Micromachining on and of Transparent Polymers for Patterning Electrodes and Growing Electrically Active Cells for Biosensor Applications
Abstract
:1. Introduction
2. Materials and Methods
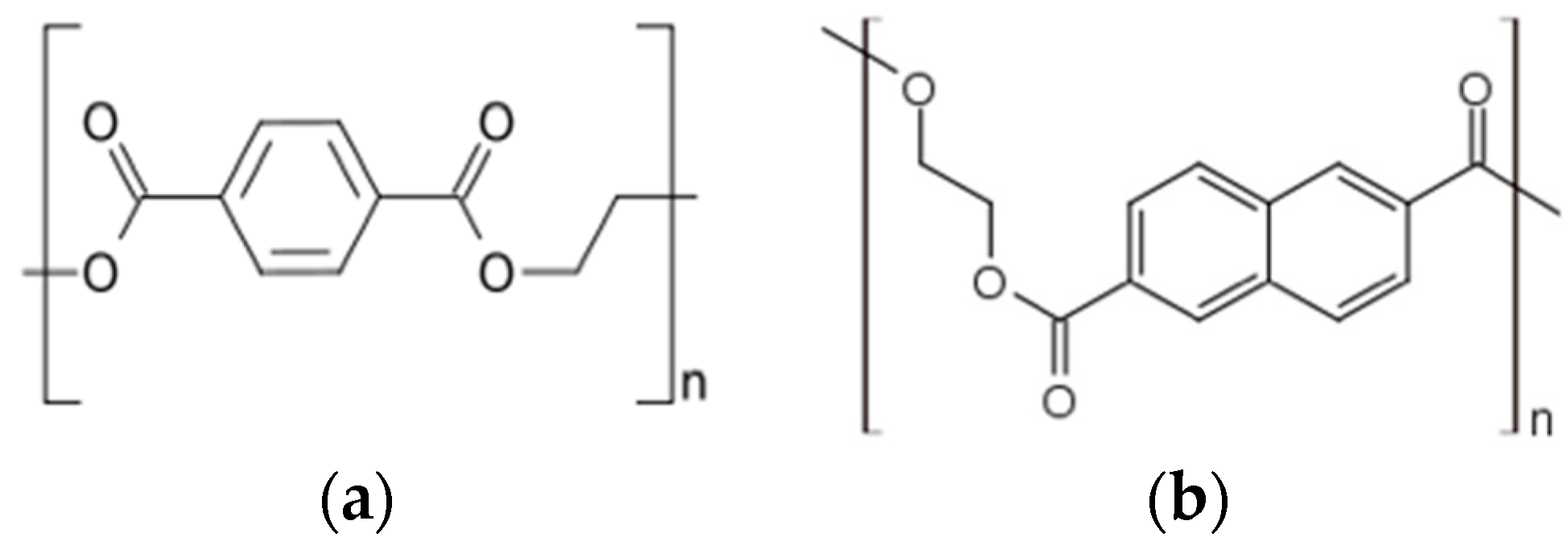
2.1. Design of PEN-Based Biosensor Arrays
2.2. Design of PET-Based Biosensor Arrays
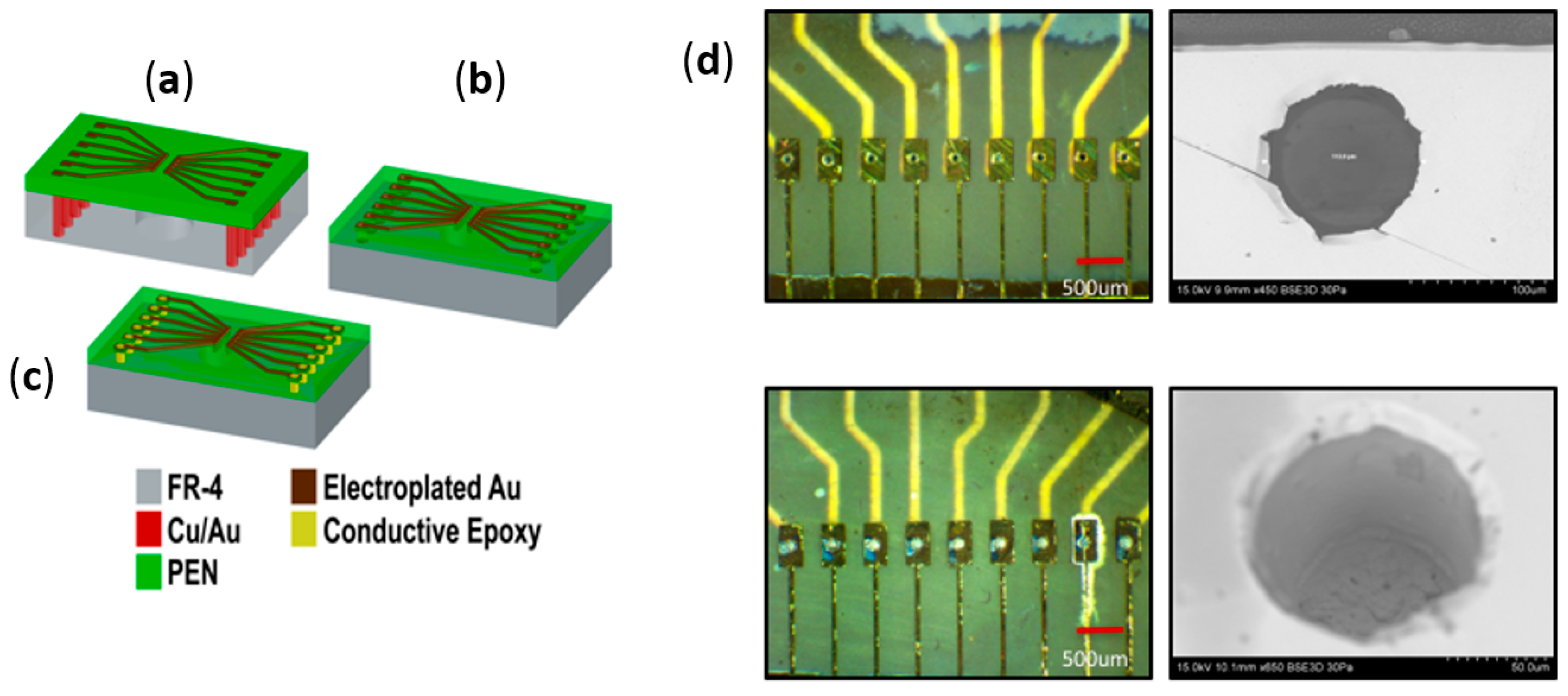
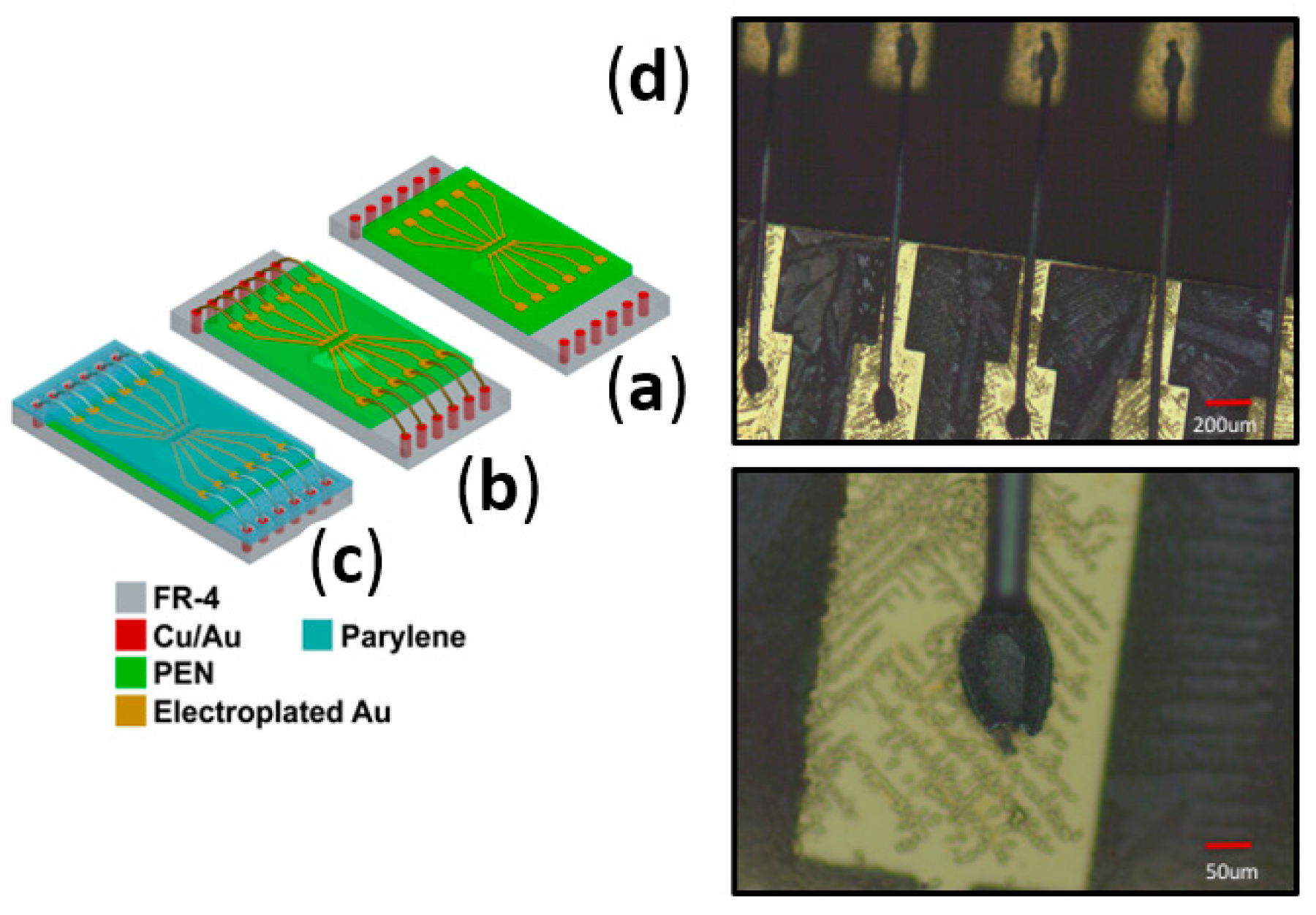
2.3. Microfabrication and Assembly Technologies for PEN-Based Biosensor Arrays
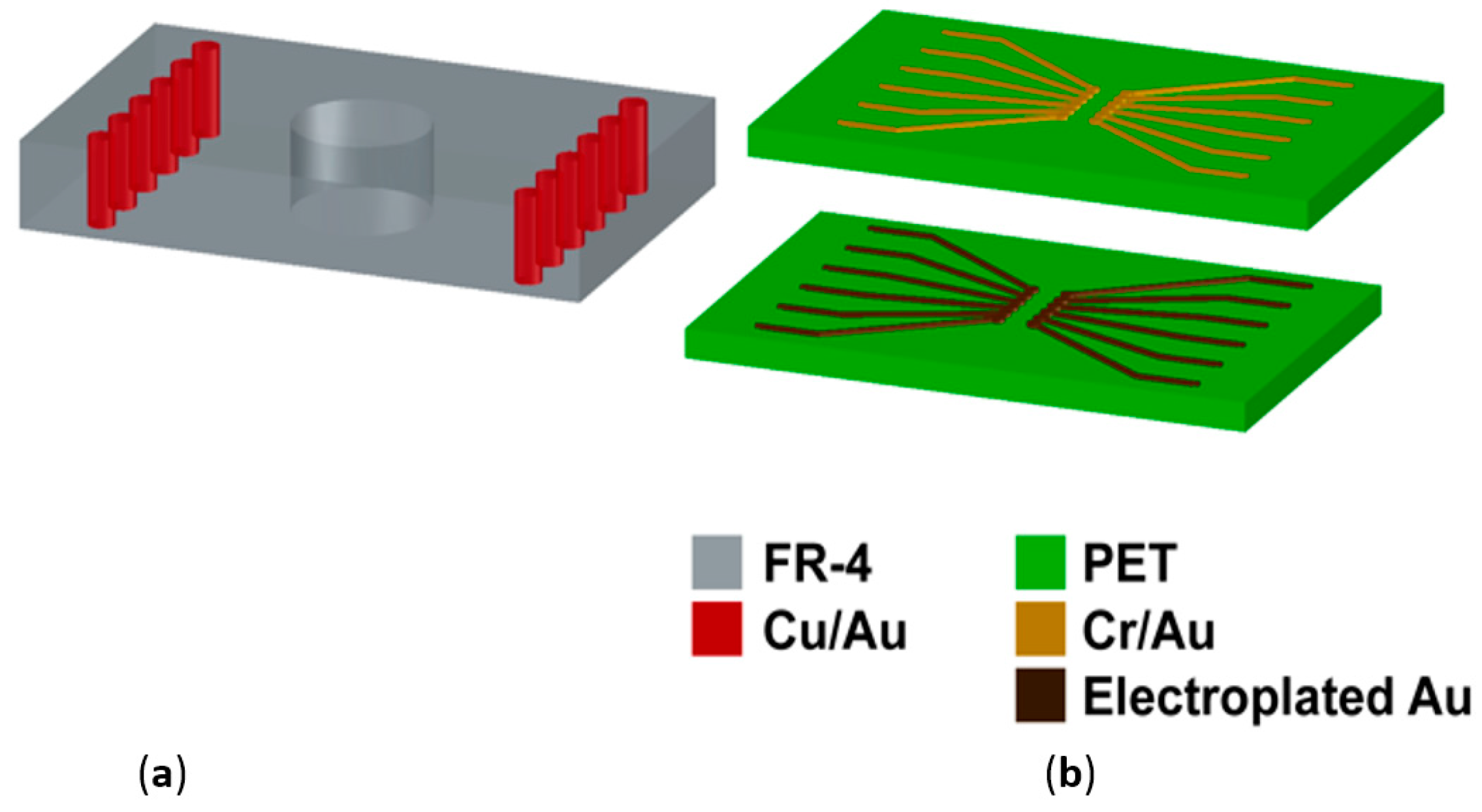
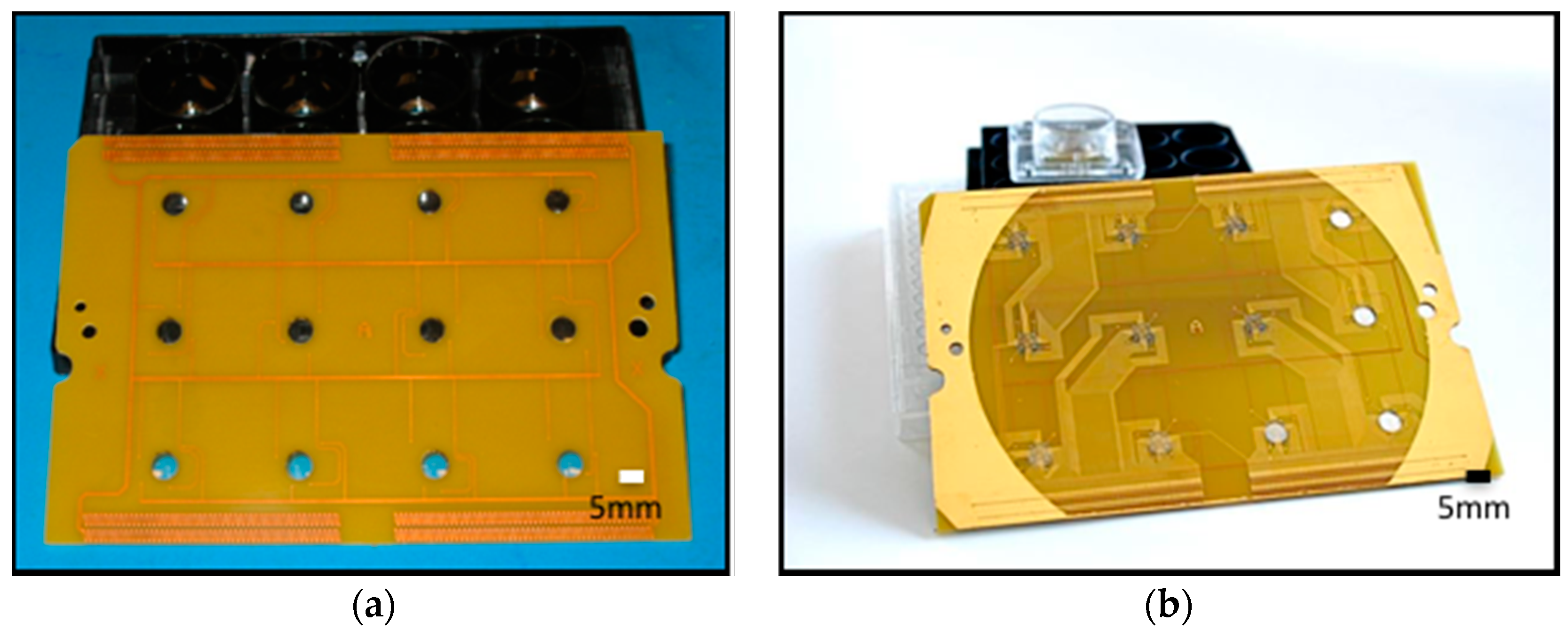
2.3.1. Printed Circuit Board (PCB) Design and Fabrication
2.3.2. Definition of Microelectrodes on PEN Substrates
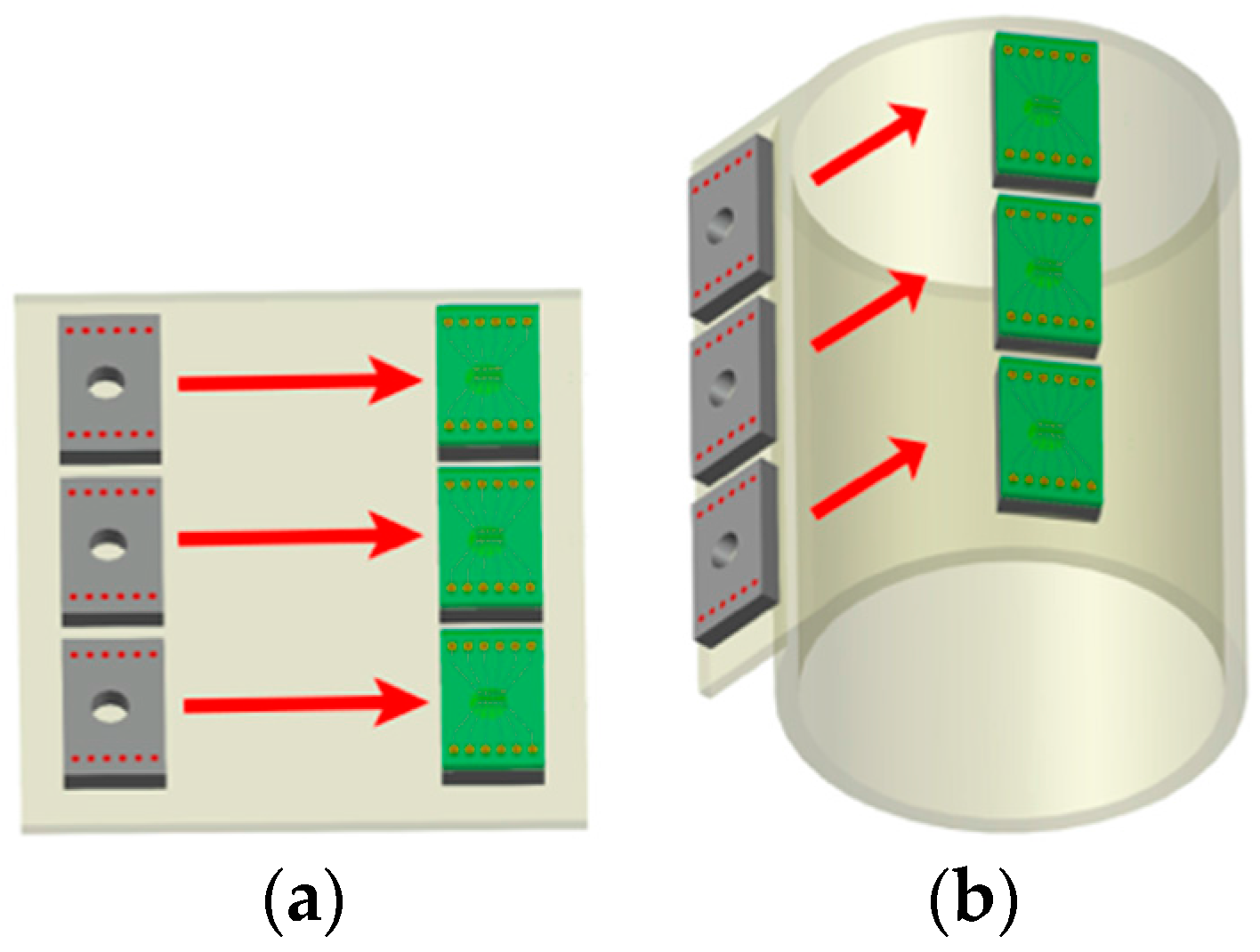
2.3.3. Inkjet-Printed Vias for Assembly of PEN Biosensors
2.3.4. Wire Bonded Interconnects for the Assembly of PEN Biosensors
2.3.5. Definition of Insulation for PEN Biosensors
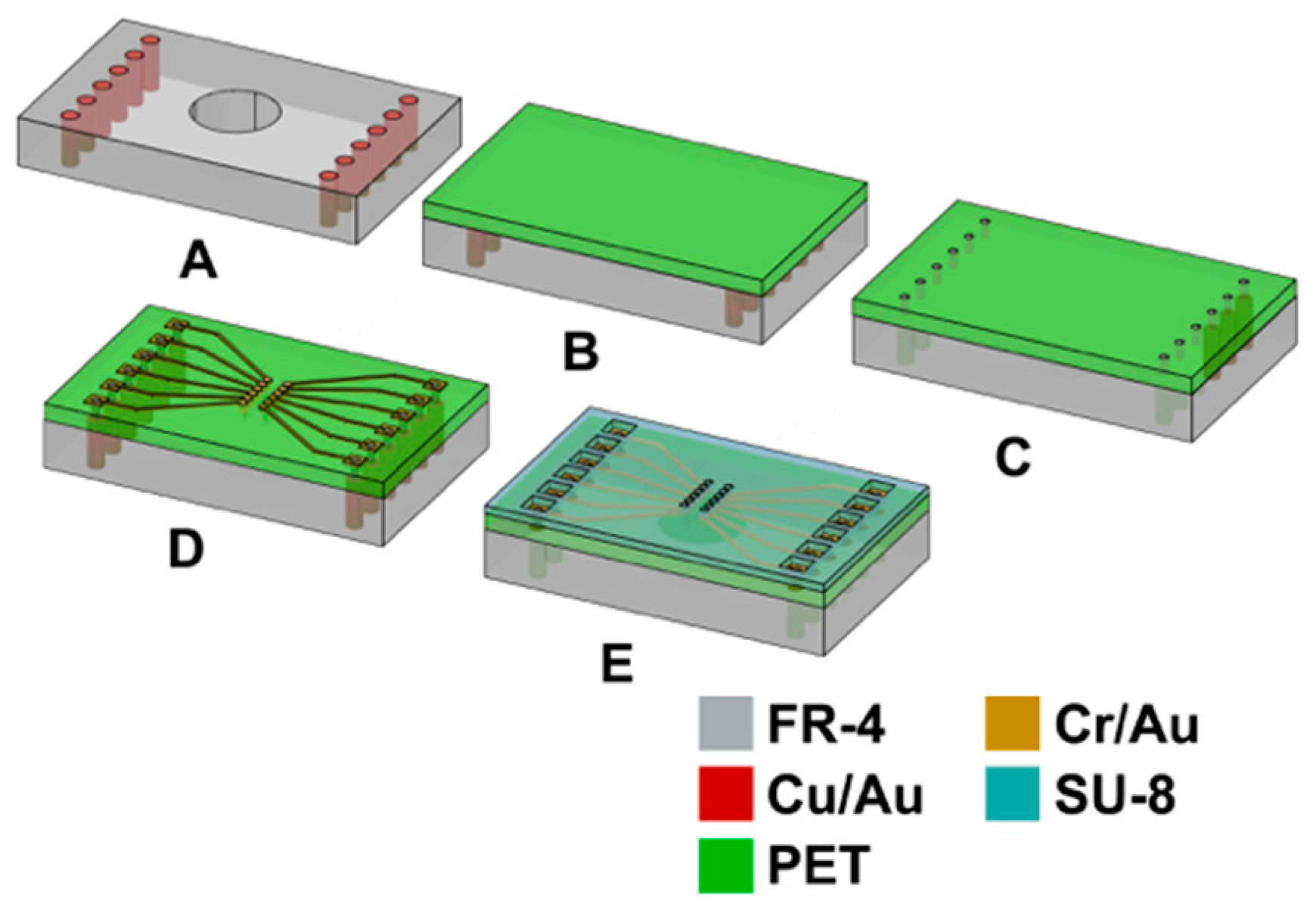
2.4. Microfabrication and Assembly Technologies for PET-Based Biosensor Arrays
2.4.1. Printed Circuit Board Design and Fabrication
2.4.2. Post Processing of PET PCB for Definition of Microelectrodes
2.4.3. Assembly Process Development for PET Biosensors
2.5. Characterization Methods for PEN-Based Biosensors
2.5.1. Metal Trace Width Measurement
2.5.2. Metal Integrity Characterization
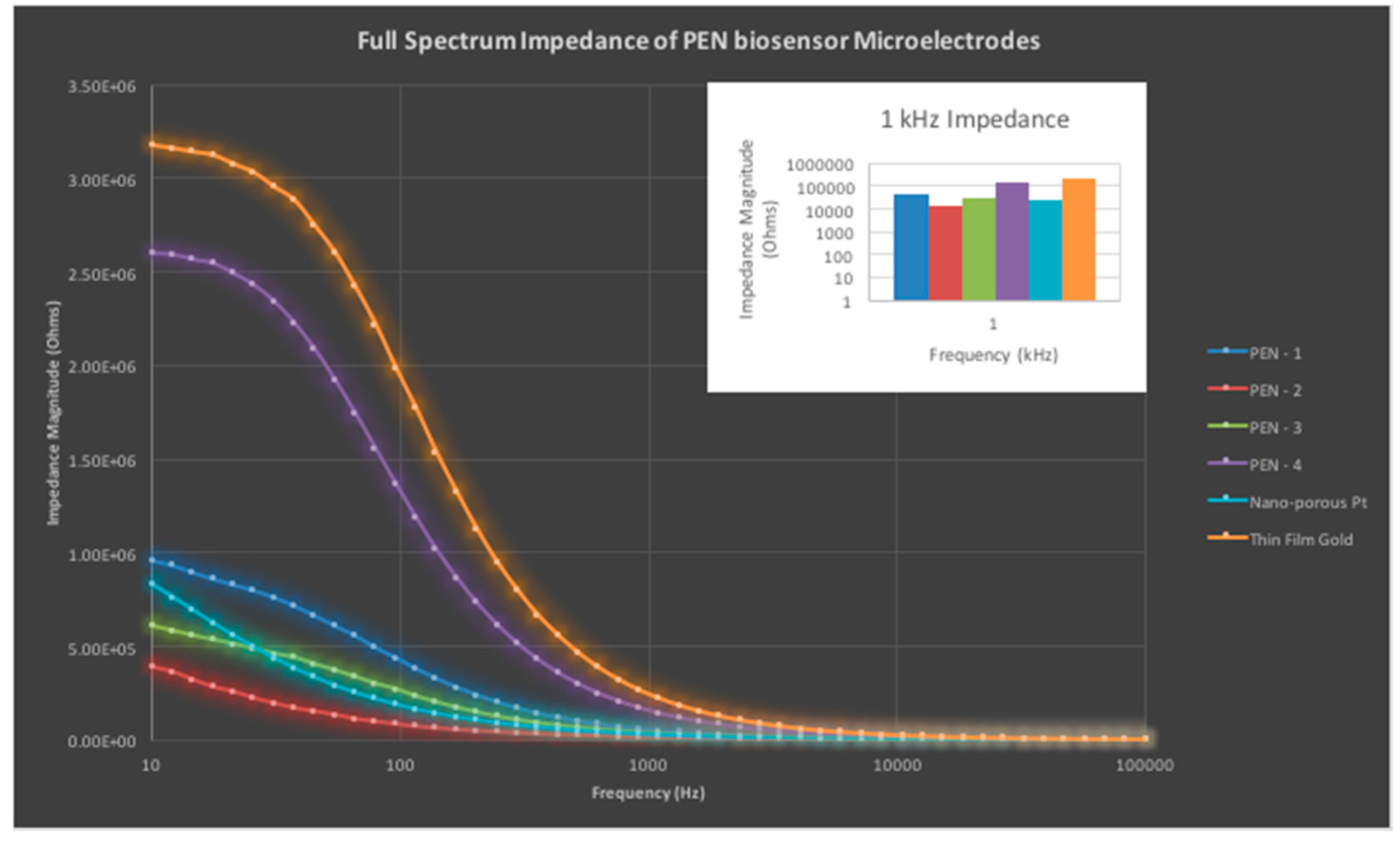
2.5.3. Impedance Characterization of PEN Biosensors
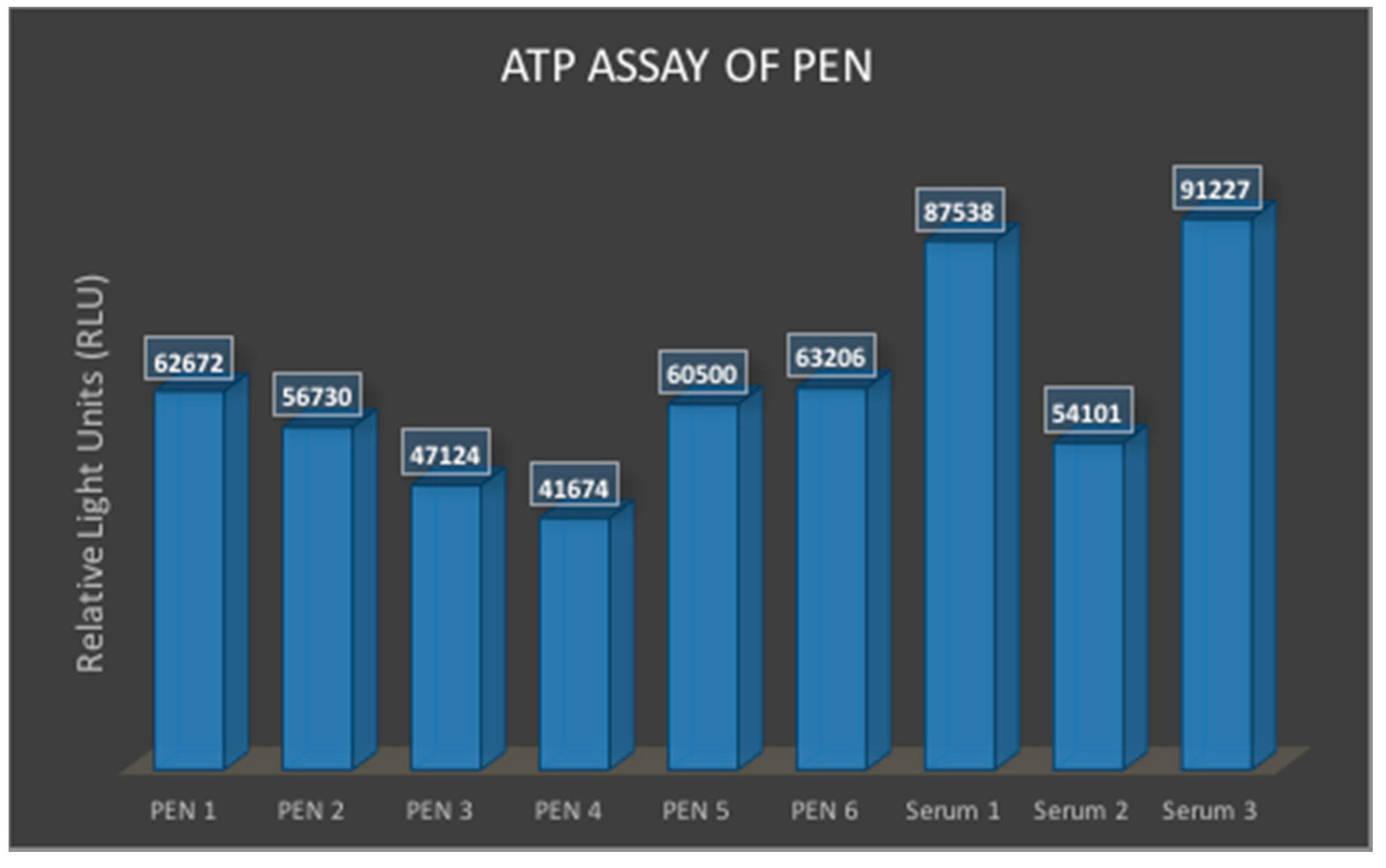
2.5.4. ATP Assay for Cytocompatibility of the PEN Biosensors
2.6. Characterization Methods for PET-Based Biosensor Arrays
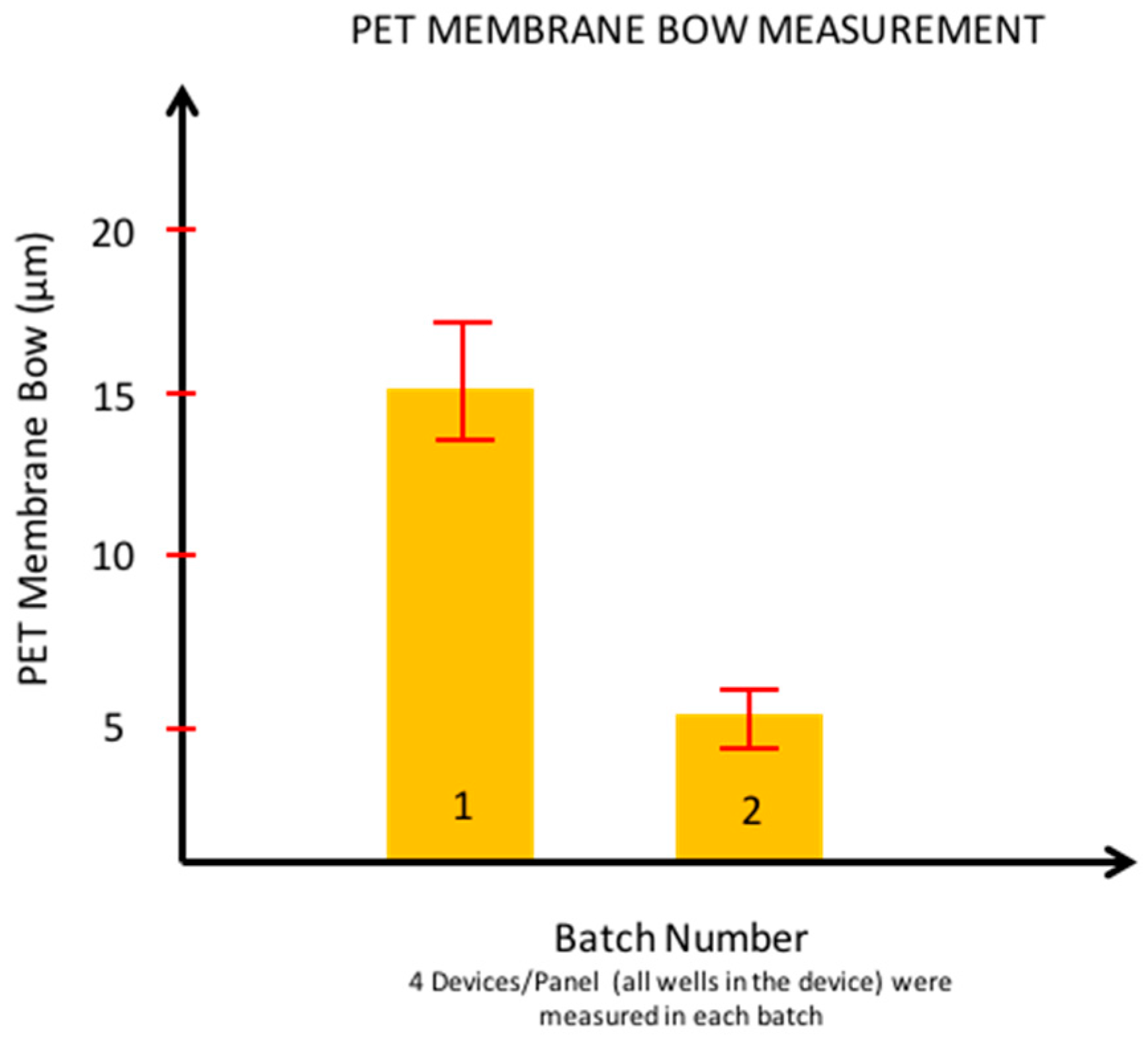
2.6.1. Membrane Bow Measurement of PET-Based Biosensors
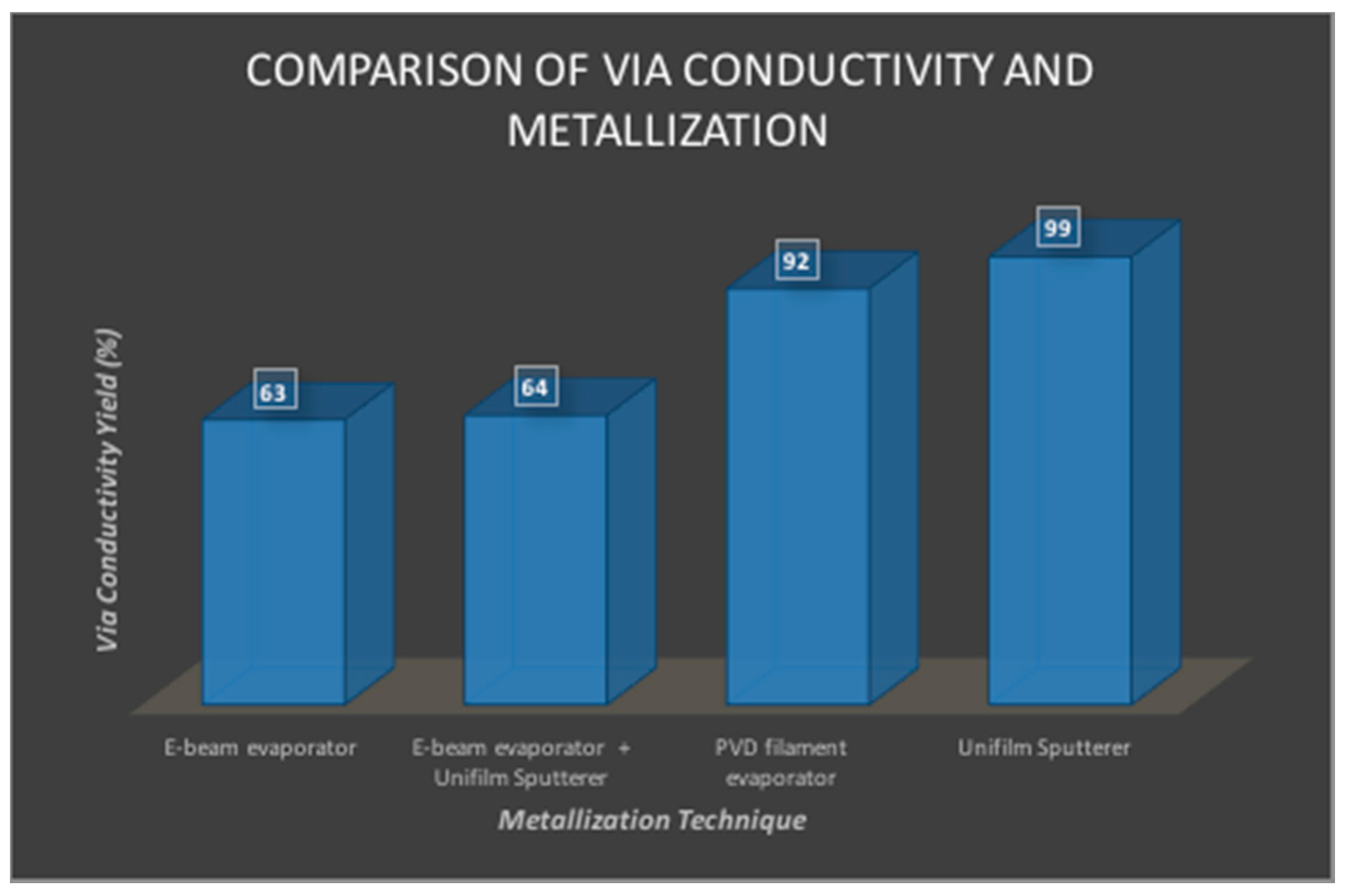
2.6.2. Via Yield Measurements
2.6.3. Metal Integrity Characterization
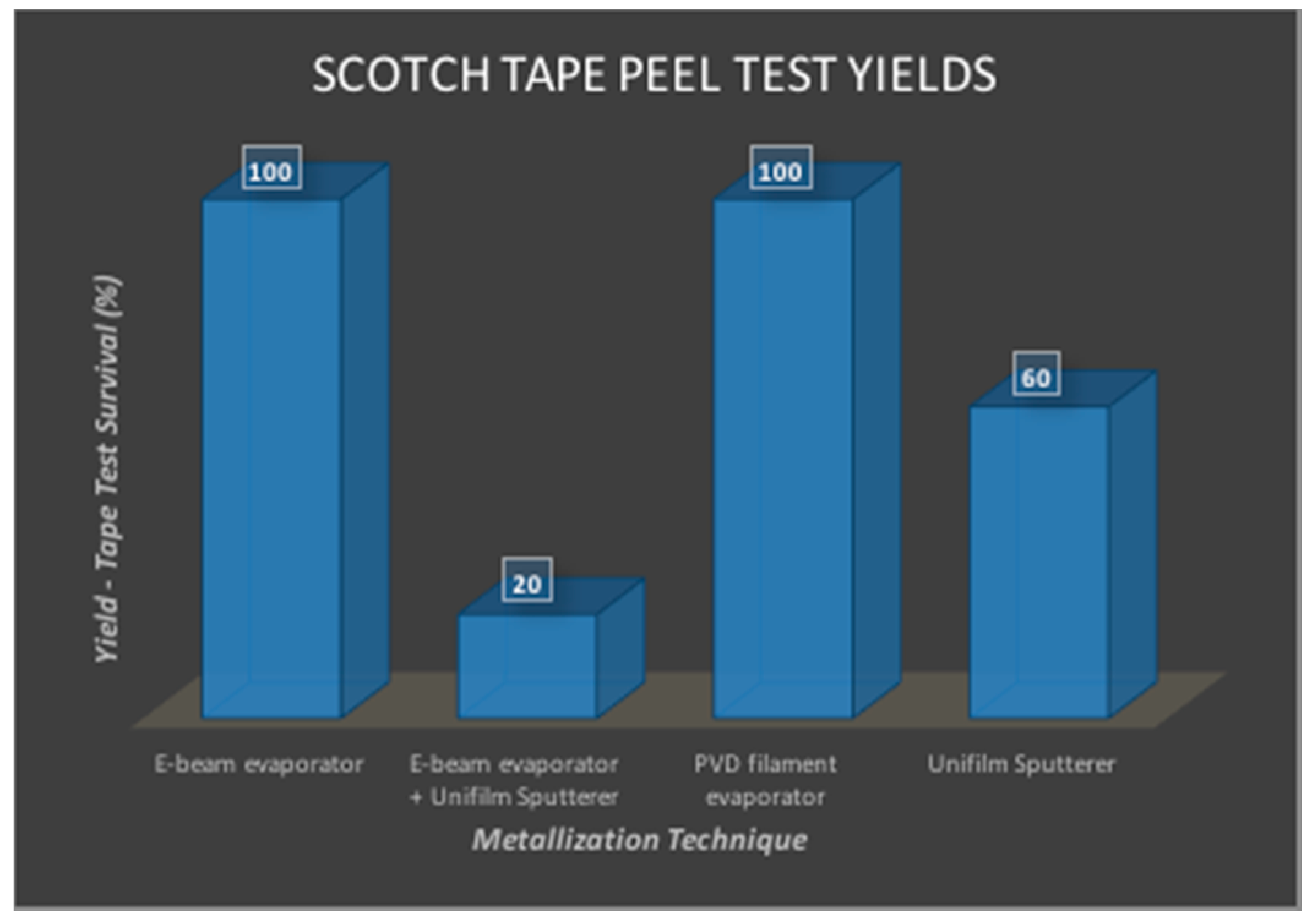
2.6.4. Nano-Porous Platinum Electroplating
2.6.5. Optical Assay for Measurement of Transparency
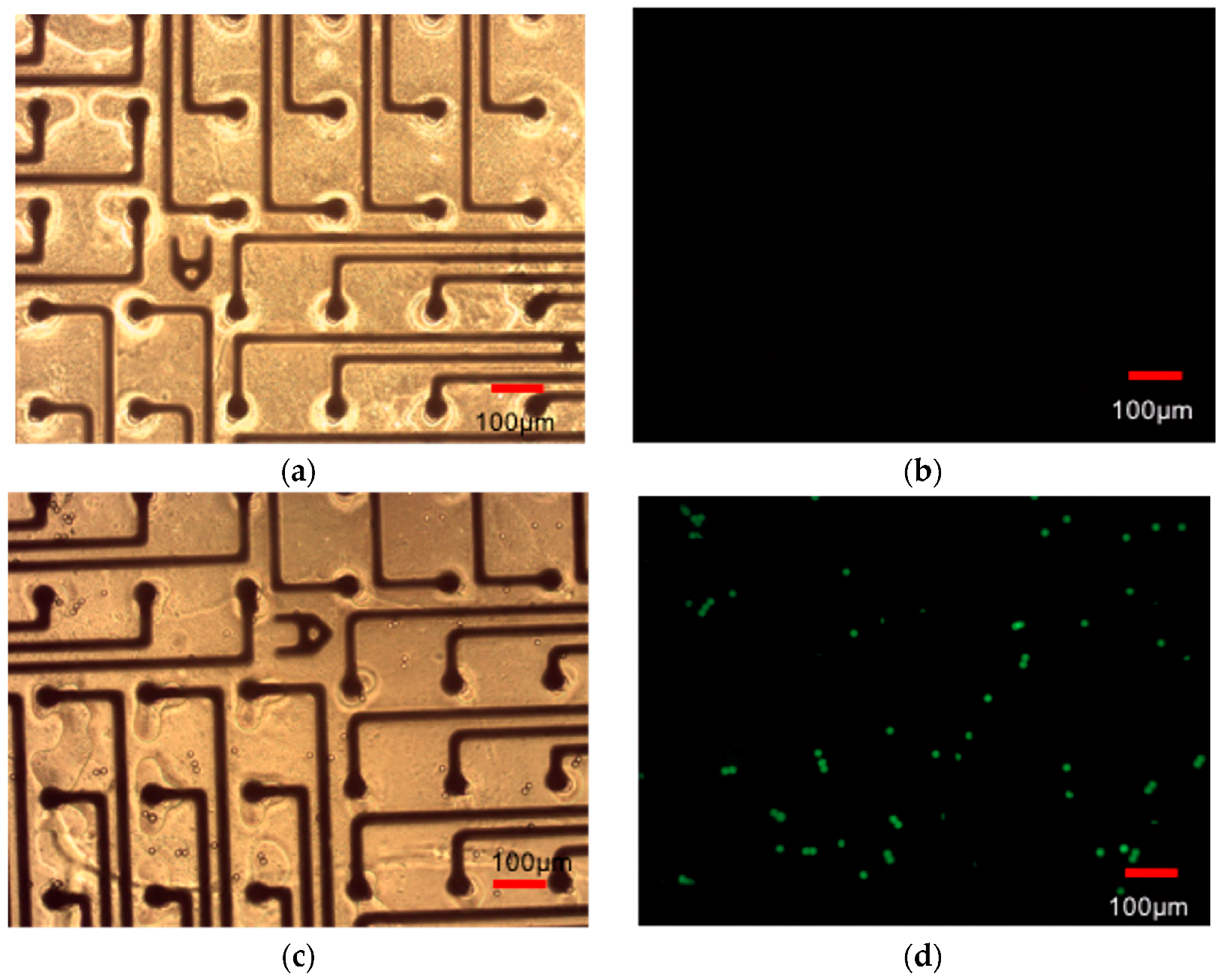
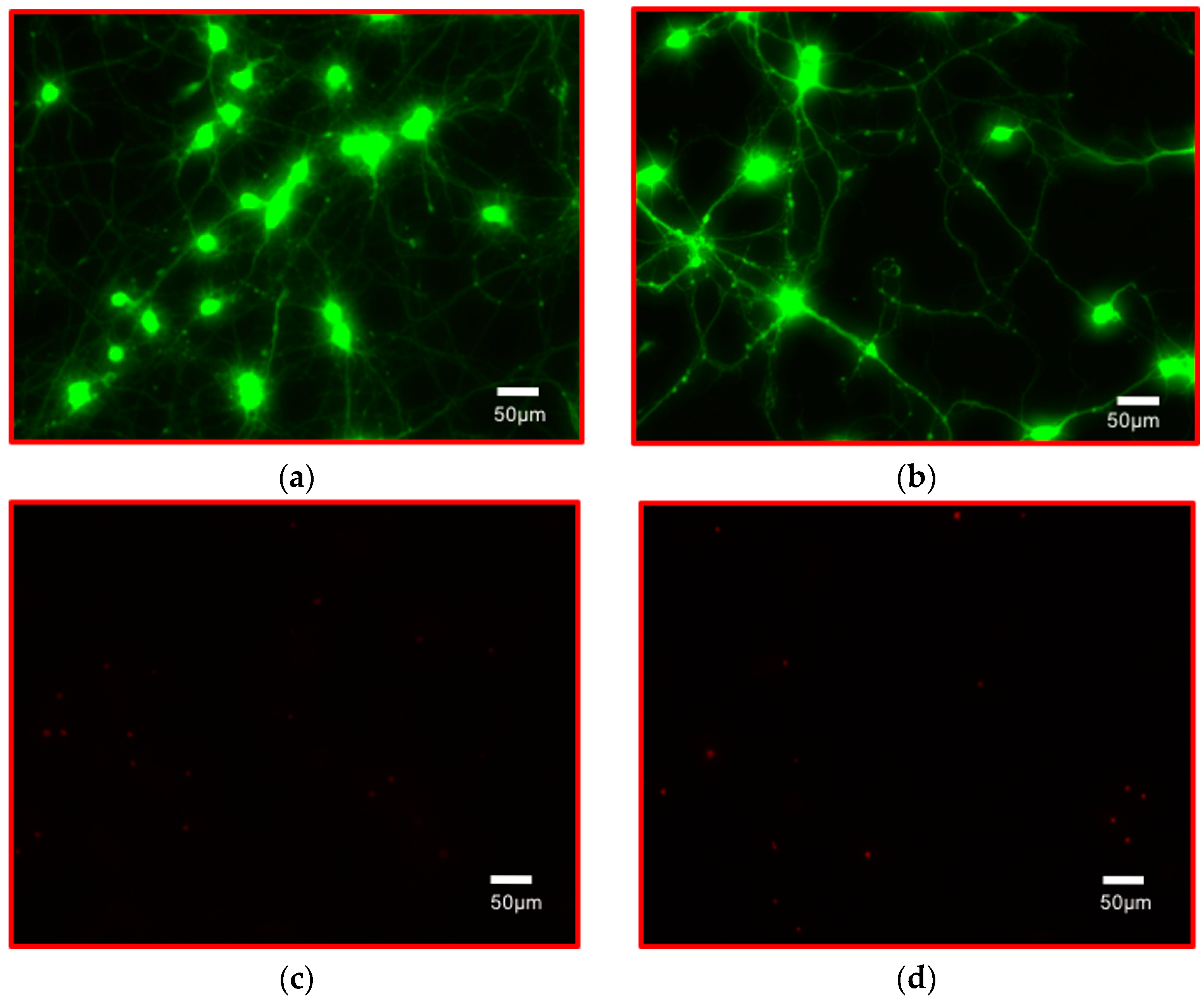
2.6.6. Growing Electrically Active Cells on PET-Based Biosensor Arrays
3. Results and Discussion
3.1. Characterization of PEN-Based Biosensor Arrays
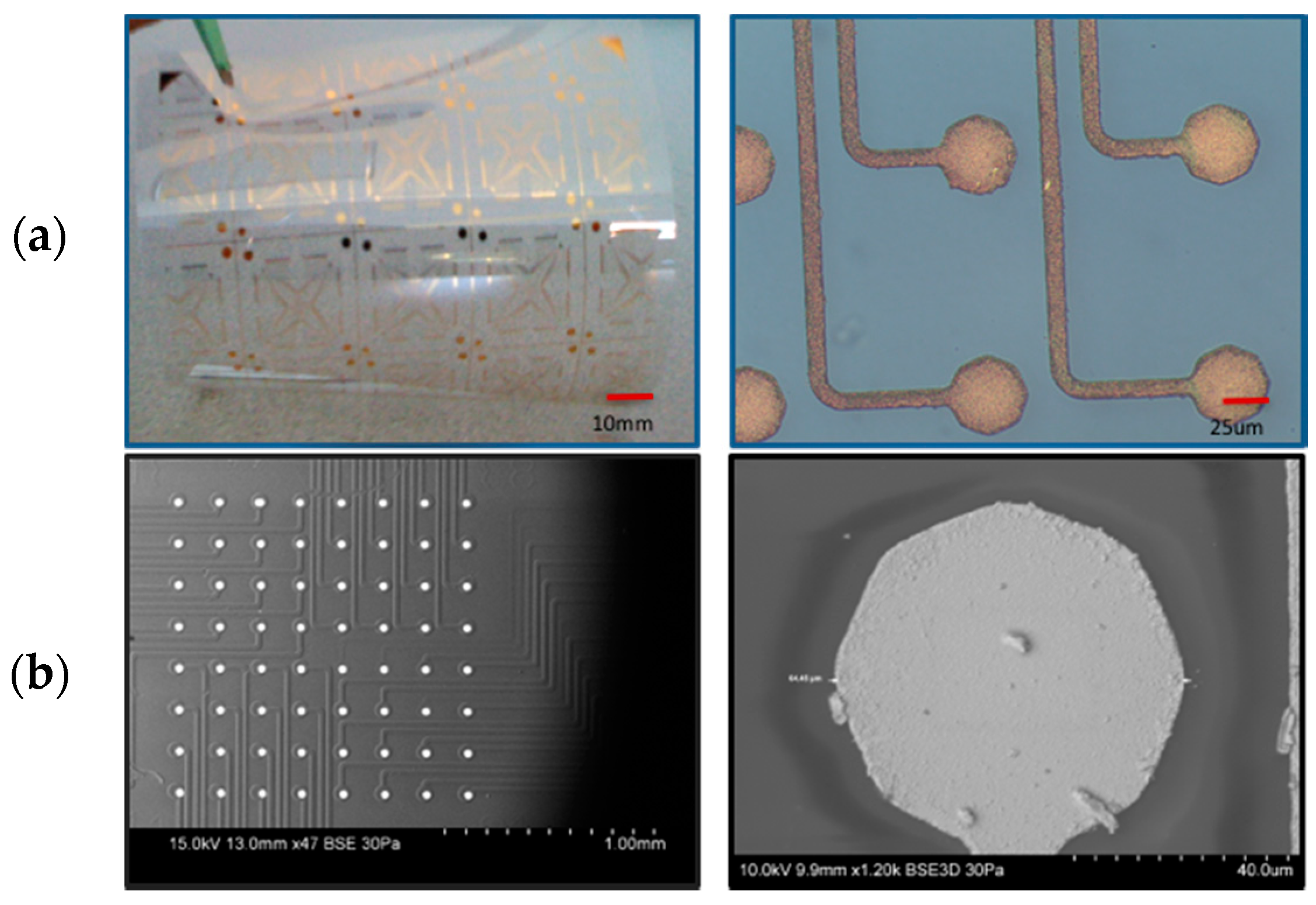
3.1.1. Metal Trace Width Characterization
3.1.2. Metal Integrity Characterization
3.1.3. Impedance Characterization of PEN Biosensors
3.1.4. ATP Assay for Cytocompatibility of PEN
3.2. Characterization of PET-Based Biosensor Arrays
3.2.1. Membrane Bow Measurement for PET-Based Biosensors
3.2.2. Via Yield Measurements
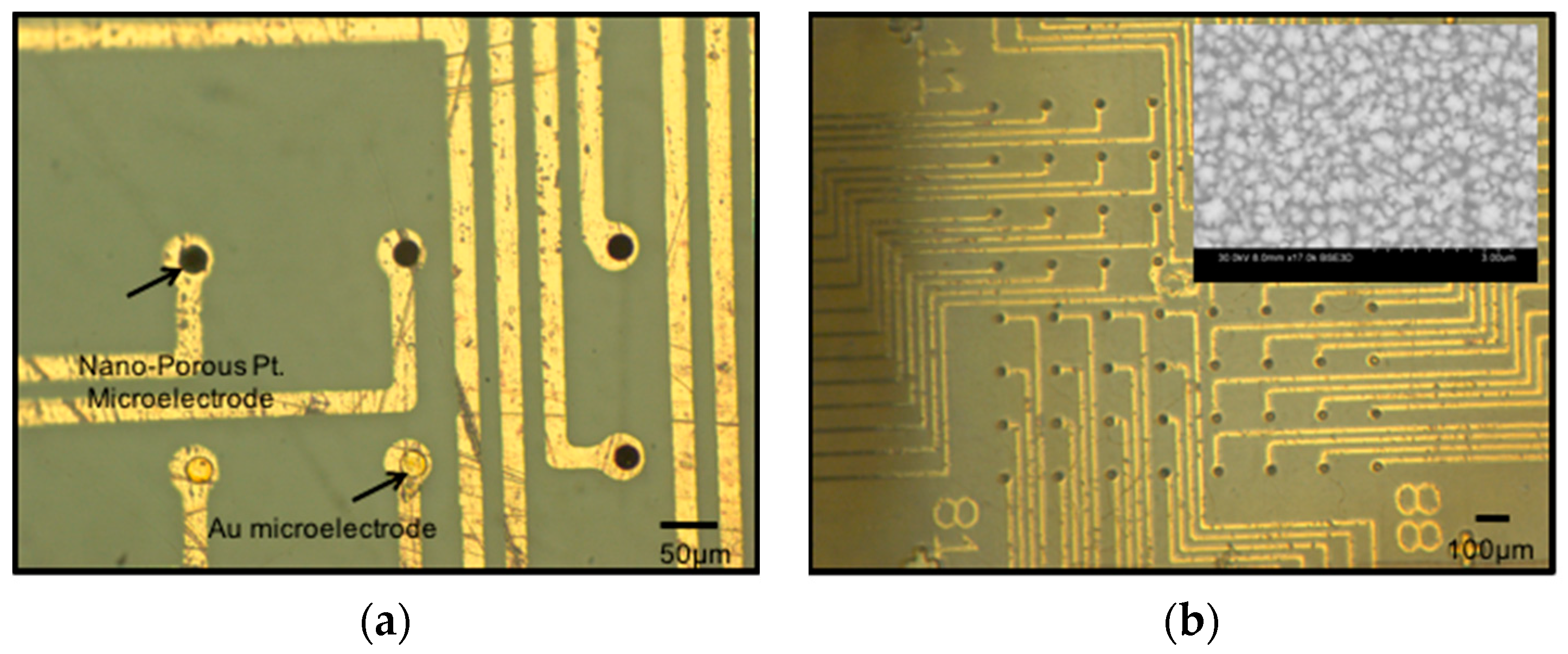
3.2.3. Metal Integrity Characterization
3.2.4. Nano-Porous Platinum Electroplating
3.2.5. Optical Assay for Measurement of Transparency
3.2.6. Growing Electrically Active Cells on PET-Based Biosensor Arrays
3.3. Discussion and Significance of the Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morin, F.; Nishimura, N.; Griscom, L.; LePioufle, B.; Fujita, H.; Takamura, Y.; Tamiya, E. Constraining the connectivity of neuronal networks cultured on microelectrode arrays with microfluidic techniques: A step towards neuron-based functional chips. Biosens. Bioelectron. 2006, 21, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.R.; McClain, M.A.; Ross, J.; LeFew, W.R.; Shafer, T.J. Evaluation of multi-well microelectrode arrays for neurotoxicity screening using a chemical training set. Neurotoxicology 2012, 33, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Lignani, G.; Ferrea, E.; Difato, F.; Amarù, J.; Ferroni, E.; Lugarà, E.; Espinoza, S.; Gainetdinov, R.R.; Baldelli, P.; Benfenati, F. Long-term optical stimulation of channelrhodopsin-expressing neurons to study network plasticity. Front. Mol. Neurosci. 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Chiappalone, M.; Bove, M.; Vato, A.; Tedesco, M.; Martinoia, S. Dissociated cortical networks show spontaneously correlated activity patterns during in vitro development. Brain Res. 2006, 1093, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, K.H.; Barker, V.N.; Fletcher, L.E.; DeBusschere, B.D.; Ghanouni, P.; Giovangrandi, L.; Kovacs, G.T. General purpose, field-portable cell-based biosensor platform. Biosens. Bioelectron. 2001, 16, 557–564. [Google Scholar] [CrossRef]
- Boppart, S.A.; Wheeler, B.C.; Wallace, C.S. A flexible perforated microelectrode array for extended neural recordings. IEEE Trans. Biomed. Eng. 1992, 39, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.A.; Springer, P.A.; Loeb, G.E.; Berwald-Netter, Y.; Okun, L.M. Miniature microelectrode array to monitor bioelectric activity of cultured cells. Exp. Cell Res. 1972, 74, 61–66. [Google Scholar] [CrossRef]
- Wise, K.D.; Sodagar, A.M.; Yao, Y.; Gulari, M.N.; Perlin, G.E.; Najafi, K. Microelectrodes, microelectronics, and implantable neural microsystems. Proc. IEEE 2008, 96, 1184–1202. [Google Scholar] [CrossRef]
- Wise, K.D. Silicon microsystems for neuroscience and neural prostheses. IEEE Eng. Med. Biol. Mag. 2005, 24, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Normann, R.A.; Greger, B.A.; House, P.; Romero, S.F.; Pelayo, F.; Fernandez, E. Toward the development of a cortically based visual neuroprosthesis. J. Neural Eng. 2009, 6, 035001. [Google Scholar] [CrossRef] [PubMed]
- Borkholder, D.A.; Bao, J.; Maluf, N.I.; Perl, E.R.; Kovacs, G.T.A. Microelectrode arrays for stimulation of neural slice preparations. J. Neurosci. Methods 1997, 77, 61–66. [Google Scholar] [CrossRef]
- Ruther, P.; Herwik, S.; Kisban, S.; Seidl, K.; Paul, O. Recent Progress in Neural Probes Using Silicon MEMS Technology. IEEE Trans. Electr. Electron. Eng. 2010, 5, 505–515. [Google Scholar] [CrossRef]
- DeBusschere, B.D.; Kovacs, G.T. Portable cell-based biosensor system using integrated CMOS cell-cartridges. Biosens. Bioelectron. 2001, 16, 543–556. [Google Scholar] [CrossRef]
- Biosystems, Maxwell. Available online: http://www.mxwbio.com (accessed on 24 February 2017).
- Microsystems, Blackrock. Available online: http://blackrockmicro.com (accessed on 24 February 2017).
- Qu, M.; Gao, X.; Hou, Y.; Shen, C.; Xu, Y.; Zhu, M.; Wang, H.; Xu, H.; Chai, G.; Zhang, Y. Influence of Cell Printing on Biological Characters of Chondrocytes. J. Biomater. Tissue Eng. 2016, 6, 107–113. [Google Scholar] [CrossRef]
- Koester, P.J.; Buehler, S.M.; Stubbe, M.; Tautorat, C.; Niendorf, M.; Baumann, W.; Gimsa, J. Modular glass chip system measuring the electric activity and adhesion of neuronal cells-application and drug testing with sodium valproic acid. Lab Chip 2010, 10, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Heuschkel, M.O.; Fejtl, M.; Raggenbass, M.; Bertrand, D.; Renaud, P. A three-dimensional multi-electrode array for multi-site stimulation and recording in acute brain slices. J. Neurosci. Methods 2002, 114, 135–148. [Google Scholar] [CrossRef]
- Systems, Multichannel. Available online: http://www.multichannelsystems.com (accessed on 24 February 2017).
- BioSystems, Axion. Available online: https://www.axionbiosystems.com (accessed on 24 February 2017).
- Berdondini, L.; Chiappalone, M.; Van Der Wal, P.D.; Imfeld, K.; de Rooij, N.F.; Koudelka-Hep, M.; Tedesco, S.; Van Pelt, J.; Le Masson, G.; Garenne, A. A microelectrode array (MEA) integrated with clustering structures for investigating in vitro neurodynamics in confined interconnected sub-populations of neurons. Sens. Actuators B Chem. 2006, 114, 530–541. [Google Scholar] [CrossRef]
- Giovangrandi, L.; Gilchrist, K.H.; Whittington, R.H.; Kovacs, G.T. Low-cost microelectrode array with integrated heater for extracellular recording of cardiomyocyte cultures using commercial flexible printed circuit technology. Sens. Actuators B Chem. 2006, 113, 545–554. [Google Scholar] [CrossRef]
- DuPont. Available online: http://www.dupont.com/products-and-services/membranes-films/polyimide-films/brands/kapton-polyimide-film.html (accessed on 24 February 2017).
- Sun, Y.; Lacour, S.P.; Brooks, R.A.; Rushton, N.; Fawcett, J.; Cameron, R.E. Assessment of the biocompatibility of photosensitive polyimide for implantable medical device use. J. Biomed. Mater. Res. Part A 2009, 90, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Rubehn, B.; Stieglitz, T. In vitro evaluation of the long-term stability of polyimide as a material for neural implants. Biomaterials 2010, 31, 3449–3458. [Google Scholar] [CrossRef] [PubMed]
- Fuentek. Available online: http://www.fuentek.com/technologies/CPI/ (accessed on 24 February 2017).
- Materials, Nexolve. Available online: http://www.nexolvematerials.com/low-cure-polyimides/cp1-polyimide (accessed on 24 February 2017).
- Films, DuPont Teijin. Available online: http://usa.dupontteijinfilms.com (accessed on 24 February 2017).
- Whelove, O.E.; Cozad, M.J.; Lee, B.D.; Sengupta, S.; Bachman, S.L.; Ramshaw, B.J.; Grant, S.A. Development and in vitro studies of a polyethylene terephthalate-gold nanoparticle scaffold for improved biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Heiduschka, P.; Thanos, S. Implantable bioelectronic interfaces for lost nerve functions. Prog. Neurobiol. 1998, 55, 433–461. [Google Scholar] [CrossRef]
- Pajouhi, H.; Mohajerzadeh, S.; Nayeri, F.; Sanaee, Z. Deep micro-machining of poly-ethylene terephthalate for plastic MEMS applications. Solid State Electron. 2010, 54, 1536–1542. [Google Scholar] [CrossRef]
- Wang, L.; Luo, J.; Yin, J.; Zhang, H.; Wu, J.; Shi, X.; Crew, E.; Xu, Z.; Rendeng, Q.; Lu, S.; et al. Flexible chemiresistor sensors: Thin film assemblies of nanoparticles on a polyethylene terephthalate substrate. J. Mater. Chem. 2010, 20, 907–915. [Google Scholar] [CrossRef]
- Tian, H.; Ma, S.; Zhao, H.M.; Wu, C.; Ge, J.; Xie, D.; Yang, Y.; Ren, T.L. Flexible electrostatic nanogenerator using graphene oxide film. Nanoscale 2013, 5, 8951–8957. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.C.; Son, P.K.; Sohn, Y.; Yi, J.; Kwon, J.H.; Gwag, J.S. Reflective Thermochromic Display on Polyethylene Naphthalate Film. J. Opt. Soc. Korea 2013, 17, 168–171. [Google Scholar] [CrossRef]
- Tortissier, G.; Ginet, P.; Daunay, B.; Jalabert, L.; Lambert, P.; Kim, B.; Fujita, H.; Toshiyoshi, H. CF4 plasma treatment-assisted inkjet printing for color pixel flexible display. J. Micromech. Microeng. 2011, 21, 105021. [Google Scholar] [CrossRef]
- Noriega, J.R.; Iyore, O.D.; Budime, C.; Gnade, B.; Vasselli, J. Characterization system for research on energy storage capacitors. Rev. Sci. Instrum. 2013, 84, 055109. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, T.; Mizuno, J.; Matsunami, S.; Edura, T.; Tsuwaki, M.; Oshima, J.; Adachi, C.; Shoji, S. Multi-color microfluidic organic light emitting device using electroluminescence and electrochemiluminescence. In Proceedings of the 2013 IEEE 26th International Conference on Micro Electro Mechanical Systems (MEMS), Taipei, Taiwan, 20–24 January 2013. [Google Scholar]
- Doran, C.; Chen, W.V.; Yu, P.K.L.; Alford, T.L.; Lau, S.S. Segmentation as a Method for the Introduction of Flexibility to Solar Cells. Mater. Express 2012, 2, 169–172. [Google Scholar] [CrossRef]
- Zardetto, V.; Brown, T.M.; Reale, A.; Di Carlo, A. Substrates for Flexible Electronics: A Practical Investigation on the Electrical, Film Flexibility, Optical, Temperature, and Solvent Resistance Properties. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 638–648. [Google Scholar] [CrossRef]
- Turnbull, L.; Liggat, J.J.; MacDonald, W.A. Thermal degradation chemistry of poly (ethylene naphthalate)—A study by thermal volatilisation analysis. Polym. Degrad. Stab. 2013, 98, 2244–2258. [Google Scholar] [CrossRef]
- Standards, ANSI/SLAS. Available online: https://www.slas.org/resources/information/industry-standards/ (accessed on 3 August 2017).
- Circuits, Innovative. Available online: http://www.innovative-circuits.com/index.html (accessed on 24 February 2017).
- Rajaraman, S.; Choi, S.O.; Shafer, R.H.; Ross, J.D.; Vukasinovic, J.; Choi, Y.; DeWeerth, S.P.; Glezer, A.; Allen, M.G. Microfabrication technologies for a coupled three-dimensional microelectrode, microfluidic array. J. Micromech. Microeng. 2006, 17, 163–171. [Google Scholar] [CrossRef]
- Herrault, F.; Ji, C.H.; Rajaraman, S.; Shafer, R.H.; Allen, M.G. Electrodeposited Metal Structures in High Aspect Ratio Cavities using Vapor Deposited Polymer Molds and Laser Micromachining. In Proceedings of the TRANSDUCERS 2007—2007 International Solid-State Sensors, Actuators and Microsystems Conference, Lyon, France, 10–14 June 2007. [Google Scholar]
- Rajaraman, S.; Ross, J.D.; Preyer, A.J. Devices, Systems and Methods for High-Throughput Electrophysiology. U.S. Patent 9,279,801, 2016. [Google Scholar]
- Crouch, S.P.M.; Kozlowski, R.; Slater, K.J.; Fletcher, J. The use of ATP bioluminescence as a measure of cell-proliferation and cytotoxicity. J. Immunol. Methods 1993, 160, 81–88. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karnati, C.; Aguilar, R.; Arrowood, C.; Ross, J.; Rajaraman, S. Micromachining on and of Transparent Polymers for Patterning Electrodes and Growing Electrically Active Cells for Biosensor Applications. Micromachines 2017, 8, 250. https://doi.org/10.3390/mi8080250
Karnati C, Aguilar R, Arrowood C, Ross J, Rajaraman S. Micromachining on and of Transparent Polymers for Patterning Electrodes and Growing Electrically Active Cells for Biosensor Applications. Micromachines. 2017; 8(8):250. https://doi.org/10.3390/mi8080250
Chicago/Turabian StyleKarnati, Chandana, Ricardo Aguilar, Colin Arrowood, James Ross, and Swaminathan Rajaraman. 2017. "Micromachining on and of Transparent Polymers for Patterning Electrodes and Growing Electrically Active Cells for Biosensor Applications" Micromachines 8, no. 8: 250. https://doi.org/10.3390/mi8080250



