Dielectrophoresis Testing of Nonlinear Viscoelastic Behaviors of Human Red Blood Cells
Abstract
:1. Introduction
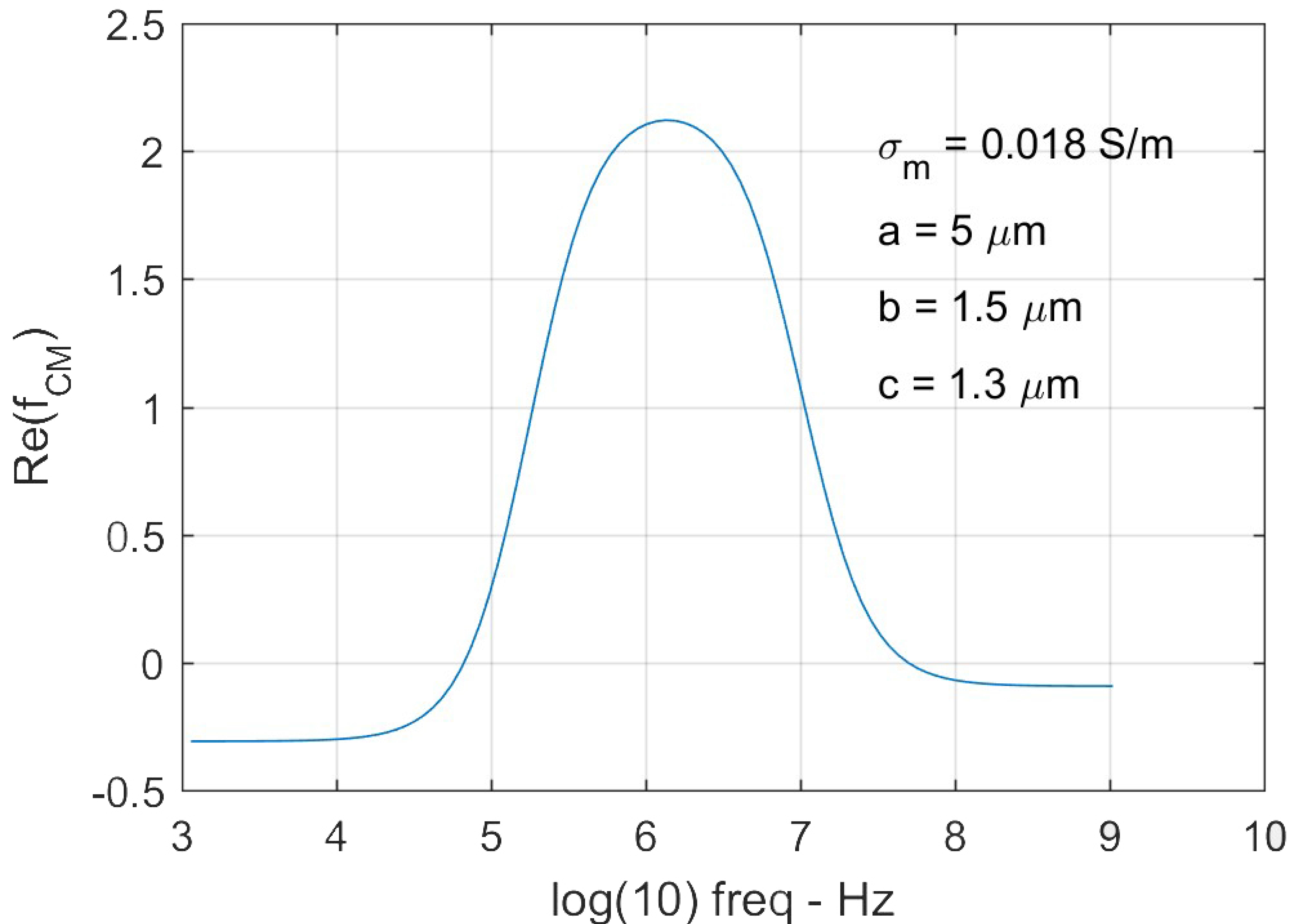
2. Materials and Methods
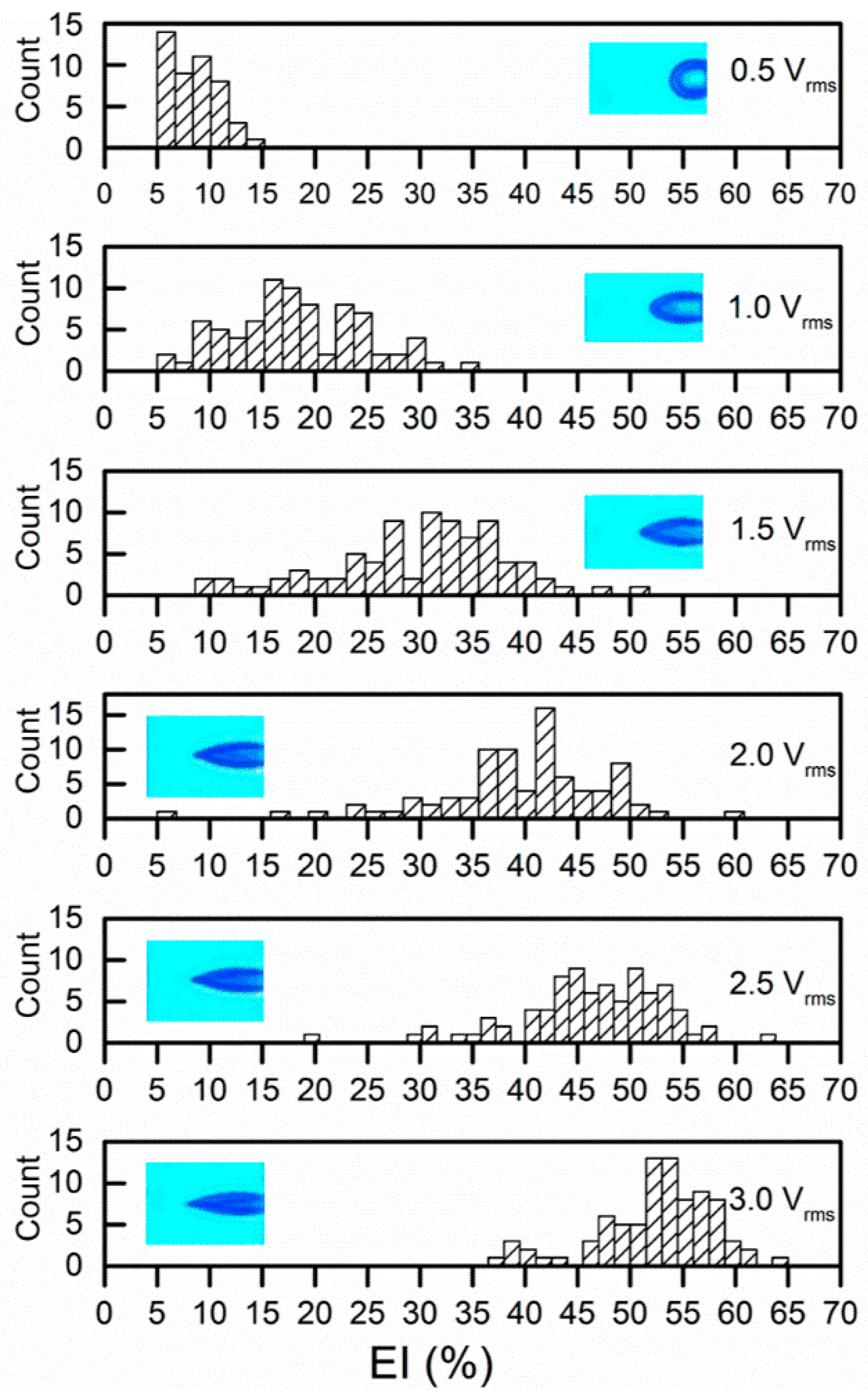
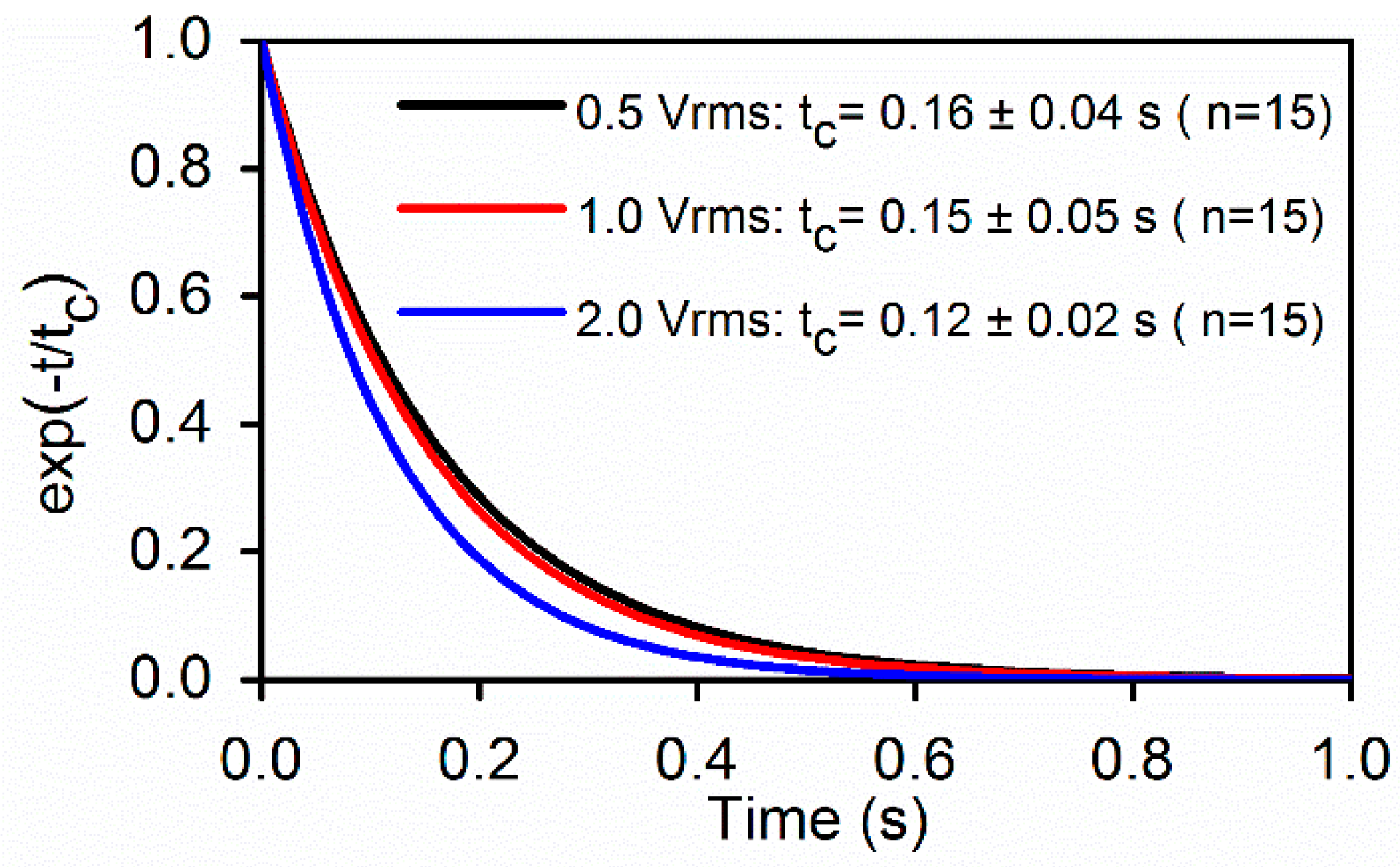
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, X.J.; Du, E.; Lei, H.; Tang, Y.H.; Dao, M.; Suresh, S.; Karniadakis, G.E. Patient-specific blood rheology in sickle-cell anaemia. Interface Focus 2016, 6, 20150065. [Google Scholar] [CrossRef] [PubMed]
- Pivkin, I.V.; Peng, Z.L.; Karniadakis, G.E.; Buffet, P.A.; Dao, M.; Suresh, S. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc. Natl. Acad. Sci. USA 2016, 113, 7804–7809. [Google Scholar] [CrossRef] [PubMed]
- Partola, K.R.; Andemariam, B.; Lykotrafitis, G. Microfluidic experimental setup for adhesion and recovery measurements of red blood cells in sickle cell disease. J. Mech. Behav. Biomed. Mater. 2017, 71, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Benoit, M.; Gottschalk, K.E. The viscoelasticity of membrane tethers and its importance for cell adhesion. Biophys. J. 2008, 95, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Puig-De-Morales-Marinkovic, M.; Turner, K.T.; Butler, J.P.; Fredberg, J.J.; Suresh, S. Viscoelasticity of the human red blood cell. Am. J. Physiol. Cell Physiol. 2007, 293, C597–C605. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, G. Biomechanical properties of red blood cells in health and disease towards microfluidics. Biomicrofluidics 2014, 8, 051501. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, K.; Park, Y. Measurement Techniques for Red Blood Cell Deformability: Recent Advances; InTech Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Nili, H.; Green, N.G. AC Electrokinetics of Nanoparticles. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 18–25. [Google Scholar]
- Morgan, H.; Green, N.G. AC Electrokinetics; Research Studies Press: Philadelphia, PA, USA, 2003. [Google Scholar]
- Doh, I.; Lee, W.C.; Cho, Y.H.; Pisano, A.P.; Kuypers, F.A. Deformation measurement of individual cells in large populations using a single-cell microchamber array chip. Appl. Phys. Lett. 2012, 100, 173702–1737023. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Dao, M.; Suresh, S. Quantitative biomechanics of healthy and diseased human red blood cells using dielectrophoresis in a microfluidic system. Extreme Mech. Lett. 2014, 1, 35–41. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, L.A.; Buschmann, M.D.; Wertheimer, M.R. Mechanical properties of mammalian cells in suspension measured by electro-deformation. J. Micromech. Microeng. 2010, 20, 065007. [Google Scholar] [CrossRef]
- Wong, P.K.; Tan, W.; Ho, C.M. Cell relaxation after electrodeformation: Effect of latrunculin A on cytoskeletal actin. J. Biomech. 2005, 38, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Abdelgawad, M.; Yu, L.M.; Shakiba, N.; Chien, W.Y.; Lu, Z.; Geddie, W.R.; Jewett, M.A.S.; Sun, Y. Electrodeformation for single cell mechanical characterization. J. Micromech. Microeng. 2011, 21, 054012. [Google Scholar] [CrossRef]
- Mills, J.P.; Qie, L.; Dao, M.; Lim, C.T.; Suresh, S. Nonlinear elastic and viscoelastic deformation of the human red blood cell with optical tweezers. Mech. Chem. Biosyst. 2004, 1, 169–180. [Google Scholar] [PubMed]
- Hochmuth, R.M. Micropipette aspiration of living cells. J. Biomech. 2000, 33, 15–22. [Google Scholar] [CrossRef]
- Qiang, Y.; Liu, J.; Du, E. Dynamic fatigue measurement of human erythrocytes using dielectrophoresis. Acta Biomater. 2017, 57, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.A.; Hochmuth, R.M. A solid-liquid composite model of the red cell membrane. J. Membr. Biol. 1976, 30, 351–362. [Google Scholar] [CrossRef]
- Engelhardt, H.; Gaub, H.; Sackmann, E. Viscoelastic properties of erythrocyte membranes in high-frequency electric fields. Nature 1984, 307, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, H.; Sackmann, E. On the Measurement of Shear Elastic-Moduli and Viscosities of Erythrocyte Plasma-Membranes by Transient Deformation in High-Frequency Electric-Fields. Biophys. J. 1988, 54, 495–508. [Google Scholar] [CrossRef]
- Waugh, R.; Evans, E.A. Viscoelastic properties of erythrocyte membranes of different vertebrate animals. Microvasc. Res. 1976, 12, 291–304. [Google Scholar] [CrossRef]
- Dao, M.; Lim, C.T.; Suresh, S. Mechanics of the human red blood cell deformed by optical tweezers. J. Mech. Phys. Solids 2003, 51, 2259–2280. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.-K. Optical tweezers for single cells. J. R. Soc. Interface 2008, 5, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, R.; Waugh, R. Erythrocyte membrane elasticity and viscosity. Annu. Rev. Physiol. 1987, 49, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Khoshmanesh, K.; Nahavandi, S.; Baratchi, S.; Mitchell, A.; Kalantar-zadeh, K. Dielectrophoretic platforms for bio-microfluidic systems. Biosens. Bioelectron. 2011, 26, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Schwan, H.; Schwarz, G. Response of nonspherical biological particles to alternating electric fields. Biophys. J. 1966, 6, 313–327. [Google Scholar] [CrossRef]
- Kakutani, T.; Shibatani, S.; Sugai, M. Electrorotation of non-spherical cells: theory for ellipsoidal cells with an arbitrary number of shells. Bioelectrochem. Bioenerg. 1993, 31, 131–145. [Google Scholar] [CrossRef]
- Ye, S.S.; Ng, Y.C.; Tan, J.; Leo, H.L.; Kim, S. Two-dimensional strain-hardening membrane model for large deformation behavior of multiple red blood cells in high shear conditions. Theor. Biol. Med. Model. 2014, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.-Z.; Kotar, J.; Yoon, G.; Cicuta, P. The nonlinear mechanical response of the red blood cell. Phys. Biol. 2008, 5, 036007. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, R.; Worthy, P.; Evans, E. Red cell extensional recovery and the determination of membrane viscosity. Biophys. J. 1979, 26, 101–114. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.L.; Chen, M.D.; Yin, D.F.; Yang, Z.; Chen, X.; Wang, Z.Y.; Xu, J.; Li, Y.Y.; Qiu, J.; et al. Electro-deformation of fused cells in a microfluidic array device. Micromachines. 2016, 7, 204. [Google Scholar] [CrossRef]
- Haque, M.M.; Moisescu, M.G.; Valkai, S.; Dér, A.; Savopol, T. Stretching of red blood cells using an electro-optics trap. Biomed. Opt. Express 2015, 6, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Dao, M.; Suresh, S.; Sow, C.; Chew, K. Large deformation of living cells using laser traps. Acta Mater. 2004, 52, 1837–1845. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lei, U. Quasistatic force and torque on ellipsoidal particles under generalized dielectrophoresis. J. Appl. Phys. 2007, 102, 094702. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, Y.; Liu, J.; Du, E. Dielectrophoresis Testing of Nonlinear Viscoelastic Behaviors of Human Red Blood Cells. Micromachines 2018, 9, 21. https://doi.org/10.3390/mi9010021
Qiang Y, Liu J, Du E. Dielectrophoresis Testing of Nonlinear Viscoelastic Behaviors of Human Red Blood Cells. Micromachines. 2018; 9(1):21. https://doi.org/10.3390/mi9010021
Chicago/Turabian StyleQiang, Yuhao, Jia Liu, and E Du. 2018. "Dielectrophoresis Testing of Nonlinear Viscoelastic Behaviors of Human Red Blood Cells" Micromachines 9, no. 1: 21. https://doi.org/10.3390/mi9010021



