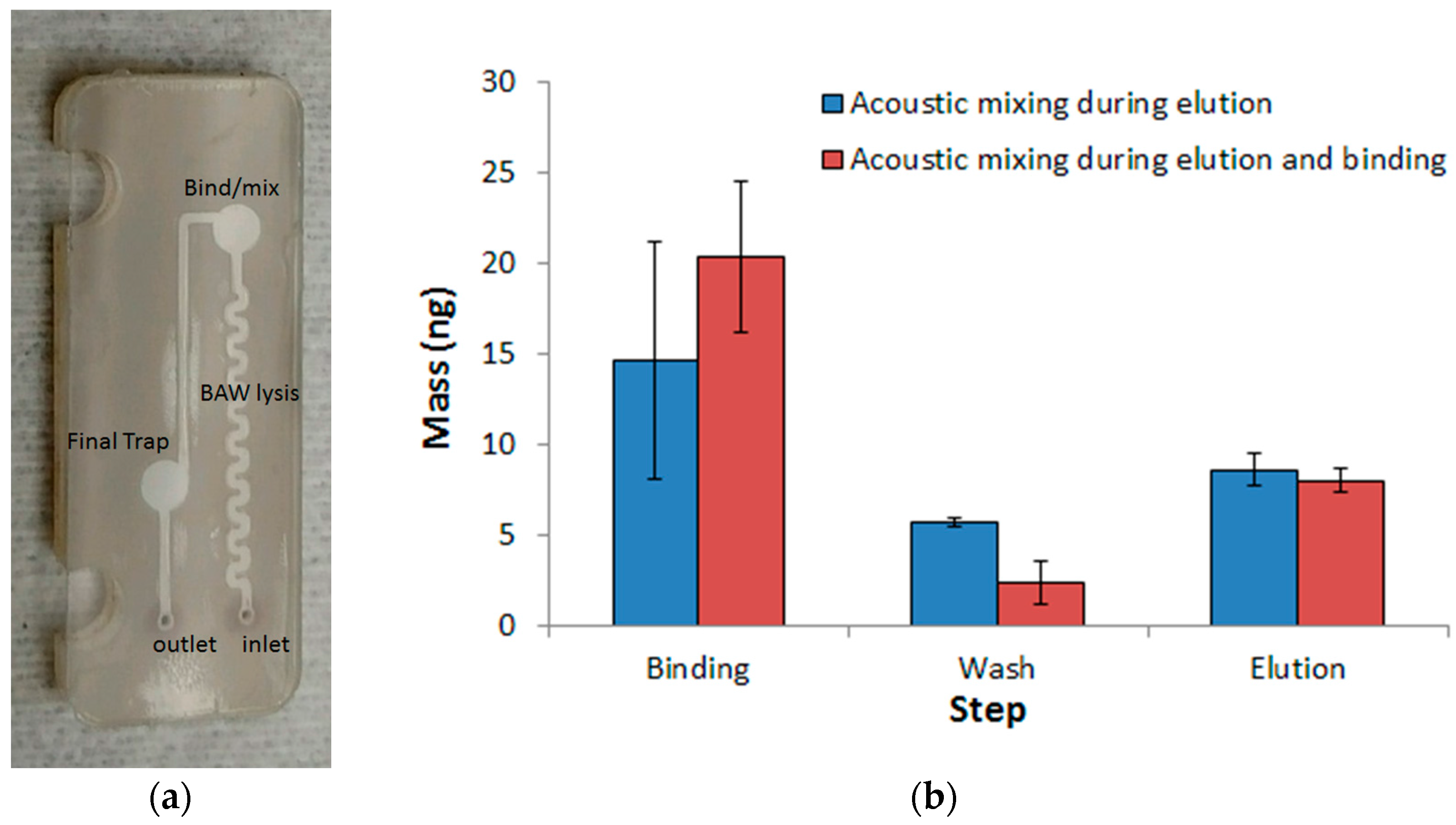
3.3. DNA Extraction Using Paramagnetic Beads with Acoustic Actuation
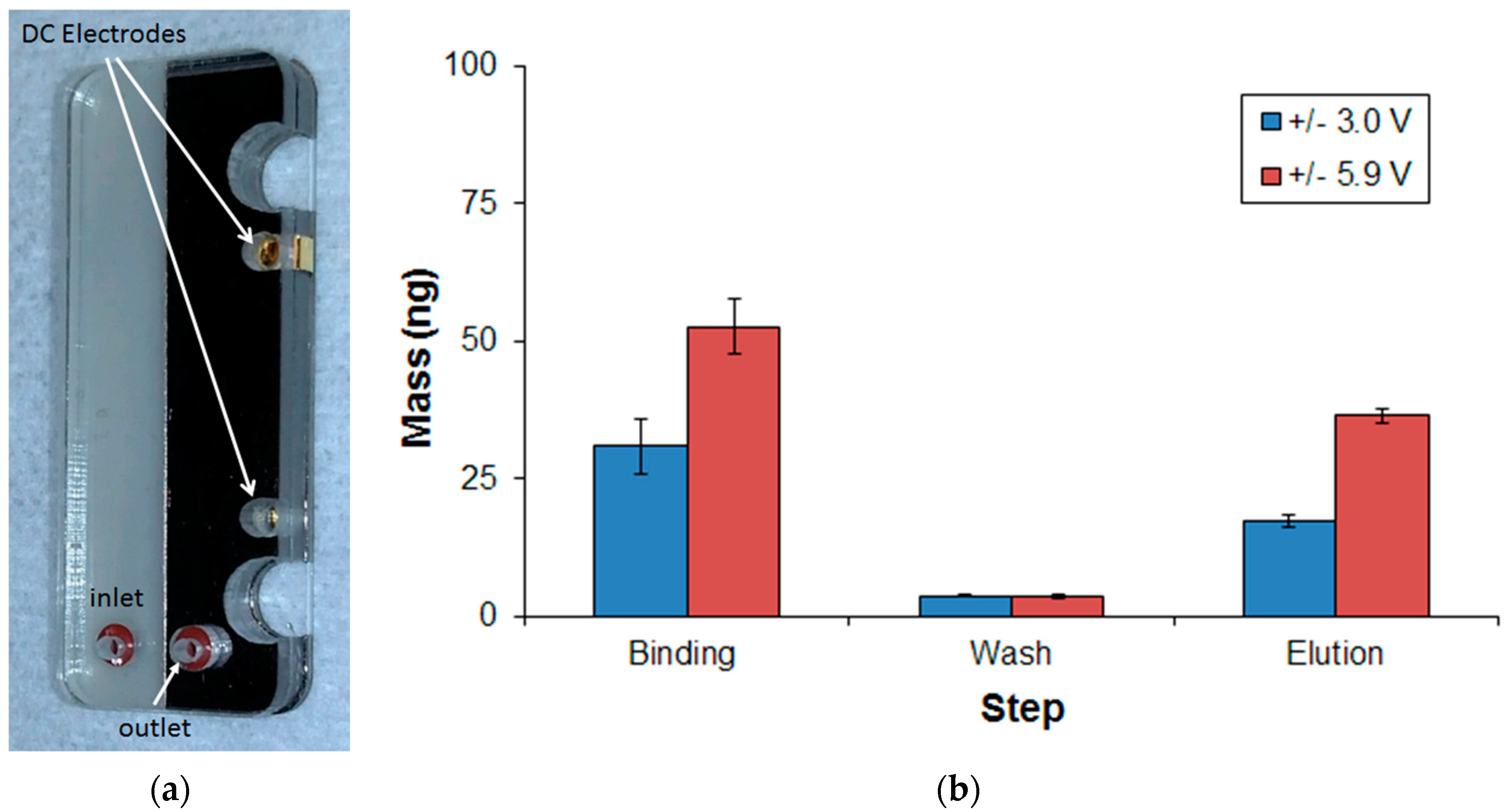
3.4. DNA Extraction Using Nafion-Based Cartridge
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix B
Appendix B.1. Fabrication of Acoustic Lysis and Paramagnetic Bead-Based Nucleic Acid Extraction Cartridges

Appendix B.2. Fabrication of Nafion-Based DNA Extraction Cartridges
References
- Linnarsson, S. Recent advances in DNA sequencing methods—General principles of sample preparation. Exp. Cell Res. 2010, 316, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Bruijns, B.; Asten, A.V.; Tiggelaar, R.; Gardeniers, H. Microfluidic devices for forensic DNA analysis: A review. Biosensors 2016, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hashimoto, K.; Yamaguchi, M. Liquid streaming by high-frequency ultrasonic waves. Jpn. J. Appl. Phys. 1996, 35, 3248–3250. [Google Scholar] [CrossRef]
- Kuske, C.R.; Banton, K.L.; Adorada, D.L.; Stark, P.C.; Hill, K.K.; Jackson, P.J. Small-scale DNA sample preparation method for field PCR detection of microbial cells and spores in soil. Appl. Environ. Microbiol. 1998, 64, 2463–2472. [Google Scholar] [PubMed]
- Oblath, E.A.; Henley, W.H.; Alarie, J.P.; Ramsey, J.M. A microfluidic chip integrating DNA extraction and realtime PCR for the detection of bacteria in saliva. Lab Chip 2013, 13, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.A.; Breadmore, M.C.; Ferrance, J.P.; Power, M.E.; Conroy, J.F.; Norris, P.M.; Landers, J.P. Toward a microchip-based solid-phase extraction method for isolation of nucleic acids. Electrophoresis 2002, 23, 727–733. [Google Scholar] [CrossRef]
- Tian, H.; Hühmer, A.F.R.; Landers, J.P. Evaluation of silica resins for direct and efficient extraction of DNA from complex biological matrices in a miniaturized format. Anal. Biochem. 2000, 283, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, S.; Yang, S.; Wang, T.-H. An all-in-one microfluidic device for parallel DNA extraction and gene analysis. Biomed. Microdevices 2010, 12, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Mauk, M.G.; Liu, C.; Sadik, M.; Bau, H.H. Microfluidic devices for nucleic acid (NA) isolation, isothermal NA amplification, and real-time detection. Methods Mol. Biol. 2015, 1256, 15–40. [Google Scholar] [PubMed]
- Zhu, K.; Jin, H.; He, Z.; Zhu, Q.; Wang, B. A continuous method for the large-scale extraction of plasmid DNA by modified boiling lysis. Nat. Protoc. 2006, 1, 3088–3093. [Google Scholar] [CrossRef] [PubMed]
- Privorotskaya, N.; Liu, Y.-S.; Lee, J.; Zeng, H.; Carlisle, J.A.; Radadia, A.; Millet, L.; Bashirbcd, R.; King, W.P. Rapid thermal lysis of cells using silicon-diamond microcantilever heaters. Lab Chip 2010, 10, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Mackey, B.M.; Miles, C.A.; Parsons, S.E.; Seymour, D.A. Thermal-denaturation of whole cells and cell components of Escherichia-coli examined by differential scanning calorimetry. J. Gen Microbiol. 1991, 137, 2361–2374. [Google Scholar] [CrossRef] [PubMed]
- Moroney, R.M.; White, R.M.; Howe, R.T. Ultrasonically induced microtransport. In Proceedings of the IEEE Micro Electro Mechanical Systems (MEMS’91), Nara, Japan, 30 January–2 February 1991; pp. 277–282. [Google Scholar]
- Ravula, S.K.; Branch, D.W.; Sigman, J.; Clem, P.G.; Brener, I. Integration of microfluidics and microacoustics components for miniature flow cytometry systems. Sens. Transducers J. 2007, 2007, 93–100. [Google Scholar]
- Andrade, M.A.; Skotis, G.D.; Ritchie, S.; Cumming, D.R.; Riehle, M.O.; Bernassau, A.L. Contactless acoustic manipulation and sorting of particles by dynamic acoustic fields. IEEE Ultrason. Ferro. Freq. Control 2016, 63, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Goto, H.; Matsumoto, M.; Maeda, R. Active micromixer for microfluidic systems using lead-zirconate-titanate (PZT)-generated ultrasonic vibration. Electrophoresis 2000, 21, 116–119. [Google Scholar] [CrossRef]
- Cular, S.; Branch, D.W.; Bhethanbotla, V.R.; Meyer, G.D.; Craighead, H.G. Removal of nonspecifically bound proteins on microarrays using surface acoustic waves. IEEE Sens. 2008, 8, 314–320. [Google Scholar] [CrossRef]
- Meyer, G.D.; Moran-Mirabal, J.M.; Branch, D.W.; Craighead, H.G.C. Nonspecific binding removal from protein microarrays using thickness shear mode resonators. IEEE Sens. 2006, 6, 254–261. [Google Scholar] [CrossRef]
- Belgrader, P.; Hansford, D.; Kovacs, G.T.A.; Venkateswaran, K.; R. Mariella, J.; Milianovich, F.; Nasarabadi, S.; Okuzumi, M.; Pourahmadi, F.; Northrup, M.A. A minisonicator to rapidly disrupt bacterial spores for DNA analysis. Anal. Chem. 1999, 71, 4232–4236. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.P.; Brown, J.; Bruckner-Lea, C.J.; Olson, L.; Posakony, G.J.; Stults, J.R.; Valentine, N.B.; Bond, L.J. Continous spore disruption using radially focused, high-frequency ultrasound. Anal. Chem. 2001, 73, 3784–3789. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, H.; Yaralioglu, G.G.; Ergun, A.S.; Degertekin, F.L.; Khuri-Yakub, B.T. Micro-fluidic channels with integrated ultrasonic transducers. In Proceedings of the 2001 IEEE Ultrasonics Symposium, Atlanta, GA, USA, 7–10 October 2001; pp. 859–862. [Google Scholar]
- Hughes, D.E.; Nyborg, W.L. Cell disruption by ultrasound. Science 1962, 138, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Esche, R. Untersuchung der schwingungskavitation in flussigkeiten. Acta Acust. United Acust. 1952, 2, 208–218. [Google Scholar]
- Wang, L.T.; Li, Y.J.; Lin, A.; Choe, Y.; Gross, M.E.; Kim, E.S. A self-focusing acoustic transducer that exploits cytoskeletal differences for selective cytolysis of cancer cells. J. Microelectromech. Syst. 2013, 22, 542–552. [Google Scholar] [CrossRef]
- Zhu, J.; Qiu, X.; Oiler, J.; Yu, C.; Wang, Z.; Lee, C.; Hsiai, T.K.; Kim, E.S.; Yu, H. Localized cell lysis by self focused acoustic transducers. Proceedings of 2009 International Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS 2009), Denver, CO, USA, 21–25 June 2009; pp. 608–611. [Google Scholar]
- Marentis, T.C.; Kusler, B.; Yaralioglu, G.G.; Liu, S.; Hæggstöm, E.O.; Kuri-Yakub, B.T. Microfluidic sonicator for real-time disruption of eukaryotic cells and bacterial spores for DNA analysis. Ultrasound Med. Biol. 2005, 31, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Tandiono, T.; Ow, D.S.; Driessen, L.; Chin, C.S.; Klaseboer, E.; Choo, A.B.; Ohl, S.W.; Ohl, C.D. Sonolysis of Escherichia coli and pichia pastoris in microfluidics. Lab Chip 2012, 12, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; White, R.M. Acoustic streaming in micromachined flexural plate wave devices: Numerical simulation and experimental verification. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Suzuki, T.; Shiokawa, S. Investigation of acoustic streaming excited by surface acoustic waves. In Proceedings of the 1995 IEEE Ultrasonics Symposium, Seattle, WA, USA, 7–10 November 1995; pp. 1081–1084. [Google Scholar]
- Wang, W.; Chen, Y.; Umar, F.; Xuan, W.; Hao, J.; Shurong, D.; Jikui, L. Ultrafast chemical-free cell lysis by high speed stream collision induced by surface acoustic waves. Appl. Phys. Lett. 2017, 110, 143504. [Google Scholar] [CrossRef]
- Reboud, J.; Bourquin, Y.; Wilson, R.; Pall, G.S.; Jiwaji, M.; Pitt, A.R.; Graham, A.; Waters, A.P.; Cooper, J.M. Shaping acoustic fields as a toolset for microfluidic manipulations in diagnostic technologies. Proc. Natl. Acad. Sci. USA 2012, 109, 15162–15167. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Reyhani, A.; Gesellchen, F.; Mampallil, D.; Wilson, R.; Reboud, J.; Ces, O.; Willison, K.R.; Cooper, J.M.; Klug, D.R. Chemical-free lysis and fractionation of cells by use of surface acoustic waves for sensitive protein assays. Anal. Chem. 2015, 87, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Branch, D.W.; Smith, G.T.; Vreeland, E.C.; Blakemore, R.; Alland, D. Nucleic acid extraction using a rapid, chemical free, ultrasonic technique for Point-of-Care diagnostics. In Proceedings of the IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 501–506. [Google Scholar]
- Valier-Brasier, T.; Conoir, J.-M.; Coulouvrat, F.; Thomas, J.-L. Sound propagation in dilute suspensions of spheres: Analytical comparison between coupled phase model and multiple scattering theory. J. Acoust. Soc. Am. 2015, 138, 2598–2612. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, J.N.; Kim, J.; Kim, J.H. Reversible capture of genomic DNA by a nafion-coated electrode. Anal. Biochem. 2008, 380, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Royer, D.; Dieulesaint, E. Elastic Waves in Solids II: Generation, Acousto-Optic Interaction, Applications; Springer: Berlin, Germany, 1999; p. 446. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branch, D.W.; Vreeland, E.C.; McClain, J.L.; Murton, J.K.; James, C.D.; Achyuthan, K.E. Rapid Nucleic Acid Extraction and Purification Using a Miniature Ultrasonic Technique. Micromachines 2017, 8, 228. https://doi.org/10.3390/mi8070228
Branch DW, Vreeland EC, McClain JL, Murton JK, James CD, Achyuthan KE. Rapid Nucleic Acid Extraction and Purification Using a Miniature Ultrasonic Technique. Micromachines. 2017; 8(7):228. https://doi.org/10.3390/mi8070228
Chicago/Turabian StyleBranch, Darren W., Erika C. Vreeland, Jamie L. McClain, Jaclyn K. Murton, Conrad D. James, and Komandoor E. Achyuthan. 2017. "Rapid Nucleic Acid Extraction and Purification Using a Miniature Ultrasonic Technique" Micromachines 8, no. 7: 228. https://doi.org/10.3390/mi8070228



