Strategy for Monitoring Cardiac Interventions with an Intelligent Robotic Ultrasound Device †
Abstract
:1. Introduction
2. Materials and Methods
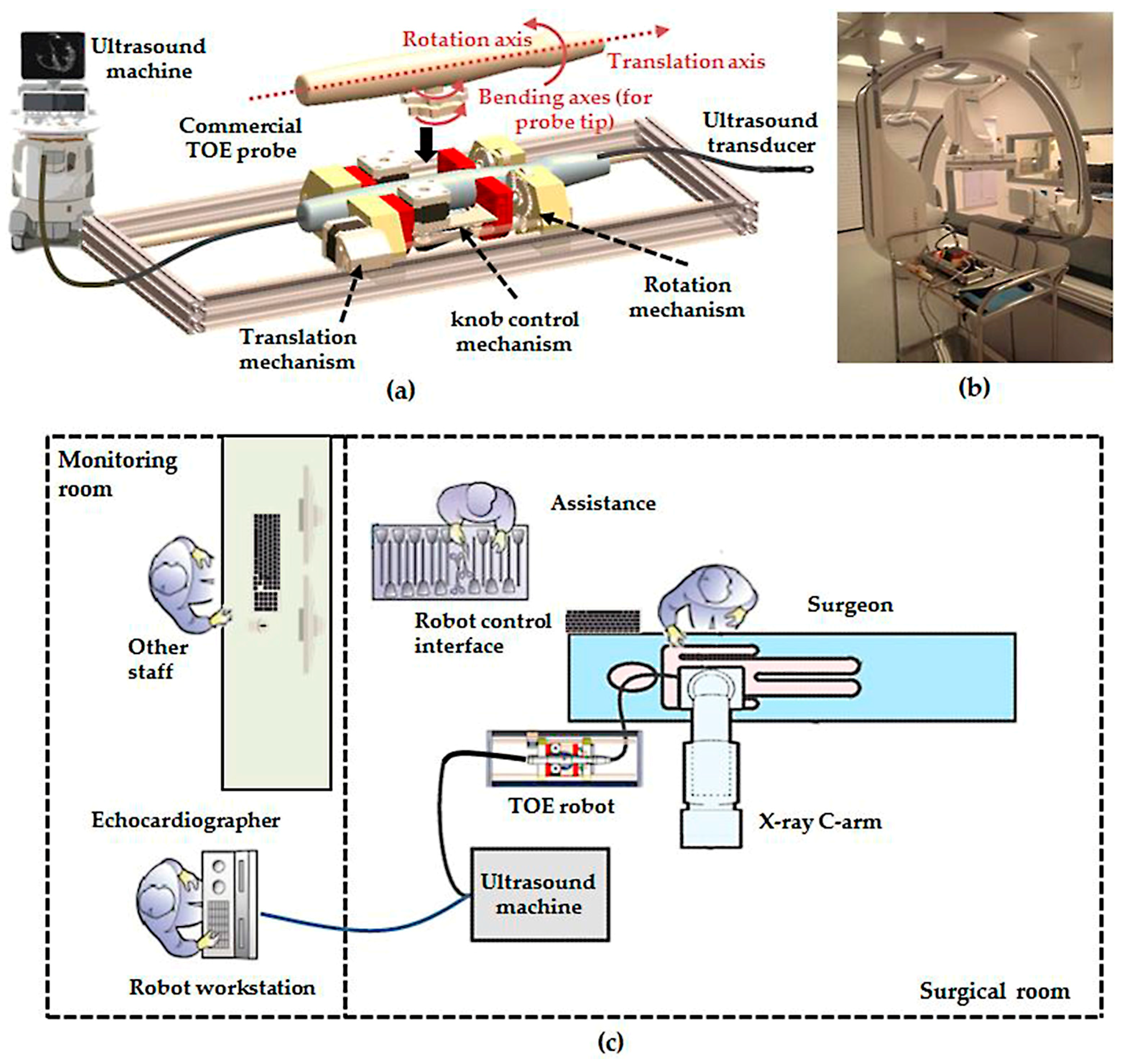
2.1. Overview of the Robotic Trans-Oesophageal Echocardiography (TOE) System
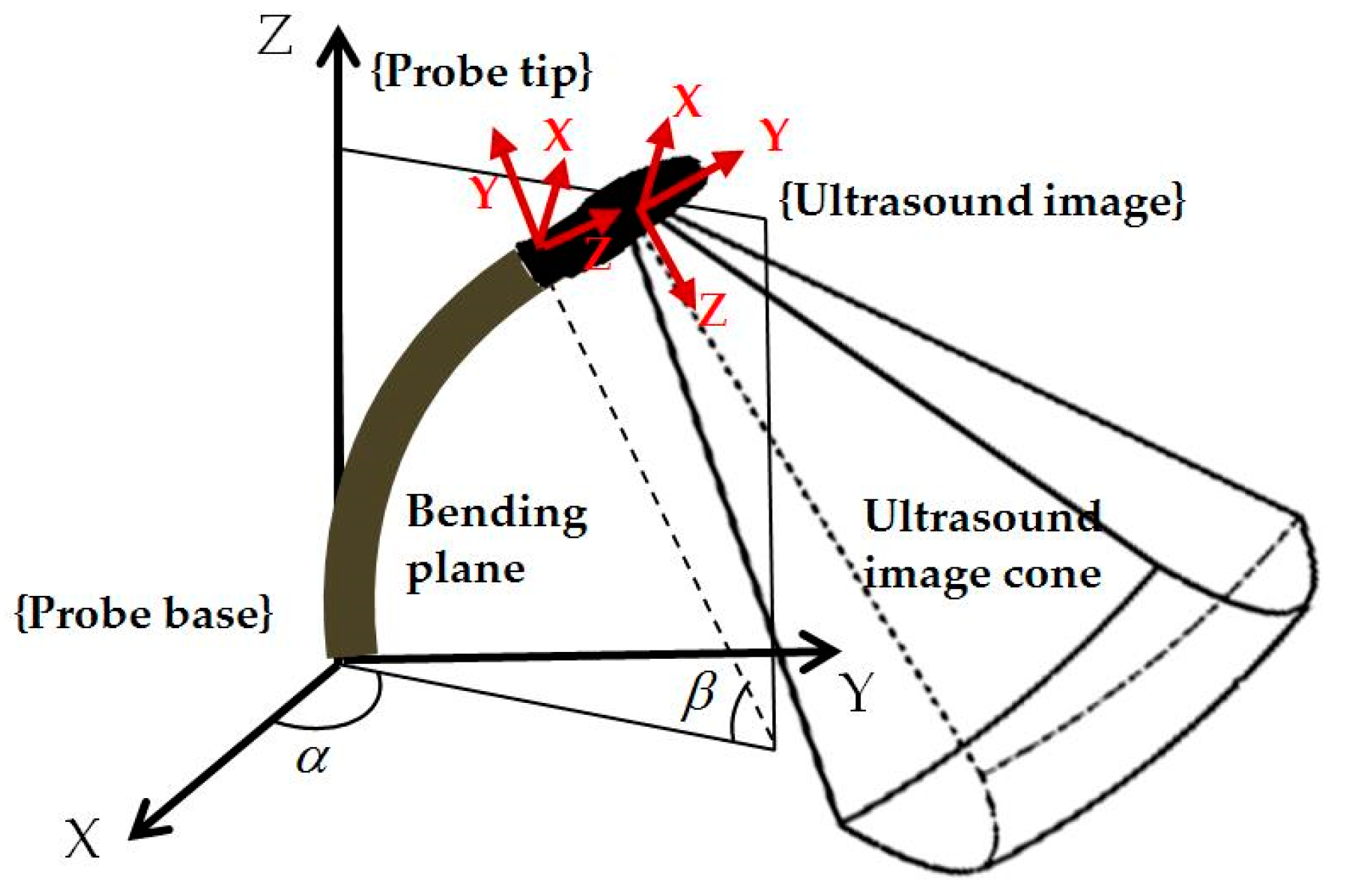
2.2. Pre-Planning Method for Initialization
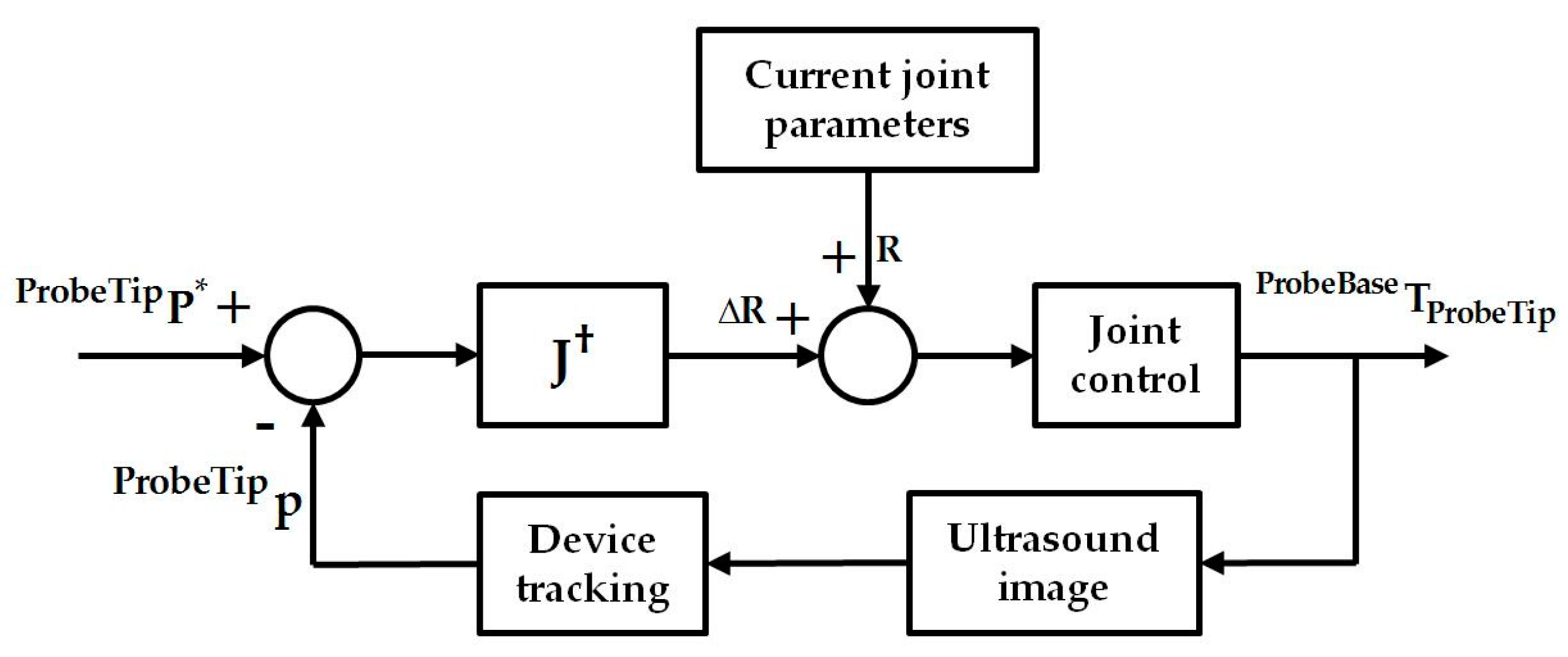
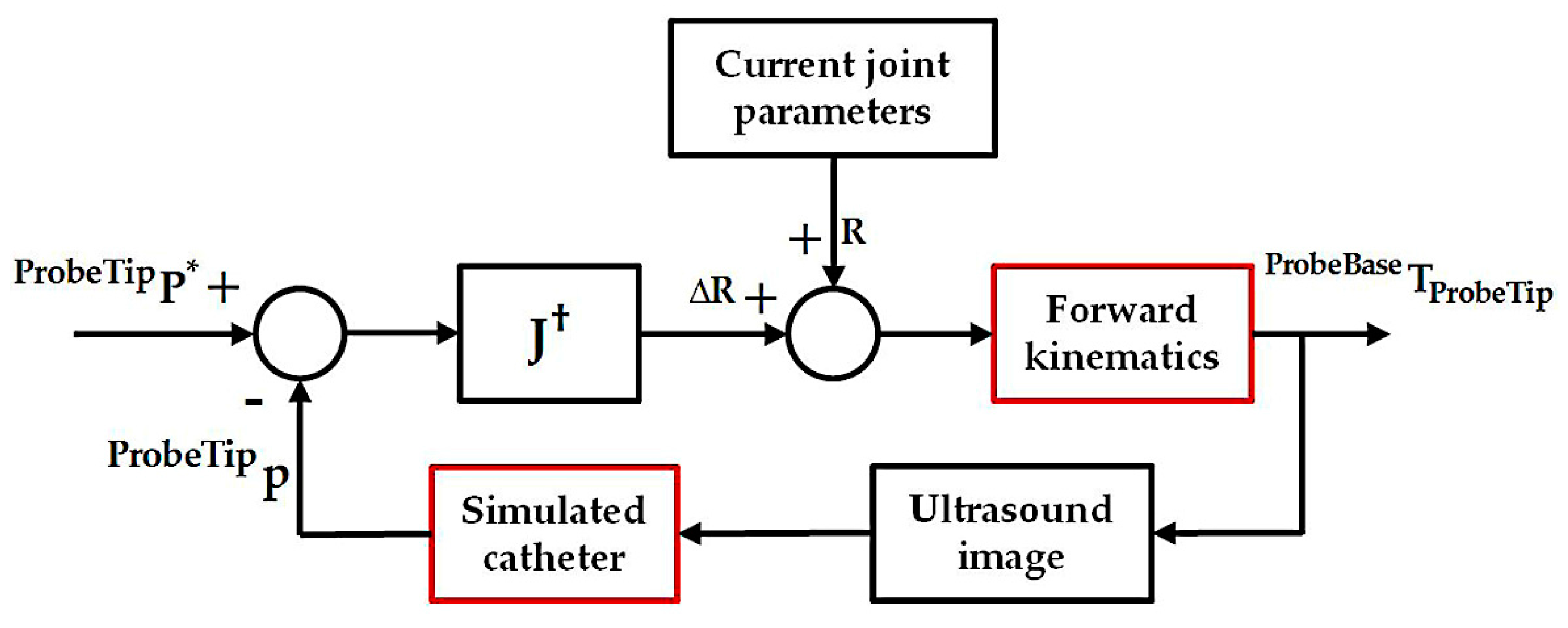
2.3. Differential Kinematics for Localized Adjustment
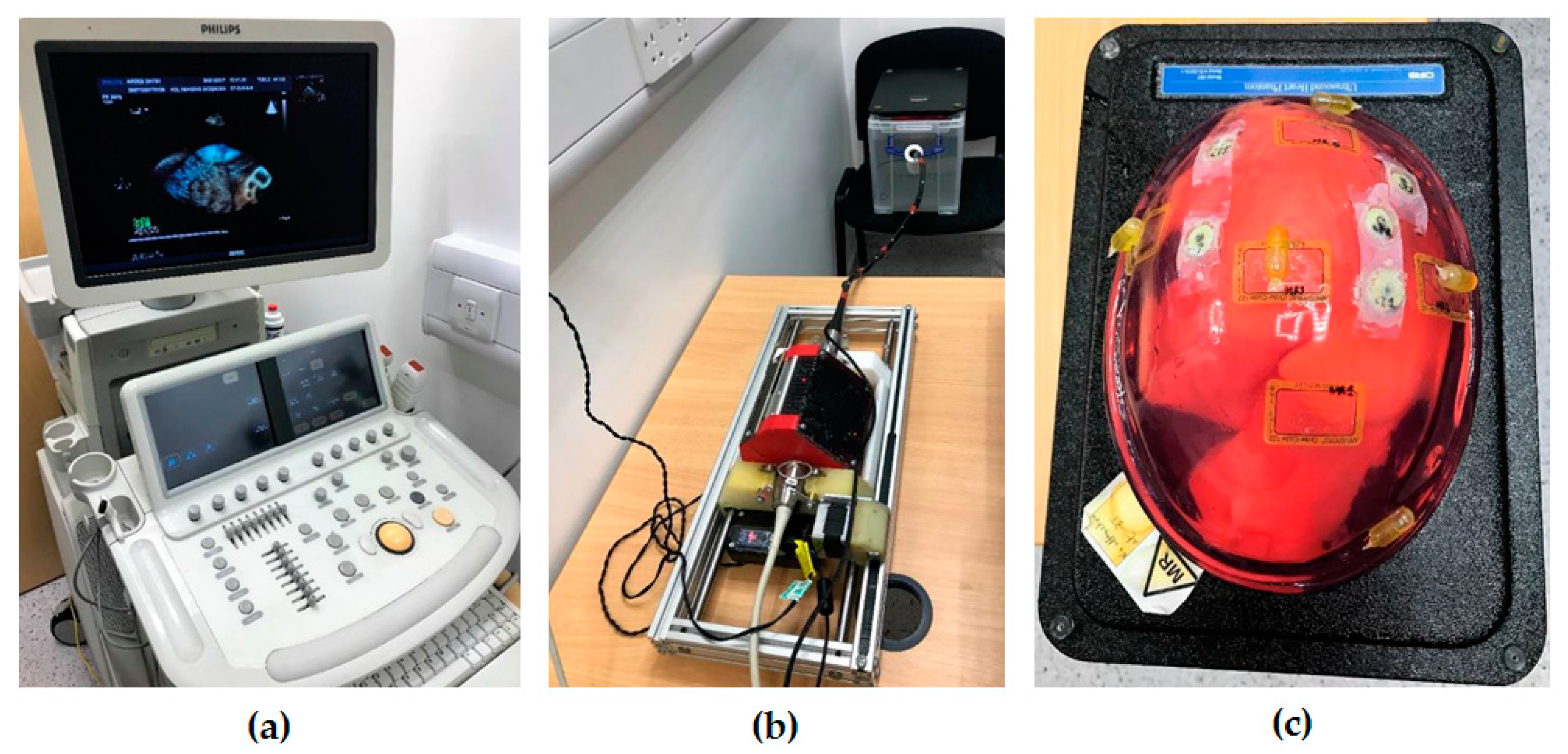
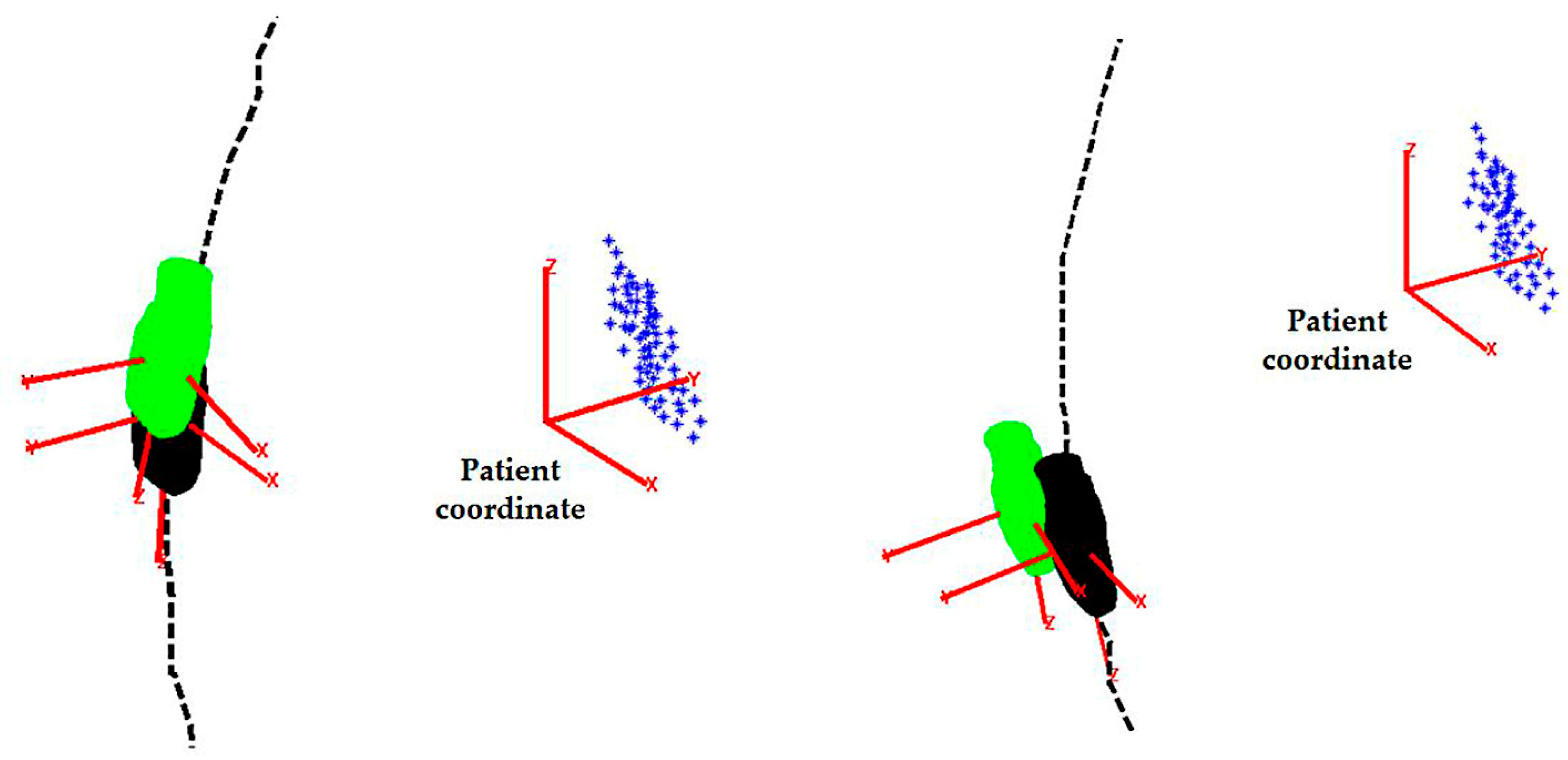
2.4. Simulation and Phantom Experiments for the Initialization Step
2.5. Simulation Experiments for the Localized Adjustment Step
3. Results and Discussion
3.1. Simulation and Phantom Experiments for the Initilization Step
3.2. Simulation Experiments for the Localized Adjustment Step
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vegas, A.; Meineri, M. Core review: Three-dimensional transesophageal echocardiography is a major advance for intraoperative clinical management of patients undergoing cardiac surgery: A core review. Anesth. Analg. 2010, 110, 1548–1573. [Google Scholar] [CrossRef] [PubMed]
- Perk, G.; Lang, R.M.; Garcia-Fernandez, M.A.; Lodato, J.; Sugeng, L.; Lopez, J.; Knight, B.P.; Messika-Zeitoun, D.; Shah, S.; Slater, J.; et al. Use of real time three-dimensional transesophageal echocardiography in intracardiac catheter based interventions. J. Am. Soc. Echocardiogr. 2009, 22, 865–882. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Abraham, T.; Adams, M.S.; Bruce, C.J.; Glas, K.E.; Lang, R.M.; Reeves, S.T.; Shanewise, J.S.; Siu, S.C.; Stewart, W. Guidelines for performing a comprehensive transesophageal echocardiographic examination: Recommendations from the American society of echocardiography and the society of cardiovascular anesthesiologists. J. Am. Soc. Echocardiogr. 2013, 26, 921–964. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.F.; Ho, S.Y.; Auricchio, A. Anatomy of right atrial structures by real-time 3d transesophageal echocardiography. JACC Cardiovasc. Imaging 2010, 3, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.F.; Nucifora, G.; Ho, S.Y. Imaging the atrial septum using real-time three-dimensional transesophageal echocardiography: Technical tips, normal anatomy, and its role in transseptal puncture. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2011, 24, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Altiok, E.; Becker, M.; Hamada, S.; Grabskaya, E.; Reith, S.; Marx, N.; Hoffmann, R. Real-time 3D tee allows optimized guidance of percutaneous edge-to-edge repair of the mitral valve. JACC Cardiovasc. Imaging 2010, 3, 1196–1198. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.F.; Pedrazzini, G.; Pasotti, E.; Petrova, I.; Drasutiene, A.; Dequarti, M.C.; Muzzarelli, S.; Moccetti, T. Role of real-time three dimensional transoesophageal echocardiography as guidance imaging modality during catheter based edge-to-edge mitral valve repair. Heart 2013, 99, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.F.; Pedrazzini, G.; Pasotti, E.; Muzzarelli, S.; Dequarti, M.C.; Murzilli, R.; Schlossbauer, S.A.; Slater, I.P.; Moccetti, T. 3D TEE during catheter-based interventions. JACC Cardiovasc. Imaging 2014, 7, 292–308. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, E.F.; Coon, P.D.; Einstein, A.J.; Mitchell, C.K.; Natello, G.W.; Palma, R.A.; Park, M.M.; Ranallo, F.; Roberts, M.L. Radiation safety for the cardiac sonographer: Recommendations of the radiation safety writing group for the council on cardiovascular sonography of the american society of echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2014, 27, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Balter, S.; Cowley, M.; Hodgson, J.; Klein, L.W. Occupational hazards of interventional cardiologists: Prevalence of orthopedic health problems in contemporary practice. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2004, 63, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.M.; Segal, J.; Borenstein, D.; Jenkins, E.; Cho, S. Prevalence of spinal disc disease among interventional cardiologists. Am. J. Cardiol. 1997, 79, 68–70. [Google Scholar] [CrossRef]
- Wang, S.; Housden, J.; Singh, D.; Althoefer, K.; Rhode, K. Design, testing and modelling of a novel robotic system for trans-oesophageal ultrasound. Int. J. Med. Robot. Comput. Assist. Surg. 2016, 12, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Ott, L.; Nageotte, F.; Zanne, P.; Mathelin, M.D. Robotic assistance to flexible endoscopy by physiological-motion tracking. IEEE Trans. Robot. 2011, 27, 346–359. [Google Scholar] [CrossRef]
- Abolmaesumi, P.; Salcudean, S.E.; Wen-Hong, Z.; Sirouspour, M.R.; DiMaio, S.P. Image-guided control of a robot for medical ultrasound. IEEE Trans. Robot. Autom. 2002, 18, 11–23. [Google Scholar] [CrossRef]
- Loschak, P.M.; Brattain, L.J.; Howe, R.D. Algorithms for automated pointing of cardiac imaging catheters. In International Workshop on Computer-Assisted and Robotic Endoscopy; Springer: Berlin, Germany, 2014; pp. 99–109. [Google Scholar]
- Wang, S.; Housden, J.; Singh, D.; Rhode, K. Automatic adjustments of a trans-oesophageal ultrasound robot for monitoring intra-operative catheters. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 012032. [Google Scholar]
- Wang, S.; Singh, D.; Johnson, D.; Althoefer, K.; Rhode, K.; Housden, R.J. Robotic ultrasound: View planning, tracking, and automatic acquisition of transesophageal echocardiography. IEEE Robot. Autom. Mag. 2016, 23, 118–127. [Google Scholar] [CrossRef]
- Gao, G.; Penney, G.; Ma, Y.; Gogin, N.; Cathier, P.; Arujuna, A.; Morton, G.; Caulfield, D.; Gill, J.; Aldo Rinaldi, C.; et al. Registration of 3D trans-esophageal echocardiography to X-ray fluoroscopy using image-based probe tracking. Med. Image Anal. 2012, 16, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, S.; Hager, G.D.; Corke, P.I. A tutorial on visual servo control. IEEE Trans. Robot. Autom. 1996, 12, 651–670. [Google Scholar] [CrossRef]
- Wang, S.; Singh, D.; Lau, D.; Reddy, K.; Althoefer, K.; Rhode, K.; Housden, R.J. Probe tracking and its application in automatic acquisition using a trans-esophageal ultrasound robot. In International Workshop on Computer-Assisted and Robotic Endoscopy; Springer: Berlin, Germany, 2016; pp. 14–23. [Google Scholar]
- Huang, J.; Triedman, J.K.; Vasilyev, N.V.; Suematsu, Y.; Cleveland, R.O.; Dupont, P.E. Imaging artifacts of medical instruments in ultrasound-guided interventions. J. Ultrasound Med. 2007, 26, 1303–1322. [Google Scholar] [CrossRef] [PubMed]
- Stoll, J.; Ren, H.; Dupont, P.E. Passive markers for tracking surgical instruments in real-time 3-D ultrasound imaging. IEEE Trans. Med. Imaging 2012, 31, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Housden, J.; Varma, N.; Ma, Y.; Rueckert, D.; Rhode, K. Catheter tracking in 3D echocardiographic sequences based on tracking in 2D X-ray sequences for cardiac catheterization interventions. In Proceedings of the 2013 IEEE 10th International Symposium on Biomedical Imaging (ISBI), San Francisco, CA, USA, 7–11 April 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 25–28. [Google Scholar]
- Wu, X.; Housden, J.; Ma, Y.; Razavi, B.; Rhode, K.; Rueckert, D. Fast catheter segmentation from echocardiographic sequences based on segmentation from corresponding X-ray fluoroscopy for cardiac catheterization interventions. IEEE Trans. Med. Imaging 2015, 34, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.T.; Wiles, A.D.; Wedlake, C.; Bainbridge, D.; Kiaii, B.; Trejos, A.L.; Patel, R.; Peters, T.M. Integration of trans-esophageal echocardiography with magnetic tracking technology for cardiac interventions. In Proceedings of the Medical Imaging 2010: Visualization, Image-Guided Procedures, and Modeling, San Diego, CA, USA, 14–16 February 2010; International Society for Optics and Photonics: Bellingham, WA, USA; p. 76252Y. [Google Scholar]
- Condino, S.; Calabrò, E.; Alberti, A.; Parrini, S.; Cioni, R.; Berchiolli, R.; Gesi, M.; Ferrari, V.; Ferrari, M. Simultaneous tracking of catheters and guidewires: Comparison to standard fluoroscopic guidance for arterial cannulation. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 53–60. [Google Scholar] [CrossRef] [PubMed]

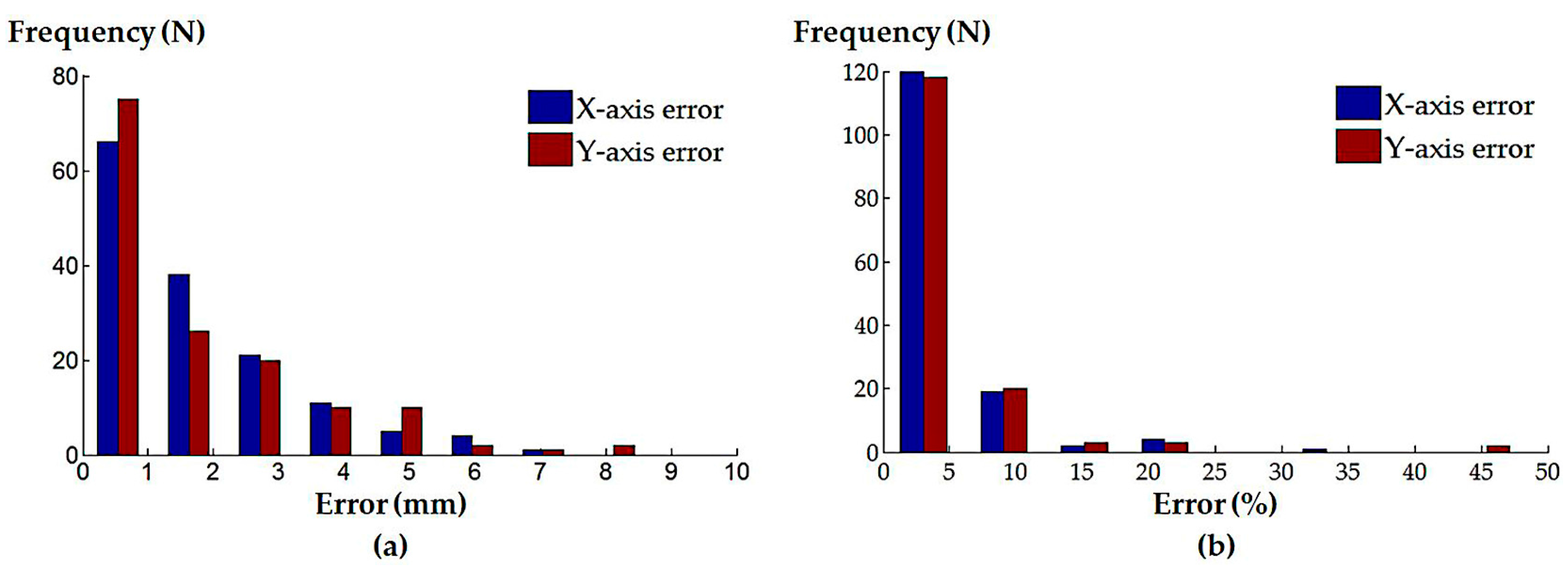

| Simulated Procedures | Axes | Absolute Error (Median ± InterQuartile Range (IQR)) | Normalized Error (Median ± IQR) |
|---|---|---|---|
| Randomly generated catheter locations | X-axis | 1.16 ± 2.09 mm | 2.78 ± 4.01% |
| Y-axis | 1.03 ± 2.42 mm | 2.18 ± 4.60% | |
| Atrial septal defect repair | X-axis | 0.05 ± 0.09 mm | 0.09 ± 0.16% |
| Y-axis | 0.05 ± 0.10 mm | 0.08 ± 0.17% | |
| Left atrial ablation | X-axis | 0.16 ± 1.87 mm | 0.29 ± 2.69% |
| Y-axis | 0.14 ± 0.38 mm | 0.26 ± 0.61% | |
| Mitral valve repair/replacement | X-axis | 0.20 ± 0.30 mm | 0.43 ± 0.54% |
| Y-axis | 0.28 ± 0.40 mm | 0.53 ± 0.85% | |
| Trans-catheter aortic valve replacement | X-axis | 0.09 ± 0.22 mm | 0.15 ± 0.38% |
| Y-axis | 0.22 ± 1.37 mm | 0.41 ± 2.23% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Housden, J.; Zar, A.; Gandecha, R.; Singh, D.; Rhode, K. Strategy for Monitoring Cardiac Interventions with an Intelligent Robotic Ultrasound Device. Micromachines 2018, 9, 65. https://doi.org/10.3390/mi9020065
Wang S, Housden J, Zar A, Gandecha R, Singh D, Rhode K. Strategy for Monitoring Cardiac Interventions with an Intelligent Robotic Ultrasound Device. Micromachines. 2018; 9(2):65. https://doi.org/10.3390/mi9020065
Chicago/Turabian StyleWang, Shuangyi, James Housden, Areeb Zar, Ruchi Gandecha, Davinder Singh, and Kawal Rhode. 2018. "Strategy for Monitoring Cardiac Interventions with an Intelligent Robotic Ultrasound Device" Micromachines 9, no. 2: 65. https://doi.org/10.3390/mi9020065





