How to Make a Fast, Efficient Bubble-Driven Micromotor: A Mechanical View
Abstract
:1. Introduction
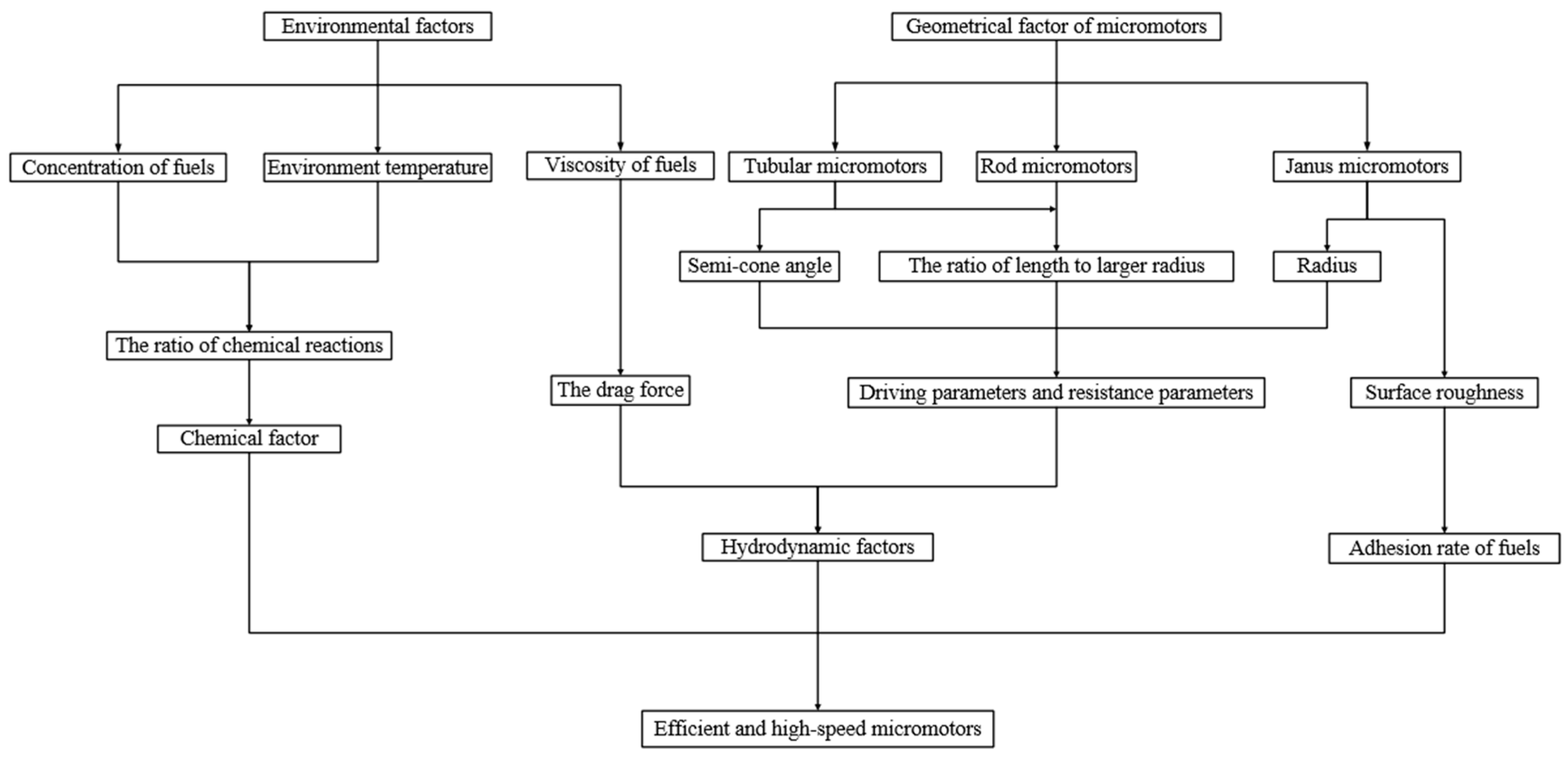
2. Environmental Factors
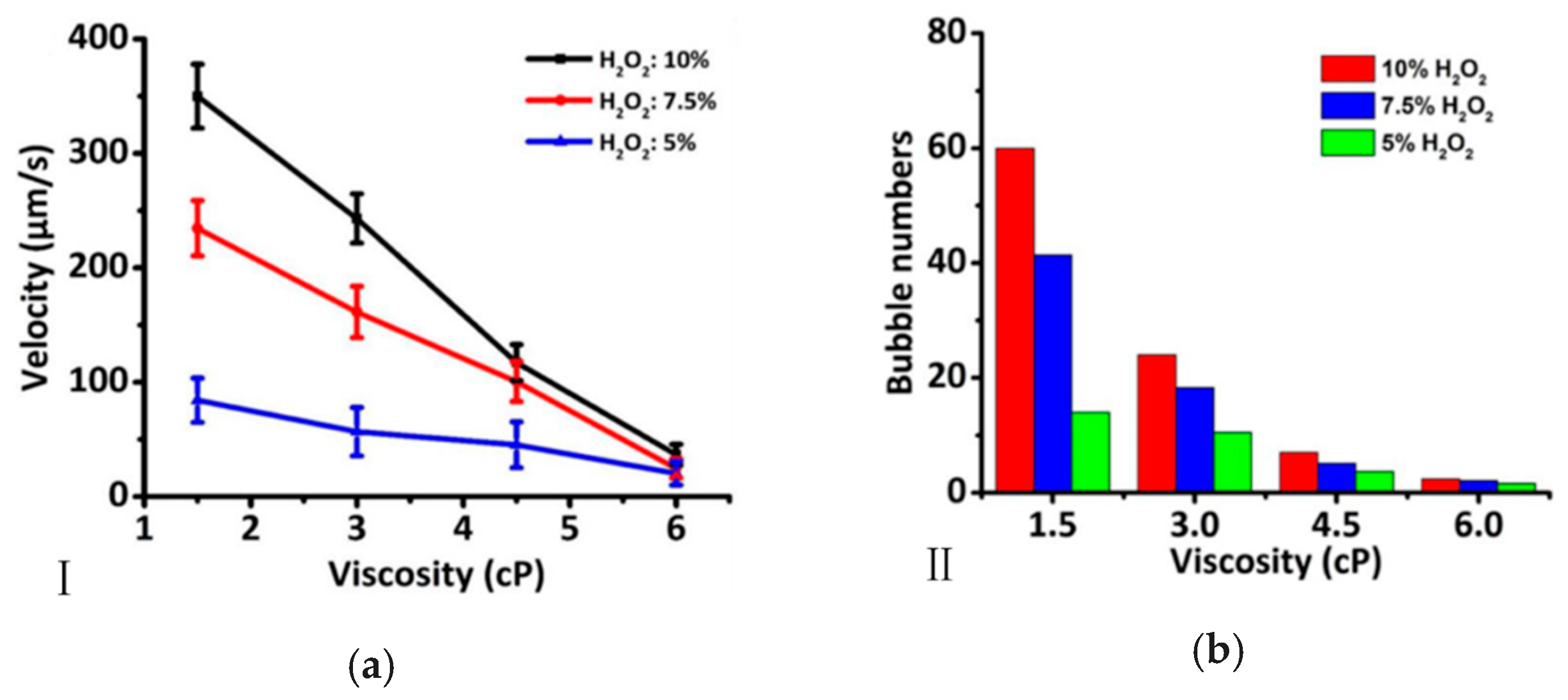
2.1. Concentration and Type of Fuel
2.2. Viscosity
2.3. Temperature
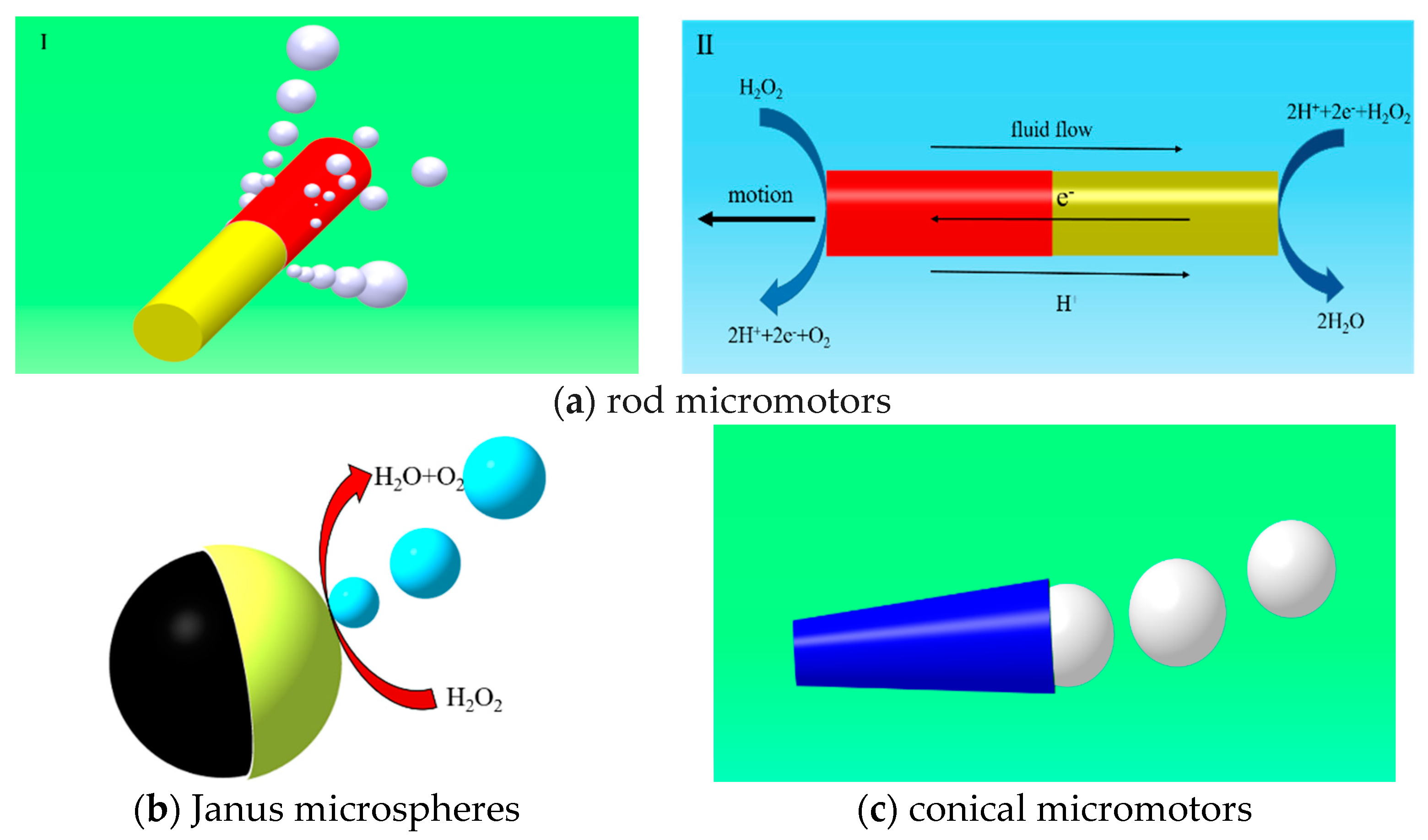
3. Geometric Design of Micromotor
3.1. Tubular and Rod Micromotors
3.1.1. Semi-Cone Angle
3.1.2. The Ratio of Length to Larger Radius
3.2. Janus Micromotors
3.3. Surface Roughness
4. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ismagilov, R.F.; Schwartz, A.; Bowden, N.; Whitesides, G.M. Autonomous movement and self-assembly. Angew. Chem. Int. Ed. 2002, 41, 41. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Kagan, D.; Hu, C.-M.J.; Campuzano, S.; Lobo-Castanon, M.J.; Lim, N.; Kang, D.Y.; Zimmerman, M.; Zhang, L.; Wang, J. Micromachine-enabled capture and isolation of cancer cells in complex media. Angew. Chem. Int. Ed. 2011, 50, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hahn, K.; Sanchez, S. Catalytic mesoporous janus nanomotors for active cargo delivery. J. Am. Chem. Soc. 2015, 137, 4976–4979. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Kagan, D.; Pak, O.S.; Clawson, C.; Campuzano, S.; Chuluun-Erdene, E.; Shipton, E.; Fullerton, E.E.; Zhang, L.; Lauga, E.; et al. Cargo-towing fuel-free magnetic nanoswimmers for targeted drug delivery. Small 2012, 8, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, J. Synthetic micro/nanomotors in drug delivery. Nanoscale 2014, 6, 10486–10494. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y.; Ng, K.W.; Zhao, Y. Integrated hollow mesoporous silica nanoparticles for target drug/sirna co-delivery. Chemistry 2013, 19, 15593–15603. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Sengupta, S.; Duan, W.; Zhang, H.; Pavlick, R.; Sen, A. Intelligent, self-powered, drug delivery systems. Nanoscale 2013, 5, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Kagan, D.; Campuzano, S.; Balasubramanian, S.; Kuralay, F.; Flechsig, G.U.; Wang, J. Functionalized micromachines for selective and rapid isolation of nucleic acid targets from complex samples. Nano Lett. 2011, 11, 2083–2087. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, J. Mesoporous silica nanoparticle based nano drug delivery systems: Synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011, 21, 5845. [Google Scholar] [CrossRef]
- Xu, T.; Soto, F.; Gao, W.; Dong, R.; Garcia-Gradilla, V.; Magana, E.; Zhang, X.; Wang, J. Reversible swarming and separation of self-propelled chemically powered nanomotors under acoustic fields. J. Am. Chem. Soc. 2015, 137, 2163–2166. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shklyaev, O.E.; Li, T.; Liu, W.; Shum, H.; Rozen, I.; Balazs, A.C.; Wang, J. Self-propelled nanomotors autonomously seek and repair cracks. Nano Lett. 2015, 15, 7077–7085. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, Y.; Okubo, K.; Yokokawa, M.; Suzuki, H. Programmed transport and release of cells by self-propelled micromotors. Langmuir 2016, 32, 9381–9388. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.G.; Fragnito, N.A.; Zhao, Y. Asymmetric Pt/Au coated catalytic micromotors fabricated by dynamic shadowing growth. Appl. Phys. Lett. 2010, 97, 253107. [Google Scholar] [CrossRef]
- Lavrentovich, O.D.; Lazo, I.; Pishnyak, O.P. Nonlinear electrophoresis of dielectric and metal spheres in a nematic liquid crystal. Nature 2010, 467, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qian, S.; Zhao, Y.; Qiao, R. Self-diffusiophoresis of janus catalytic micromotors in confined geometries. Langmuir 2016, 32, 5580–5592. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Duan, W.; Ahmed, S.; Mallouk, T.E.; Sen, A. Small power: Autonomous nano- and micromotors propelled by self-generated gradients. Nano Today 2013, 8, 531–554. [Google Scholar] [CrossRef]
- Wang, S.; Wu, N. Selecting the swimming mechanisms of colloidal particles: Bubble propulsion versus self-diffusiophoresis. Langmuir 2014, 30, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, M.; Kong, F.; Cui, H.; Silber-Li, Z. Visualization and measurement of the self-propelled and rotational motion of the janus microparticles. J. Vis. 2015, 18, 425–435. [Google Scholar] [CrossRef]
- Mei, Y.; Huang, G.; Solovev, A.A.; Ureña, E.B.; Mönch, I.; Ding, F.; Reindl, T.; Fu, R.K.Y.; Chu, P.K.; Schmidt, O.G. Versatile approach for integrative and functionalized tubes by strain engineering of nanomembranes on polymers. Adv. Mater. 2008, 20, 4085–4090. [Google Scholar] [CrossRef]
- Solovev, A.A.; Mei, Y.; Bermudez Urena, E.; Huang, G.; Schmidt, O.G. Catalytic microtubular jet engines self-propelled by accumulated gas bubbles. Small 2009, 5, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Maria-Hormigos, R.; Jurado-Sanchez, B.; Vazquez, L.; Escarpa, A. Carbon allotrope nanomaterials based catalytic micromotors. Chem. Mater. 2016, 28, 8962–8970. [Google Scholar] [CrossRef]
- Gao, W.; Sattayasamitsathit, S.; Orozco, J.; Wang, J. Highly efficient catalytic microengines: Template electrosynthesis of polyaniline/platinum microtubes. J. Am. Chem. Soc. 2011, 133, 11862–11864. [Google Scholar] [CrossRef] [PubMed]
- Ozin, G.A.; Manners, I.; Fournier-Bidoz, S.; Arsenault, A. Dream nanomachines. Adv. Mater. 2005, 17, 3011–3018. [Google Scholar] [CrossRef]
- Paxton, W.F.; Sen, A.; Mallouk, T.E. Motility of catalytic nanoparticles through self-generated forces. Chemistry 2005, 11, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.S.; Orozco, J.; Wu, Z.; Malone, C.D.; Yi, B.; Gao, W.; Eghtedari, M.; Wang, J.; Mattrey, R.F. Bubble_toward in vivo detection of hydrogen peroxide with ultrasound molecular imaging. Biomaterials 2013, 34, 8918–8924. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Pei, A.; Feng, X.; Hennessy, C.; Wang, J. Organized self-assembly of janus micromotors with hydrophobic hemispheres. J. Am. Chem. Soc. 2013, 135, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Fukai, S. Controlled motion of janus particles in periodically phase-separating binary fluids. Soft Matter 2015, 11, 3470–3479. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Wang, W.; Bai, L.; Gentekos, D.T.; Hoyos, M.; Mallouk, T.E. Density and shape_density and shape effects in the acoustic propulsion of bimetallic nanorod motors. ACS Nano 2016, 10, 4763–4769. [Google Scholar] [CrossRef] [PubMed]
- Ezhilan, B.; Gao, W.; Pei, A.; Rozen, I.; Dong, R.; Jurado-Sanchez, B.; Wang, J.; Saintillan, D. Motion-based threat detection using microrods: Experiments and numerical simulations. Nanoscale 2015, 7, 7833–7840. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Liu, M.; Liu, L.; Sun, Y.; Zhang, H.; Dong, B. R_dual-fuel-driven bactericidal micromotor. Nano-Micro. Lett. 2015, 8, 157–164. [Google Scholar] [CrossRef]
- Gao, W.; Sattayasamitsathit, S.; Wang, J. Catalytically propelled micro-/nanomotors: How fast can they move? Chem. Rec. 2012, 12, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Manjare, M.; Zhao, Y. Catalytic nanoshell micromotors. J. Phys. Chem. C 2013, 117, 21590–21596. [Google Scholar] [CrossRef]
- Wang, H.; Pumera, M. Fabrication of micro/nanoscale motors. Chem. Rev. 2015, 115, 8704–8735. [Google Scholar] [CrossRef] [PubMed]
- Delezuk, J.A.; Ramirez-Herrera, D.E.; Esteban-Fernandez de Avila, B.; Wang, J. Chitosan-based water-propelled micromotors with strong antibacterial activity. Nanoscale 2017, 9, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Uygun, A.; Wang, J. Hydrogen-bubble-propelled zinc-based microrockets in strongly acidic media. J. Am. Chem. Soc. 2012, 134, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Angsantikul, P.; Liu, W.; Esteban-Fernandez de Avila, B.; Thamphiwatana, S.; Xu, M.; Sandraz, E.; Wang, X.; Delezuk, J.; Gao, W.; et al. Micromotors spontaneously neutralize gastric acid for pH-responsive payload release. Angew. Chem. 2017, 56, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Ibele, M.E.; Wang, Y.; Kline, T.R.; Mallouk, T.E.; Sen, A. Hydrazine fuels for bimetallic catalytic microfluidic pumping. J. Am. Chem. Soc. 2007, 129, 7762–7763. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Li, J.; Han, J.; Xu, K.-X.; Huang, Z.-X.; Hu, J.; Sun, J.-J. The role of hydrazine in mixed fuels (H2O2/N2H4) for Au–Fe/Ni nanomotors. RSC Adv. 2015, 5, 71139–71143. [Google Scholar] [CrossRef]
- Zong, Y.W.; Liu, J.; Liu, R.; Guo, H.L.; Yang, M.C.; Li, Z.Y.; Chen, K. An optically driven bistable janus rotor with patterned metal coatings. ACS Nano 2015, 9, 10844–10851. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, Q.; Gao, W.; Pei, A.; Ren, B. Highly efficient light-driven TiO2-Au janus micromotors. ACS Nano 2016, 10, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Hu, Y.; Wu, Y.; Gao, W.; Ren, B.; Wang, Q.; Cai, Y. Visible-light-driven bioi-based janus micromotor in pure water. J. Am. Chem. Soc. 2017, 139, 1722–1725. [Google Scholar] [CrossRef] [PubMed]
- Baraban, L.; Makarov, D.; Streubel, R.; Monch, I.; Grimm, D.; Sanchez, S.; Schmidt, O.G. Catalytic janus motors on microfluidic chip: Deterministic motion for targeted cargo delivery. ACS Nano 2012, 6, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Löwen, H.; Menzel, A.M. Dynamics of a linear magnetic “microswimmer molecule”. EPL (Europhys. Lett.) 2016, 113, 58003. [Google Scholar] [CrossRef]
- Bellouard, Y.; Jin, D.; Yu, J.; Chan, K.H.; Zhang, L. Self-propelled magnesium based micromotors: Synthesis and magnetic steering. MATEC Web Conf. 2015, 32, 04004. [Google Scholar]
- Ghosh, A.; Fischer, P. Controlled propulsion of artificial magnetic nanostructured propellers. Nano Lett. 2009, 9, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.X.; Liu, Y.; Oh, H.; Gargava, A.; Kendall, E.; Nie, Z.; DeVoe, D.L.; Raghavan, S.R. Catalytic propulsion and magnetic steering of soft, patchy microcapsules: Ability to pick-up and drop-off microscale cargo. ACS Appl. Mater. Interfaces 2016, 8, 15676–15683. [Google Scholar] [CrossRef] [PubMed]
- Angelani, L.; Di Leonardo, R. Numerical modeling of bacteria propelled micromotors. Comput. Phys. Commun. 2011, 182, 1970–1973. [Google Scholar] [CrossRef]
- Mashimo, T. Micro ultrasonic motor using a cube with a side length of 0.5 mm. IEEE Asme Trans. Mech. 2016, 21, 1189–1192. [Google Scholar] [CrossRef]
- Mallouk, T.E.; Sen, A. Powering nanorobots. Sci. Am. 2009, 300, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, J. The environmental impact of micro/nanomachines: A review. ACS Nano 2014, 8, 3170–3810. [Google Scholar] [CrossRef] [PubMed]
- Orozco, J.; Mercante, L.A.; Pol, R.; Merkoci, A. Graphene-based janus micromotors for the dynamic removal of pollutants. J. Mater. Chem. A 2016, 4, 3371–3378. [Google Scholar] [CrossRef]
- Gao, W.; Dong, R.F.; Thamphiwatana, S.; Li, J.X.; Gao, W.W.; Zhang, L.F.; Wang, J. Artificial micromotors in the mouse's stomach: A step toward in vivo use of synthetic motors. ACS Nano 2015, 9, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Pumera, M. Concentric bimetallic microjets by electrodeposition. RSC Adv. 2013, 3, 3963. [Google Scholar] [CrossRef]
- Gao, W.; Pei, A.; Wang, J. Water-driven micromotors. ACS Nano 2012, 6, 8432–8438. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.; Chen, C.; Ma, H.; Yin, Y.; Wu, Q.; Guan, J. Self-propelled micromotors driven by the magnesium-water reaction and their hemolytic properties. Angew. Chem. Int. Ed. Engl. 2013, 52, 7208–7212. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Singh, V.V.; Sattayasamitsathit, S.; Orozco, J.; Kaufmann, K.; Dong, R.F.; Gao, W.; Jurado-Sanchez, B.; Fedorak, Y.; Wang, J. Water-driven micromotors for rapid photocatalytic degradation of biological and chemical warfare agents. ACS Nano 2014, 8, 11118–11125. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Sanchez, B.; Sattayasamitsathit, S.; Gao, W.; Santos, L.; Fedorak, Y.; Singh, V.V.; Orozco, J.; Galarnyk, M.; Wang, J. Self-propelled activated carbon janus micromotors for efficient water purification. Small 2015, 11, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, J.; Feng, X.; Zeng, W.; Liu, R.; Xiujing Lin, X.L.; Ma, Y.; Wang, L. Self-propelled manganese oxide-based catalytic micromotors for drug delivery. RSC Adv. 2016, 6, 65624–65630. [Google Scholar] [CrossRef]
- Wang, H.; Moo, J.G.; Pumera, M. From nanomotors to micromotors: The influence of the size of an autonomous bubble-propelled device upon its motion. ACS Nano 2016, 10, 5041–5050. [Google Scholar] [CrossRef] [PubMed]
- Moo, J.G.; Wang, H.; Pumera, M. Influence of pH on the motion of catalytic janus particles and tubular bubble-propelled micromotors. Chemistry 2016, 22, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, G.; Pumera, M. Beyond platinum: Bubble-propelled micromotors based on Ag and MnO2 catalysts. J. Am. Chem. Soc. 2014, 136, 2719–2722. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, T.; Li, L.; Wang, J.; Song, W.; Zhang, G. Microrocket based viscometer. ECS J. Solid State Sci. Technol. 2015, 4, S3020–S3023. [Google Scholar] [CrossRef]
- Zhao, G.; Nguyen, N.T.; Pumera, M. Reynolds numbers influence the directionality of self-propelled microjet engines in the 10−4 regime. Nanoscale 2013, 5, 7277–7283. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Li, T.; Song, W.; Zhang, G. Hydrodynamics and propulsion mechanism of self-propelled catalytic micromotors: Model and experiment. Soft Matter 2014, 10, 7511–7518. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, B.; Folio, D.; Ferreira, A.; IEEE. Catalytic tubular microjet propulsion model for endovascular navigation. In 2015 IEEE International Conference on Robotics and Automation; IEEE Computer Society: Los Alamitos, CA, USA, 2015; pp. 3537–3542. [Google Scholar]
- Agrawal, A.; Dey, K.K.; Paul, A.; Basu, S.; Chattopadhyay, A. Chemical locomotives based on polymer supported catalytic nanoparticles. J. Phys. Chem. C 2008, 112, 2797–2801. [Google Scholar] [CrossRef]
- Sanchez, S.; Ananth, A.N.; Fomin, V.M.; Viehrig, M.; Schmidt, O.G. Superfast motion of catalytic microjet engines at physiological temperature. J. Am. Chem. Soc. 2011, 133, 14860–14863. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Manjare, M.; Zhao, Y.; Qiao, R. On the peculiar bubble formation, growth, and collapse behaviors in catalytic micro-motor systems. Microfluid. Nanofluid. 2017, 21, 6. [Google Scholar] [CrossRef]
- Hayakawa, M.; Onoe, H.; Nagai, K.; Takinoue, M. Influence of asymmetry and driving forces on the propulsion of bubble-propelled catalytic micromotors. Micromachines 2016, 7, 229. [Google Scholar] [CrossRef]
- Parmar, J.; Vilela, D.; Sanchez, S. Tubular microjets: Fabrication, factors affecting the motion and mechanism of propulsion. Eur. Phys. J. Spec. Top. 2016, 225, 2255–2267. [Google Scholar] [CrossRef]
- Wang, J. Self-propelled affinity biosensors: Moving the receptor around the sample. Biosens. Bioelectron. 2016, 76, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sen, A. Autonomous nanomotor based on copper-platinum segmented nanobattery. J. Am. Chem. Soc. 2011, 133, 20064–20067. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hernandez, R.M.; Bartlett, D.J.; Bingham, J.M.; Kline, T.R.; Sen, A.; Mallouk, T.E. Bipolar electrochemical mechanism for the propulsion of catalytic nanomotors in hydrogen peroxide solutions. Langmuir 2006, 22, 10451–10456. [Google Scholar] [CrossRef] [PubMed]
- Soto, F.; Martin, A.; Ibsen, S.; Vaidyanathan, M.; Garcia-Gradilla, V.; Levin, Y.; Escarpa, A.; Esener, S.C.; Wang, J. Acoustic microcannons: Toward advanced microballistics. ACS Nano 2016, 10, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Moo, J.G.; Pumera, M. Tissue cell assisted fabrication of tubular catalytic platinum microengines. Nanoscale 2014, 6, 11359–11363. [Google Scholar] [CrossRef] [PubMed]
- Magdanz, V.; Guix, M.; Hebenstreit, F.; Schmidt, O.G. Dynamic polymeric microtubes for the remote-controlled capture, guidance, and release of sperm cells. Adv. Mater. 2016, 28, 4084–4089. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.; Wang, H.; Pumera, M. Beyond platinum: Silver-catalyst based bubble-propelled tubular micromotors. Chem. Commun. (Camb.) 2016, 52, 4333–4336. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Garg, A.; Campbell, A.I.; Howse, J.; Sen, A.; Velegol, D.; Golestanian, R.; Ebbens, S.J. Boundaries can steer active janus spheres. Nat. Commun. 2015, 6, 8999. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.J.; Schofield, J.; Kapral, R. A microscopic model for chemically-powered janus motors. Soft Matter 2016, 12, 5581–5589. [Google Scholar] [CrossRef] [PubMed]
- Manjare, M.; Ting Wu, Y.; Yang, B.; Zhao, Y.P. Hydrophobic catalytic janus motors: Slip boundary condition and enhanced catalytic reaction rate. Appl. Phys. Lett. 2014, 104, 054102. [Google Scholar] [CrossRef]
- Gao, W.; Feng, X.; Pei, A.; Kane, C.R.; Tam, R.; Hennessy, C.; Wang, J. Bioinspired helical microswimmers based on vascular plants. Nano Lett. 2014, 14, 305–310. [Google Scholar] [CrossRef] [PubMed]
- De Cristofaro, S.; Stefanini, C.; Pak, N.N.; Susilo, E.; Carrozza, M.C.; Dario, P. Electromagnetic wobble micromotor for microrobots actuation. Sens. Actuators A 2010, 161, 234–244. [Google Scholar] [CrossRef]
- Walker, D.; Kubler, M.; Morozov, K.I.; Fischer, P.; Leshansky, A.M. Optimal length of low reynolds number nanopropellers. Nano Lett. 2015, 15, 4412–4416. [Google Scholar] [CrossRef] [PubMed]
- Nourhani, A.; Lammert, P.E. Geometrical performance of self-phoretic colloids and microswimmers. Phys. Rev. Lett. 2016, 116, 178302. [Google Scholar] [CrossRef] [PubMed]
- MITROVIC, J. Formation of a liquid jet after detachment of a vapour bubble. Pergamon 1997, 40, 4309–4317. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Li, T.; Song, W.; Zhang, G. A unified model of drag force for bubble-propelled catalytic micro/nano-motors with different geometries in low reynolds number flows. J. Appl. Phys. 2015, 117, 104308. [Google Scholar] [CrossRef]
- Wang, Z.; Chi, Q.; Liu, L.; Liu, Q.; Bai, T.; Wang, Q. A viscosity-based model for bubble-propelled catalytic micromotors. Micromachines 2017, 8, 198. [Google Scholar] [CrossRef]
- Gao, W.; D’Agostino, M.; Garcia-Gradilla, V.; Orozco, J.; Wang, J. Multi-fuel driven janus micromotors. Small 2013, 9, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Wu, Z.G.; Lin, X.K.; He, Q.; Li, J.B. Autonomousmovement of controllable assembled janus capsulemotors. ACS Nano 2012, 6, 10910–10916. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chiang, T.Y.; Velegol, D.; Mallouk, T.E. Understanding the efficiency of autonomous nano- and microscale motors. J. Am. Chem. Soc. 2013, 135, 10557–10565. [Google Scholar] [CrossRef] [PubMed]
- Laocharoensuk, R.; Burdick, J.; Wang, J. Carbon-nanotuble-induced acceleration of catalytic nanomotors. ACS Nano 2008, 2, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Liu, M.; Liu, L.; Sun, Y.; Li, M.; Wang, D.; Zhang, H.; Dong, B. Shape-controlled fabrication of the polymer-based micromotor based on the polydimethylsiloxane template. Langmuir 2015, 31, 11914–11920. [Google Scholar] [CrossRef] [PubMed]
- Manjare, M.; Yang, B.; Zhao, Y.P. Bubble-propelled microjets: Model and experiment. J. Phys. Chem. C 2013, 117, 4657–4665. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.; Ye, M.; Li, M.; Liu, R.; Mei, Y. Dynamics of catalytic tubular microjet engines: Dependence on geometry and chemical environment. Nanoscale 2011, 3, 5083–5089. [Google Scholar] [CrossRef] [PubMed]
- Shklyaev, S. Janus droplet as a catalytic micromotor. EPL (Europhys. Lett.) 2015, 110, 54002. [Google Scholar] [CrossRef]
- Manjare, M.; Yang, B.; Zhao, Y.P. Bubble driven quasioscillatory translational motion of catalytic micromotors. Phys. Rev. Lett. 2012, 109, 128305. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.G.; Zhao, Y.P. Autonomously motile catalytic nanomotors by bubble propulsion. Appl. Phys. Lett. 2009, 94, 163104. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Lin, X.; Dai, L.; He, Q. Light-activated janus self-assembled capsule micromotors. Coll. Surfaces A 2015, 482, 92–97. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Z.; Lin, X.; He, Q. Catalytic polymer multilayer shell motors for separation of organics. Chemistry 2016, 22, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jannasch, A.; Albrecht, U.R.; Hahn, K.; Miguel-Lopez, A.; Schaffer, E.; Sanchez, S. Enzyme-powered hollow mesoporous janus nanomotors. Nano Lett. 2015, 15, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Katuri, J.; Zeng, Y.; Zhao, Y.; Sanchez, S. Surface conductive graphene-wrapped micromotors exhibiting enhanced motion. Small 2015, 11, 5023–5027. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Chen, C.; Liu, Z.; Liu, P.; Zheng, N. A cationic surfactant assisted selective etching strategy to hollow mesoporous silica spheres. Nanoscale 2011, 3, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Sanchez, B.; Wang, J.; Escarpa, A. Ultrafast nanocrystals decorated micromotors for on-site dynamic chemical processes. ACS Appl. Mater. Interfaces 2016, 8, 19618–19625. [Google Scholar] [CrossRef] [PubMed]

| Type of the Micromotors | Morphology | Condition | Schematic | Length | Radius | Max. Speed |
|---|---|---|---|---|---|---|
| Janus micromotors [88] | Al/Pd bimetallic Janus micromotors | Strong acid (HCl, 100 mM) and strong base (NaOH, 100 mM) | 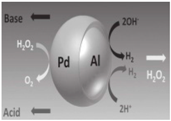 | 2.5–15 μm | 200 μm/s | |
| Tubular micomotors [52] | PEDOT/Zn tubular micromotors | Under the gastric acidic condition (pH up to 2) at phsiological temperature | 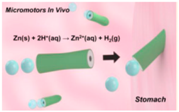 | 20 μm | 5 μm | 60 μm/s |
| Conical micromotors [58] | PEDOT/MnO2 tubular micromotors | In very low levels of hydrogen peroxide down to 0.4% | 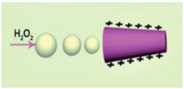 | 8 μm | 1 μm | 318.8 μm/s |
| Tubular micromotors [59] | Pure platinum micro/nanotubes | At 1–3% concentration of hydrogen peroxide. |  | 15.68 μm | 4.66 μm | 379.77 μm/s |
| Tubular microengines [75] | Tubular microengines using fruit tissue cells as the support of the deposited metal layer | In 3% concentration of hydrogen peroxide |  | 50 μm | 1.5 μm | 1000 μm/s |
| Shell micromotors [32] | Au/Ag/Pt nanoshell micromotors | In 5% concentration of hydrogen peroxide | 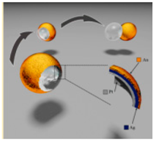 | 1–15 μm | 100 μm/s | |
| Conical micromotors [64] | PEDOT/platinum multilayer conical micromotors | In 1% concentration of hydrogen peroxide containing 1% Triton X-100 |  | 100 μm | 10 μm | 100 μm/s |
| Janus micromotots [54] | Al–Ga/Ti Janus microparticles | Pure water | 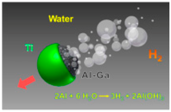 | 10 μm | 3000 μm/s | |
| Janus capsule micromotors [89] | Partially coated dendritic platinum nanoparticles | At 30% concentration of hydrogen peroxide | 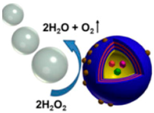 | 8 μm | 1000 μm/s | |
| Rod micromotors [90] | Catalytic Pt–Au nanorod motors | At 3% concentration of hydrogen peroxide |  | 100 μm | 1 μm | 50 μm/s |
| Carbon nanotube [91] | Au/Pt carbon nanotubes | At 15 wt % aqueous H2O2 fuel | 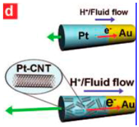 | 1 μm | 0.11 μm | 50–60 μm/s (up to above 200 μm/s) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Bai, T.; Chi, Q.; Wang, Z.; Xu, S.; Liu, Q.; Wang, Q. How to Make a Fast, Efficient Bubble-Driven Micromotor: A Mechanical View. Micromachines 2017, 8, 267. https://doi.org/10.3390/mi8090267
Liu L, Bai T, Chi Q, Wang Z, Xu S, Liu Q, Wang Q. How to Make a Fast, Efficient Bubble-Driven Micromotor: A Mechanical View. Micromachines. 2017; 8(9):267. https://doi.org/10.3390/mi8090267
Chicago/Turabian StyleLiu, Lisheng, Tao Bai, Qingjia Chi, Zhen Wang, Shuang Xu, Qiwen Liu, and Qiang Wang. 2017. "How to Make a Fast, Efficient Bubble-Driven Micromotor: A Mechanical View" Micromachines 8, no. 9: 267. https://doi.org/10.3390/mi8090267




