Investigation on Single-Molecule Junctions Based on Current–Voltage Characteristics
Abstract
:1. Introduction
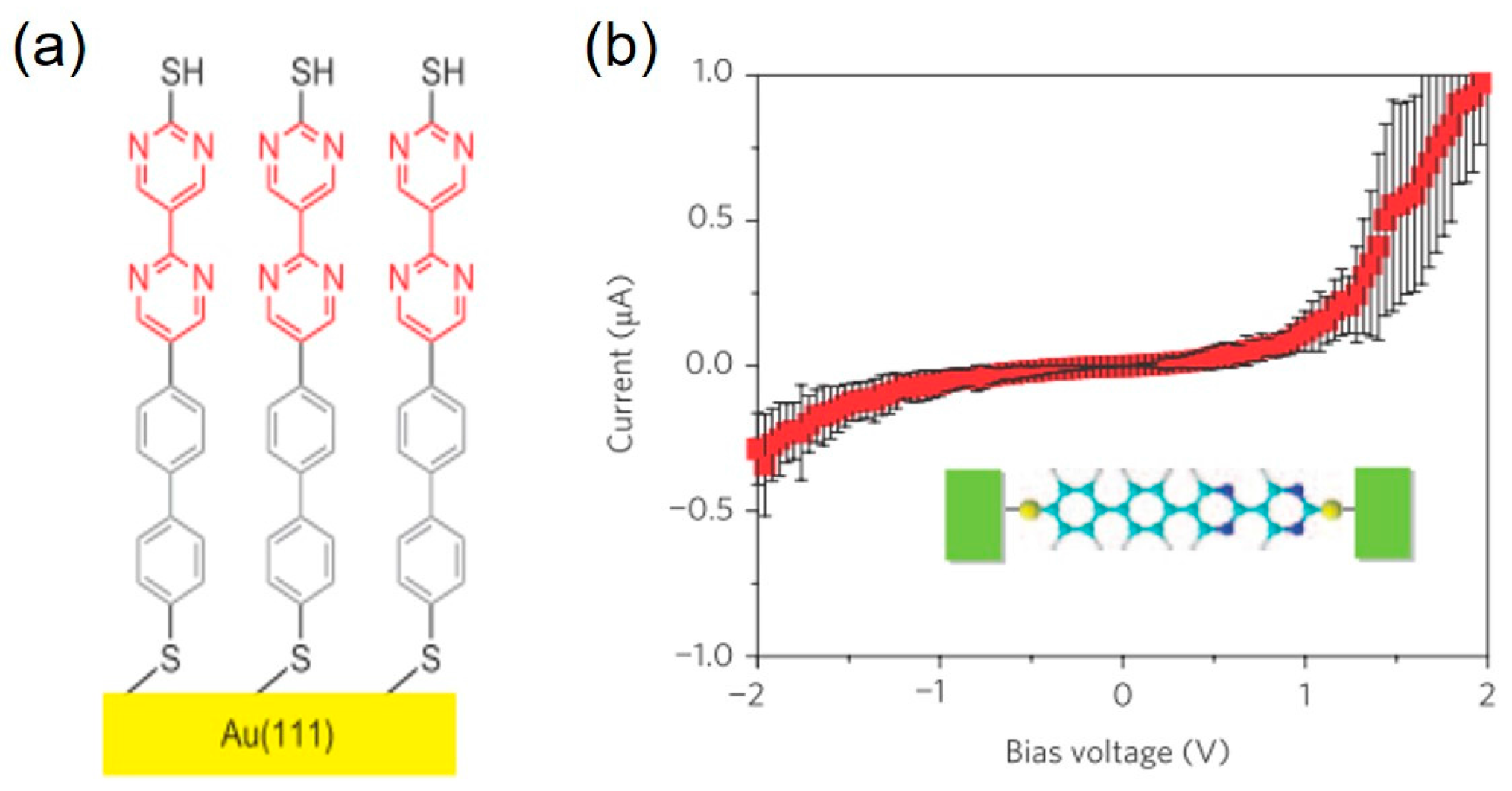
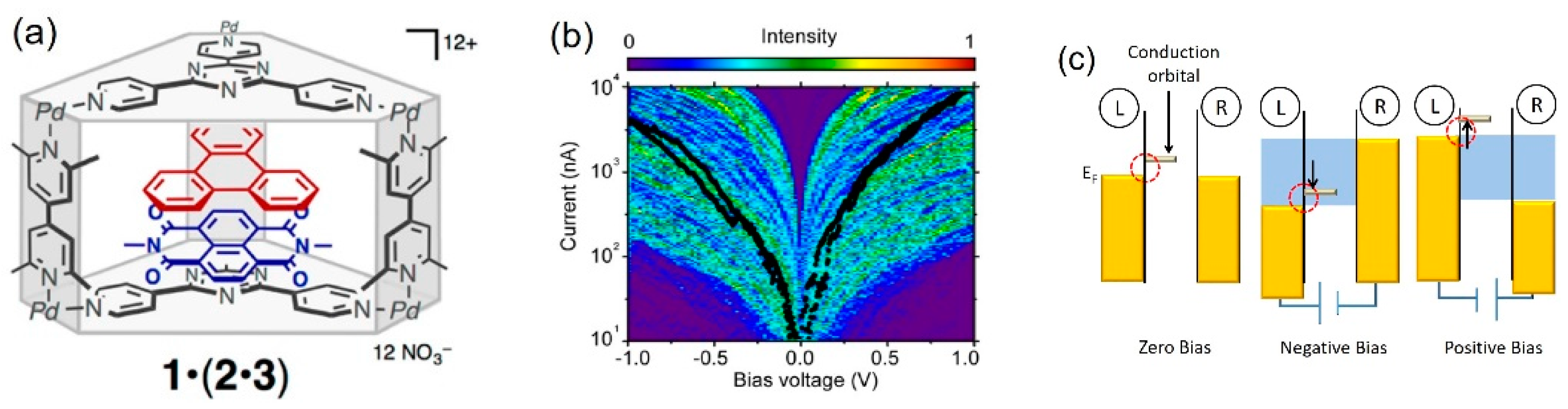
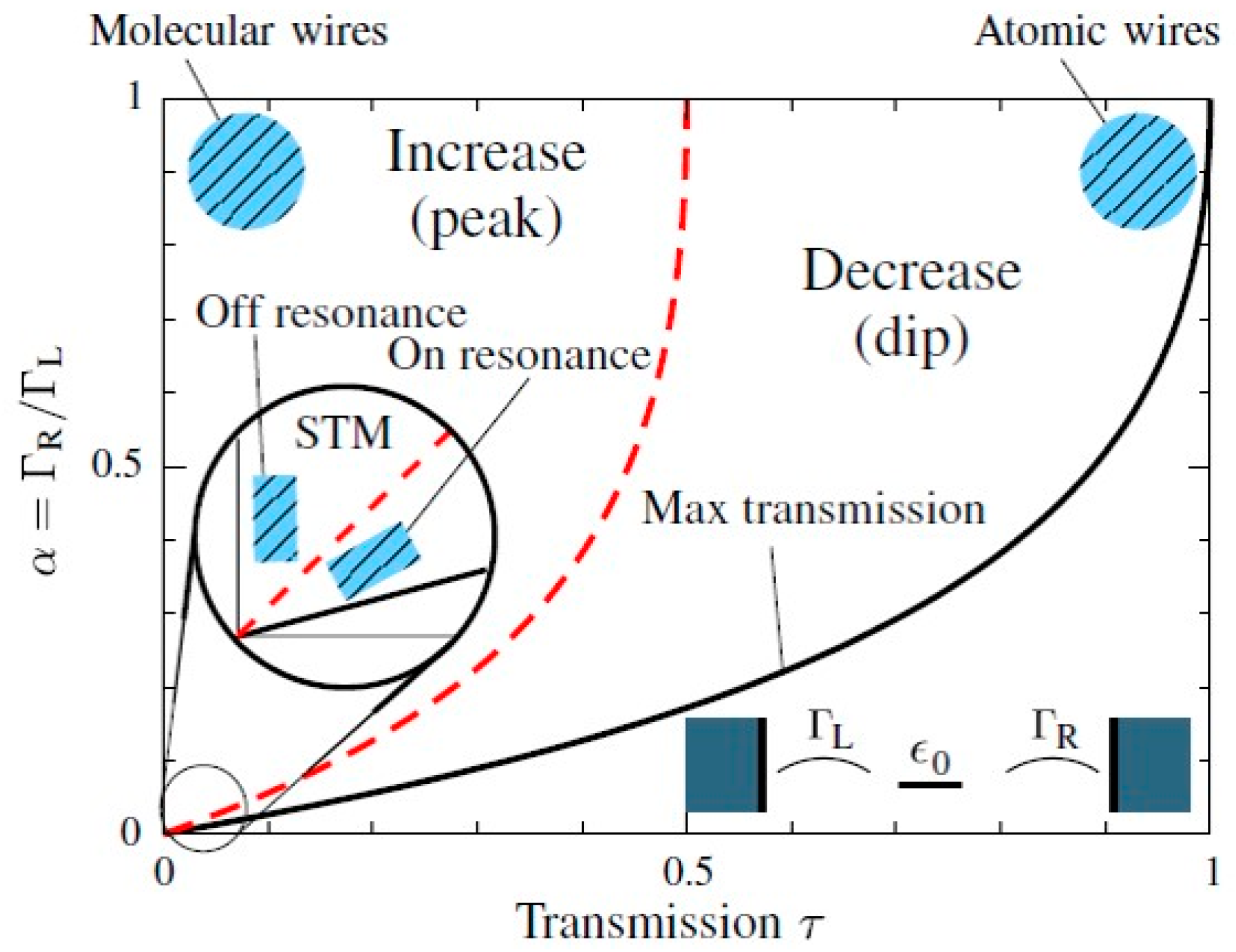
2. Diode Characteristics
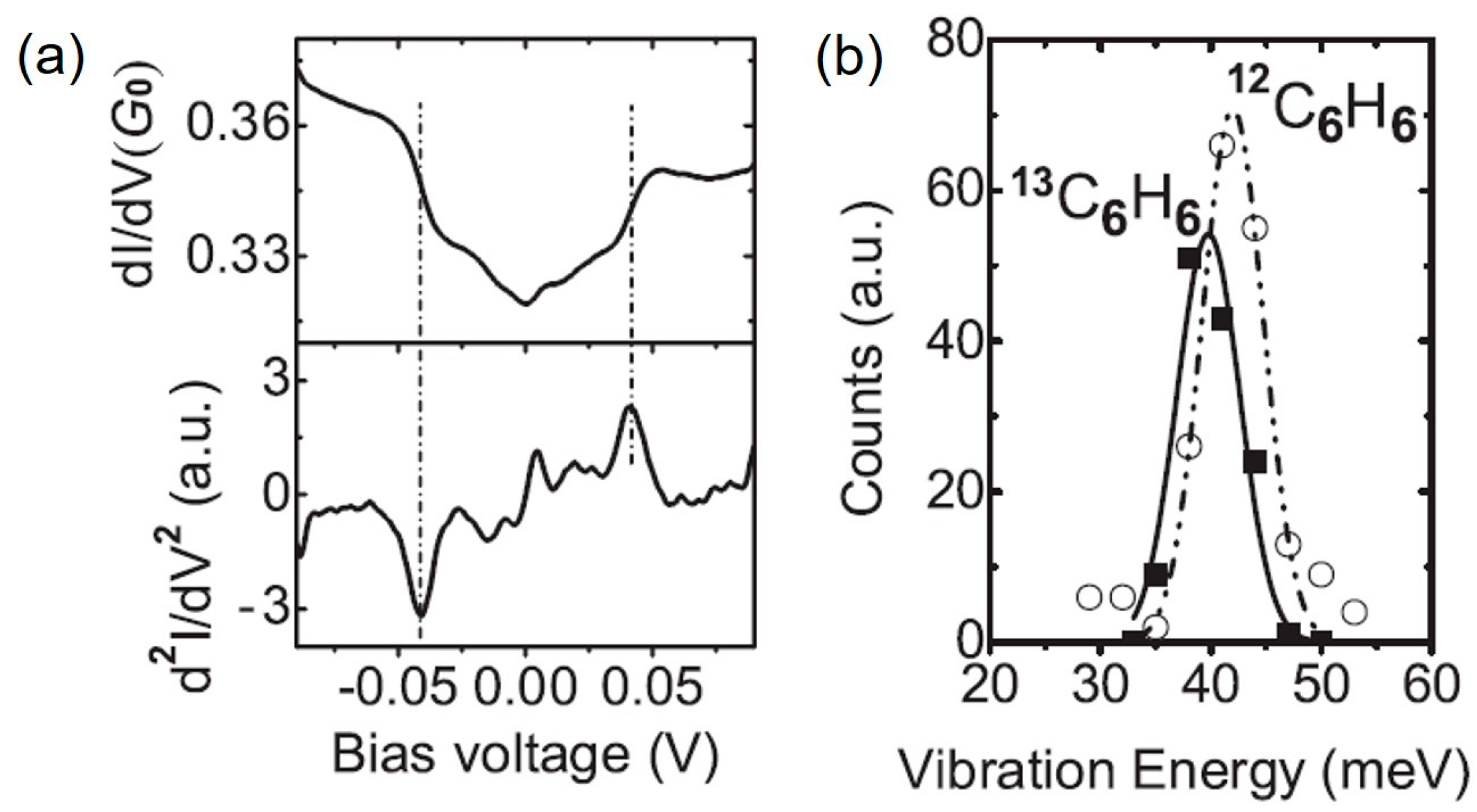
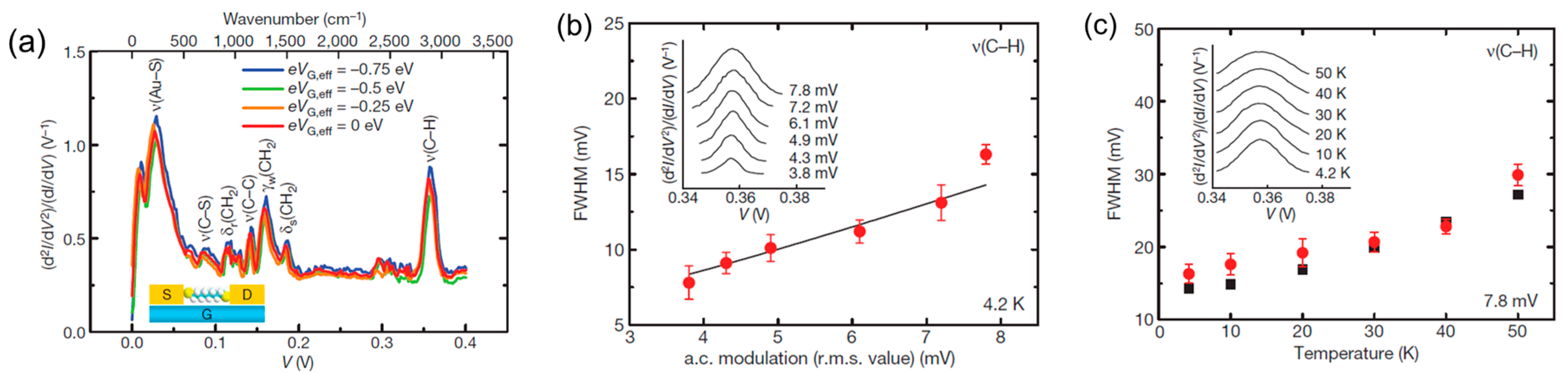
3. Vibrational Mode
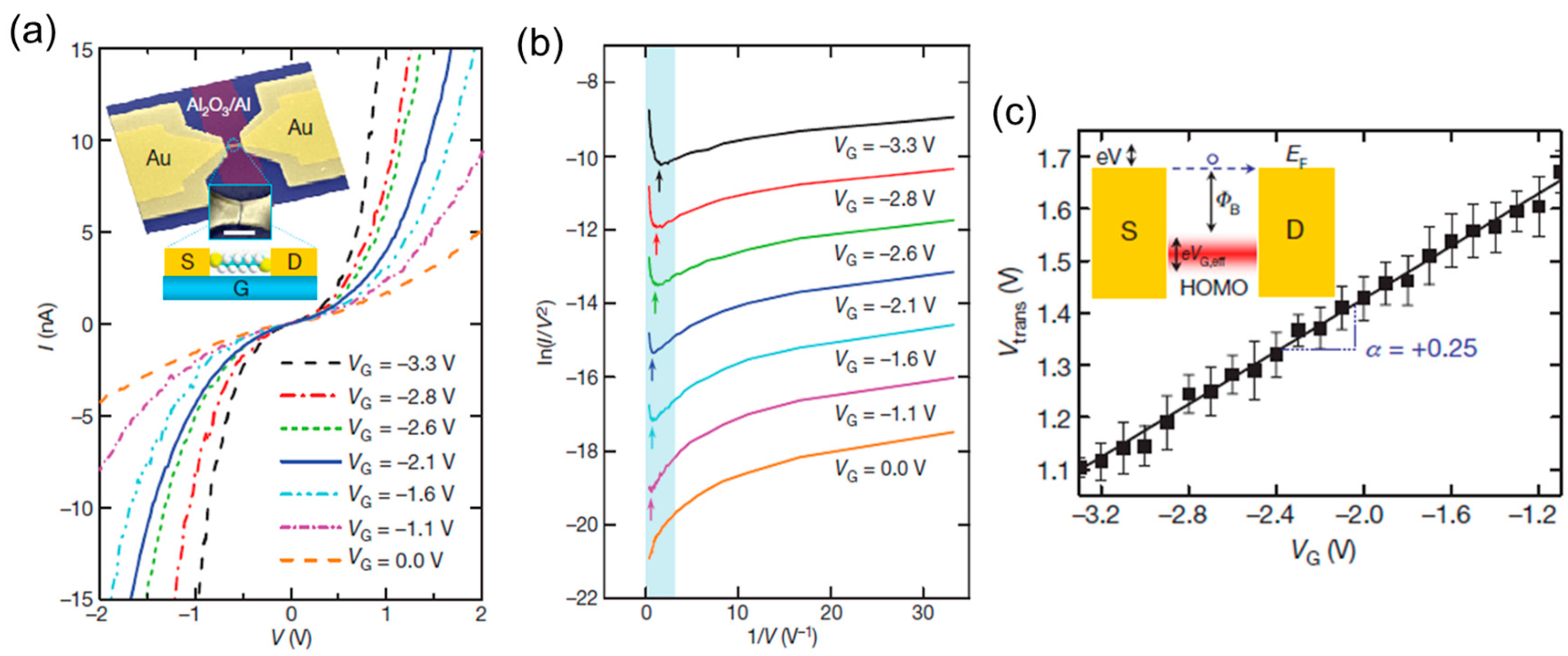
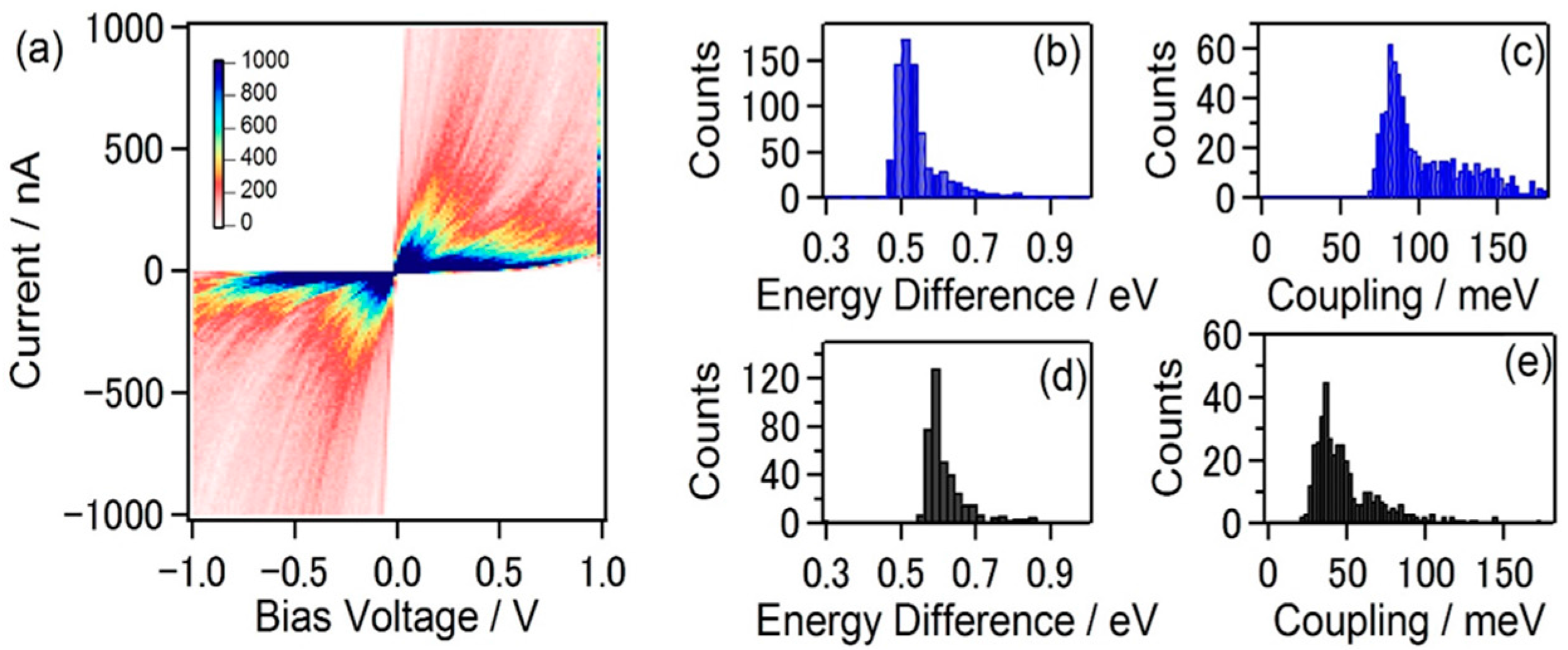
4. Electronic Structure
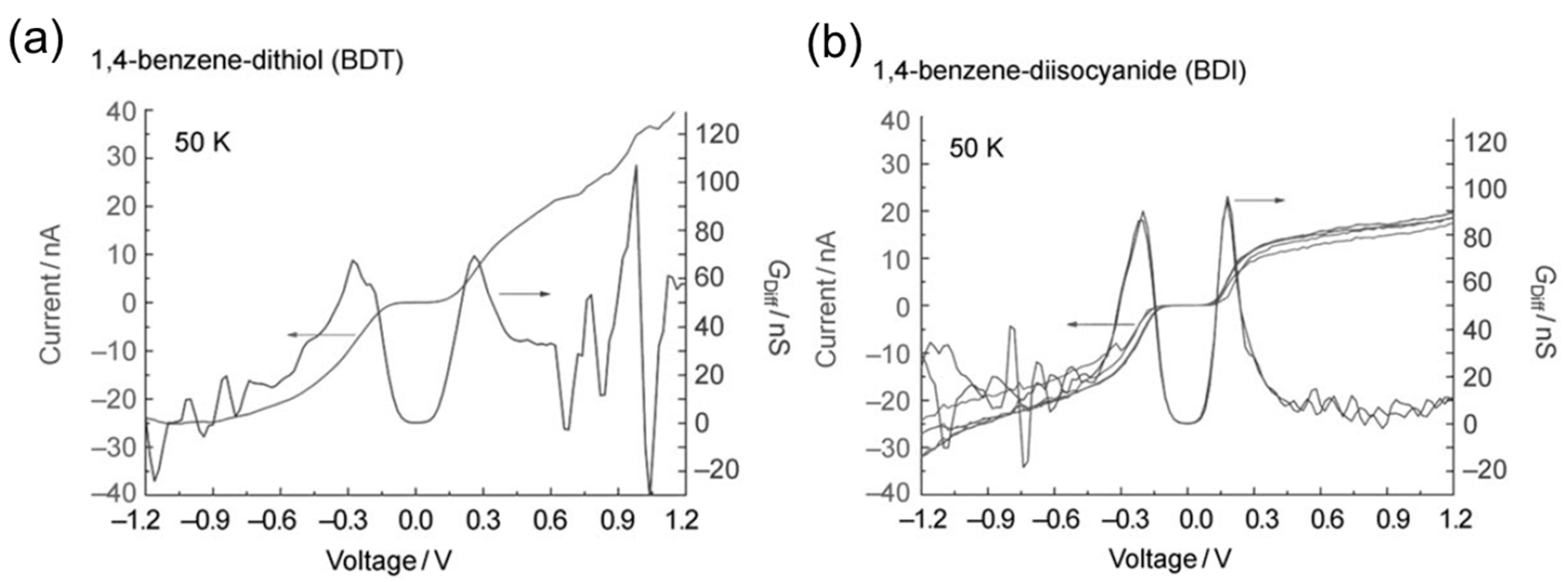
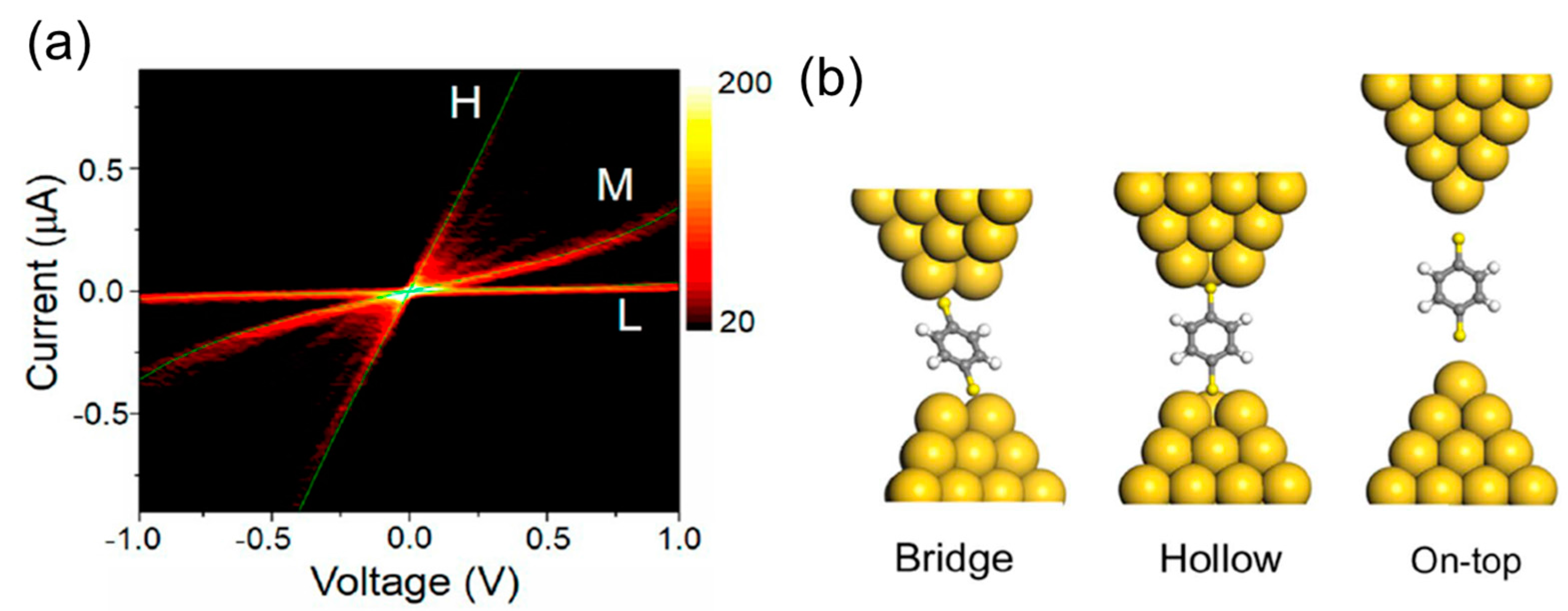
5. Interface Structure
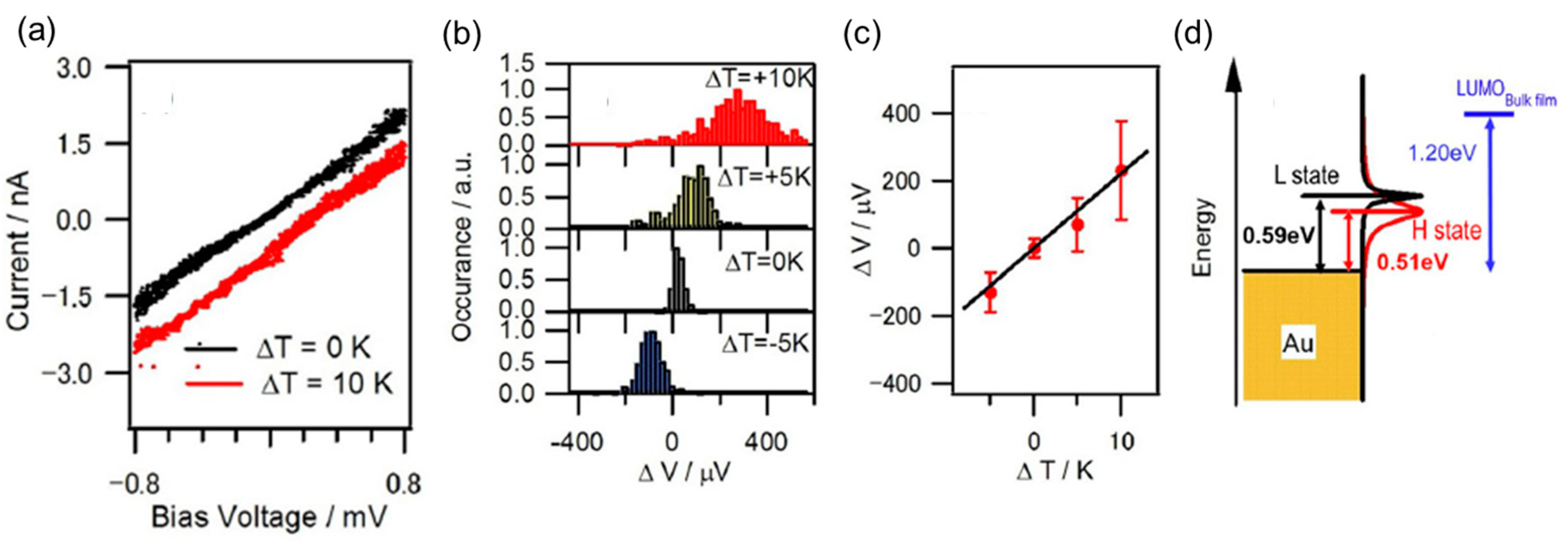
6. Type of Charge Carriers
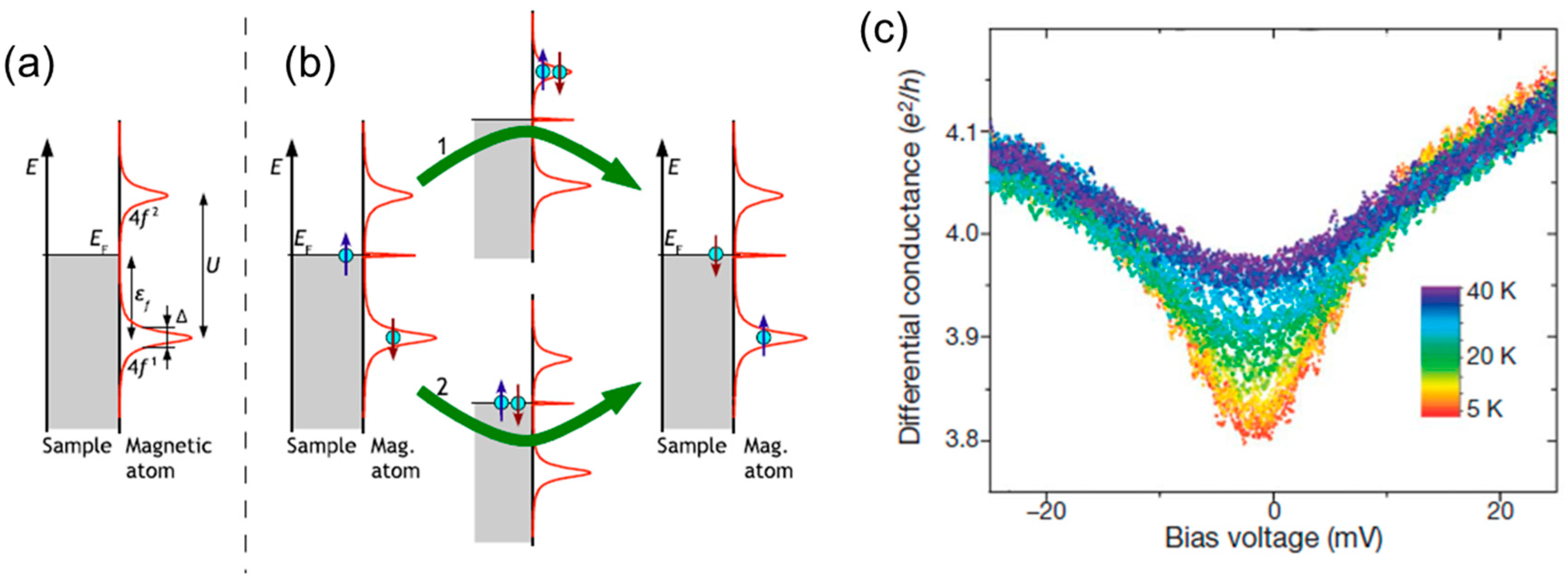
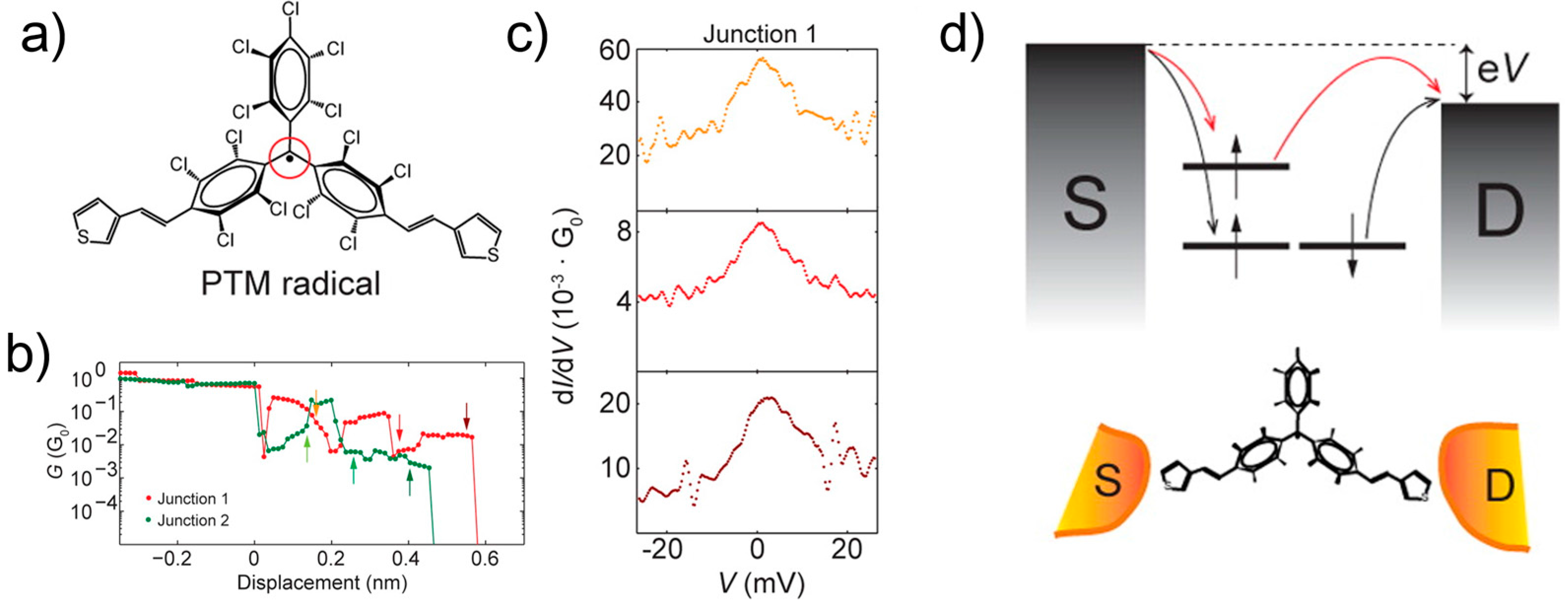
7. Spin State
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reed, M.A.; Zhou, C.; Muller, C.J.; Burgin, T.P.; Tour, J.M. Conductance of a molecular junction. Science 1997, 278, 252–254. [Google Scholar] [CrossRef]
- Agraït, N. Quantum properties of atomic-sized conductors. Phys. Rep. 2003, 377, 81–279. [Google Scholar] [CrossRef]
- Aradhya, S.V.; Venkataraman, L. Single-molecule junctions beyond electronic transport. Nat. Nanotechnol. 2013, 8, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.J. Electron transport in molecular junctions. Nat. Nanotechnol. 2006, 1, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Komoto, Y.; Fujii, S.; Iwane, M.; Kiguchi, M. Single-molecule junctions for molecular electronics. J. Mater. Chem. C 2016, 4, 8842–8858. [Google Scholar] [CrossRef]
- Muller, C.J.; Ruitenbeek, J.M.V.; Jongh, L.J.D. Experimental observation of the transition from weak link to tunnel junction. Physica C 1992, 191, 485–504. [Google Scholar] [CrossRef]
- Xu, B.; Tao, N.J. Measurement of single-molecule resistance by repeated formation of molecular junctions. Science 2003, 301, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Smit, R.H.M.; Noat, Y.; Untiedt, C.; Lang, N.D.; van Hemert, M.C.; van Ruitenbeek, J.M. Measurement of the conductance of a hydrogen molecule. Nature 2002, 419, 906–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiguchi, M.; Stadler, R.; Kristensen, I.S.; Djukic, D.; van Ruitenbeek, J.M. Evidence for a single hydrogen molecule connected by an atomic chain. Phys. Rev. Lett. 2007, 98, 146802. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Marques-Gonzalez, S.; Shin, J.Y.; Shinokubo, H.; Masuda, T.; Nishino, T.; Arasu, N.P.; Vazquez, H.; Kiguchi, M. Highly-conducting molecular circuits based on antiaromaticity. Nat. Commun. 2017, 8, 15984. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, M.; Inatomi, J.; Takahashi, Y.; Tanaka, R.; Osuga, T.; Murase, T.; Fujita, M.; Tada, T.; Watanabe, S. Highly conductive [3×n] gold-ion clusters enclosed within self-assembled cages. Angew. Chem. Int. Ed. Engl. 2013, 52, 6202–6205. [Google Scholar] [CrossRef] [PubMed]
- Lafferentz, L.; Ample, F.; Yu, H.; Hecht, S.; Joachim, C.; Grill, L. Conductance of a single conjugated polymer as a continuous function of its length. Science 2009, 323, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Ziatdinov, M.; Higashibayashi, S.; Sakurai, H.; Kiguchi, M. Bowl inversion and electronic switching of buckybowls on gold. J. Am. Chem. Soc. 2016, 138, 12142–12149. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kim, Y.; Jang, Y.H.; Jeong, H.; Reed, M.A.; Lee, T. Observation of molecular orbital gating. Nature 2009, 462, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Diez-Perez, I.; Hihath, J.; Lee, Y.; Yu, L.; Adamska, L.; Kozhushner, M.A.; Oleynik, I.I.; Tao, N. Rectification and stability of a single molecular diode with controlled orientation. Nat. Chem. 2009, 1, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Migliore, A.; Xin, N.; Huang, S.; Wang, J.; Yang, Q.; Wang, S.; Chen, H.; Wang, D.; Feng, B.; et al. Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity. Science 2016, 352, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, M.; Ohto, T.; Fujii, S.; Sugiyasu, K.; Nakajima, S.; Takeuchi, M.; Nakamura, H. Single molecular resistive switch obtained via sliding multiple anchoring points and varying effective wire length. J. Am. Chem. Soc. 2014, 136, 7327–7332. [Google Scholar] [CrossRef] [PubMed]
- Quek, S.Y.; Kamenetska, M.; Steigerwald, M.L.; Choi, H.J.; Louie, S.G.; Hybertsen, M.S.; Neaton, J.B.; Venkataraman, L. Mechanically controlled binary conductance switching of a single-molecule junction. Nat. Nanotechnol. 2009, 4, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, M.; Rahong, S.; Iizumi, Y.; Okazaki, T.; Taniguchi, M.; Kawai, T. Single-molecule sensing electrode embedded in-plane nanopore. Sci. Rep. 2011, 1, 46. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; He, J.; Chang, S.; Zhang, P.; Liang, F.; Li, S.; Tuchband, M.; Fuhrmann, A.; Ros, R.; Lindsay, S. Identifying single bases in a DNA oligomer with electron tunnelling. Nat. Nanotechnol. 2010, 5, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiang, L.; Palma, J.L.; Asai, Y.; Tao, N. Thermoelectric effect and its dependence on molecular length and sequence in single DNA molecules. Nat. Commun. 2016, 7, 11294. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Palma, J.L.; Bruot, C.; Mujica, V.; Ratner, M.A.; Tao, N. Intermediate tunnelling-hopping regime in DNA charge tranport. Nat. Chem. 2015, 7, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Palma, J.L.; Li, Y.; Mujica, V.; Ratner, M.A.; Tao, N. Gate-controlled conductance switching in DNA. Nat. Commun. 2017, 8, 14471. [Google Scholar] [CrossRef] [PubMed]
- Bruot, C.; Palma, J.L.; Xiang, L.; Mujica, V.; Ratner, M.A.; Tao, N. Piezoresistivity in single DNA molecules. Nat. Commun. 2015, 6, 8032. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, M.L.; Martin, C.A.; Smit, R.H.; Guedon, C.M.; Baart, T.A.; van der Molen, S.J.; van Ruitenbeek, J.M. Transition voltage spectroscopy and the nature of vacuum tunneling. Nano Lett. 2011, 11, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Ternes, M.; Heinrich, A.J.; Schneider, W.D. Spectroscopic manifestations of the kondo effect on single adatoms. J. Phys. Condens. Matter 2009, 21, 053001. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Tada, T.; Komoto, Y.; Osuga, T.; Murase, T.; Fujita, M.; Kiguchi, M. Rectifying electron-transport properties through stacks of aromatic molecules inserted into a self-assembled cage. J. Am. Chem. Soc. 2015, 137, 5939–5947. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, B.; Xia, J.; Adak, O.; Dell, E.J.; Liu, Z.F.; Taylor, J.C.; Neaton, J.B.; Campos, L.M.; Venkataraman, L. Single-molecule diodes with high rectification ratios through environmental control. Nat. Nanotechnol. 2015, 10, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Lortscher, E.; Gotsmann, B.; Lee, Y.; Yu, L.; Rettner, C.; Riel, H. Transport properties of a single-molecule diode. ACS Nano 2012, 6, 4931–4939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lo, W.Y.; Cai, Z.; Li, L.; Yu, L. Molecular rectification tuned by through-space gating effect. Nano Lett. 2017, 17, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, M.; Frederiksen, T.; Ueba, H.; Lorente, N.; Brandbyge, M. Unified description of inelastic propensity rules for electron transport through nanoscale junctions. Phys. Rev. Lett. 2008, 100, 226604. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, M.; Tal, O.; Wohlthat, S.; Pauly, F.; Krieger, M.; Djukic, D.; Cuevas, J.C.; van Ruitenbeek, J.M. Highly conductive molecular junctions based on direct binding of benzene to platinum electrodes. Phys. Rev. Lett. 2008, 101, 046801. [Google Scholar] [CrossRef] [PubMed]
- Tal, O.; Krieger, M.; Leerink, B.; van Ruitenbeek, J.M. Electron-vibration interaction in single-molecule junctions: From contact to tunneling regimes. Phys. Rev. Lett. 2008, 100, 196804. [Google Scholar] [CrossRef] [PubMed]
- Nakazumi, T.; Kaneko, S.; Matsushita, R.; Kiguchi, M. Electric conductance of single ethylene and acetylene molecules bridging between Pt electrodes. J. Phys. Chem. C 2012, 116, 18250–18255. [Google Scholar] [CrossRef]
- Kiguchi, M.; Hashimoto, K.; Ono, Y.; Taketsugu, T.; Murakoshi, K. Formation of a Pd atomic chain in a hydrogen atmosphere. Phys. Rev. B 2010, 81, 195401. [Google Scholar] [CrossRef]
- Kim, Y.; Pietsch, T.; Erbe, A.; Belzig, W.; Scheer, E. Benzenedithiol: A broad-range single-channel molecular conductor. Nano Lett. 2011, 11, 3734–3738. [Google Scholar] [CrossRef] [PubMed]
- Hihath, J.; Arroy, C.R.; Rubio-Bollinger, G.; Tao, N.; Agraït, N. Study of electron-phonon interactions in a single molecule covalently connected to two electrodes. Nano Lett. 2008, 8, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Long, D.P.; Lazorcik, J.L.; Mantooth, B.A.; Moore, M.H.; Ratner, M.A.; Troisi, A.; Yao, Y.; Ciszek, J.W.; Tour, J.M.; Shashidhar, R. Effects of hydration on molecular junction transport. Nat. Mater. 2006, 5, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Kiguchi, M.; Takase, M.; Nagasawa, F.; Nabika, H.; Ikeda, K.; Uosaki, K.; Ueno, K.; Misawa, H.; Murakoshi, K. Single molecule dynamics at a mechanically controllable break junction in solution at room temperature. J. Am. Chem. Soc. 2013, 135, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Murai, D.; Marques-Gonzalez, S.; Nakamura, H.; Komoto, Y.; Fujii, S.; Nishino, T.; Ikeda, K.; Tsukagoshi, K.; Kiguchi, M. Site-selection in single-molecule junction for highly reproducible molecular electronics. J. Am. Chem. Soc. 2016, 138, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Huisman, E.H.; Guedon, C.M.; van Wees, B.J.; van der Molen, S.J. Interpretation of transition voltage spectroscopy. Nano Lett. 2009, 9, 3909–3913. [Google Scholar] [CrossRef] [PubMed]
- Zotti, L.A.; Kirchner, T.; Cuevas, J.C.; Pauly, F.; Huhn, T.; Scheer, E.; Erbe, A. Revealing the role of anchoring groups in the electricalconduction through single-molecule junctions. Small 2010, 6, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.G. Generalized formula for the electric tunnel effect between similar electrodes separated by a thin insulating film. J. Appl. Phys. 1963, 34, 1793–1803. [Google Scholar] [CrossRef]
- Vilan, A. Analyzing molecular current-voltage characteristics with the simmons tunneling model: Scaling and linearization. J. Phys. Chem. C 2007, 111, 4431–4444. [Google Scholar] [CrossRef]
- Dorneles, L.S.; Schaefer, D.M.; Carara, M.; Schelp, L.F. The use of simmons’ equation to quantify the insulating barrier parameters in Al/AlOx/Al tunnel junctions. Appl. Phys. Lett. 2003, 82, 2832. [Google Scholar] [CrossRef]
- Wang, W.; Lee, T.; Reed, M.A. Electron tunnelling in self-assembled monolayers. Rep. Prog. Phys. 2005, 68, 523–544. [Google Scholar] [CrossRef]
- Engelkes, V.B.; Beebe, J.M.; Frisbie, C.D. Length-dependent transport in molecular junctions based on SAMs of alkanethiols and alkanedithiols: Effect of metal work function and applied bias on tunneling efficiency and contact resistance. J. Am. Chem. Soc. 2004, 126, 14287–14296. [Google Scholar] [CrossRef] [PubMed]
- Leary, E.; Zalinge, H.V.; Higgins, S.J.; Nichols, R.J.; de Biani, F.F.; Leoni, P.; Marchettic, L.; Zanellob, P. A molecular wire incorporating a robust hexanuclear platinum cluster. Phys. Chem. Chem. Phys. 2009, 11, 5198–5202. [Google Scholar] [CrossRef] [PubMed]
- Ho Choi, S.; Kim, B.; Frisbie, C.D. Electrical resistance of long conjugated molecular wires. Science 2008, 320, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Beebe, J.M.; Kim, B.; Gadzuk, J.W.; Frisbie, C.D.; Kushmerick, J.G. Transition from direct tunneling to field emission in metal-molecule-metal junctions. Phys. Rev. Lett. 2006, 97, 026801. [Google Scholar] [CrossRef] [PubMed]
- Lçrtscher, E.C.; Cho, C.J.; Mayor, M.; Tschudy, M.; Rettner, C.; Riel, H. Influence of the anchor group on charge transport through single-molecule junctions. ChemPhysChem 2011, 12, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Komoto, Y.; Isshiki, Y.; Fujii, S.; Nishino, T.; Kiguchi, M. Evaluation of the electronic structure of single-molecule junctions based on current–voltage and thermopower measurements: Application to C60. Chem. Asian J. 2017, 12, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Komoto, Y.; Fujii, S.; Nakamura, H.; Tada, T.; Nishino, T.; Kiguchi, M. Resolving metal-molecule interfaces at single-molecule junctions. Sci. Rep. 2016, 6, 26606. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Jang, S.-Y.; Segalman, R.A.; Majumdar, A. Thermoelectricity in molecular junctions. Science 2007, 315, 1568–1571. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Ohto, T.; Yamada, R.; Tada, H. Thermopower of benzenedithiol and C60 molecular junctions with Ni and Au electrodes. Nano Lett. 2014, 14, 5276–5280. [Google Scholar] [CrossRef] [PubMed]
- Widawsky, J.R.; Chen, W.; Vázquez, H.; Kim, T.; Breslow, R.; Hybertsen, M.S.; Venkataraman, L. Length-dependent thermopower of highly conducting Au−C bonded single molecule junctions. Nano Lett. 2013, 13, 2889–2894. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Darancet, P.; Widawsky, R.J.; Kotiuga, M.; Quek, S.Y.; Neaton, J.B.; Venkataraman, L. Determination of energy level alignment and coupling strength in 4,4′-bipyridine single-molecule junctions. Nano Lett. 2014, 14, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Fano, U. Effects of configuration interaction on intensities and phase shifts. Phys. Rev. 1961, 124, 1866–1878. [Google Scholar] [CrossRef]
- Calvo, M.R.; Fernandez-Rossier, J.; Palacios, J.J.; Jacob, D.; Natelson, D.; Untiedt, C. The Kondo effect in ferromagnetic atomic contacts. Nature 2009, 458, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Frisenda, R.; Gaudenzi, R.; Franco, C.; Mas-Torrent, M.; Rovira, C.; Veciana, J.; Alcon, I.; Bromley, S.T.; Burzuri, E.; van der Zant, H.S. Kondo effect in a neutral and stable all organic radical single molecule break junction. Nano Lett. 2015, 15, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Kisslinger, F.; Ballmann, S.; Schramm, F.; Chandrasekar, R.; Bodenstein, T.; Fuhr, O.; Secker, D.; Fink, K.; Ruben, M.; et al. Switching of a coupled spin pair in a single-molecule junction. Nat. Nanotechnol. 2013, 8, 575–579. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isshiki, Y.; Matsuzawa, Y.; Fujii, S.; Kiguchi, M. Investigation on Single-Molecule Junctions Based on Current–Voltage Characteristics. Micromachines 2018, 9, 67. https://doi.org/10.3390/mi9020067
Isshiki Y, Matsuzawa Y, Fujii S, Kiguchi M. Investigation on Single-Molecule Junctions Based on Current–Voltage Characteristics. Micromachines. 2018; 9(2):67. https://doi.org/10.3390/mi9020067
Chicago/Turabian StyleIsshiki, Yuji, Yuya Matsuzawa, Shintaro Fujii, and Manabu Kiguchi. 2018. "Investigation on Single-Molecule Junctions Based on Current–Voltage Characteristics" Micromachines 9, no. 2: 67. https://doi.org/10.3390/mi9020067