Stroke Management: An Emerging Role of Nanotechnology
Abstract
:1. Introduction
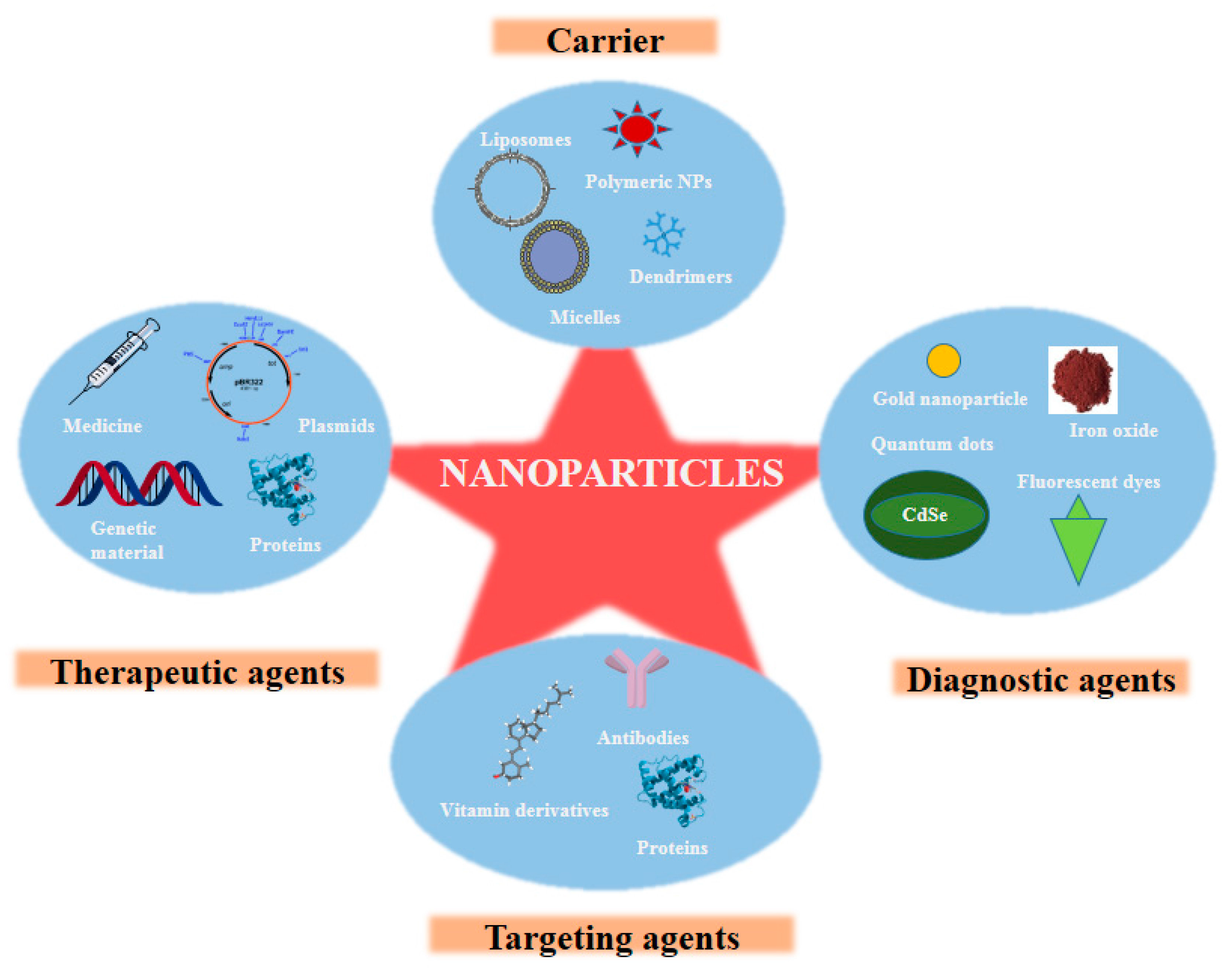
2. Advent of Nanotechnology
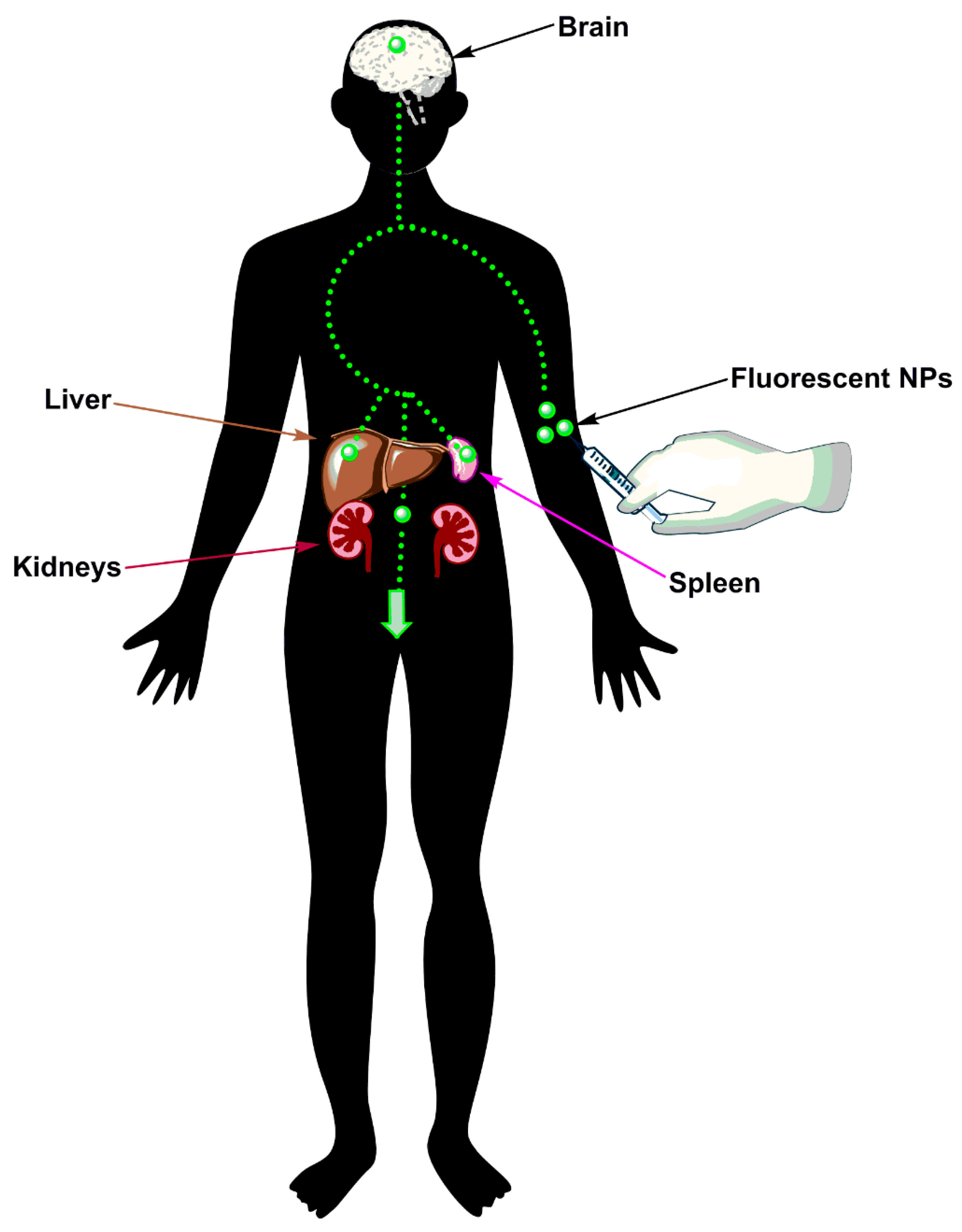
3. Nanotechnology for the Diagnosis of Stroke
3.1. Perfluorocarbon Nanoparticles (PFC-NPs)
3.2. Iron Oxide NPs
3.3. Gold NPs (GNPs)
3.4. Polymeric NPs
3.5. Quantum Dots
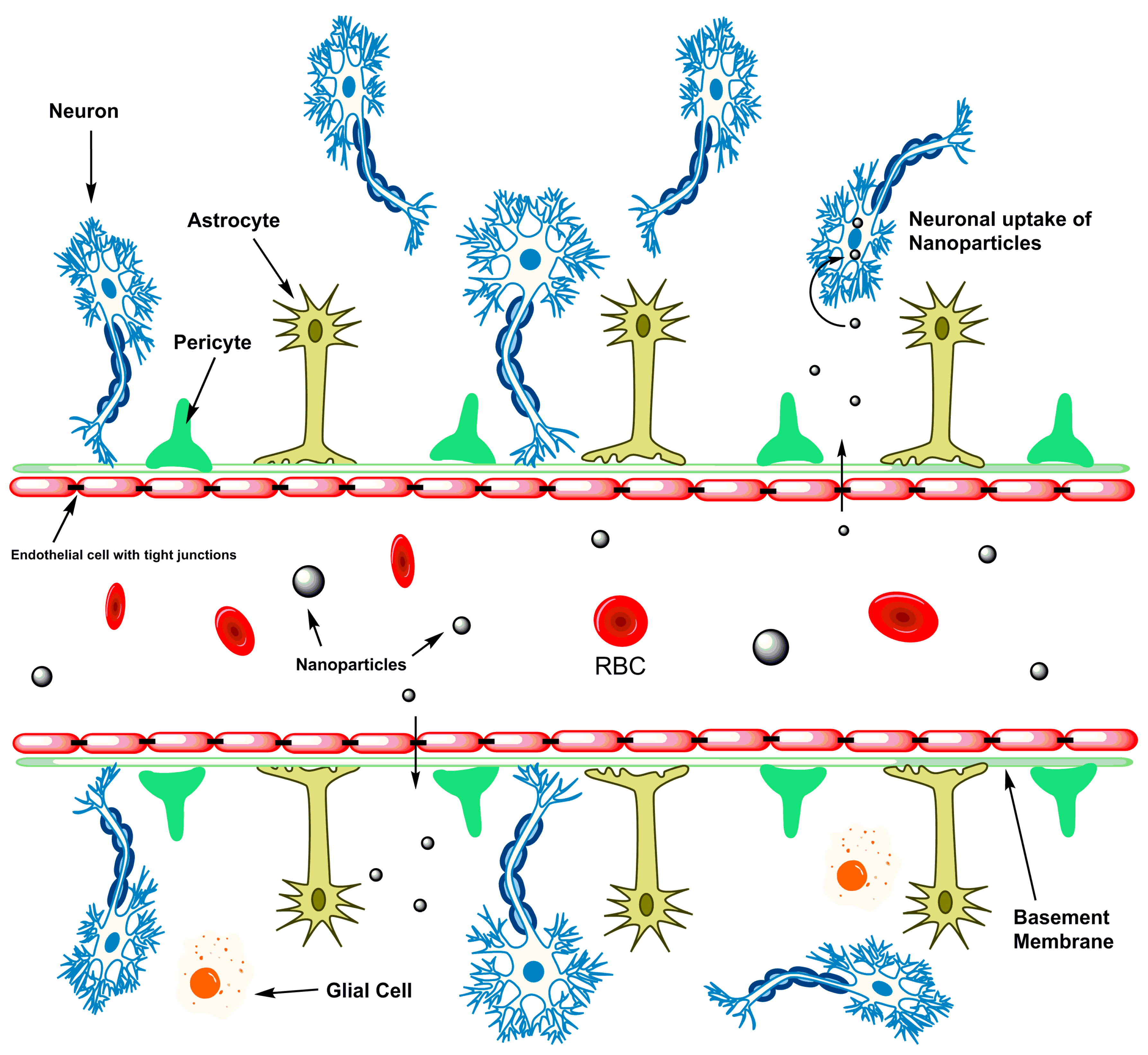
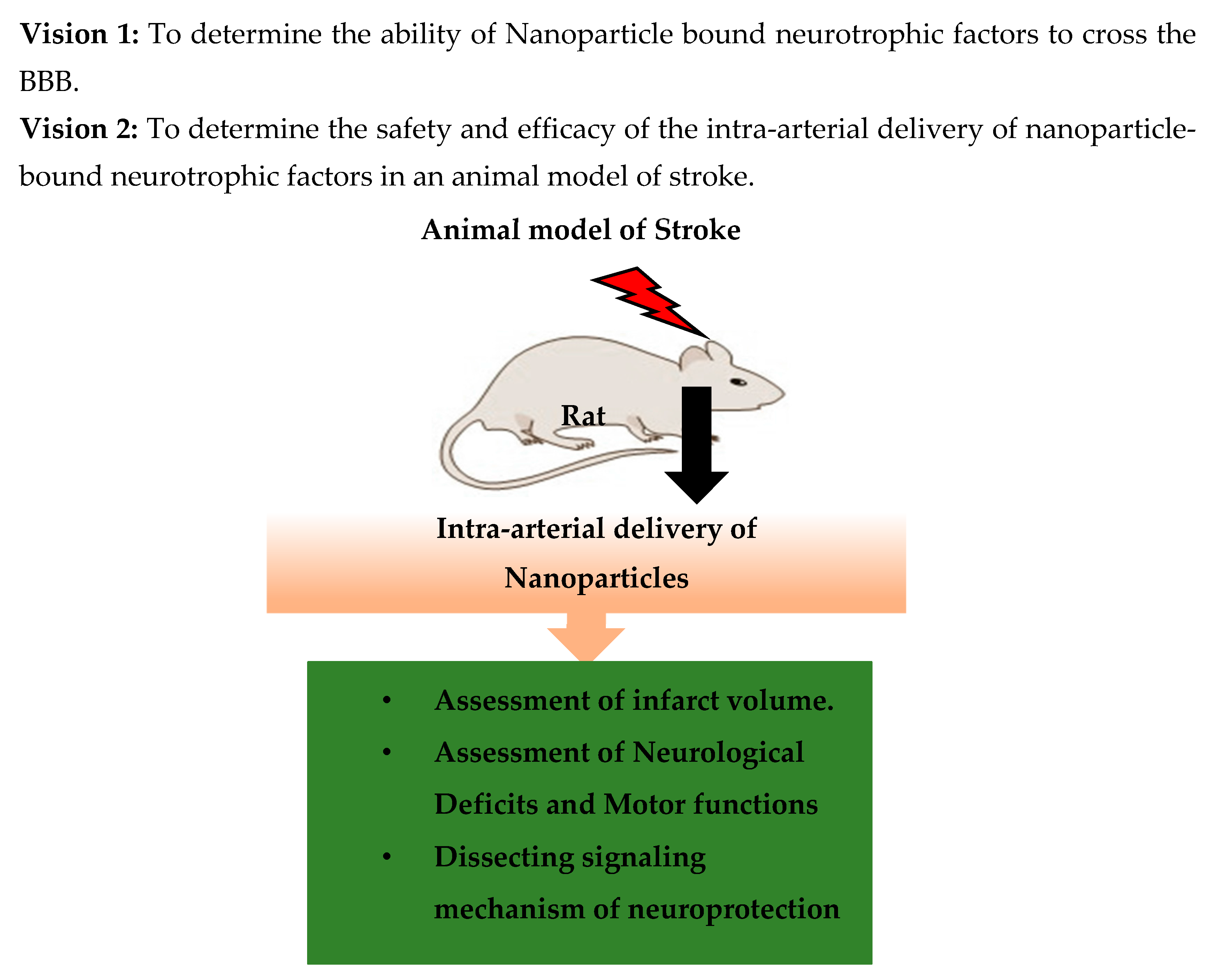
4. Stroke Therapy Using Nanotechnology
5. Challenges
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Strong, K.; Mathers, C.; Bonita, R. Preventing stroke: Saving lives around the world. Lancet Neurol. 2007, 6, 182–187. [Google Scholar] [CrossRef]
- Feigin, V.L.; Roth, G.A.; Naghavi, M.; Parmar, P.; Krishnamurthi, R.; Chugh, S.; Mensah, G.A.; Norrving, B.; Shiue, I.; Ng, M. Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet Neurol. 2016, 15, 913–924. [Google Scholar] [CrossRef]
- Powell, J.L.; Cook, I.G. Global ageing in comparative perspective: A critical discussion. Int. J. Sociol. Soc. Policy 2009, 29, 388–400. [Google Scholar] [CrossRef]
- Esiri, M. Ageing and the brain. J. Pathol. 2007, 211, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Hubbard, B.; Coghill, G.; Slidders, W. The effect of advanced old age on the neurone content of the cerebral cortex: Observations with an automatic image analyser point counting method. J. Neurol. Sci. 1983, 58, 235–246. [Google Scholar] [CrossRef]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global burden of stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Hankey, G. Preventable stroke and stroke prevention. J. Thromb. Haemost. 2005, 3, 1638–1645. [Google Scholar] [CrossRef]
- Candelario-Jalil, E. Injury and repair mechanisms in ischemic stroke: Considerations for the development of novel neurotherapeutics. Curr. Opin. Investig. Drugs 2009, 10, 644–654. [Google Scholar] [PubMed]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Macrez, R.; Ali, C.; Toutirais, O.; Le Mauff, B.; Defer, G.; Dirnagl, U.; Vivien, D. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol. 2011, 10, 471–480. [Google Scholar] [CrossRef]
- Fisher, C.M. Pathological observations in hypertensive cerebral hemorrhage. J. Neuropathol. Exp. Neurol. 1971, 30, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Keep, R.F.; Hoff, J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006, 5, 53–63. [Google Scholar] [CrossRef]
- Huang, F.-P.; Xi, G.; Keep, R.F.; Hua, Y.; Nemoianu, A.; Hoff, J.T. Brain edema after experimental intracerebral hemorrhage: Role of hemoglobin degradation products. J. Neurosurg. 2002, 96, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Felberg, R.A.; Grotta, J.C.; Shirzadi, A.L.; Strong, R.; Narayana, P.; Hill-Felberg, S.J.; Aronowski, J. Cell death in experimental intracerebral hemorrhage: The “black hole” model of hemorrhagic damage. Ann. Neurol. 2002, 51, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.B. Modern medical management of acute ischemic stroke. Methodist DeBakey Cardiovasc. J. 2014, 10, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Saha, S. Nanotechnology for the detection and therapy of stroke. Adv. Healthc. Mater. 2014, 3, 1703–1720. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.N. Nanotechnology in herbal medicines and cosmetics. Int. J. Res. Ayurveda Pharm. 2013, 4, 472–474. [Google Scholar] [CrossRef]
- Oberdörster, G.; Stone, V.; Donaldson, K. Toxicology of nanoparticles: A historical perspective. Nanotoxicology 2007, 1, 2–25. [Google Scholar] [CrossRef]
- Poole, C.P., Jr.; Owens, F.J. Introduction to Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef]
- Kubinová, Š.; Syková, E. Nanotechnology for treatment of stroke and spinal cord injury. Nanomedicine 2010, 5, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L. Time is brain—Quantified. Stroke 2006, 37, 263–266. [Google Scholar] [CrossRef]
- Mc Carthy, D.J.; Malhotra, M.; O’mahony, A.M.; Cryan, J.F.; O’driscoll, C.M. Nanoparticles and the blood-brain barrier: Advancing from in vitro models towards therapeutic significance. Pharm. Res. 2015, 32, 1161–1185. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yantsen, E.; Larson, T.; Karpiouk, A.B.; Sethuraman, S.; Su, J.L.; Sokolov, K.; Emelianov, S.Y. Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques. Nano Lett. 2008, 9, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Sharp, F.R. Blood biomarkers of ischemic stroke. Neurotherapeutics 2011, 8, 349–360. [Google Scholar] [CrossRef]
- Gilmore, J.L.; Yi, X.; Quan, L.; Kabanov, A.V. Novel nanomaterials for clinical neuroscience. J. Neuroimmune Pharmacol. 2008, 3, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Morawski, A.M.; Winter, P.M.; Crowder, K.C.; Caruthers, S.D.; Fuhrhop, R.W.; Scott, M.J.; Robertson, J.D.; Abendschein, D.R.; Lanza, G.M.; Wickline, S.A. Targeted nanoparticles for quantitative imaging of sparse molecular epitopes with MRI. Magn. Reson. Med. 2004, 51, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.M.; Caruthers, S.D.; Allen, J.S.; Cai, K.; Williams, T.A.; Lanza, G.M.; Wickline, S.A. Molecular imaging of angiogenic therapy in peripheral vascular disease with αvβ3-integrin-targeted nanoparticles. Magn. Reson. Med. 2010, 64, 369–376. [Google Scholar] [PubMed]
- Caruthers, S.D.; Cyrus, T.; Winter, P.M.; Wickline, S.A.; Lanza, G.M. Anti-angiogenic perfluorocarbon nanoparticles for diagnosis and treatment of atherosclerosis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.S.; Varallyay, C.G.; Dosa, E.; Gahramanov, S.; Hamilton, B.; Rooney, W.D.; Muldoon, L.L.; Neuwelt, E.A. Superparamagnetic iron oxide nanoparticles: Diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J. Cereb. Blood Flow Metab. 2010, 30, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.L.; Kim, P.E.; Law, M.; Liu, C.Y.; Apuzzo, M.L. Visualizing the future: Enhancing neuroimaging with nanotechnology. World Neurosurg. 2011, 75, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Etame, A.B.; Diaz, R.J.; O’Reilly, M.A.; Smith, C.A.; Mainprize, T.G.; Hynynen, K.; Rutka, J.T. Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using mri-guided focused ultrasound. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Egashira, K.; Masuda, S.; Funakoshi, K.; Zhao, G.; Kimura, S.; Matoba, T.; Sueishi, K.; Endo, Y.; Kawashima, Y. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology. JACC Cardiovasc. Interv. 2009, 2, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, Y.; Cao, M.; Chen, M.; Lin, B.; Duan, X.; Zhang, F.; Mao, J.; Shuai, X.; Shen, J. A novel polymeric micelle used for in vivo mr imaging tracking of neural stem cells in acute ischemic stroke. RSC Adv. 2017, 7, 15041–15052. [Google Scholar] [CrossRef]
- Duan, X.; Wang, Y.; Zhang, F.; Lu, L.; Cao, M.; Lin, B.; Zhang, X.; Mao, J.; Shuai, X.; Shen, J. Superparamagnetic iron oxide-loaded cationic polymersomes for cellular MR imaging of therapeutic stem cells in stroke. J. Biomed. Nanotechnol. 2016, 12, 2112–2124. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Y.; Zhang, F.; Zhang, X.; Lu, L.; Shuai, X.; Shen, J. In vivo monitoring of neural stem cells after transplantation in acute cerebral infarction with dual-modal MR imaging and optical imaging. Biomaterials 2014, 35, 4627–4635. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Controll. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Hara, H.; Friedlander, R.M.; Gagliardini, V.; Ayata, C.; Fink, K.; Huang, Z.; Shimizu-Sasamata, M.; Yuan, J.; Moskowitz, M.A. Inhibition of interleukin 1β converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc. Natl. Acad. Sci. USA 1997, 94, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Psarros, C.; Lee, R.; Margaritis, M.; Antoniades, C. Nanomedicine for the prevention, treatment and imaging of atherosclerosis. Maturitas 2012, 73, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.; Mansour, M.M.; Kazmierczak, S.C. From diagnostics to therapy: Prospects of quantum dots. Clin. Biochem. 2007, 40, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Derfus, A.M.; Chan, W.C.; Bhatia, S.N. Intracellular delivery of quantum dots for live cell labeling and organelle tracking. Adv. Mater. 2004, 16, 961–966. [Google Scholar] [CrossRef]
- Gaudin, A.; Yemisci, M.; Eroglu, H.; Lepetre-Mouelhi, S.; Turkoglu, O.F.; Dönmez-Demir, B.; Caban, S.; Sargon, M.F.; Garcia-Argote, S.; Pieters, G. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat. Nanotechnol. 2014, 9, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, J.; Yoon, O.J.; Kim, H.W.; Kim, D.H.; Lee, W.B.; Lee, N.-E.; Bonventre, J.V.; Kim, S.S. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat. Nanotechnol. 2011, 6, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Haddon, R.C.; Rao, A.M. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J. Mol. Neurosci. 2000, 14, 175–182. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Jiang, Q.; Zhang, R.; Zhang, L.; Wang, L.; Zhang, L.; Arniego, P.; Ho, K.L.; Chopp, M. Magnetic resonance imaging and neurosphere therapy of stroke in rat. Ann. Neurol. 2003, 53, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, Z.G.; Ding, G.L.; Zhang, L.; Ewing, J.R.; Wang, L.; Zhang, R.; Li, L.; Lu, M.; Meng, H. Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using mri. Neuroimage 2005, 28, 698–707. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.L.; Waid, M.C.; Shi, R.; Webster, T.J. Decreased functions of astrocytes on carbon nanofiber materials. Biomaterials 2004, 25, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly (l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef] [PubMed]
- Corey, J.M.; Lin, D.Y.; Mycek, K.B.; Chen, Q.; Samuel, S.; Feldman, E.L.; Martin, D.C. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J. Biomed. Mater. Res. Part A 2007, 83, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Tysseling-Mattiace, V.M.; Sahni, V.; Niece, K.L.; Birch, D.; Czeisler, C.; Fehlings, M.G.; Stupp, S.I.; Kessler, J.A. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J. Neurosci. 2008, 28, 3814–3823. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.; Dargusch, R.; Raitano, J.; Chan, S.-W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 2006, 342, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Onizawa, S.; Aoshiba, K.; Kajita, M.; Miyamoto, Y.; Nagai, A. Platinum nanoparticle antioxidants inhibit pulmonary inflammation in mice exposed to cigarette smoke. Pulm. Pharmacol. Ther. 2009, 22, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Klohs, J.; Steinbrink, J.; Bourayou, R.; Mueller, S.; Cordell, R.; Licha, K.; Schirner, M.; Dirnagl, U.; Lindauer, U.; Wunder, A. Near-infrared fluorescence imaging with fluorescently labeled albumin: A novel method for non-invasive optical imaging of blood-brain barrier impairment after focal cerebral ischemia in mice. J. Neurosci. Methods 2009, 180, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Kitazato, K.T.; Uno, M.; Tada, Y.; Kinouchi, T.; Shimada, K.; Nagahiro, S. Edaravone, a free radical scavenger, inhibits mmp-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke 2009, 40, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, M.; Miyamoto, Y.; Yamashita, T.; Deguchi, K.; Ohta, Y.; Abe, K. Strong neuroprotection with a novel platinum nanoparticle against ischemic stroke-and tissue plasminogen activator-related brain damages in mice. Neuroscience 2012, 221, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.; Britton, G.L.; Kim, H.; Cattano, D.; Aronowski, J.; Grotta, J.; McPherson, D.D.; Huang, S.L. Therapeutic time window and dose dependence of xenon delivered via echogenic liposomes for neuroprotection in stroke. CNS Neurosci. Ther. 2013, 19, 773–784. [Google Scholar] [CrossRef]
- Karatas, H.; Aktas, Y.; Gursoy-Ozdemir, Y.; Bodur, E.; Yemisci, M.; Caban, S.; Vural, A.; Pinarbasli, O.; Capan, Y.; Fernandez-Megia, E. A nanomedicine transports a peptide caspase-3 inhibitor across the blood-brain barrier and provides neuroprotection. J. Neurosci. 2009, 29, 13761–13769. [Google Scholar] [CrossRef]
- Gaudin, A.; Andrieux, K.; Couvreur, P. Nanomedicines and stroke: Toward translational research. J. Drug Deliv. Sci. Technol. 2015, 30, 278–299. [Google Scholar] [CrossRef]
- De Boer, A.; Gaillard, P. Drug targeting to the brain. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R. The blood-brain barrier in health and disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef]
- Bhaskar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; de la Fuente, J.M.; Grazú, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010, 7, 3. [Google Scholar] [CrossRef]
- Shcharbina, N.; Shcharbin, D.; Bryszewska, M. Nanomaterials in stroke treatment. Stroke 2013, 44, 2351–2355. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Hussain, S.; Schlager, J.; Ali, S.F.; Sharma, A. Influence of nanoparticles on blood-brain barrier permeability and brain edema formation in rats. In Brain Edema XIV; Springer: Vienna, Austria, 2010; pp. 359–364. [Google Scholar]
- Bosi, S.; Feruglio, L.; Da Ros, T.; Spalluto, G.; Gregoretti, B.; Terdoslavich, M.; Decorti, G.; Passamonti, S.; Moro, S.; Prato, M. Hemolytic effects of water-soluble fullerene derivatives. J. Med. Chem. 2004, 47, 6711–6715. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, A.; Sharma, V. Toxicity assessment of nanomaterials: Methods and challenges. Anal. Bioanal. Chem. 2010, 398, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Wu, X.Y.; Bendayan, R. Nanotechnological advances for the delivery of cns therapeutics. Adv. Drug Deliv. Rev. 2012, 64, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmah, D.; Saraf, J.; Kaur, H.; Pravalika, K.; Tekade, R.K.; Borah, A.; Kalia, K.; Dave, K.R.; Bhattacharya, P. Stroke Management: An Emerging Role of Nanotechnology. Micromachines 2017, 8, 262. https://doi.org/10.3390/mi8090262
Sarmah D, Saraf J, Kaur H, Pravalika K, Tekade RK, Borah A, Kalia K, Dave KR, Bhattacharya P. Stroke Management: An Emerging Role of Nanotechnology. Micromachines. 2017; 8(9):262. https://doi.org/10.3390/mi8090262
Chicago/Turabian StyleSarmah, Deepaneeta, Jackson Saraf, Harpreet Kaur, Kanta Pravalika, Rakesh Kumar Tekade, Anupom Borah, Kiran Kalia, Kunjan R. Dave, and Pallab Bhattacharya. 2017. "Stroke Management: An Emerging Role of Nanotechnology" Micromachines 8, no. 9: 262. https://doi.org/10.3390/mi8090262



