Development of an AVF Stenosis Assessment Tool for Hemodialysis Patients Using Robotic Ultrasound System
Abstract
:1. Introduction
2. Methodology
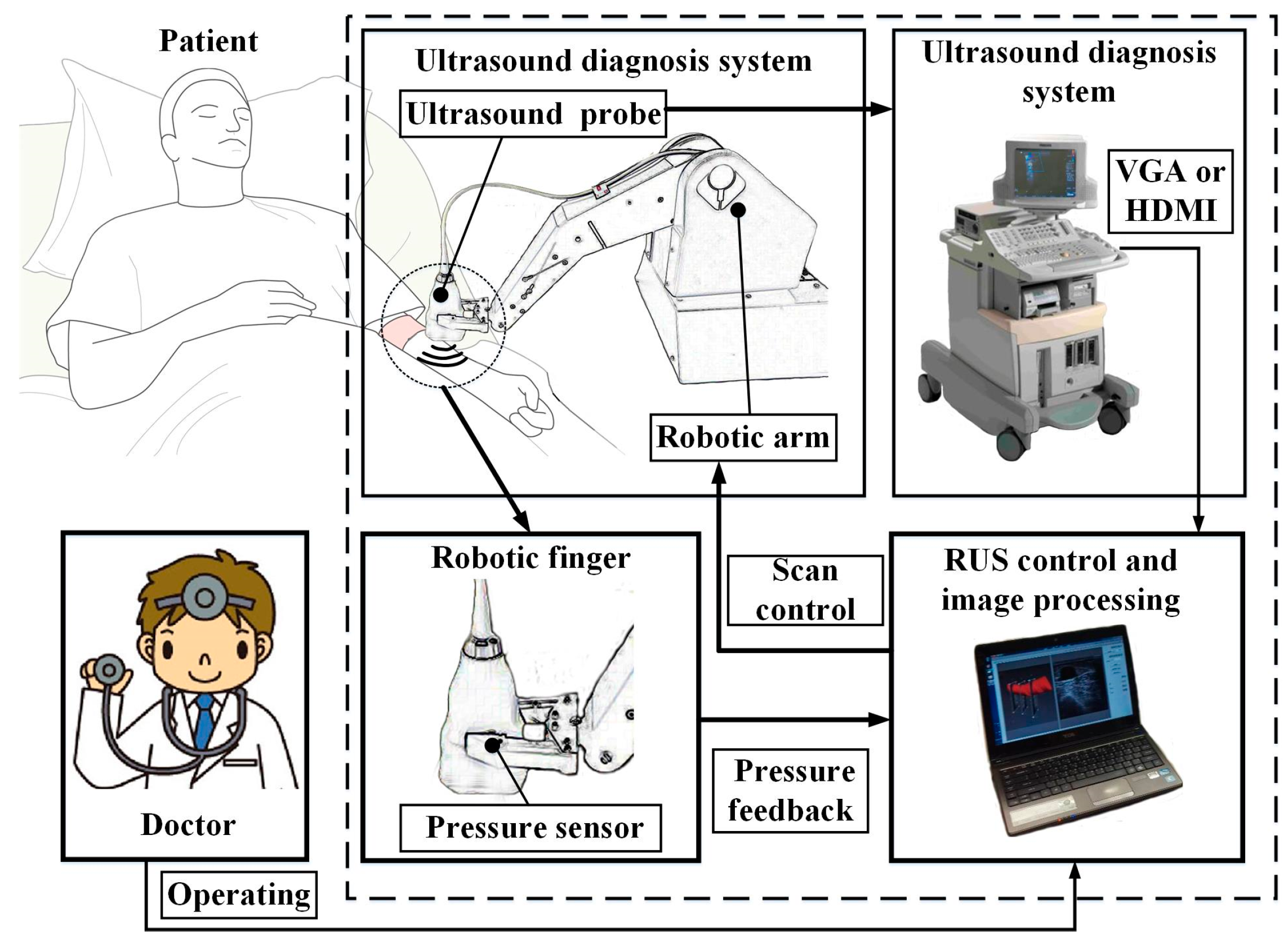
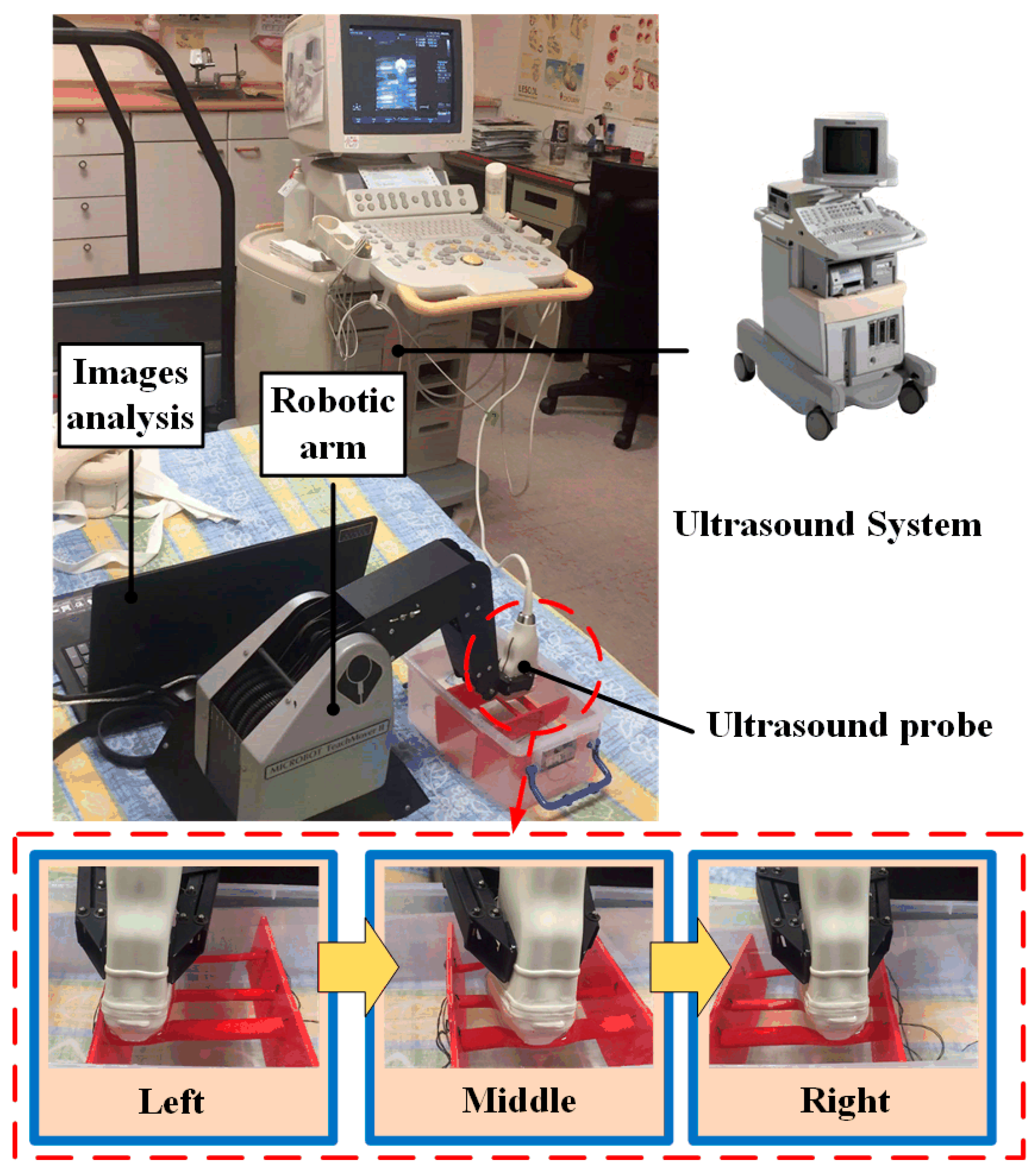
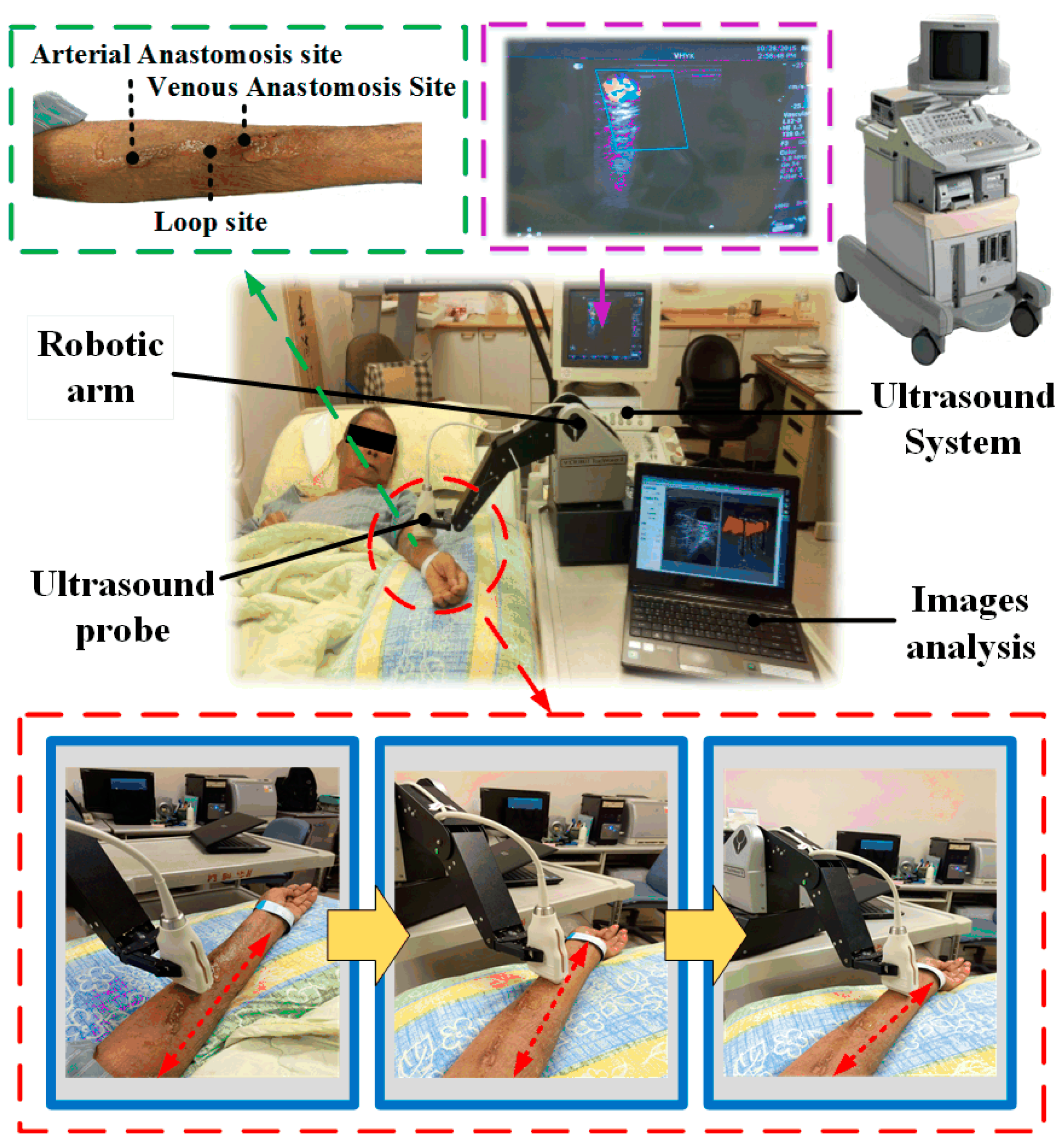
2.1. Architecture of the Robotic Ultrasound System (RUS)
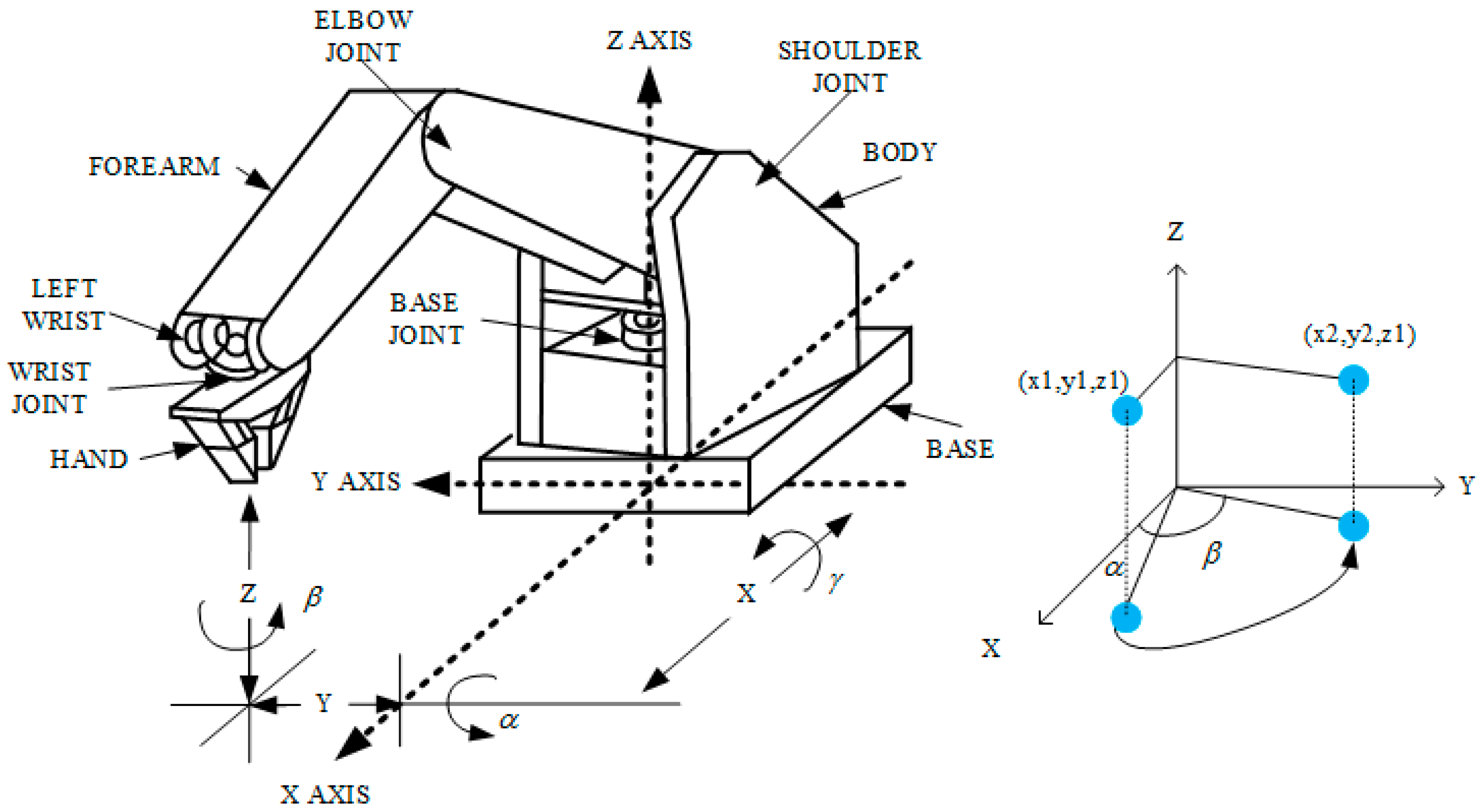
2.2. Robotic Arm
2.3. Pressure Sensing Module
2.4. Ultrasound Device
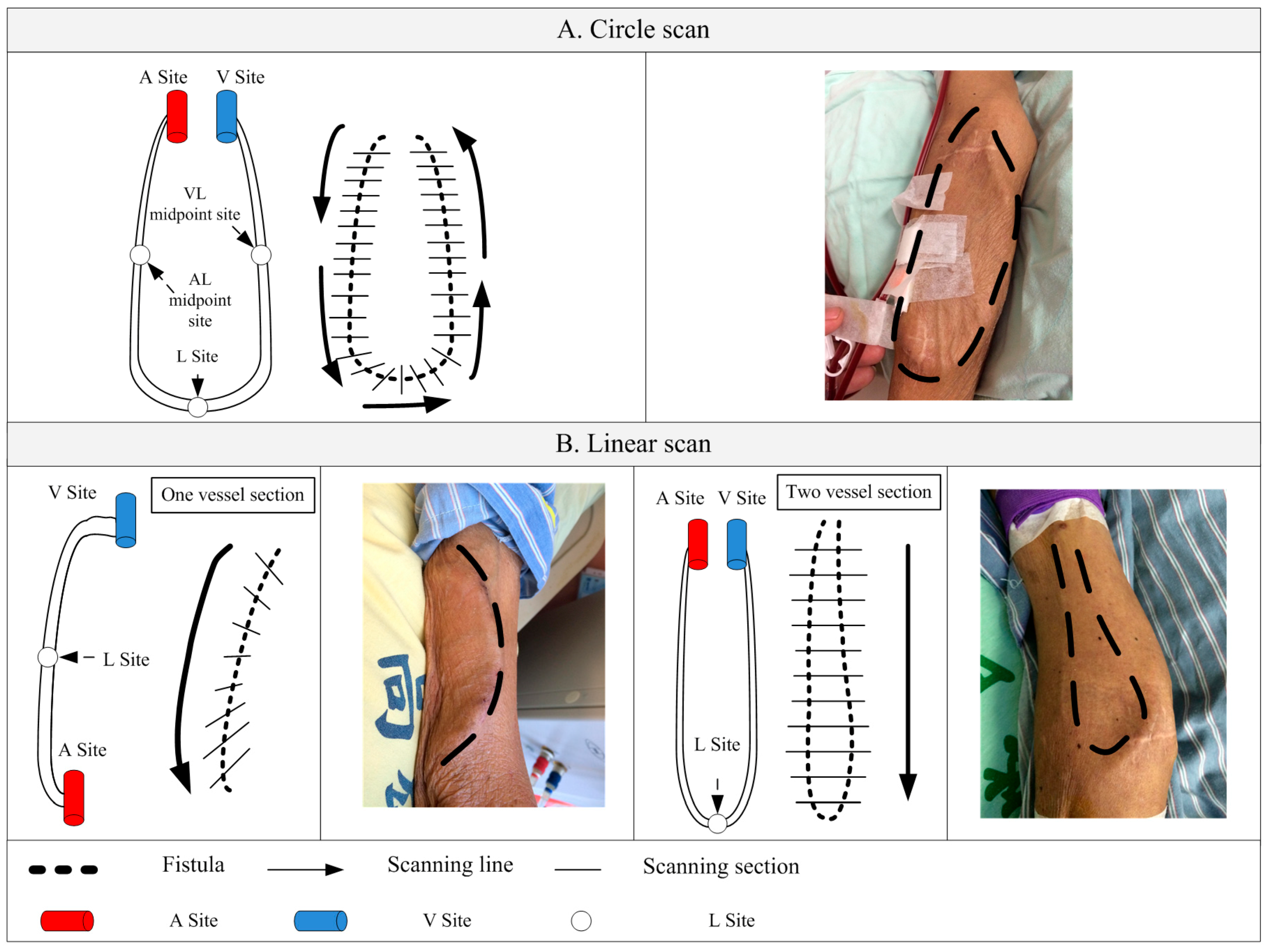
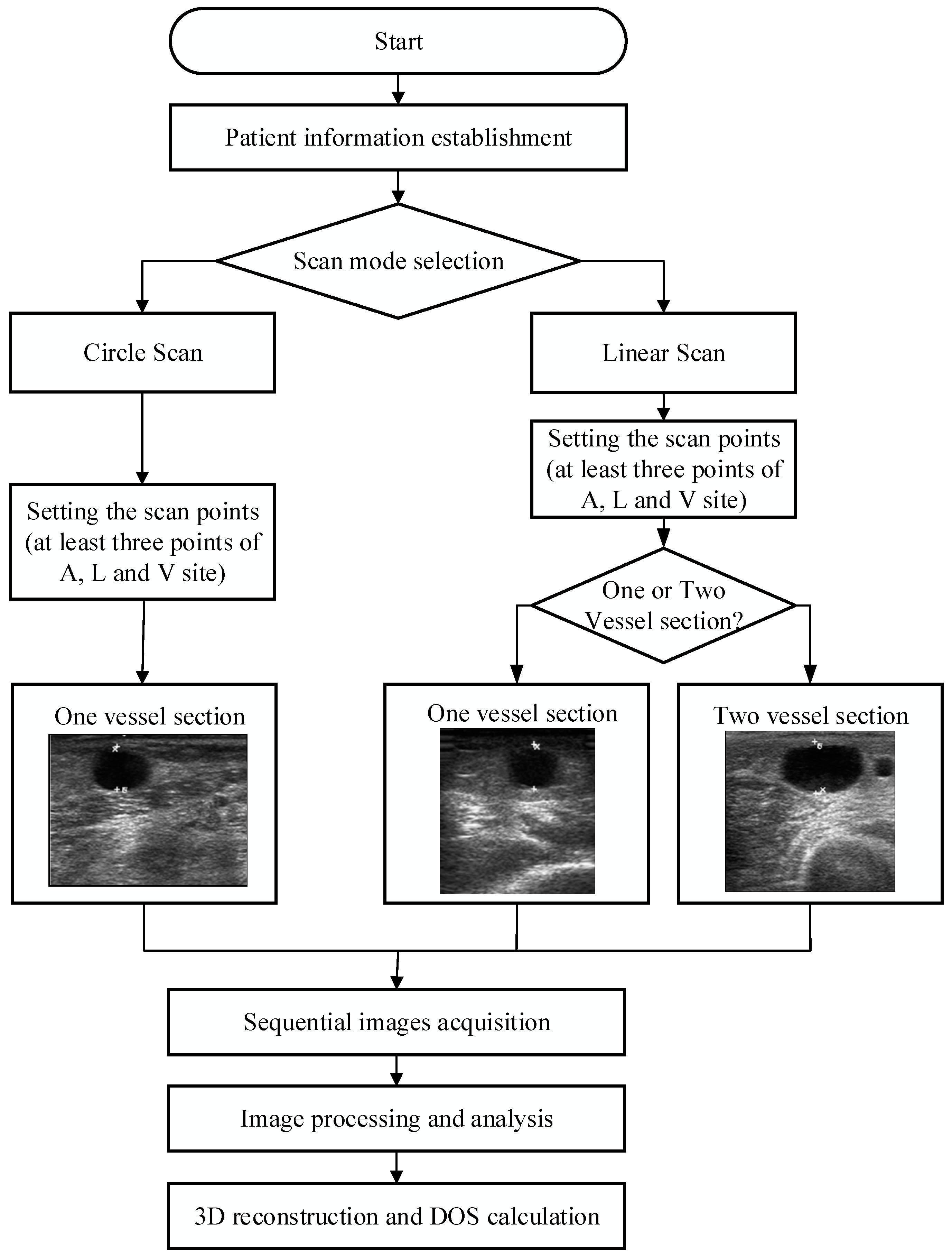
2.5. Scanning Methods
2.6. Scan Path Computation
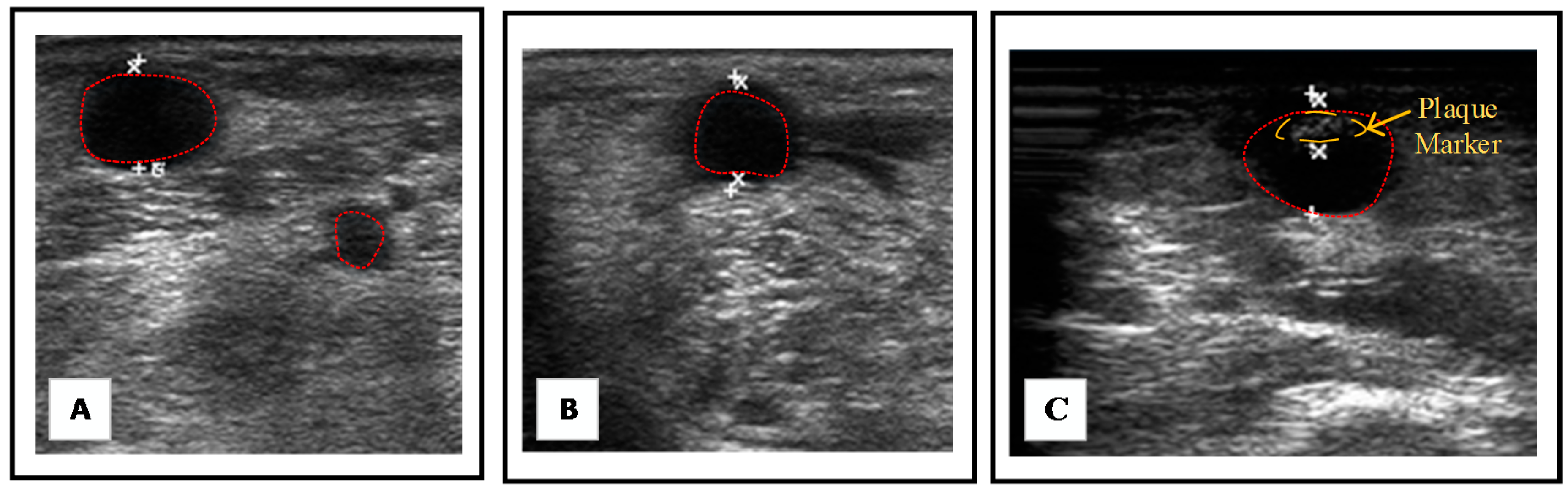
2.7. B-Mode Image Processing and Reconstruction
3. Experimental Design
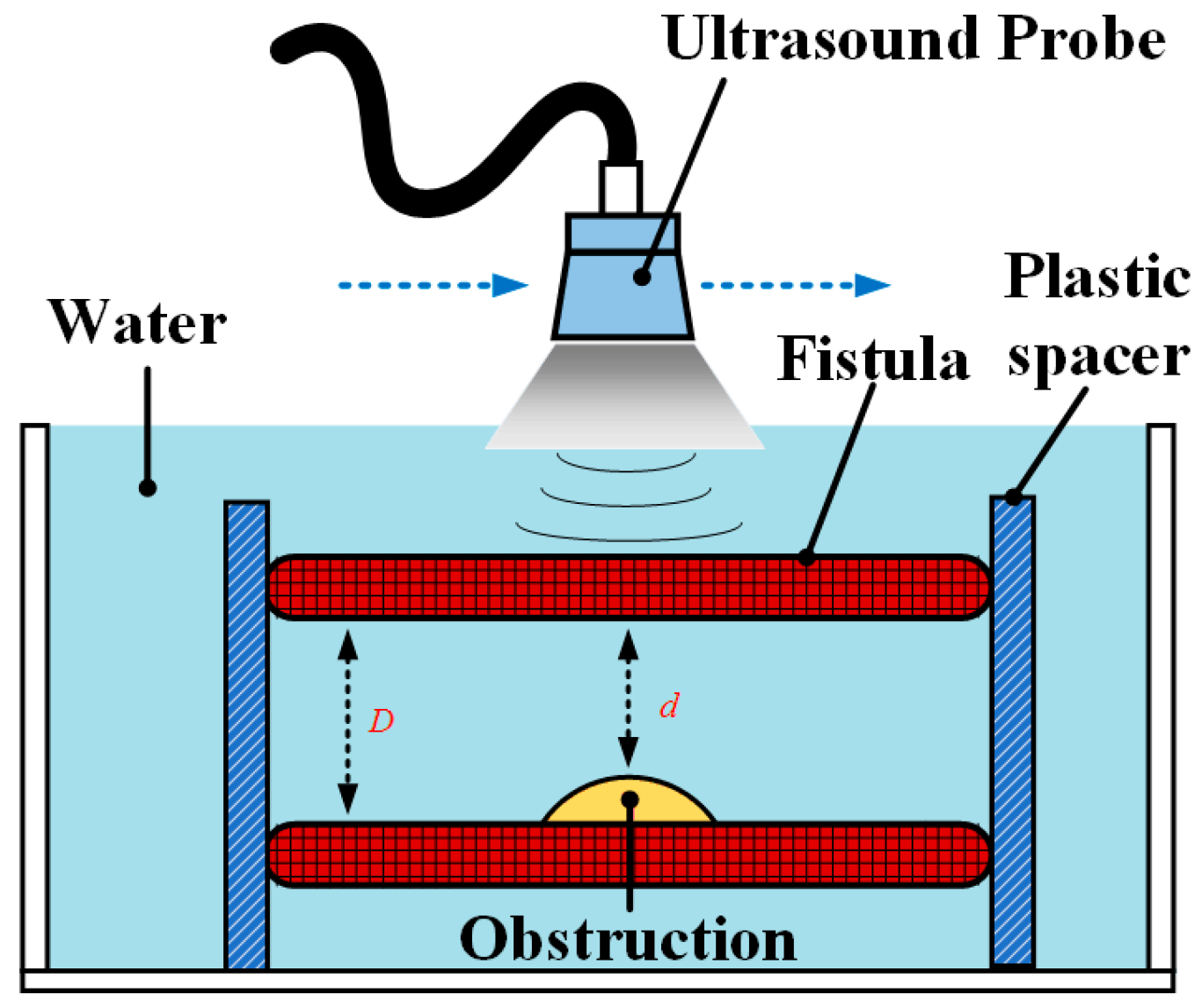
3.1. Phantom Test
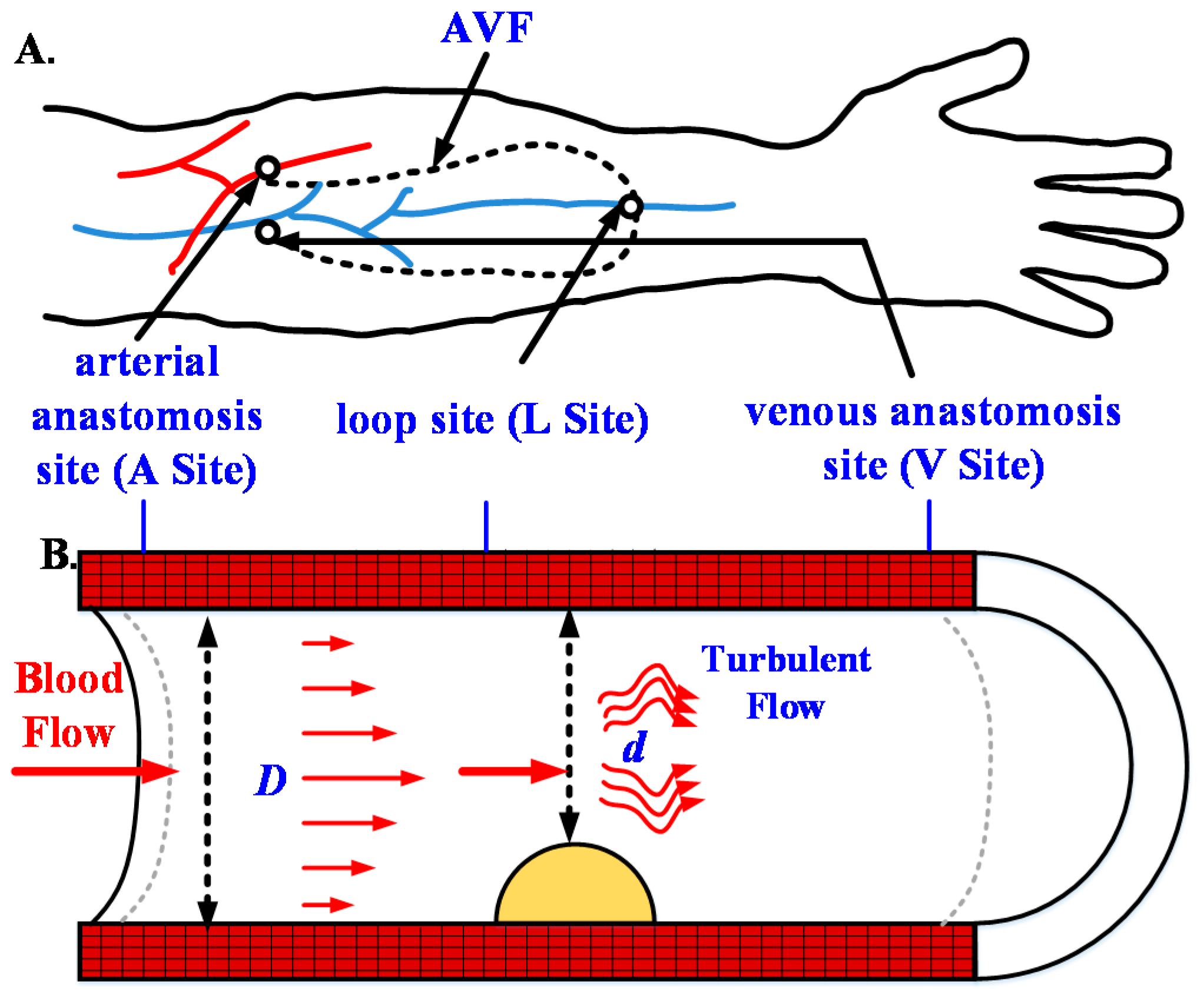
3.1.1. Design of Three Types of Fistula Phantoms
3.1.2. Verification and Comparison of the RUS with the CBCT
3.2. Clinical Trials
4. Results
4.1. Results of Phantom Test
4.2. Results of Clinical Trials
5. Conclusions and Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Al Ismaili, F.; Al Salmi, I.; Al Maimani, Y.; Metry, A.M.; Al Marhoobi, H.; Hola, A.; Pisoni, R.L. Epidemiological transition of end-stage kidney disease in oman. Kidney Int. Rep. 2017, 2, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C.; Chen, S.C.; Hwang, S.J.; Chang, J.M.; Lin, M.Y.; Chen, H.C. Prevalence and risk factors for CKD in spouses and relatives of hemodialysis patients. Am. J. Kidney Dis. 2010, 55, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.Y.S.; Lai, S.K.; Leung, K.M.; Leung, G.K.K.; Chow, K.W. Influence of the aspect ratio on the endovascular treatment of intracranial aneurysms: A computational investigation. J. Biomed. Sci. Eng. 2012, 5, 422–431. [Google Scholar] [CrossRef]
- Farghaly, A.; Marakbi, A. Balloon angioplasty in the treatment of stenosis of brachio-cephalic and brachio-basilicarteriovenous fistulae for haemodialysis. Kasr El-Aini J. Surg. 2011, 12, 1–4. [Google Scholar]
- Liang, A.; Wang, Y.; Han, G.; Truong, L.; Cheng, J. Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am. J. Physiol. Renal Physiol. 2013, 304, 1413–1414. [Google Scholar] [CrossRef] [PubMed]
- Dember, L.M.; Imrey, P.B.; Beck, G.J.; Cheung, A.K.; Himmelfarb, J.; Huber, T.S.; Kusek, J.W.; Roy-Chaudhury, P.; Vazquez, M.A.; Alpers, C.E.; et al. Objectives and design of the hemodialysis fistula maturation study. Am. J. Kidney. Dis. 2014, 63, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.K.; Ahanchi, S.S.; Dexter, D.J.; Glickman, M.H.; Panneton, J.M. Patient compliance limits the efforts of quality improvement initiatives on arteriovenous fistula maturation. J. Vasc. Surg. 2015, 61, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Nakadate, R.; Solis, J.; Takanishi, A.; Sugawara, M.; Niki, K.; Minagawa, E. Development of the ultrasound probe holding robot WTA-1RII and an automated scanning algorithm based on ultrasound image feedback. In ROMANSY 18 Robot Design, Dynamics and Control; Springer: Vienna, Austria, 2010; pp. 359–366. [Google Scholar]
- Harrison, G.; Harris, A. Work-related musculoskeletal disorders in ultrasound: Can you reduce risk? Ultrasound 2015, 23, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Oleynikov, D.; Gould, J.; Azagury, D.; Sandler, B.; Hutter, M.; Ross, S.; Haas, E.; Brody, F.; Satava, R. SAGES TAVAC safety and effectiveness analysis: Da Vinci® Surgical System (Intuitive Surgical, Sunnyvale, CA). Surg. Endosc. 2015, 29, 2873–2884. [Google Scholar] [CrossRef] [PubMed]
- Freschi, C.; Ferrari, V.; Melfi, F.; Ferrari, M.; Mosca, F.; Cuschieri, A. Technical review of the da Vinci surgical telemanipulator. Int. J. Med. Robot. Comput. Assist. Surg. 2013, 9, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, G.R.; Wolfsberger, S.; Lama, S.; Zarei-Nia, K. The evolution of neuroArm. Neurosurgery 2013, 72, A27–A32. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, D.R.; Cleary, K.; Wilson, E.; Azizi-Koutenaei, B.; Monfaredi, R. Robotic arm–assisted sonography: Review of technical developments and potential clinical applications. Am. J. Roentgenol. 2017, 208, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Priester, A.M.; Natarajan, S.; Culjat, M.O. Robotic ultrasound systems in medicine. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Nakadate, R.; Solis, J.; Takanishi, A.; Minagawa, E.; Sugawara, M.; Niki, K. Out-of-plane visual servoing method for tracking the carotid artery with a robot-assisted ultrasound diagnostic system. In Proceedings of the 2011 IEEE International Conference on Robotics and Automation (ICRA), Shanghai, China, 9–13 May 2011. [Google Scholar]
- Mustafa, A.S.B.; Ishii, T.; Matsunaga, Y.; Nakadate, R.; Ishii, H.; Ogawa, K.; Saito, A.; Sugawara, M.; Niki, K.; Takanishi, A. Development of robotic system for autonomous liver screening using ultrasound scanning device. In Proceedings of the 2013 IEEE International Conference on Robotics and Biomimetics (ROBIO), Shenzhen, China, 12–14 December 2013. [Google Scholar]
- Janvier, M.A.; Merouche, S.; Allard, L.; Soulez, G.; Cloutier, G. A 3-D ultrasound imaging robotic system to detect and quantify lower limb arterial stenoses: In vivo feasibility. Ultrasound Med. Biol. 2014, 40, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Chadli, S.; Ababou, N.; Ababou, A.; Djelal, N.; Saadia, N. A removable device for axial force and orientation measurement on medical ultrasound probe. In Proceedings of the 2012 9th IEEE International Multi-Conference on Systems, Signals and Devices (SSD), Chemnitz, Germany, 20–23 March 2012. [Google Scholar]
- Du, Y.C.; Stephanus, A. A novel classification technique of arteriovenous fistula stenosis evaluation using bilateral PPG analysis. Micromachines 2016, 7, 147. [Google Scholar] [CrossRef]
- MICROBOT TeachMover II Instruction Manual. Available online: http://www.theoldrobots.com/book71/Teachmover_II.pdf (accessed on 28 January 2018).
- Uneo Inc. Available online: http://www.uneotech.com/uneo/ (accessed on 28 January 2018).
- Liu, B.; Cheng, H.D.; Huang, J.; Tian, J.; Tang, X.; Liu, J. Fully automatic and segmentation-robust classification of breast tumors based on local texture analysis of ultrasound images. Pattern Recognit. 2010, 43, 280–298. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Song, H.; Zhou, W. Active contours with selective local or global segmentation: A new formulation and level set method. Image Vis. Comput. 2010, 28, 668–676. [Google Scholar] [CrossRef]
- Guzman, P.; Ros, R.; Ros, E. Artery segmentation in ultrasound images based on an evolutionary scheme. Informatics 2014, 1, 52–71. [Google Scholar] [CrossRef]
- Leon, C.; Asif, A. Arteriovenous access and hand pain: The distal hypoperfusion ischemic syndrome. Clin. J. Am. Soc. Nephrol. 2007, 2, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Nikitichev, D.I.; Barburas, A.; McPherson, K.; Mari, J.M.; West, S.J.; Desjardins, A.E. Construction of 3-Dimensional printed ultrasound phantoms with wall-less vessels. J. Ultrasound Med. 2016, 35, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Van Canneyt, K.; Pourchez, T.; Eloot, S.; Guillame, C.; Bonnet, A.; Segers, P.; Verdonck, P. Hemodynamic impact of anastomosis size and angle in side-to-end arteriovenous fistulae: A computer analysis. J. Vasc. Access 2010, 11, 52–58. [Google Scholar] [CrossRef] [PubMed]



| Phantom Test | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Design | CBCT | RUS | Error (%) | |||||||||
| Type/Class | d | D | DOS | d | D | DOS | d | D | DOS | Design and CBCT | CBCT and RUS | |
| A | I | 9 | 10 | 19.0% | 8.8 | 9.8 | 20.6% | 8.7 | 9.8 | 21.2% | 1.6% | 0.6% |
| II | 7.8 | 10 | 40.2% | 7.5 | 9.8 | 42.6% | 7.3 | 9.7 | 43.4% | 2.4% | 0.7% | |
| III | 4.5 | 10 | 80.2% | 4.3 | 10 | 81.5% | 4.2 | 9.8 | 81.6% | 1.3% | 1.0% | |
| B | I | 7 | 8 | 23.0% | 7 | 7.9 | 21.5% | 7.1 | 8.1 | 23.2% | 1.5% | 1.7% |
| II | 6.2 | 8 | 40.0% | 6.2 | 7.9 | 38.4% | 6 | 7.8 | 40.8% | 1.6% | 2.4% | |
| III | 3.5 | 8 | 80.9% | 3.3 | 7.8 | 82.2% | 3.1 | 7.9 | 84.6% | 1.3% | 2.4% | |
| C | I | 4.5 | 5 | 19.0% | 4.2 | 4.8 | 23.4% | 3.9 | 4.5 | 24.9% | 4.4% | 1.5% |
| II | 3.9 | 5 | 39.2% | 3.6 | 4.8 | 43.3% | 3.5 | 4.7 | 44.5% | 4.1% | 2.2% | |
| III | 2.2 | 5 | 80.6% | 1.8 | 4.6 | 84.7% | 1.6 | 4.5 | 87.4% | 4.1% | 2.7% | |
| Measured by RUS | Measured by Doctor | Average Error (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | D | d | DOSRUS | Variation | D | d | DOSDoc | Variation | |
| 1 | 76.8 | 23.6 | 90.6 | 1.2% | 75.2 | 19.8 | 93.0 | 4.7% | 2.4 |
| 2 | 88.3 | 44.4 | 74.7 | 0.6% | 86.8 | 41.7 | 76.9 | 6.8% | 2.9 |
| 3 | 128.0 | 96.0 | 43.8 | 1.8% | 125.7 | 88 | 50.9 | 17.1% | 7.1 |
| 4 | 108.0 | 85.1 | 37.9 | 2.1% | 107.2 | 81.8 | 41.8 | 9.6% | 3.9 |
| 5 | 96.1 | 81.2 | 28.6 | 1.1% | 94.8 | 78.4 | 31.6 | 8.8% | 3.0 |
| 6 | 170.0 | 143.4 | 28.8 | 2.2% | 169.8 | 140.3 | 31.7 | 12.9% | 2.9 |
| 7 | 93.2 | 77.2 | 31.3 | 1.4% | 90.1 | 74.2 | 32.1 | 7.0% | 2.5 |
| 8 | 113.0 | 92.8 | 32.6 | 1.6% | 109.2 | 85.1 | 39.2 | 11.1% | 6.6 |
| 9 | 98.5 | 79.8 | 34.4 | 1.5% | 97.8 | 73.4 | 43.7 | 3.6% | 9.3 |
| 10 | 96.0 | 52.2 | 70.4 | 2.0% | 93.1 | 48.6 | 72.7 | 2.4% | 2.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.-C.; Shih, J.-B.; Wu, M.-J.; Chiou, C.-Y. Development of an AVF Stenosis Assessment Tool for Hemodialysis Patients Using Robotic Ultrasound System. Micromachines 2018, 9, 51. https://doi.org/10.3390/mi9020051
Du Y-C, Shih J-B, Wu M-J, Chiou C-Y. Development of an AVF Stenosis Assessment Tool for Hemodialysis Patients Using Robotic Ultrasound System. Micromachines. 2018; 9(2):51. https://doi.org/10.3390/mi9020051
Chicago/Turabian StyleDu, Yi-Chun, Jheng-Bang Shih, Ming-Jui Wu, and Chung-Yi Chiou. 2018. "Development of an AVF Stenosis Assessment Tool for Hemodialysis Patients Using Robotic Ultrasound System" Micromachines 9, no. 2: 51. https://doi.org/10.3390/mi9020051





