Design, Preparation and Performance Study of On-Chip Flow-Through Amperometric Sensors with an Integrated Ag/AgCl Reference Electrode
Abstract
:1. Introduction
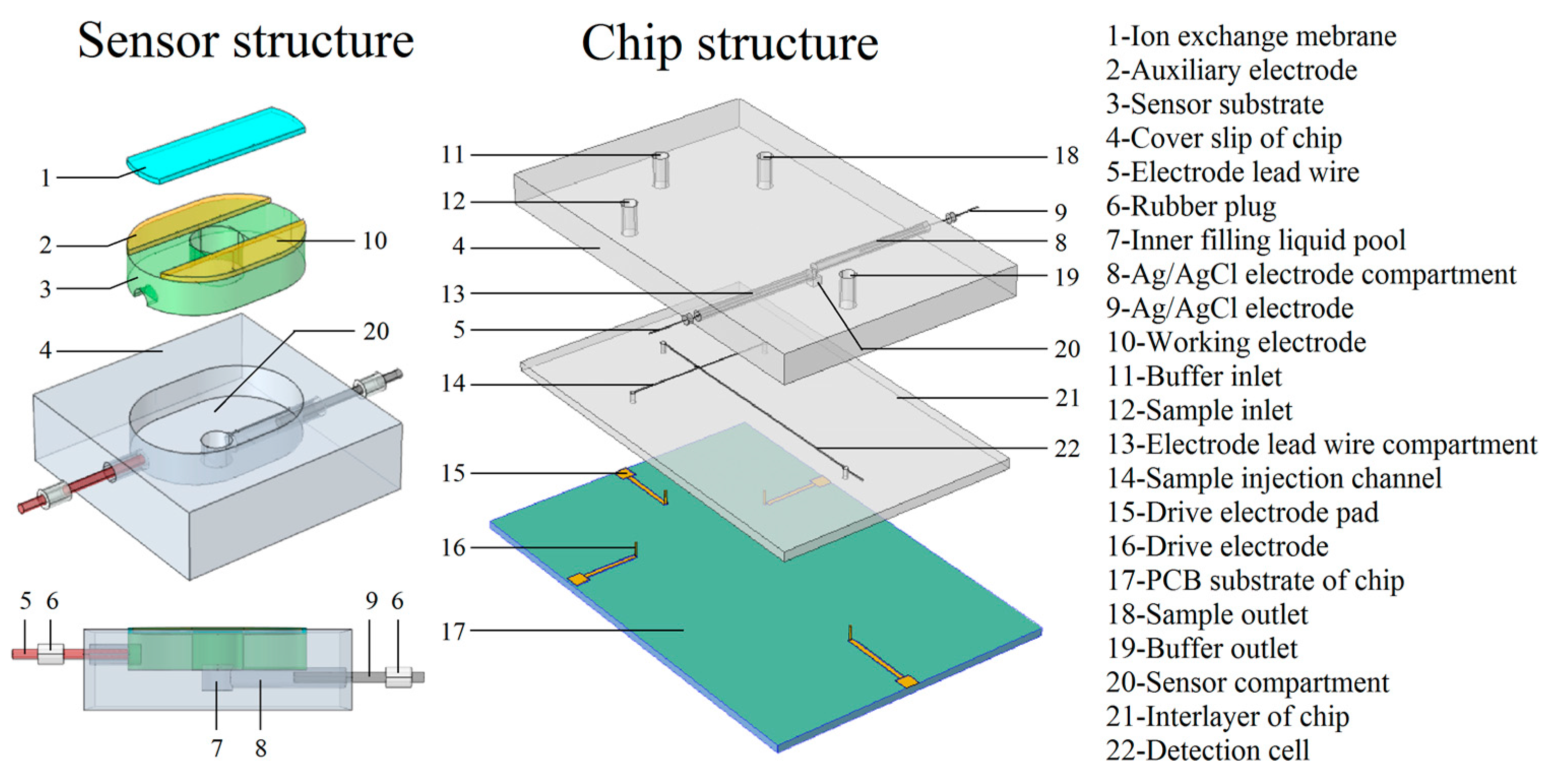
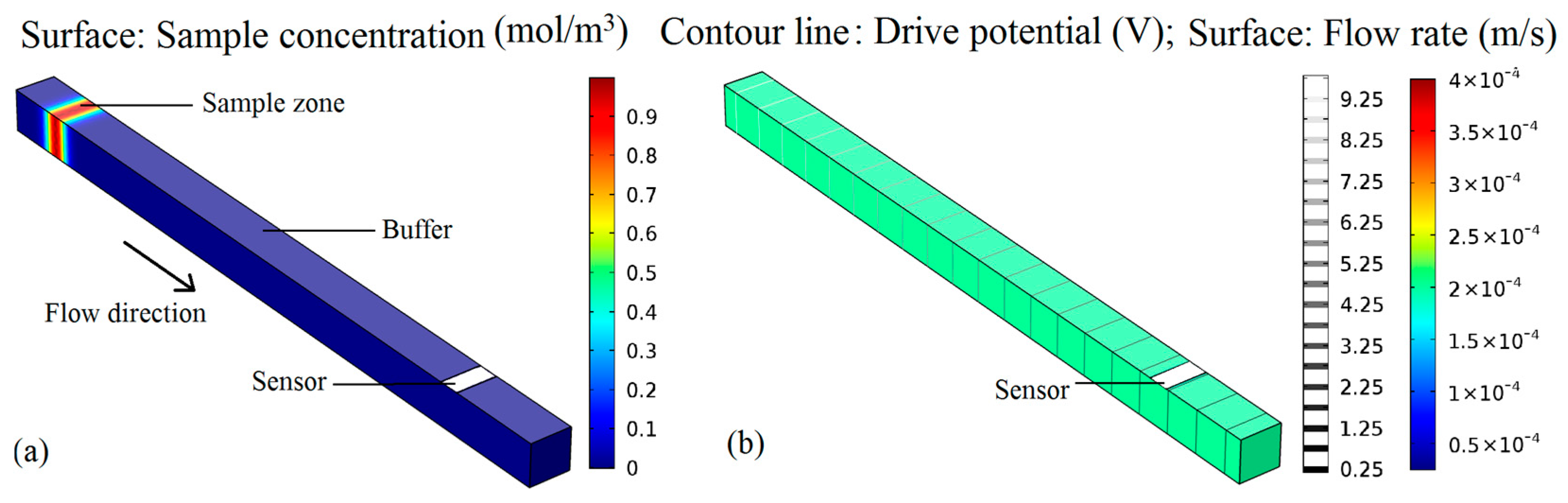
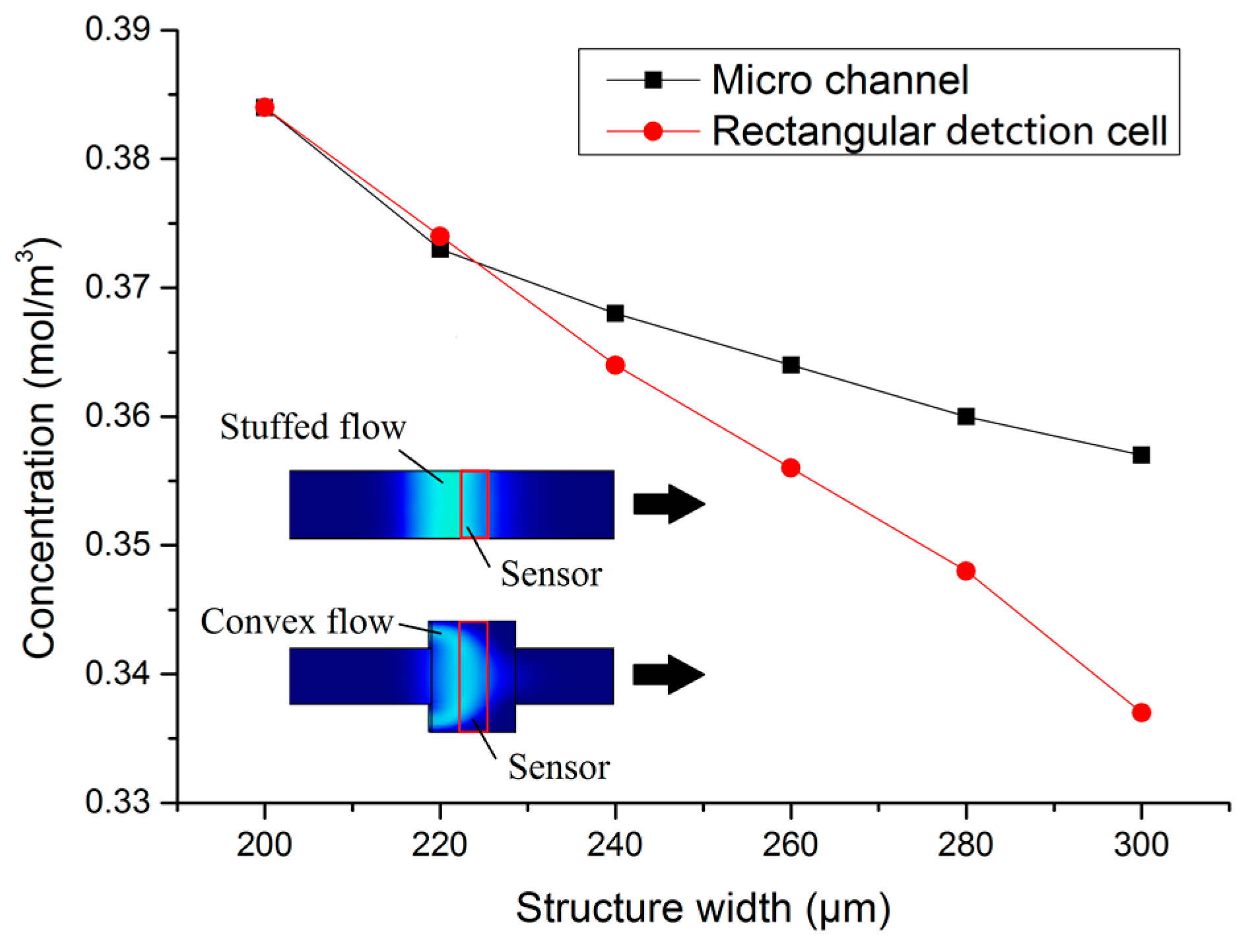
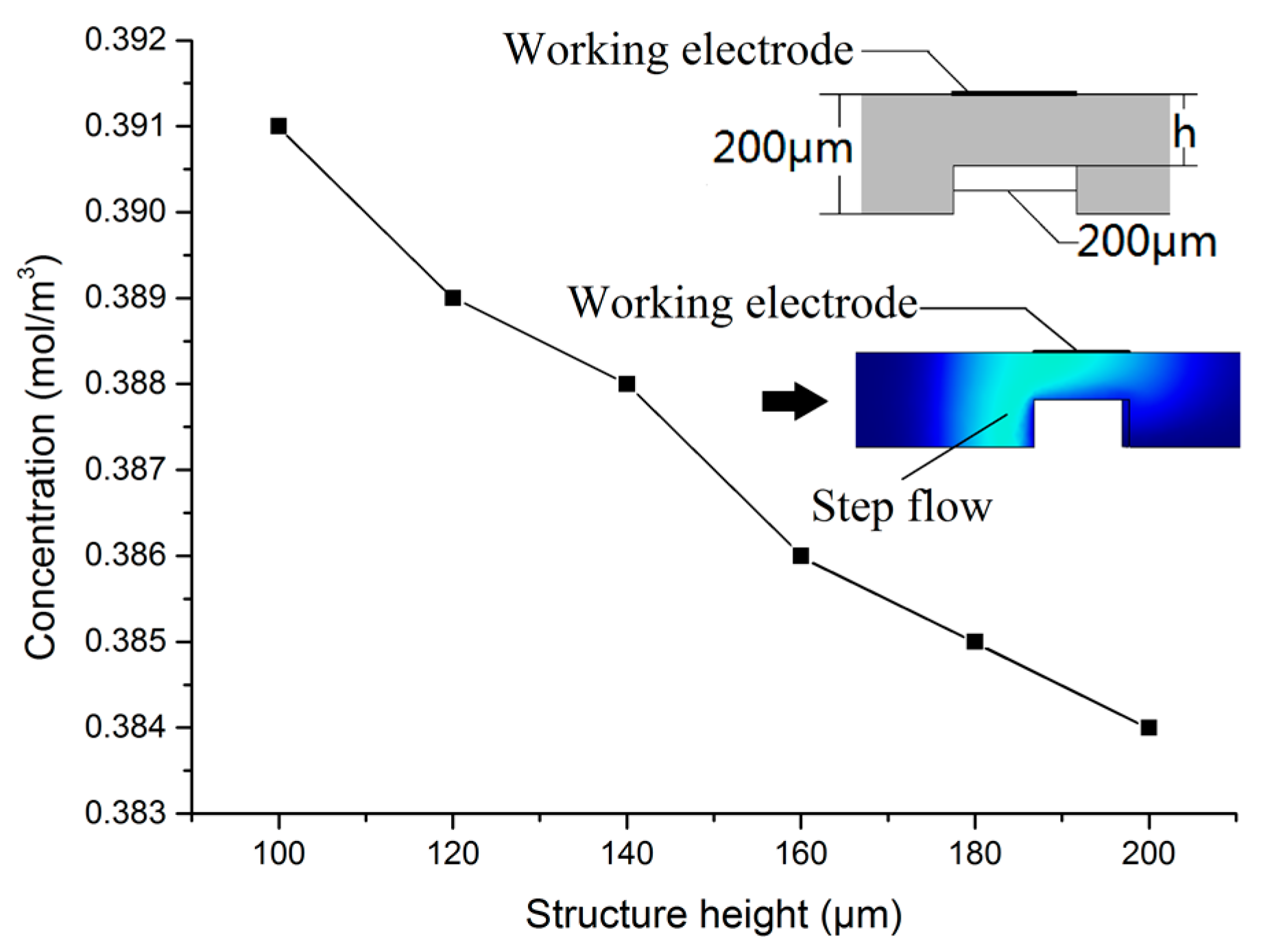
2. Sensor Design and Optimization
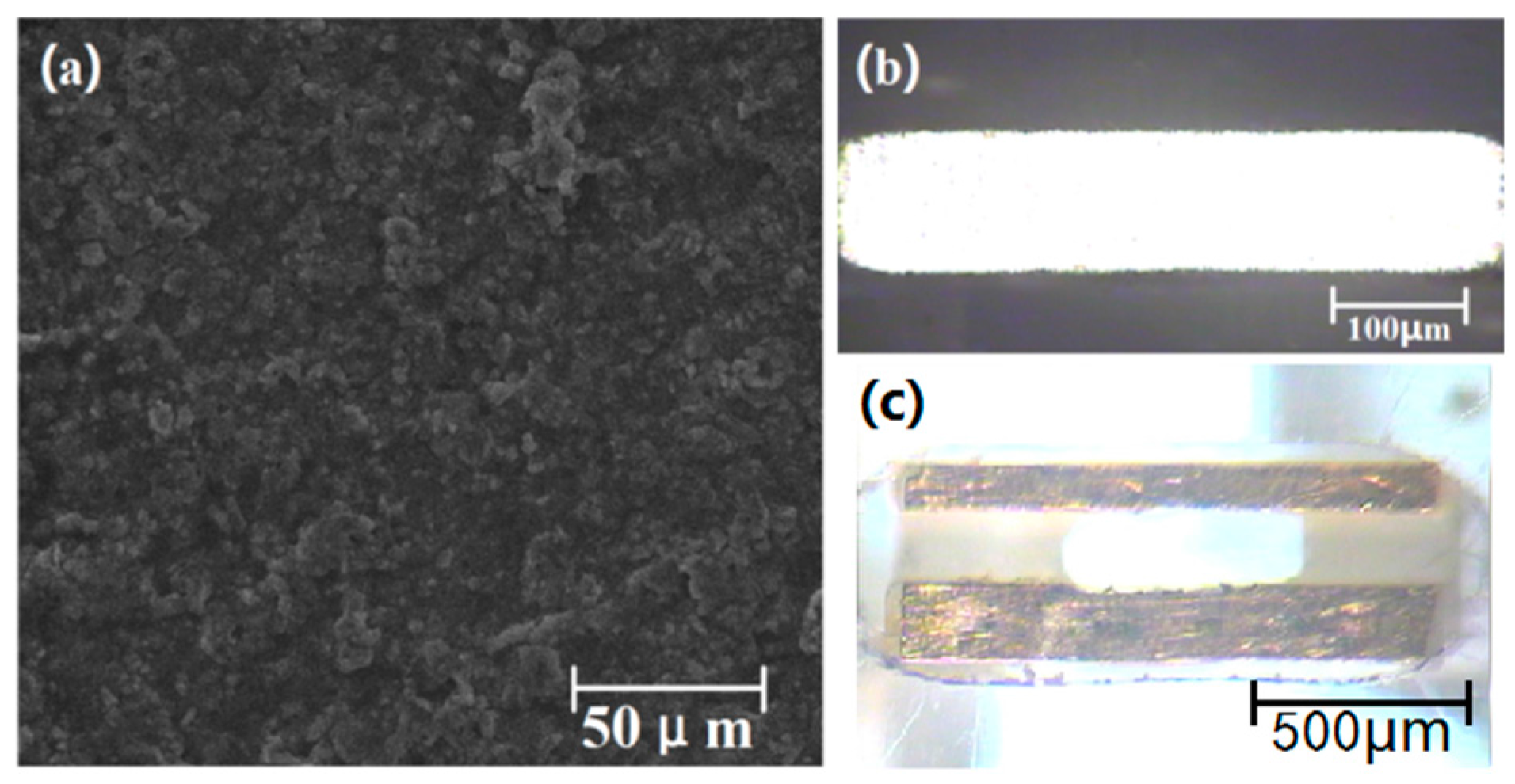
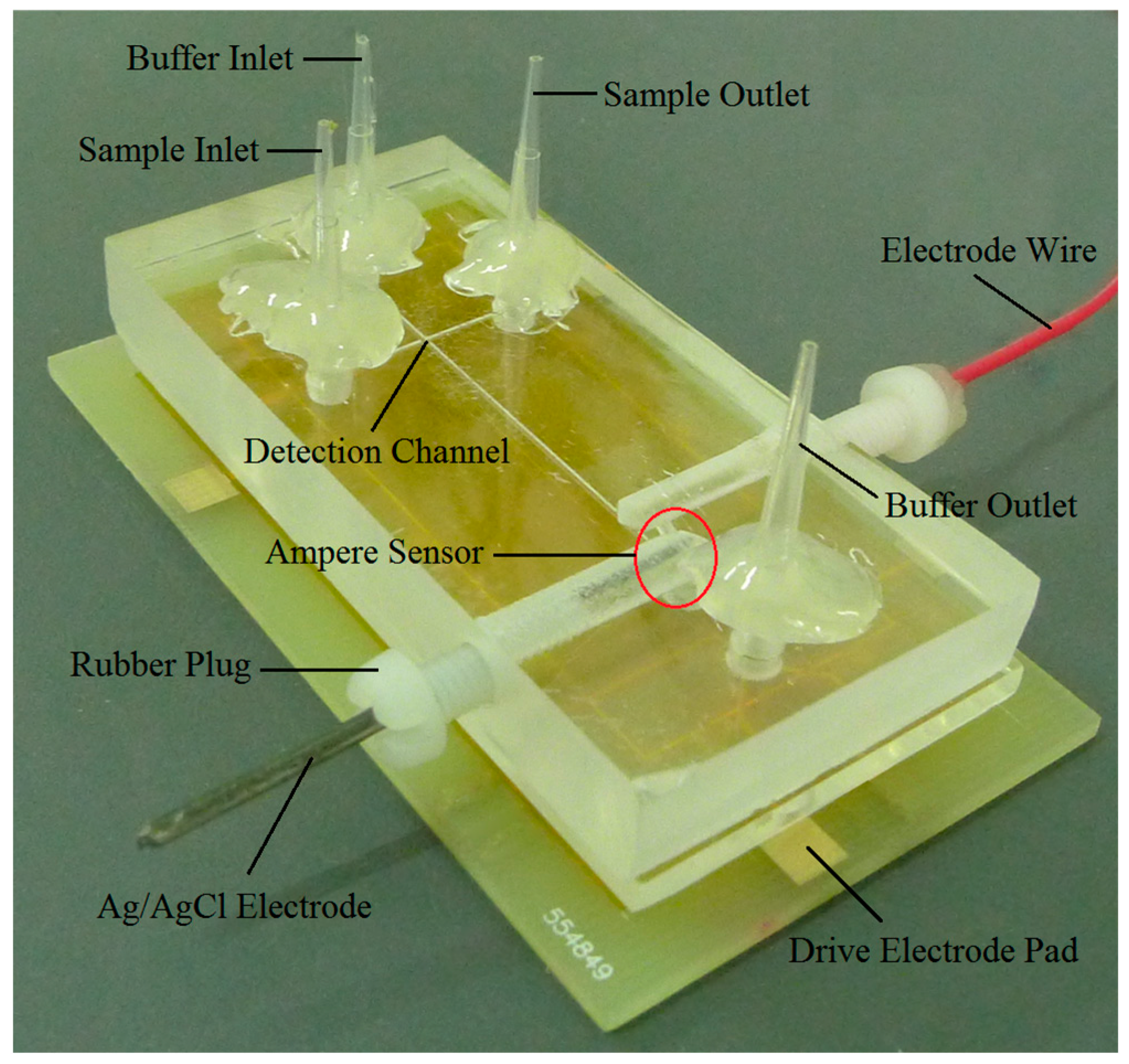
3. Sensor and Microfluidic Chip Preparation
4. Results and Discussion
- Detection of reference electrode stability. Three self-made Ag/AgCl reference electrodes were labelled as 1#, 2# and 3#, then immersed into KCl solution (0.0l mol/L) at room temperature (25 °C). The stability of the electrodes was measured using an electrochemical workstation. The test results are displayed in Figure 8, The potential drifts of the three electrodes are all within 0.10 mV, indicating good stability for all three self-made Ag/AgCl reference electrodes.
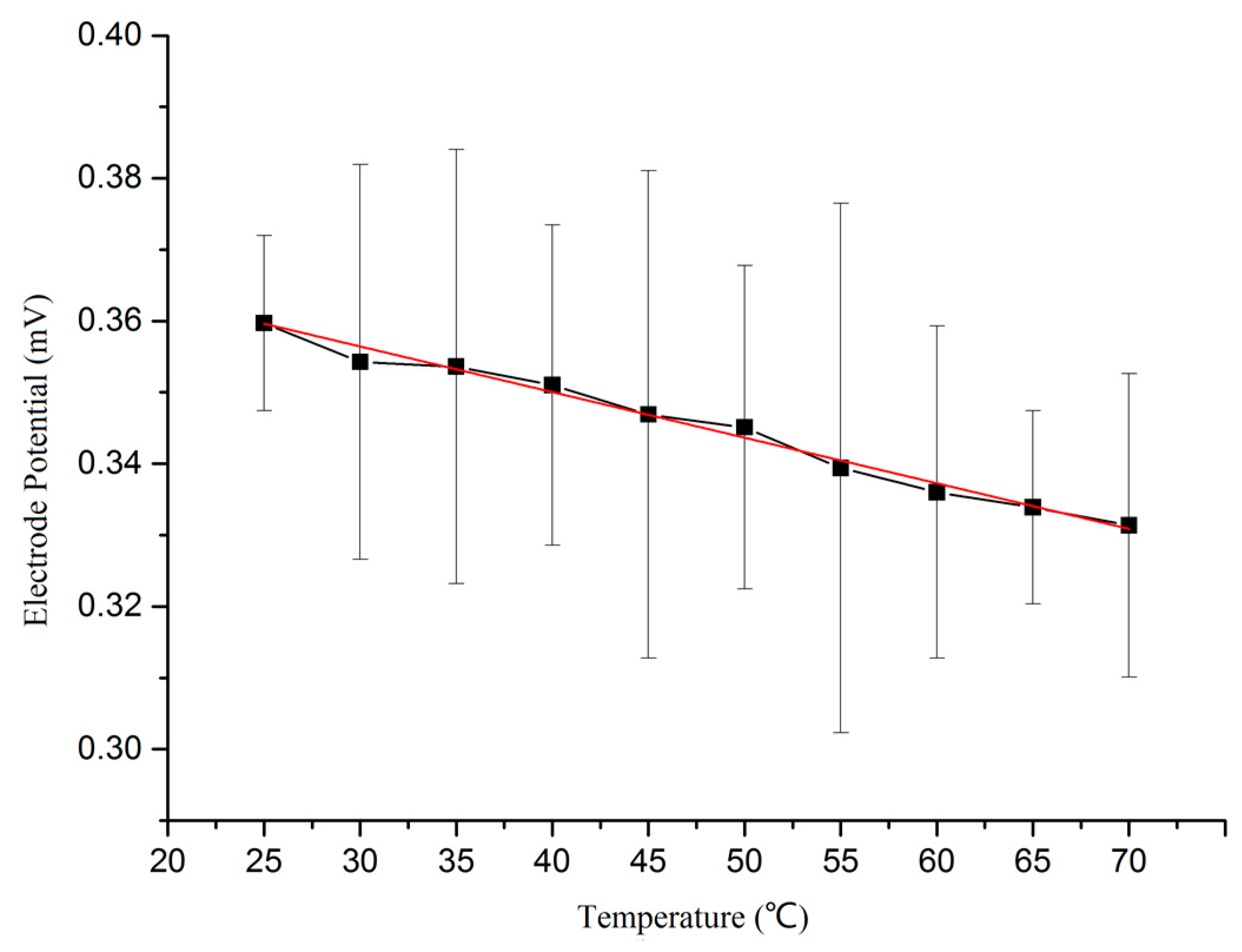
- Detection of the temperature response. A testing reference electrode was immersed into KCl solution (0.0l mol/L), and the temperature was adjusted using an oven. The potential of the self-made Ag/AgCl reference electrode versus a standard saturated calomel electrode (SCE) was measured using an electrochemical workstation. The testing curve is shown in Figure 9. It can be seen from the curve that the electrode potential is inversely proportional to the temperature. The electrode potential was reduced from 0.359 mV to 0.331 mV, when the temperature rose from 25 °C to 70 °C. The expression EAg/AgCl = 0.376 − 6.389 × 10−4·T that was obtained by using the software Origin9.0 (OriginLab, Hampton, MA, USA). The electrode reaction of Ag/AgCl can be written as:
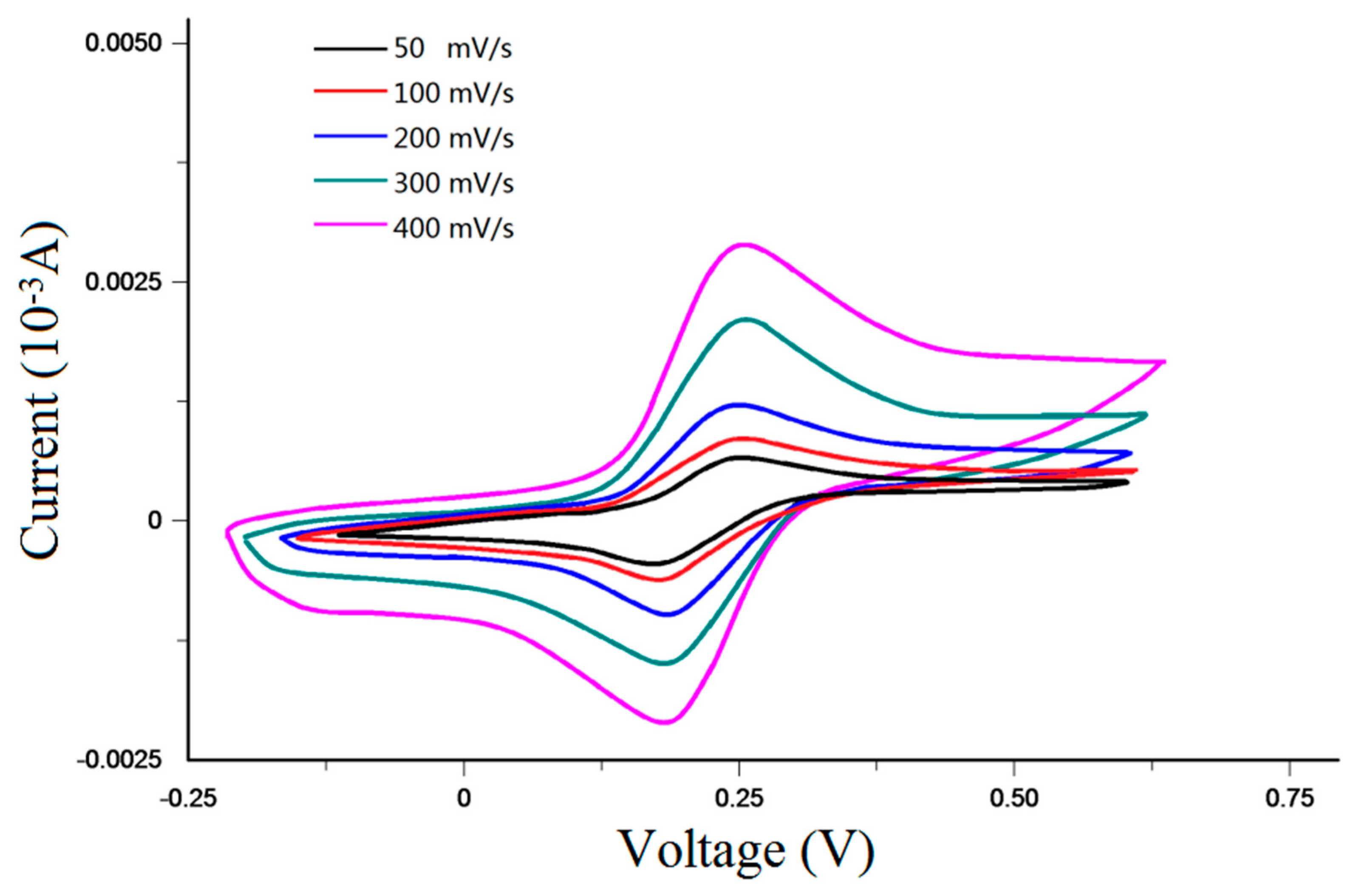
- Cyclic voltammetry testing. The testing solution consisted of KCl (0.1 mol/L), K4Fe(CN)6 (1 × 10−3 mol/L) and K3Fe(CN)6 (1 × 10−3 mol/L). PB buffer solution was used to adjust the testing solution pH to 5. Next, the testing solution was injected into the detection channel of the microfluidic chip, and care was taken to ensure that the integrated sensor was connected to the appropriate port of the electrochemical workstation. The start scan potential of the electrochemical workstation was −200 mV, the ending scan potential of the electrochemical workstation was 600 mV, and the scan interval was 2 mV. The cyclic voltammetry curves of the sensor for scan rates of 50, 100, 200, 300, 400 and 500 mV/s are shown in Figure 10. As shown in the figure, the oxidation/reduction peak of the sensor is approximately symmetrical, which indicates that the amperometric sensor has good reversibility.
- The detection of heavy metal ions in water. Tricine-MES mixed solution was used as a buffer solution (20 mol/L, pH = 5), Mg2+ and Pb2+ mixed solutions of different concentrations were used as samples. The drive electrode pads of the chip were connected to a voltage power supply, and the injection voltage was set as +1000 V, while the separation voltage was set as +1200 V. The integrated sensor was connected to the appropriate port of the electrochemical workstation and the detection potential of the electrochemical workstation was set as −0.4 V. Due to the electroosmotic flow that was generated by the external electric field, the sample zone, sandwiched between the buffer solutions, moves toward the amperometric sensor. The speeds of Pb2+ and Mg2+ are different under the same driving electric field intensity. Therefore, after passing though the same length of detection channel, Pb2+ and Mg2+ ions reach the sensor surface at different times. When Pb2+ and Mg2+ ions arrive at the sensor surface, a redox reaction occurs on the golden working electrode, causing the peak current to be generated. The peak current intensity is positively correlated with Pb2+ and Mg2+ concentration, so the ion concentrations can be determined from the peak current intensity. The detection curves of Mg2+ and Pb2+ at different concentrations are shown in Figure 11. It can be seen that the sensor and the chip can achieve the separation and detection of Mg2+ and Pb2+ ions, and the detection signal intensity was proportional to the sample concentration. When the concentration of Mg2+ and Pb2+ were both 2 mmol/L, the peak currents were 3.496 nA and 1.273 nA, respectively. When the concentration of Mg2+ and Pb2+ were both 1 mmol/L, the peak currents were 2.147 nA and 0.721 nA, respectively. When the concentration of Mg2+ and Pb2+ were both 0.5 mmol/L, the peak currents were 1.398 nA and 0.467 nA, respectively. When the signal-to-noise ratio was 3, the detection limit of Mg2+ was 52 μmol/L (the standard deviation of 5 times detection was 2.43%) and the detection limit of Pb2+ was 84 μmol/L (the standard deviation of 5 times detection was 3.51%).
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fernandez, R.E.; Rohani, A.; Farmehini, V.; Swami, N.S. Review: Microbial analysis in dielectrophoretic microfluidic systems. Anal. Chim. Acta 2017, 966, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, J.; Noon, B.; Willems, D.; Budeski, K.; Bothner, B. Analysis of raw biofluids by mass spectrometry using microfluidic diffusion-based separation. Anal. Methods 2017, 9, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Mansor, M.A.; Takeuchi, M.; Nakajima, M.; Hasegawa, Y.; Ahmad, M.R. Electrical impedance spectroscopy for detection of cells in suspensions using microfluidic device with integrated microneedles. Appl. Sci. 2017, 7, 170–179. [Google Scholar] [CrossRef]
- Han, Z.; Chang, Y.Y.; Au, S.W.N.; Zheng, B. Measuring rapid kinetics by a potentiometric method in droplet-based microfluidic devices. Chem. Commun. 2012, 48, 1601–1603. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, A.J.; Breadmore, M.C.; Guijt, R.M. In-plane alloy electrodes for capacitively coupled contactless conductivity detection in poly(methylmethacrylate) electrophoretic chips. Electrophoresis 2013, 34, 2980. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, X. A portable paper-based microfluidic platform for multiplexed electrochemical detection of human immunodeficiency virus and hepatitis C virus antibodies in serum. Biomicrofluidics 2016, 10, 12–22. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Bishop, G.W.; Bhakta, S.; El-Sawy, A.; Suib, S.L.; Rusling, J.F. Fe3O4 nanoparticles on graphene oxide sheets for isolation and ultrasensitive amperometric detection of cancer biomarker proteins. Biosens. Bioelectron. 2017, 91, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Polk, B.J.; Stelzenmuller, A.; Mijares, G.; MacCrehan, W.; Gaitan, M. Ag/AgCl microelectrodes with improved stability for microfluidics. Sens. Actuators B Chem. 2006, 114, 239–247. [Google Scholar] [CrossRef]
- Schöning, M.J.; Jacobs, M.; Muck, A.; Knobbe, D.T.; Wang, J.; Chatrathi, M.; Spillmann, S. Amperometric PDMS/glass capillary electrophoresis-based biosensor microchip for catechol and dopamine detection. Sens. Actuators B Chem. 2005, 108, 688–694. [Google Scholar] [CrossRef]
- Dias, A.A.; Cardoso, T.M.; Cardoso, R.M.; Duarte, L.C.; Muñoz, R.A.; Richter, E.M.; Coltro, W.K. Paper-based enzymatic reactors for batch injection analysis of glucose on 3D printed cell coupled with amperometric detection. Sens. Actuators B Chem. 2016, 226, 196–203. [Google Scholar] [CrossRef]
- Zhu, G.; Song, Q.; Liu, W.; Yan, X.; Xiao, J.; Chen, C. A gold nanoparticle-modified indium tin oxide microelectrode for in-channel amperometric detection in dual-channel microchip electrophoresis. Anal. Methods 2017. [Google Scholar] [CrossRef]
- Vandaveer, W.R.; Pasas, S.A.; Martin, R.S.; Lunte, S.M. Recent developments in amperometric detection for microchip capillary electrophoresis. Electrophoresis 2015, 23, 3667–3677. [Google Scholar] [CrossRef]
- Wongkaew, N.; Kirschbaum, S.E.; Surareungchai, W.; Durst, R.A.; Baeumner, A.J. A novel three-electrode system fabricated on polymethyl methacrylate for on-chip electrochemical detection. Electroanalysis 2012, 24, 1903–1908. [Google Scholar] [CrossRef]
- Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A. Microfabricated Reference Electrodes and their Biosensing Applications. Sensors 2010, 10, 1679–1715. [Google Scholar] [CrossRef] [PubMed]
- Turyan, I.; Khatwani, N.; Sosic, Z.; Jayawickreme, S.; Mandler, D. A novel approach for oxidation analysis of therapeutic proteins. Anal. Biochem. 2016, 494, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Zhao, Y.; Chu, J.; Liu, S.Y.; Li, W.W.; Liu, G.; Tian, Y.C.; Xiong, Y.; Yu, H.Q. Fabrication and characterization of an innovative integrated solid-state microelectrode. Electrochim. Acta 2010, 55, 5984–5989. [Google Scholar] [CrossRef]
- Lan, W.Y. The Design and Application of Amperometric Detection on Microfluidic Chip; Ph.D. Dissertation, Chongqing University, Chongqing, China, 2004. [Google Scholar]
- Liu, X.; Zhang, H.; Han, X.; Zhao, L. Simulation of backward facing step flow at the outlet of micro-channel and optimal design of amperometric detection chip. In Proceedings of the 8th Annual IEEE International Conference on Nano/micro Engineered and Molecular Systems, DuShu Lake Hotel, SuZhou, China; IEEE: New York, NY, USA.
- Zhang, H.; Liu, X.; Li, T.; Han, X. Miscible organic solvents soak bonding method use in a PMMA multilayer microfluidic device. Micromachines 2014, 5, 1416–1428. [Google Scholar] [CrossRef]
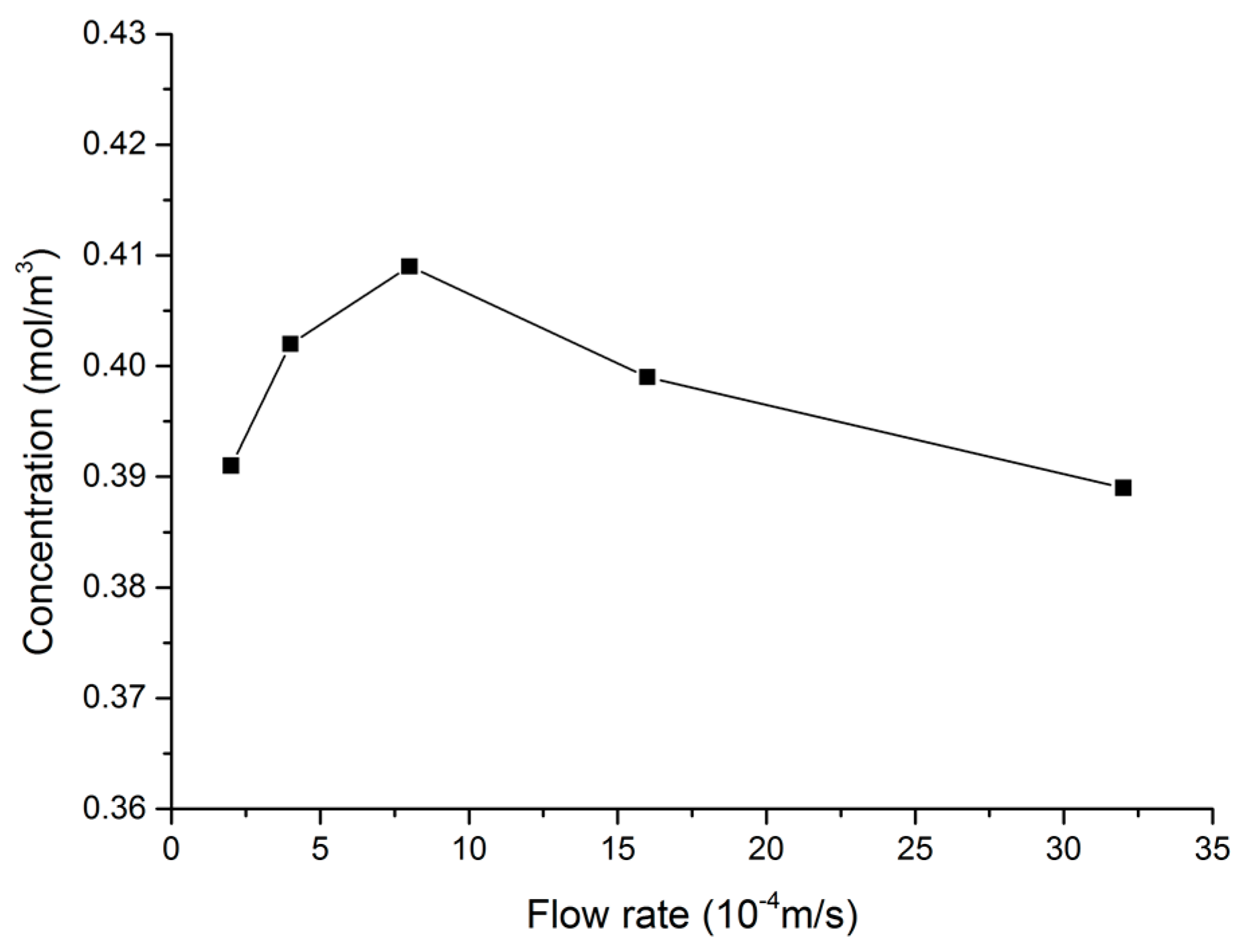


| Name | Value (Unit) | Name | Value (Unit) |
|---|---|---|---|
| Drive potential | 10 (V) | Density | 1000 (kg/m3) |
| Dielectric constant | 7.08 × 10−10 (F/m) | Channel length | 3.5 × 10−4 (m) |
| Wall potential | −0.01 (V) | Channel cross section | 2 × 10−5 (m) by 2 × 10−5 (m) |
| Diffusion coefficient | 1 × 10−11 (m2/s) | Sample concentration | 1 (mol/L) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chuai, R.; Li, X.; Zhang, B. Design, Preparation and Performance Study of On-Chip Flow-Through Amperometric Sensors with an Integrated Ag/AgCl Reference Electrode. Micromachines 2018, 9, 114. https://doi.org/10.3390/mi9030114
Zhang H, Chuai R, Li X, Zhang B. Design, Preparation and Performance Study of On-Chip Flow-Through Amperometric Sensors with an Integrated Ag/AgCl Reference Electrode. Micromachines. 2018; 9(3):114. https://doi.org/10.3390/mi9030114
Chicago/Turabian StyleZhang, He, Rongyan Chuai, Xin Li, and Bing Zhang. 2018. "Design, Preparation and Performance Study of On-Chip Flow-Through Amperometric Sensors with an Integrated Ag/AgCl Reference Electrode" Micromachines 9, no. 3: 114. https://doi.org/10.3390/mi9030114






