Sinusoidal Microchannel with Descending Curves for Varicose Veins Implantation
Abstract
:1. Introduction
1.1. Sclerotherapy and Foam Sclerotherapy of Large Veins
1.2. Laser Surgeries and Radiofrequency
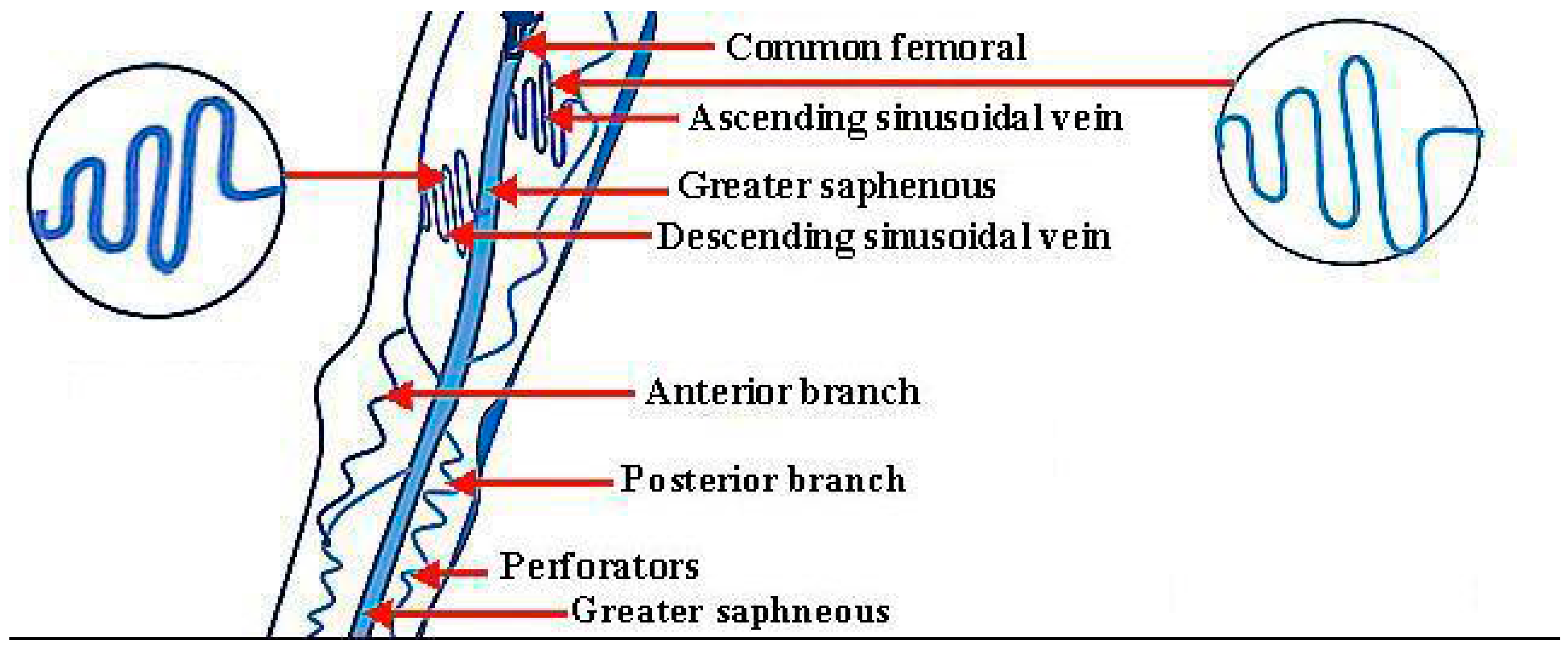
1.3. Vein Ligation and Stripping
1.4. Ambulatory Phlebectomy
1.5. Endoscopic Vein Surgery
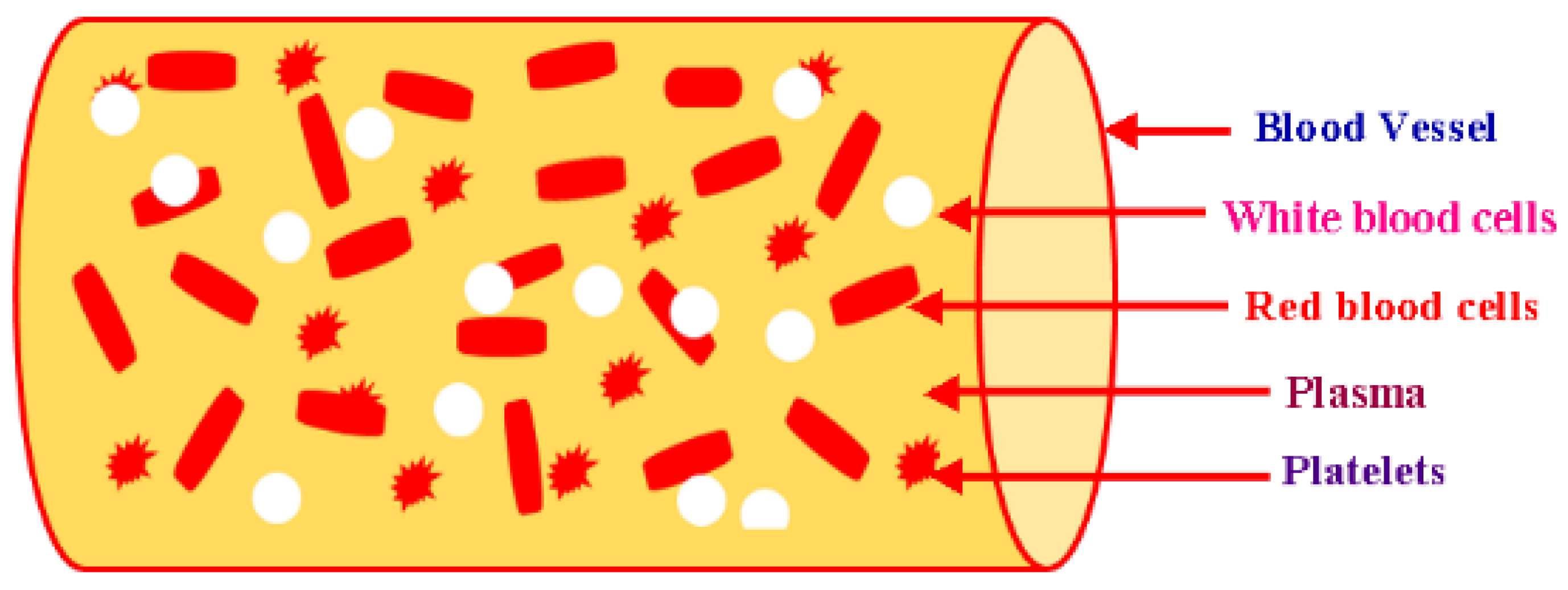
2. Blood Rheology
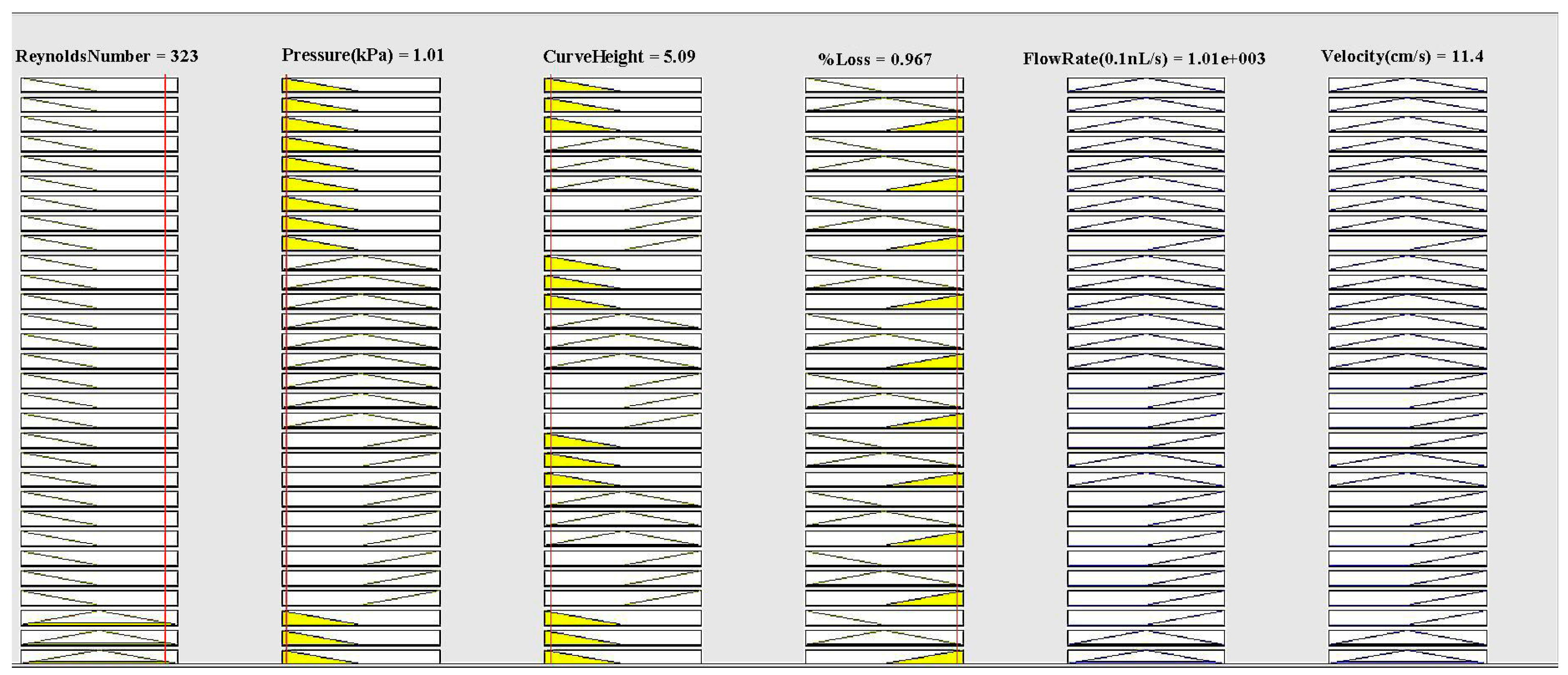
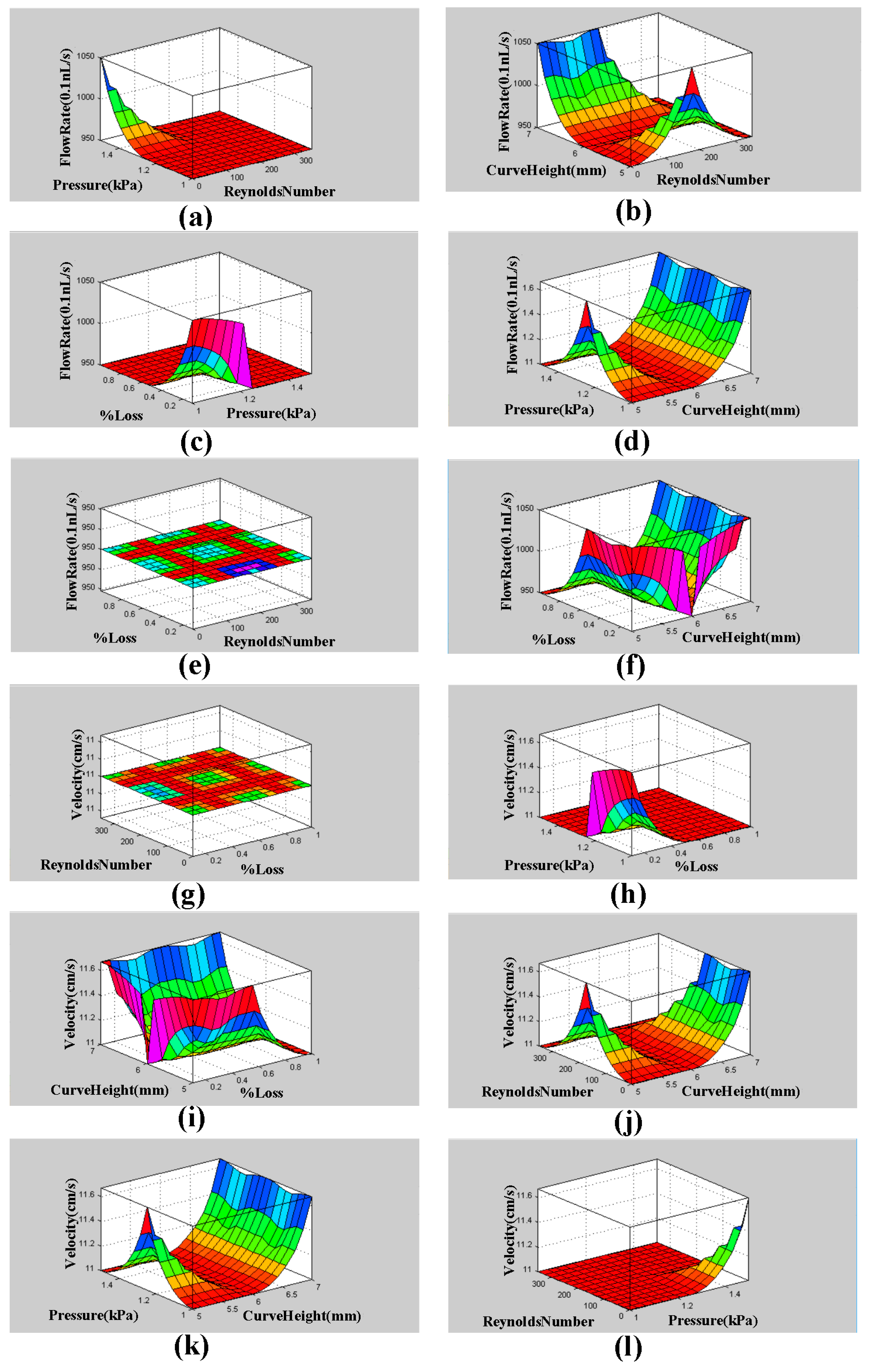
3. Fuzzy Logic MATLAB Simulation for Descending Sinusoidal Microchannel (DSMC)
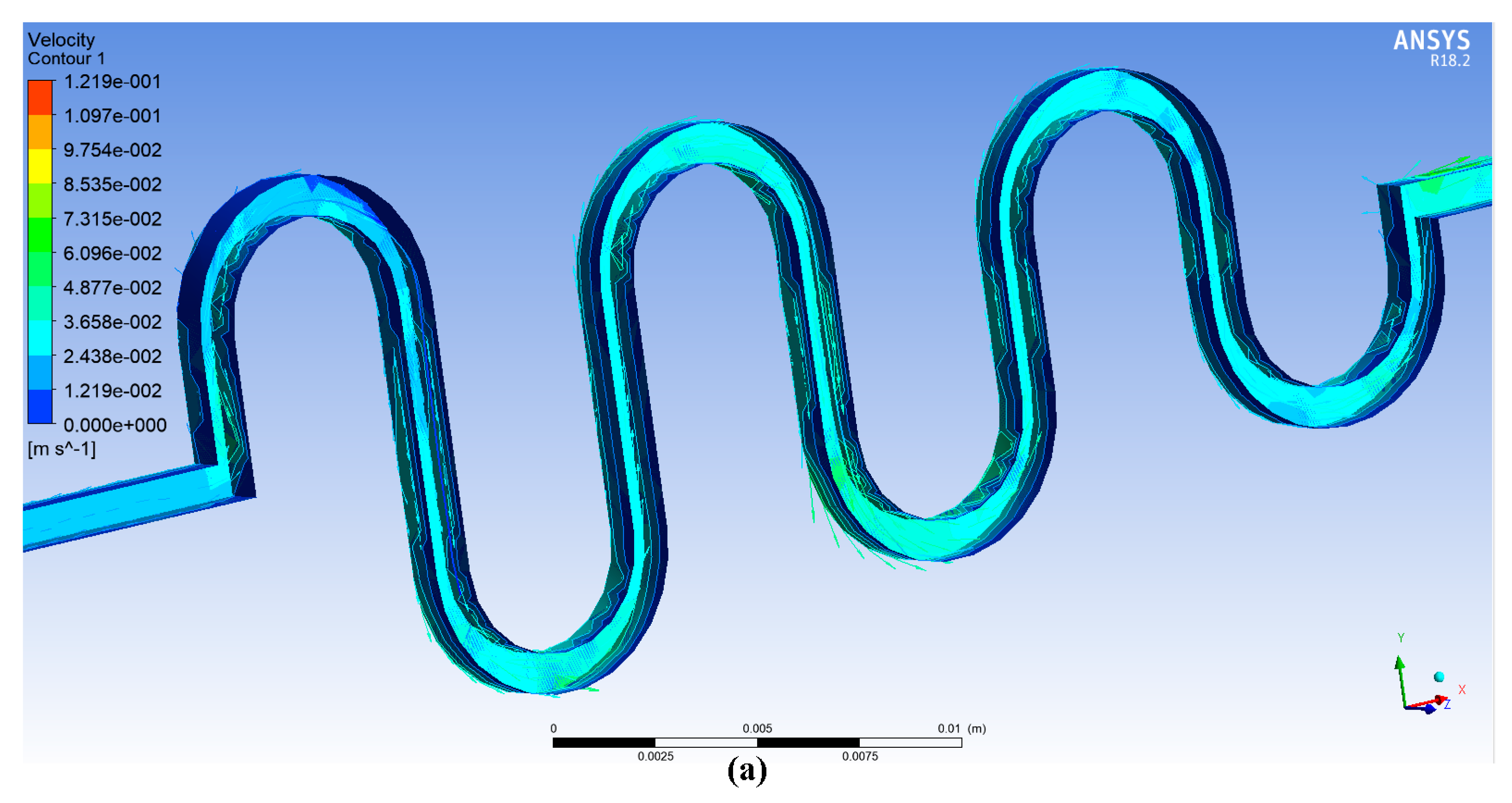
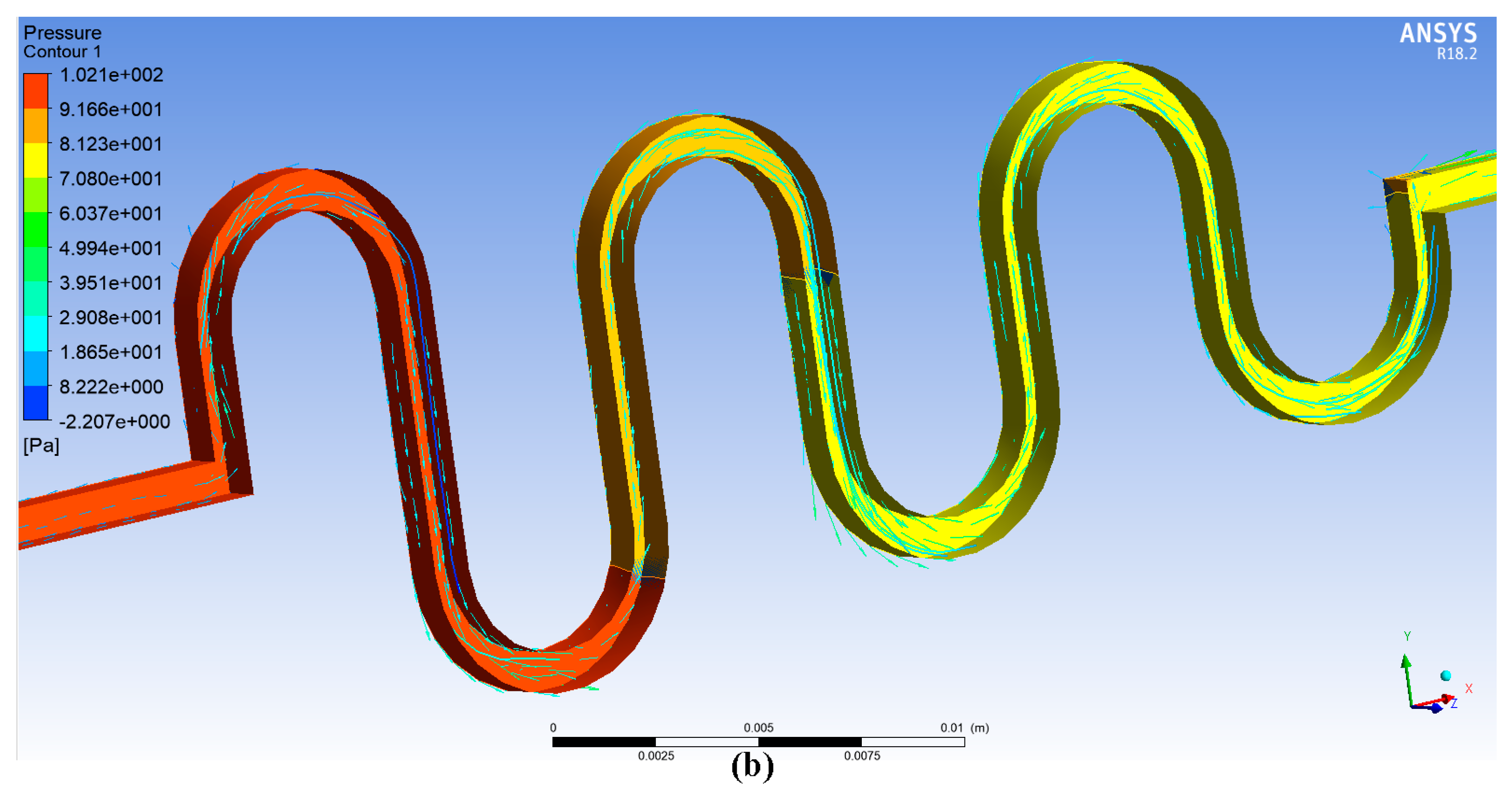
4. ANSYS Fluent Simulation for DSMC
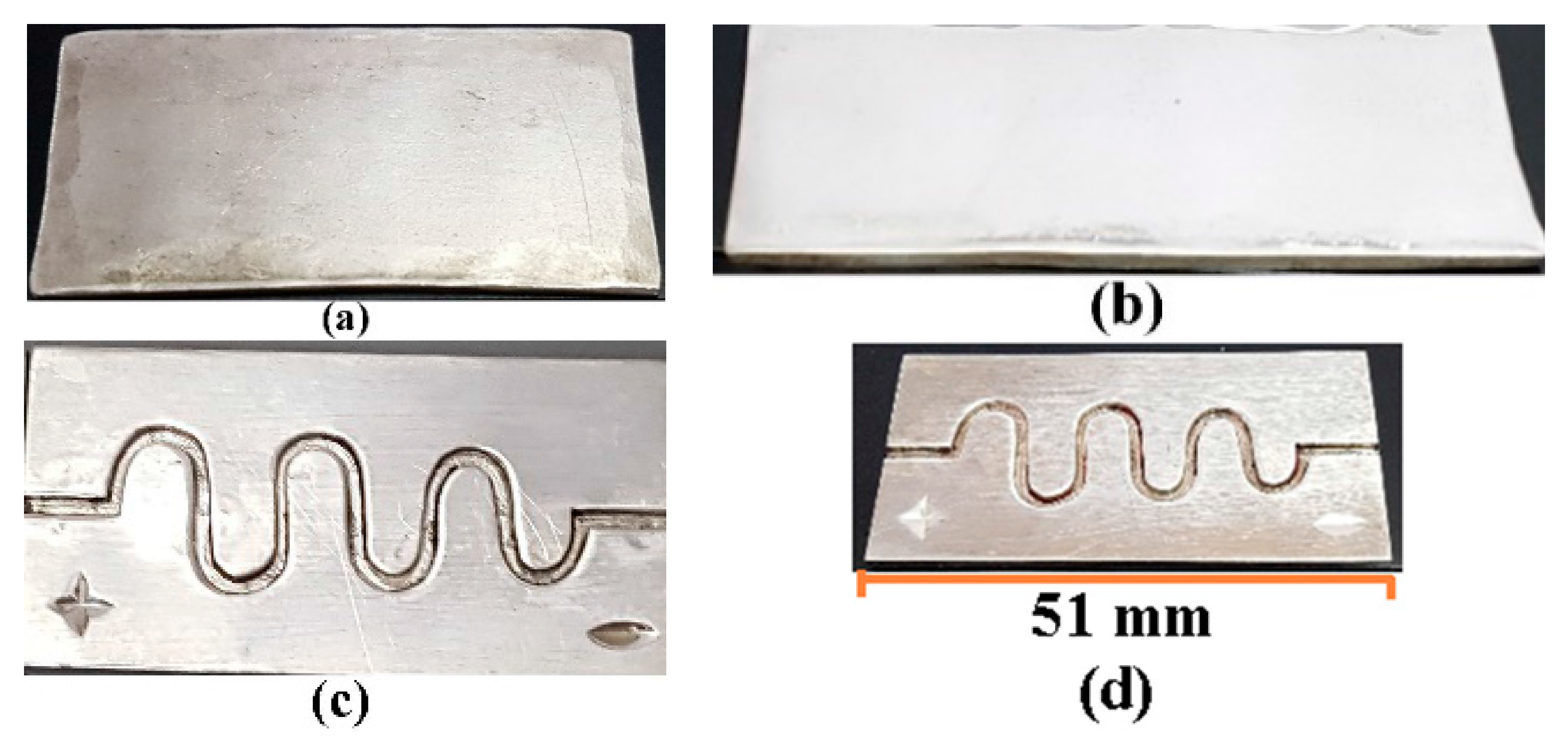
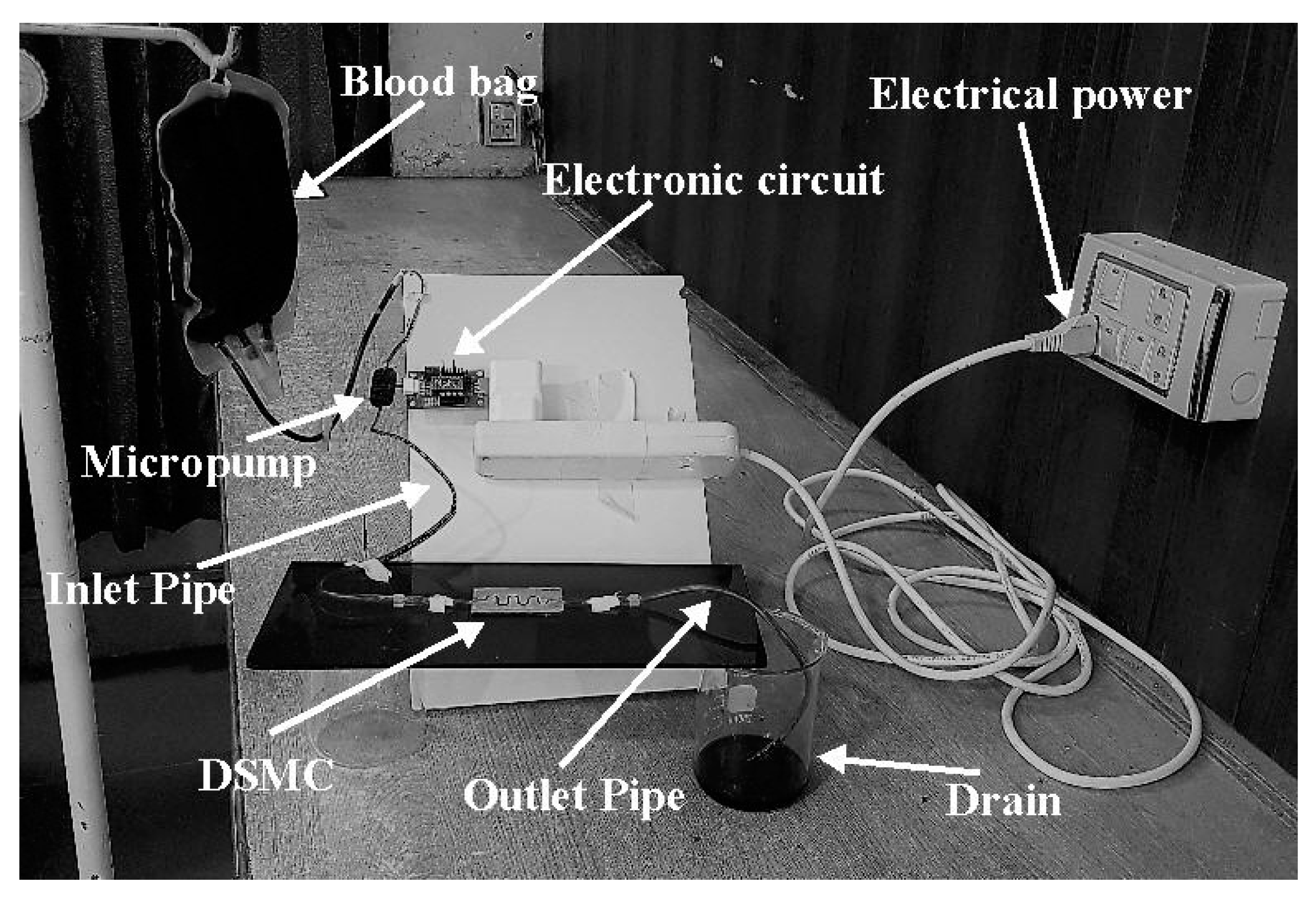
5. Fabrication of DSMC and Experimental Setup
6. Results and Discussion
6.1. Experimental Results
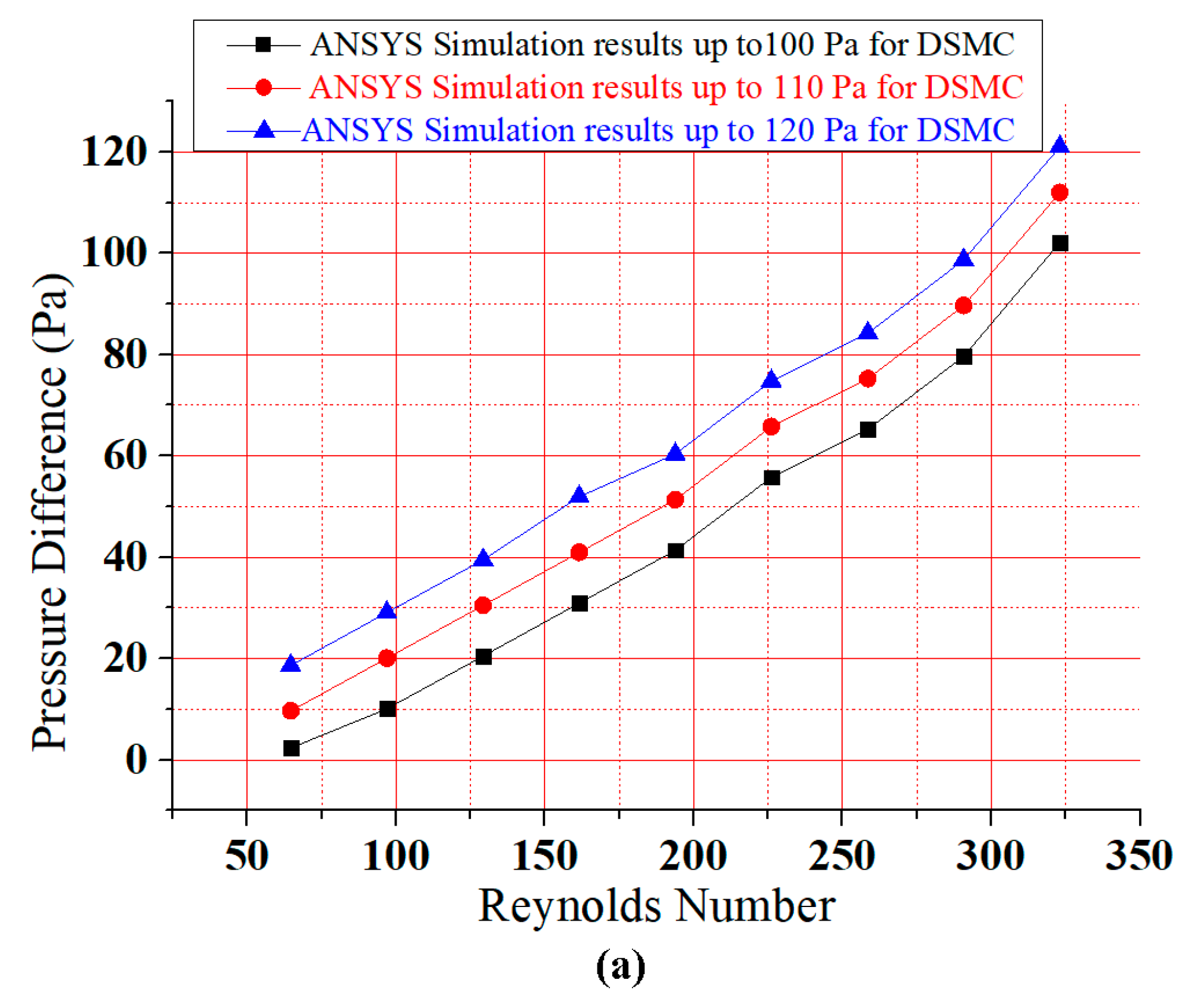
6.2. Dean Number
6.3. The Role of Diameter and Viscosity of the Vein and Tissue Damage with the Flow of Blood
6.4. Implantable Devices in Humans
6.5. Comparison of ASMC and DSMC
6.6. Results Comparison
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Anwar, M.A.; Adesina-Georgiadis, K.N.; Spagou, K.; Vorkas, P.A.; Li, J.V.; Shalhoub, J.; Holmes, E.; Davies, A.H. A comprehensive characterisation of the metabolic profile of varicose veins; implications in elaborating plausible cellular pathways for disease pathogenesis. Sci. Rep. 2017, 7, 2989. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.J.; Tayyaba, S.; Ashraf, M.W.; Hossain, M.K.; Uddin, M.J.; Afzulpurkar, N. Simulation, fabrication and analysis of silver based ascending sinusoidal microchannel (ASMC) for implant of varicose veins. Micromachines 2017, 8, 278. [Google Scholar] [CrossRef]
- Hirai, M.; Naiki, K.; Nakayama, R. Prevalence and risk factors of varicose veins in Japanese women. Angiology 1990, 41, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Sansilvestri-Morel, P.; Rupin, A.; Badier-Commander, C.; Kern, P.; Fabiani, J.; Verbeuren, T.J.; Vanhoutte, P.M. Imbalance in the synthesis of collagen type I and collagen type III in smooth muscle cells derived from human varicose veins. J. Vasc. Res. 2001, 38, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Tessari, L.; Cavezzi, A.; Frullini, A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol. Surg. 2001, 27, 58–60. [Google Scholar] [PubMed]
- Blomgren, L.; Johansson, G.; Dahlberg-Åkerman, A.; Norén, A.; Brundin, C.; Nordström, E.; Bergqvist, D. Recurrent varicose veins: Incidence, risk factors and groin anatomy. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Lawaetz, M.; Serup, J.; Lawaetz, B.; Bjoern, L.; Blemings, A.; Eklof, B.; Rasmussen, L. Comparison of endovenous radiofrequency ablation, laser ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Extended 5-year follow-up of a RCT. Int. Angiol. 2017, 53, e14. [Google Scholar] [CrossRef]
- Belcaro, G.; Dugall, M.; Luzzi, R.; Corsi, M.; Ledda, A.; Ricci, A.; Pellegrini, L.; Cesarone, M.R.; Hosoi, M.; Errichi, B.M. Management of varicose veins and chronic venous insufficiency in a comparative registry with nine venoactive products in comparison with stockings. Int. J. Angiol. 2017, 26, 170–178. [Google Scholar] [PubMed]
- Dabbs, E.; Nemchand, J.L.; Whiteley, M.S. Suprapubic varicose vein formation during pregnancy following pre-pregnancy pelvic vein embolisation with coils, without any residual pelvic venous reflux or obstruction. SAGE Open Med. Case Rep. 2017, 5, 2050313X17724712. [Google Scholar] [CrossRef] [PubMed]
- Bothra, N.; Panda, L.; Sheth, J.; Tripathy, D. Role of intralesional bleomycin sclerotherapy as the sole or adjunct treatment of superficial ocular adnexal lymphatic malformations. Eye 2017, 32, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.S. Duplex ultrasound guided catheter directed foam sclerotherapy for treatment of axial varicose veins of the lower limbs and its preliminary results. Int. J. Surg. Res. 2017, 6, 1–4. [Google Scholar]
- Pires, M.F.B.; Nogueira, R.F.; Navarro, T.P. Chronic Venous Disease and Varicose Veins, in Vascular Diseases for the Non-Specialist; Springer: Berlin, Germany, 2017; pp. 167–181. [Google Scholar]
- Rao, S.V.S.; Datta, A.S.; Anvesh, D. Spectrum of chronic venous insufficiency of lower limbs-A katuri perspective. J. Evid. Based Med. Healthc. 2017, 4, 1246–1253. [Google Scholar]
- Argyriou, C.; Papasideris, C.; Antoniou, G.A.; Georgakarakos, E.; Papanas, N.; Lazarides, M.K.; Georgiadis, G.S. The effectiveness of various interventions versus standard stripping in patients with varicose veins in terms of quality of life. Phlebology 2017. [Google Scholar] [CrossRef] [PubMed]
- Hager, E.S.; Ozvath, K.J.; Dillavou, E.D. Evidence summary of combined saphenous ablation and treatment of varicosities versus staged phlebectomy. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Zafarghandi, M.R.; Farsavian, H.; Davoudi, M.; Farsavian, A.A. Outcome of ultrasonography-guided foam sclerotherapy versus stab avulsion ambulatory phlebectomy in the treatment of varicosis in small veins of the leg: A single blinded randomized clinical trial. Iran. J. Radiol. 2017, in press. [Google Scholar] [CrossRef]
- Rajam, V.; Kathaperumal, K.D.A.; Hemant, U.; Rajavelu, N. Study of clinical outcomes of subfascial perforator ligation surgery in perforator incompetence. J. Evid. Based Med. Healthc. 2017, 4, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-N.; Hsieh, T.-Y.; Huang, S.-H.; Liu, C.-M.; Chang, K.-P.; Lin, S.-D. Management of venous ulcers according to their anatomical relationship with varicose veins. Phlebology 2017, 33, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.; Ramesh, R.; Aravind, T. Analysis of different size microchannel through particle tracing for biomolecule separation. J. Comput. Theor. Nanosci. 2017, 14, 3351–3355. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, P.; Bian, S.; Shi, G.; Liu, P.; Zong, S.; Wang, W. A novel bioMEMS device for efficient on-chip single cell loading and 3D rotation. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017. [Google Scholar]
- Wang, S.-L.; Fan, S.-K. DNA stretching in surfactant-stabilized microchannel. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017. [Google Scholar]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ohta, A.T. Editorial for the Special Issue on Microdevices and Microsystems for Cell Manipulation; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2017. [Google Scholar]
- Birch, C.; Landers, J.P. Electrode materials in microfluidic systems for the processing and separation of DNA: A mini review. Micromachines 2017, 8, 76. [Google Scholar] [CrossRef]
- Pinto, E.; Faustino, V.; Rodrigues, R.O.; Pinho, D.; Garcia, V.; Miranda, J.M.; Lima, R. A rapid and low-cost nonlithographic method to fabricate biomedical microdevices for blood flow analysis. Micromachines 2014, 6, 121–135. [Google Scholar] [CrossRef]
- Sundell, G.; Dahlin, C.; Andersson, M.; Thuvander, M. The bone-implant interface of dental implants in humans on the atomic scale. Acta Biomater. 2017, 48, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Küçük, M.; Çömlekoğlu, M.E.; Zor, M. The effect of crown geometry on stress distribution of a single implant restoration: A finite element analysis. Turkiye Klinikleri J. Dent. Sci. 2010, 16, 136–141. [Google Scholar]
- Dai, P.; Wang, B.; Bao, C.; Ju, Y. Constructing a computer model of the human eye based on tissue slice images. J. Biomed. Imaging 2010, 2010, 15. [Google Scholar] [CrossRef] [PubMed]
- Todd, C.; Naghdy, F. Virtual cochlear implant insertion for medical education. In Proceedings of the Eurohaptics Conference, 2005 and Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems, Pisa, Italy, 18–20 March 2005. [Google Scholar]
- Zhang, W.; Li, Z.; Gilles, M.; Wu, D. Mechanical simulation of neural electrode-brain tissue interface under various micromotion conditions. J. Med. Biol. Eng. 2014, 34, 386–392. [Google Scholar] [CrossRef]
- Karanasiou, G.S.; Sakellarios, A.I.; Tripoliti, E.E.; Petrakis, E.G.M.; Zervakis, M.E.; Migliavacca, F.; Dubini, G.; Dordoni, E.; Michalis, L.K.; Fotiadis, D.I. Modeling stent deployment in realistic arterial segment geometries: The effect of the plaque composition. In Proceedings of the 2013 IEEE 13th International Conference on Bioinformatics and Bioengineering (BIBE), Chania, Greece, 10–13 November 2013. [Google Scholar]
- Shah, Y.; Bhave, A.; Sonetha, V. Fatigue analysis of the knee joint. Procedia Comput. Sci. 2015, 45, 250–255. [Google Scholar] [CrossRef]
- Kim, S.; Feinberg, B.; Kant, R.; Chui, B.; Goldman, K.; Park, J.; Moses, W.; Blaha, C.; Iqbal, Z.; Chow, C. Diffusive silicon nanopore membranes for hemodialysis applications. PLoS ONE 2016, 11, e0159526. [Google Scholar] [CrossRef] [PubMed]
- Zak, J.; Hadas, Z.; Dusek, D.; Pekarek, J.; Svatos, V.; Janak, L.; Prasek, J.; Hubalek, J. The charge push-through electronics design for fully implantable artificial cochlea powered by energy harvesting technologies. Microsyst. Technol. 2016, 22, 1709–1719. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Blood rheology and hemodynamics. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Skalak, R.; Keller, S.; Secomb, T. Mechanics of blood flow. J. Biomech. Eng. 1981, 103, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, P.; Fraser, K.H.; Griffith, B.P.; Wu, Z.J. Comparison of fluid dynamic numerical models for a clinical ventricular assist device and experimental validation. Artif. Organs 2013, 37, 380. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.-C.; Hsiao, H.-D.; Chen, P.-Y.; Tu, C.-Y.; Tseng, T.-I.; Ho, Y.-J. Study of pre-and post-stent implantation in the trachea using computational fluid dynamics. J. Med. Biol. Eng. 2014, 34, 150–156. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, J.L.; Itatani, K.; Miyaji, K.; Umezu, M. Computational hemodynamic analysis in congenital heart disease: Simulation of the Norwood procedure. Ann. Biomed. Eng. 2010, 38, 2302–2313. [Google Scholar] [CrossRef] [PubMed]
- Heck, M.L.; Yen, A.; Snyder, T.A.; O'Rear, E.A.; Papavassiliou, D.V. Flow-field simulations and hemolysis estimates for the food and drug administration critical path initiative centrifugal blood pump. Artif. Organs 2017, 41, E129–E140. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.A. Computational fluid dynamics as a tool for testing functional and ecological hypotheses in fossil taxa. Palaeontology 2017. [Google Scholar] [CrossRef]
- Cherng, W.-J.; Dong, Z.-S.; Chou, C.-C.; Yeh, C.-H.; Pan, Y.-H. Hydrodynamic simulation of an orbital shaking test for the degradation assessment of blood-contact biomedical coatings. Micromachines 2017, 8, 132. [Google Scholar] [CrossRef]
- Elblbesy, M.A.; Hereba, A.T. Computation of the coefficients of the power law model for whole blood and their correlation with blood parameters. Appl. Phys. Res. 2016, 8. [Google Scholar] [CrossRef]
- Chiam, Z.L.; Lee, P.S.; Singh, P.K.; Mou, N. Investigation of fluid flow and heat transfer in wavy micro-channels with alternating secondary branches. Int. J. Heat Mass Transf. 2016, 101, 1316–1330. [Google Scholar] [CrossRef]
- Folch, A. Introduction to bioMEMS; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Nicolaides, A.N.; Zukowski, A.J. The value of dynamic venous pressure measurements. World J. Surg. 1986, 10, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.-C. Biomechanics: Circulation; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Smieško, V.; Kožík, J.; Doležel, S. Role of endothelium in the control of arterial diameter by blood flow. J. Vasc. Res. 1985, 22, 247–251. [Google Scholar]
- Letcher, R.L.; Chien, S.; Pickering, T.G.; Sealey, J.E.; Laragh, J.H. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects: Role of fibrinogen and concentration. Am. J. Med. 1981, 70, 1195–1202. [Google Scholar] [CrossRef]
- Fu, G.-X.; J, M.; Han, L.-Z.; Xu, C.-Z.; Pan, F.-F.; Hu, T.-J.; Zhong, Y. Erythrocyte rheological properties but not whole blood and plasma viscosity are associated with severity of hypertension in older people. Zeitschrift für Gerontologie und Geriatrie 2017, 50, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Rajagopal, K.R. A short review of advances in the modelling of blood rheology and clot formation. Fluids 2017, 2, 35. [Google Scholar] [CrossRef]
- Bento, D.; Sousa, L.; Yaginuma, T.; Garcia, V.; Lima, R.; Miranda, J.M. Microbubble moving in blood flow in microchannels: Effect on the cell-free layer and cell local concentration. Biomed. Microdevices 2017, 19, 6. [Google Scholar] [CrossRef] [PubMed]


| References | Medical Device | Organ | Material/Type | Software |
|---|---|---|---|---|
| Afzal et al. [2] | ASMC | Varicose vein | Silver | ANSYS, MATLAB |
| Sundell et al. [26] | Dental implant | Human jaw | Titanium | IVAS 3.6.6 software |
| Küçük et al. [27] | Cardiovascular implant | Heart | Electronics | Corrosion Analyzer |
| Dai et al. [28] | Intraocular lenses | Eyes | Plastic | ANSYS |
| Todd and Naghdy [29] | Cochlear implant | Ears | Electronics | ANSYS |
| Zhang et al. [30] | Intracranial electroencephalogram electrodes | Brain | Electronics | Finite element simulation |
| Georgia et al. [31] | Heart stent | Heart | Metal or plastic | ANSYS |
| Shah et al. [32] | Knee implant | Knee | Titanium and Stainless Steel | ANSYS |
| Kim et al. [33] | Wearable artificial kidney (WAK) | Kidney | Charcoal, activated carbon, and zeolite | ANSYS Fluent |
| Zak et al. [34] | Artificial dermis | Skin | Donated skin tissues and polymers | ANSYS |
| Model | Flow Rate in 0.1 nL/s | Velocity in cm/s |
|---|---|---|
| Mamdani’s value | 1001.2 | 11.5 |
| MATLAB simulation | 1001.0 | 11.4 |
| Difference | 0.2 | 0.1 |
| Error percentage | 0.02% | 0.85% |
| MATLAB Results | ANSYS Results | Experimental Results |
|---|---|---|
| Reynolds Number = 323 | Reynolds Number = 323 | Reynolds Number = 323 |
| Flow Rate = 1001.0 (0.1 nL/s) | Flow Rate = 1015.3 (0.1 nL/s) | Flow Rate = 995.3 (0.1 nL/s) |
| Velocity = 11.4 cm/s | Velocity = 12.19 cm/s | Velocity = 12.2 cm/s |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, M.J.; Ashraf, M.W.; Tayyaba, S.; Hossain, M.K.; Afzulpurkar, N. Sinusoidal Microchannel with Descending Curves for Varicose Veins Implantation. Micromachines 2018, 9, 59. https://doi.org/10.3390/mi9020059
Afzal MJ, Ashraf MW, Tayyaba S, Hossain MK, Afzulpurkar N. Sinusoidal Microchannel with Descending Curves for Varicose Veins Implantation. Micromachines. 2018; 9(2):59. https://doi.org/10.3390/mi9020059
Chicago/Turabian StyleAfzal, Muhammad Javaid, Muhammad Waseem Ashraf, Shahzadi Tayyaba, M. Khalid Hossain, and Nitin Afzulpurkar. 2018. "Sinusoidal Microchannel with Descending Curves for Varicose Veins Implantation" Micromachines 9, no. 2: 59. https://doi.org/10.3390/mi9020059






