A Label Free Disposable Device for Rapid Isolation of Rare Tumor Cells from Blood by Ultrasounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Working Principle for High-Throughput Separation
2.2. Experimental
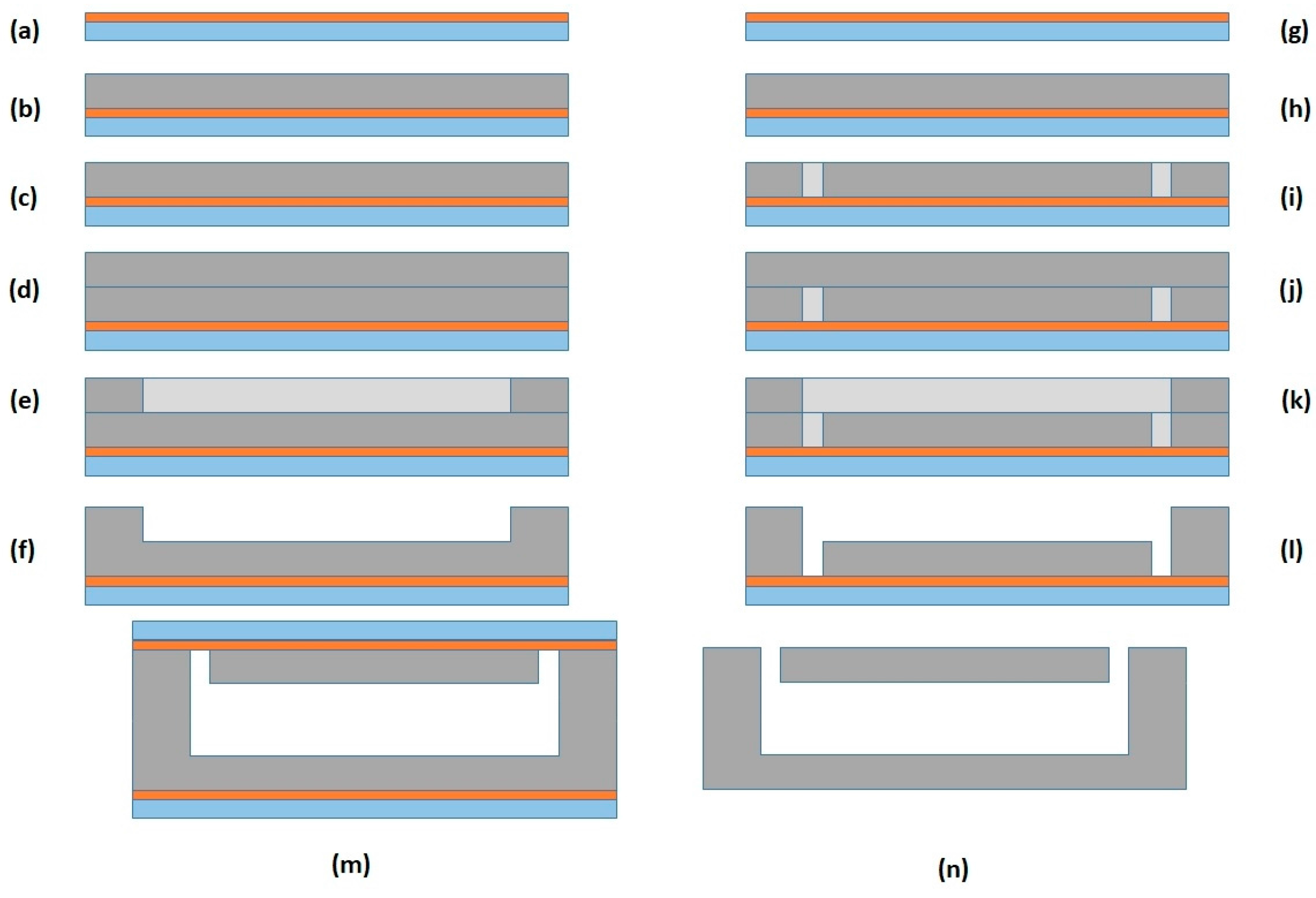
Acoustophoretic Device
3. Results
3.1. Calibration of the Device with Bead Suspensions
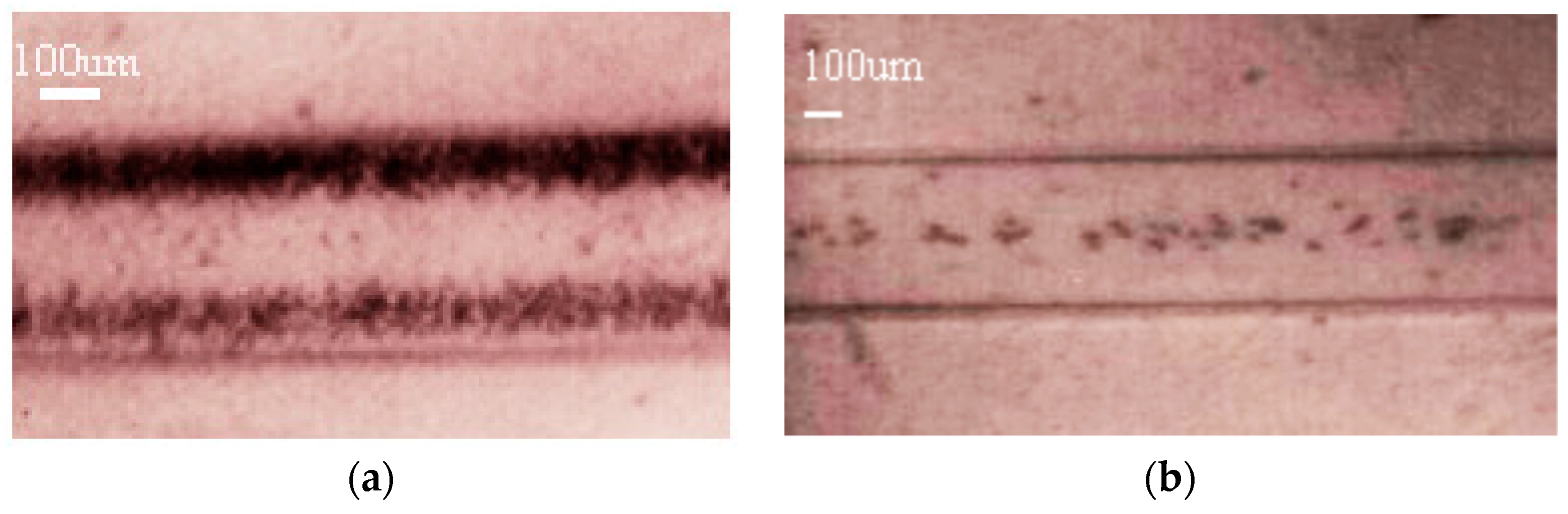
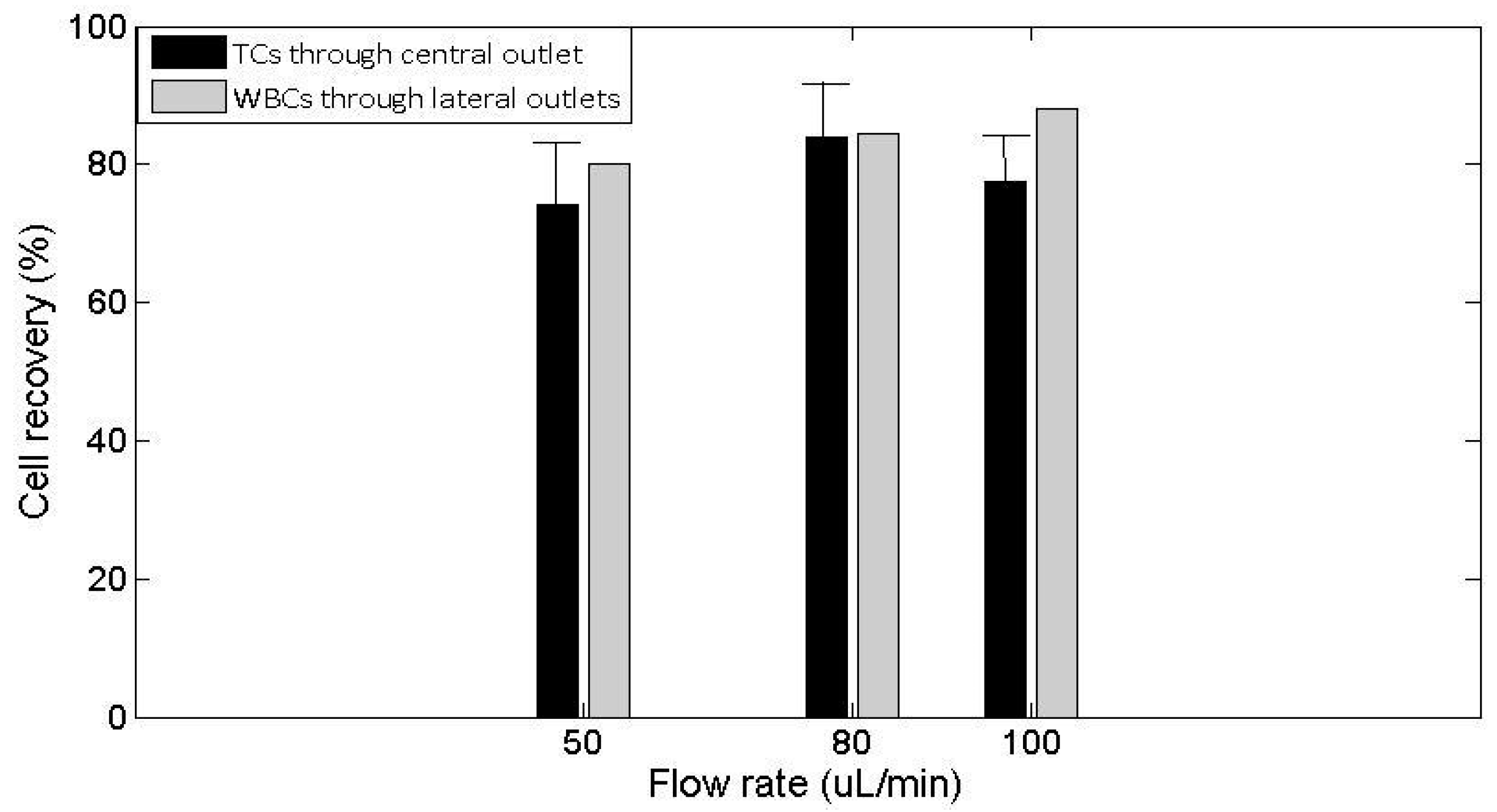
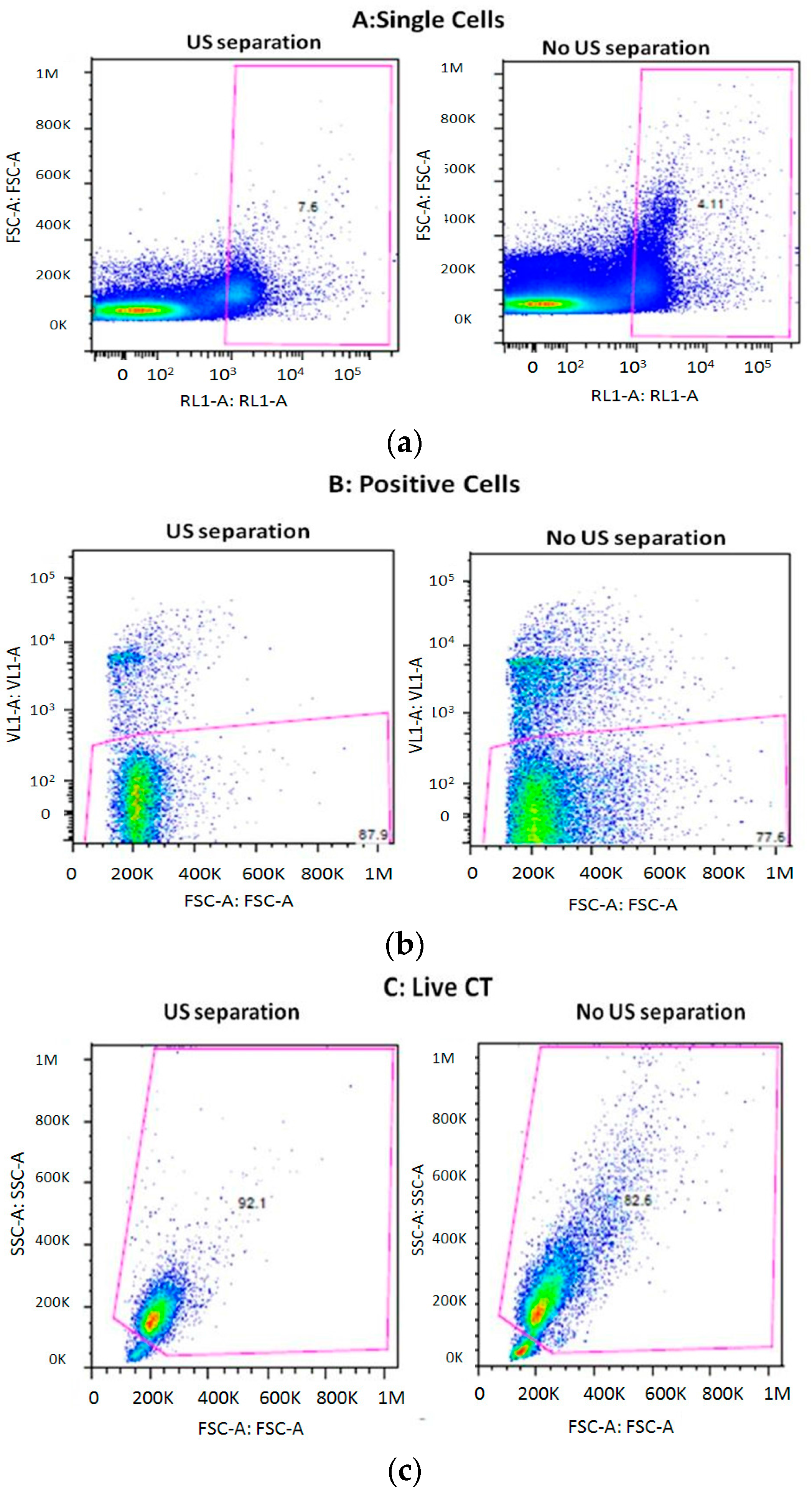
3.2. Actuation of the Ultrasounds on Tumor Cells and White Blood Cells
3.3. Cell Viability after Separation through the THINUS-Chip
4. Discussion and Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hou, J.M.; Krebs, M.; Ward, T.; Sloane, R.; Priest, L.; Hughes, A.; Clack, G.; Ranson, M.; Blackhall, F.; Dive, C. Circulating tumor cells as a window on metastasis biology in lung cancer. Am. J. Pathol. 2011, 178, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, L.A.; Williams, H.; Frankow, A.; Ellis, H.P.; Haynes, H.R.; Perks, C.; Holly, J.M.P.; Kurian, K.M. Current understanding of circulating tumor cells—Potential value in malignancies of the central nervous system. Front. Neurol. 2015, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Green, B.T.; Hughes, A.; King, M. Circulating tumor cells: The substrate of personalized medicine? Front. Oncol. 2012, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Circulating tumor cells: Liquid biopsy of cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Tsai, W.S.; Chang, Y.H.; Chou, T.Y.; Pang, S.T.; Lin, P.H.; Tsai, C.M.; Chang, Y.C. Identifying cancer origin using circulating tumor cells. Cancer Biol. Ther. 2016, 17, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Martinez, S.R.; Goto, Y.; Koyanagi, K.; Kitago, M.; Shingai, T.; Elashoff, D.A.; Ye, X.; Singer, F.R.; Giuliano, A.E. Detection of Circulating Tumor Cells in Early-Stage Breast Cancer Metastasis to Axillary Lymph Nodes. Clin. Cancer Res. 2007, 13, 4105–4110. [Google Scholar] [CrossRef] [PubMed]
- Joosse, S.A.; Gorges, T.M.; Pantel, K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2014, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Song, S.; Choi, C.; Park, J. Microfluidic Self-Sorting of Mammalian Cells to Achieve Cell Cycle Synchrony by Hydrophoresis. Anal. Chem. 2009, 81, 1964–1968. [Google Scholar] [CrossRef] [PubMed]
- Inglis, D.W. Efficient microfluidic particle separation arrays. Appl. Phys. Lett. 2009, 94, 013510–013513. [Google Scholar] [CrossRef]
- Tan, S.J.; Yobas, L.; Lee, G.Y.H.; Ong, C.N.; Lim, C.T. Microdevice for the isolation and enumeration of cancer cells from blood. Biomed. Microdevices 2009, 11, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.C.; Mach, A.J.; Di Carlo, D. High-throughput size-based rare cell enrichment using microscale vortices. Biomicrofluidics 2011, 5, 0222061. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-C.M.D.; Wang, L.; Yu, F.-L. Recent Advances and Prospects in the Isolation by Size of Epithelial Tumor Cells (ISET) Methodology. Technol. Cancer Res. Treat. 2013, 12, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Sollier, E.; Go, D.E.; Che, J.; Gossett, D.R.; O’Byrne, S.; Weaver, W.M.; Kummer, N.; Rettig, M.; Goldman, J.; Nickols, N.; et al. Size-selective collection of circulating tumor cells using Vortex technology. Lab Chip 2014, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Ogunwobi, O.O.; Chen, T.; Zhang, J.; George, T.J.; Liu, C.; Fan, Z.H. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip 2014, 14, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Weaver, W.M.; Mach, A.J.; Hur, S.C.; Kwong Tse, H.T.; Lee, W.; Amini, H.; Di Carlo, D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 3249–3267. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.W.; Warkiani, M.E.; Khoo, B.L.; Li, Z.R.; Soo, R.A.; Tan, D.S.; Lim, W.T.; Han, J.; Bhagat, A.A.; Lim, C.T. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 2013, 3, 1259. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Guan, G.; Luan, K.B.; Lee, W.C.; Bhagat, A.A.; Chaudhuri, P.K.; Tan, D.S.; Lim, W.T.; Lee, S.C.; Chen, P.C.; et al. Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip 2014, 14, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Khoo, B.L.; Warkiani, M.E.; Tan, D.S.; Bhagat, A.A.; Irwin, D.; Lau, D.P.; Lim, A.S.; Lim, K.H.; Krisna, S.S.; Lim, W.T.; et al. Clinical validation of an ultra high-throughput spiral microfluidics for the detection and enrichment of viable circulating tumor cells. PLoS ONE 2014, 9, e99409. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.A.S.; Hou, H.W.; Li, L.D.; Lim, C.T.; Han, J. Pinched flow coupled shear-modulated inertial microfluidics for high-throughput rare blood cell separation. Lab Chip 2011, 11, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Sarioglu, F.; Aceto, N.; Kojic, N.; Donaldson, M.C.; Zeinali, M.; Hamza, B.; Engstrom, A.; Zhu, H.; Sundaresan, T.K.; Miyamoto, D.T.; et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat. Methods 2015, 12, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, J.K.; Choi, S. Continuous sorting and washing of cancer cells from blood cells by hydrophoresis. BioChip J. 2016, 10, 81–87. [Google Scholar] [CrossRef]
- Stott, S.L.; Hsu, C.H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yu, V.; Dhar, M.; Renier, C.; Matsumoto, M.; Heirich, K.; Garon, E.B.; Goldman, J.; Rao, J.; Sledge, G.W.; et al. Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic Vortex technology. Oncotarget 2016, 7, 12748–12760. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.; Lakshmi, R.L.; Chen, P.F.; Lim, W.T.; Yobas, L.; Lim, C.T. Versatile label free biochip for the detection of circulating tumor cells from peripheral blood in cancer patients. Biosens. Bioelectron. 2010, 26, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.S.; Zhao, Y.X.; Schiro, P.G.; Ng, L.Y.; Lim, D.S.W.; Shelby, J.P.; Chiu, D.T. Deformability considerations in filtration of biological cells. Lab Chip 2010, 10, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Dessitter, I.; Guerrouahena, B.S.; Benali-Furet, N.; Weschler, J.; Janne, P.A.; Kuang, Y.; Yanagita, M.; Wuang, L.; Berkowitz, J.A.; Distel, R.J.; et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011, 31, 427–442. [Google Scholar]
- Zheng, S.; Lin, H.; Liu, J.Q.; Balic, M.; Datar, R.; Cote, R.J.; Tai, Y.C. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J. Chromatogr. A 2007, 1162, 154–161. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Fernández, L.J.; López, N.; Berganzo, J.; Martin, A.; Gómez, T.; Bouali, M.; Soto, J.L.; Carrato, A. Ultrasonic extraction of circulating tumor cells from peripheral blood-a noninvasive method to keep the cells viable for later biomolecular analysis. In Proceedings of the 6th International Conference on Microtechnologies in Medicine and Biology, Lucerne, Switzerland, 4–6 May 2011; Volume 83, pp. 60–61. [Google Scholar]
- Augustsson, P.; Magnusson, C.; Nordin, M.; Lilja, H.; Laurell, T. Microfluidic, label-free enrichment of prostate cancer cells in blood based on acoustophoresis. Anal. Chem. 2012, 84, 7954–7962. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Peng, Z.; Lin, S.-C.S.; Geri, M.; Li, S.; Li, P.; Chen, Y.; Subra Suresh, M.D.; Huang, T.J. Cell separation using tilted-angle standing surface acoustic waves. Proc. Natl. Acad. Sci. USA 2015, 111, 12992–12997. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mao, Z.; Peng, L.; Zhou, Y.; Chen, P.-H.; Huang, C.I.; Truica, J.J.; Drabick, S.W.; El-Deiry, M.; Dao, S.; Suresh, S.; Huang, T.J. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef] [PubMed]
- Petersson, F.; Nilsson, A.; Jonsson, H.; Laurell, T. Carrier Medium Exchange through Ultrasonic Particle Switching in Microfluidic Channels. Anal. Chem. 2005, 77, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Fernández, L.J.; Gómez, T.; Berganzo, J.; Soto, J.L.; Carrato, A. A polymeric chip for micromanipulation and particle sorting by ultrasounds based on a multilayer configuration. Sens. Actuators B Chem. 2010, 144, 310–317. [Google Scholar] [CrossRef]
- Hulström, J.; Manneberg, O.; Dopf, K.; Hertz, H.; Brismar, H.; Wiklund, M. Proliferation and viability of adherent cells manipulated by standing-wave ultrasound in a microfluidic chip. Ultrasound Med. Biol. 2007, 33, 145–151. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Tijero, M.; Martin, A.; Acosta, V.; Berganzo, J.; Castillejo, A.; Bouali, M.M.; Soto, J.L. Optimizing polymer Lab-on-Chip platforms for ultrasonic manipulation: Influence of the substrate. Micromachines 2015, 6, 574–591. [Google Scholar] [CrossRef]
- Mindlin, R.D.; Schaknow, A.; Deresiewicz, H. Flexural vibration of rectangular plates. ASME J. Appl. Mech. 1956, 23, 430–436. [Google Scholar]
- Sadrnejad, S.A.; Saedi Daryan, A.; Ziaei, M. Vibration Equations of Thick Rectangular Plates Using Mindlin Plate Theory. J. Comput. Sci. 2009, 5, 838–842. [Google Scholar] [CrossRef]
- Gor’kov, L. On the Forces Acting on a Small Particle in an Acoustical Field in an Ideal Fluid. Sov. Phys. Acoust. 1962, 6, 773–775. [Google Scholar]
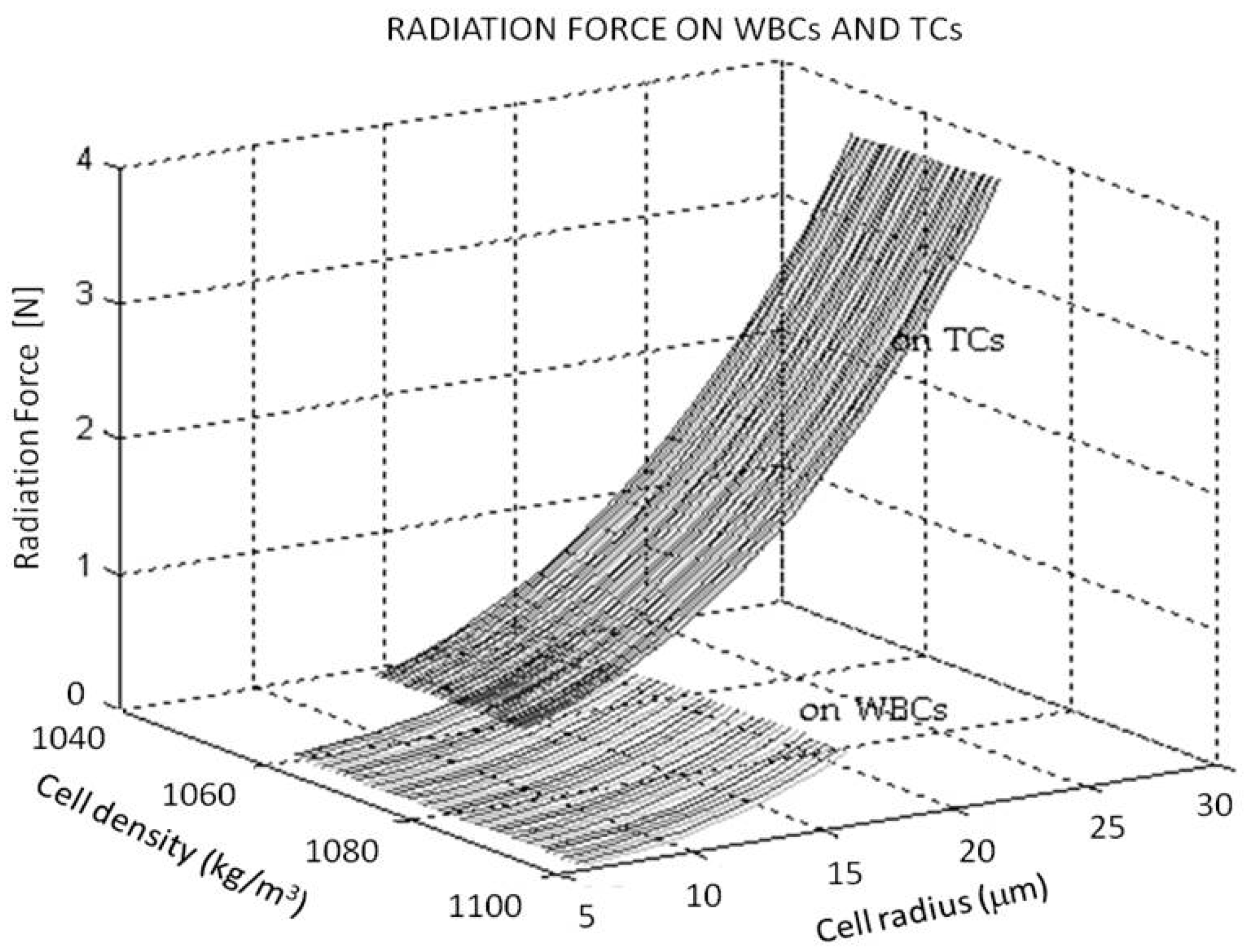
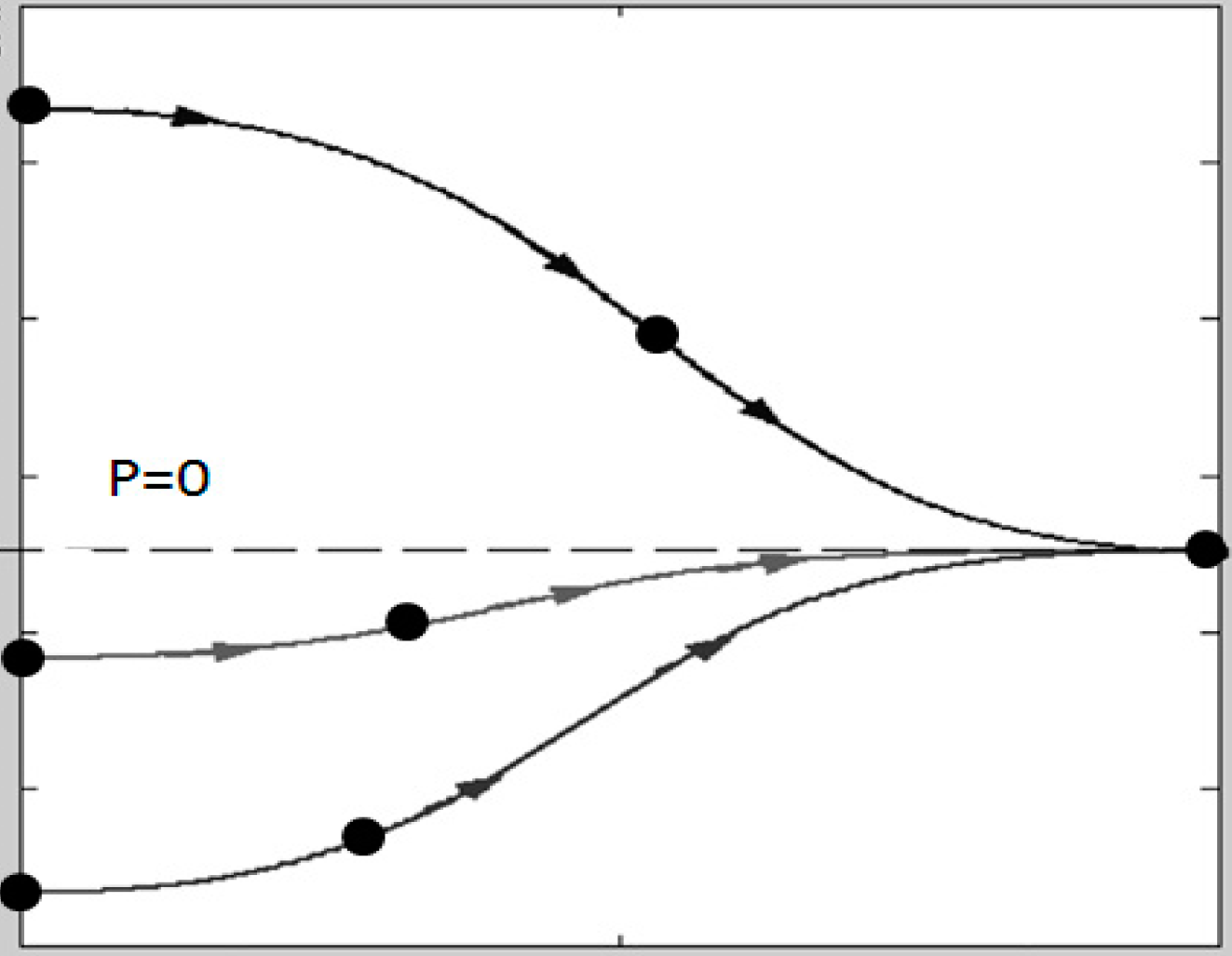

| Cells | Density ρ (kg/m3) | Size (µm) |
|---|---|---|
| WBCs | 1060 ≤≤ 1100 | 7 ≤ RL ≤ 15 7–8 μm and 12–15 μm Lymphocytes,10–12 μm Neutrophils and Eosinophils, 12–15 μm Basophils |
| Pancreas CTs | 1050 ≤≤ 1080 | 10 ≤ RCT ≤ 25 |
| Chip | Frequency | Advantages | Disadvantages |
| BAW devices chip resonance | Not restrictions |
|
|
| BAW devices channel resonance | Not restrictions |
|
|
| SAW devices | >9 MHz |
|
|
| THINUS Plate-like devices | Not restrictions |
|
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, I.; Earl, J.; Fernández, L.J.; Sainz, B.; Pinto, A.; Monge, R.; Alcalá, S.; Castillejo, A.; Soto, J.L.; Carrato, A. A Label Free Disposable Device for Rapid Isolation of Rare Tumor Cells from Blood by Ultrasounds. Micromachines 2018, 9, 129. https://doi.org/10.3390/mi9030129
González I, Earl J, Fernández LJ, Sainz B, Pinto A, Monge R, Alcalá S, Castillejo A, Soto JL, Carrato A. A Label Free Disposable Device for Rapid Isolation of Rare Tumor Cells from Blood by Ultrasounds. Micromachines. 2018; 9(3):129. https://doi.org/10.3390/mi9030129
Chicago/Turabian StyleGonzález, Itziar, Julie Earl, Luis J. Fernández, Bruno Sainz, Alberto Pinto, Rosa Monge, Sonia Alcalá, Adela Castillejo, Jose L. Soto, and Alfredo Carrato. 2018. "A Label Free Disposable Device for Rapid Isolation of Rare Tumor Cells from Blood by Ultrasounds" Micromachines 9, no. 3: 129. https://doi.org/10.3390/mi9030129






