Optofluidic Tunable Lenses for In-Plane Light Manipulation
Abstract
:1. Introduction
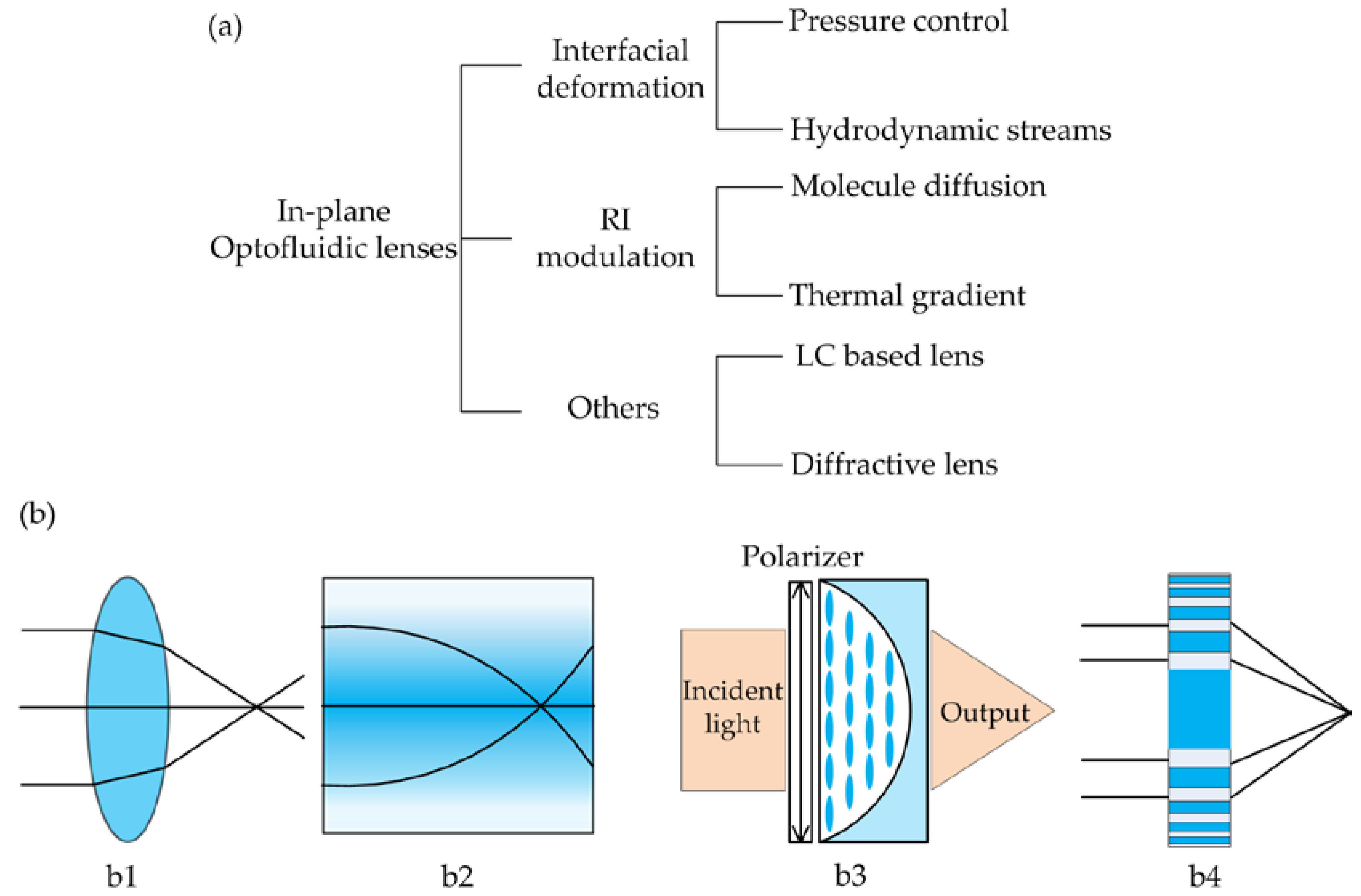
2. Classification of In-Plane Optofluidic Lenses
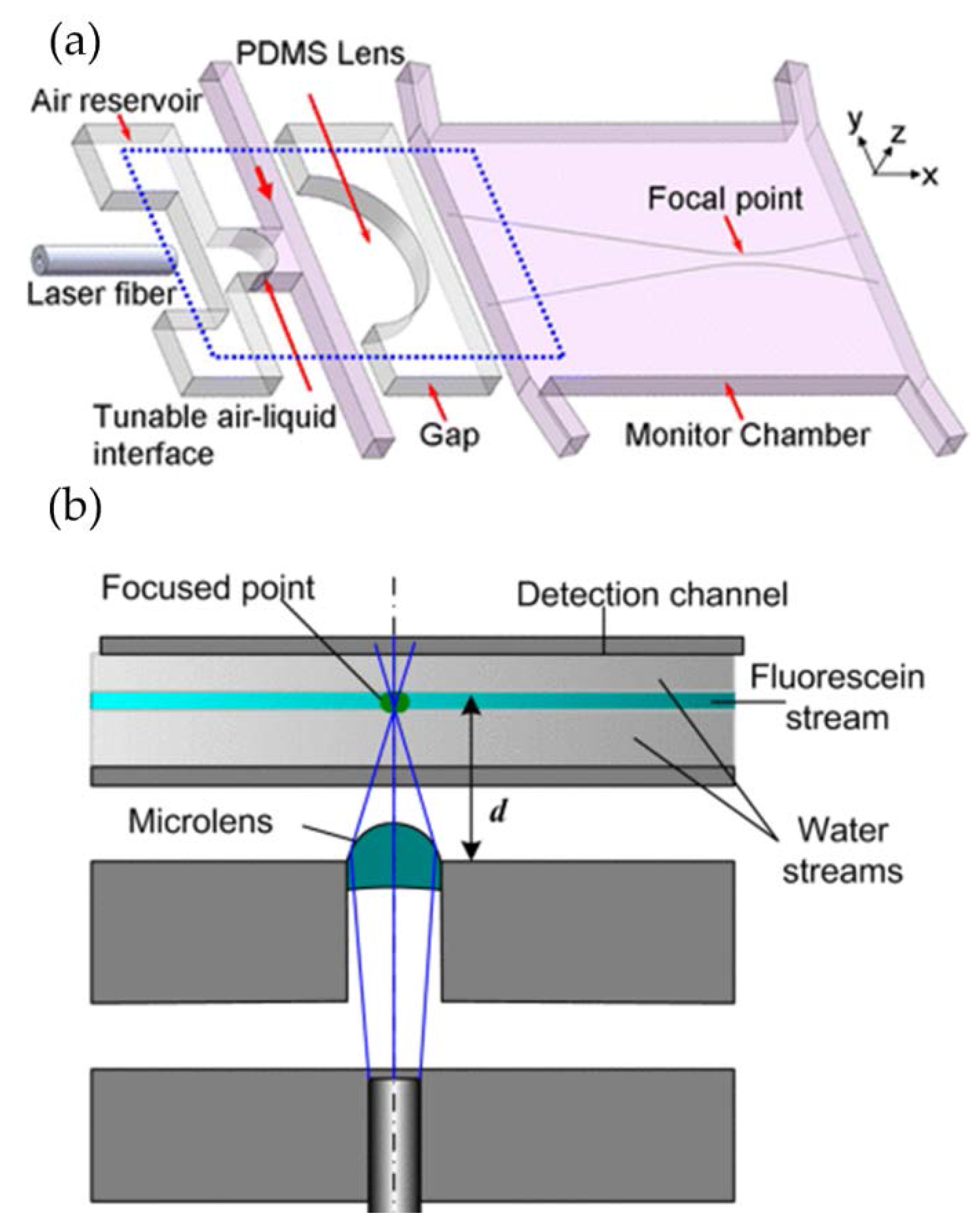
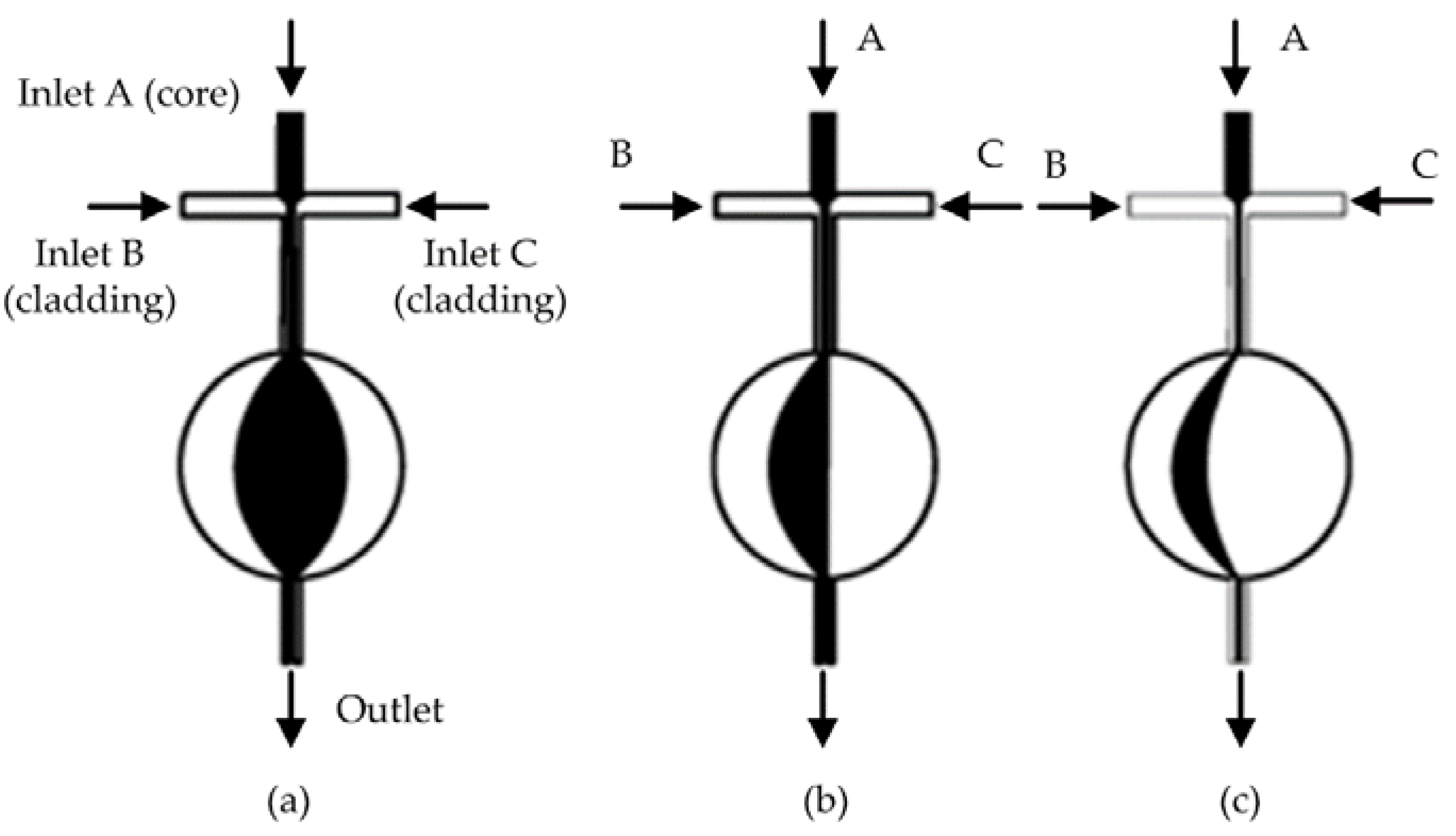
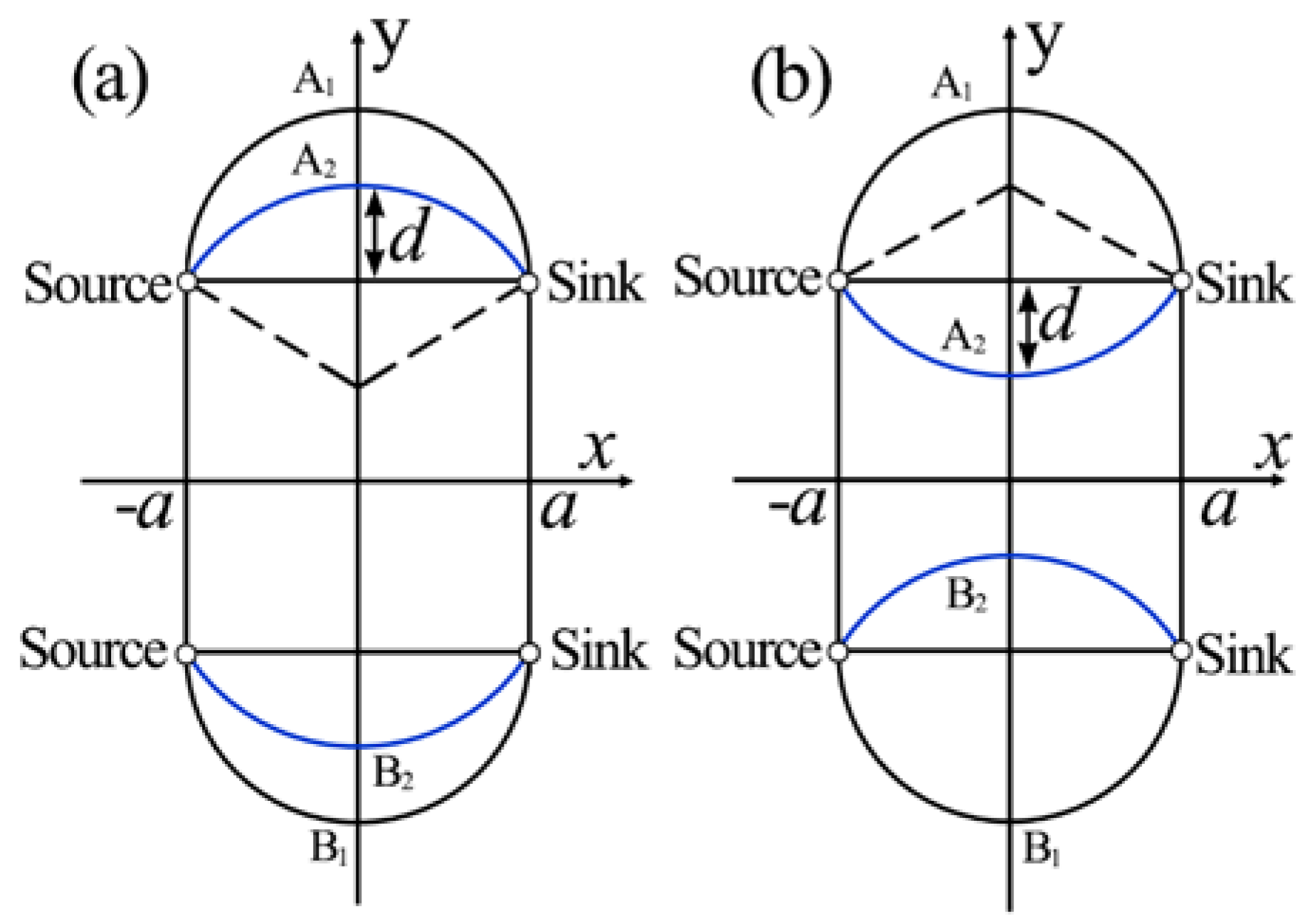
2.1. Interfacial Deformation
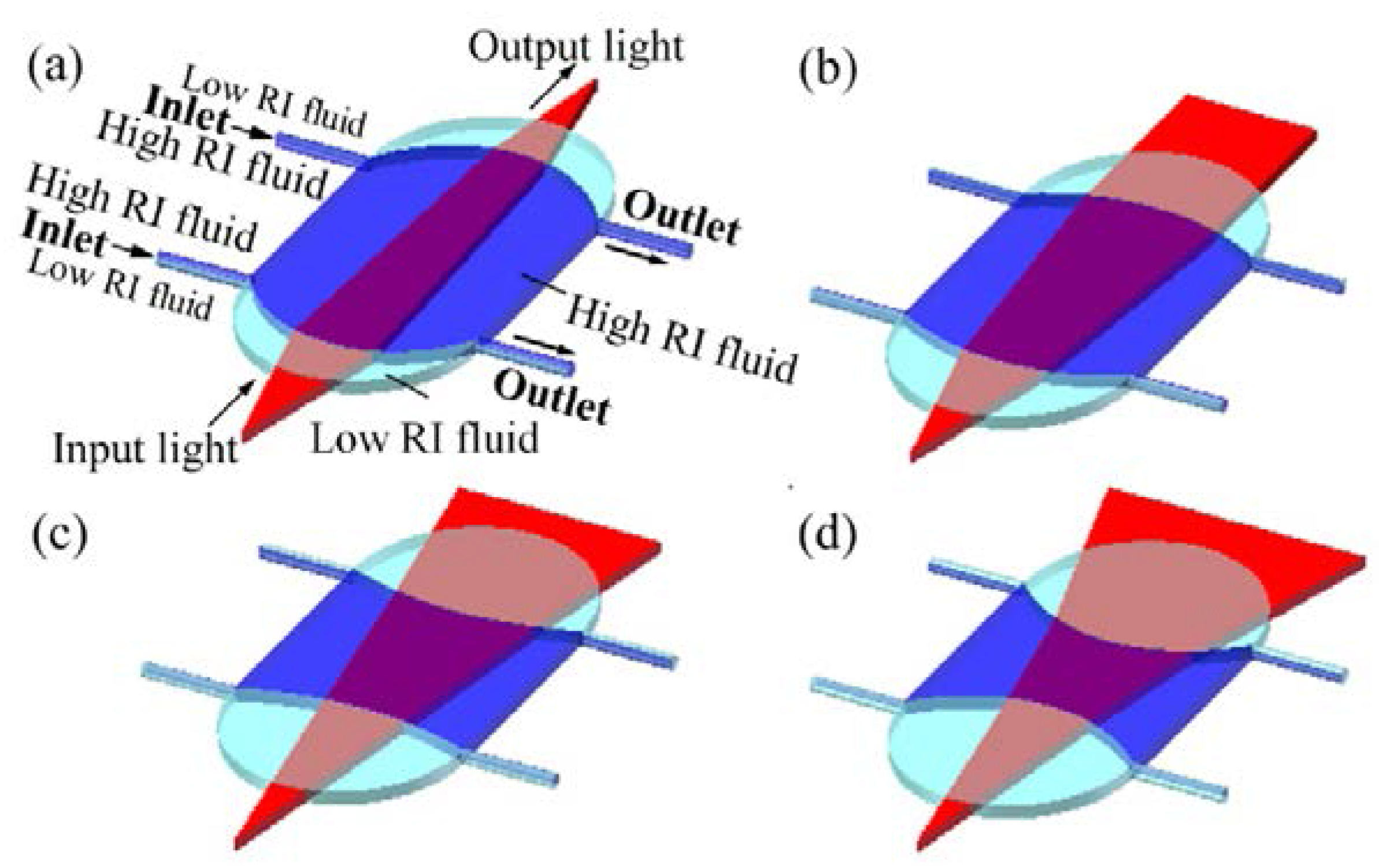
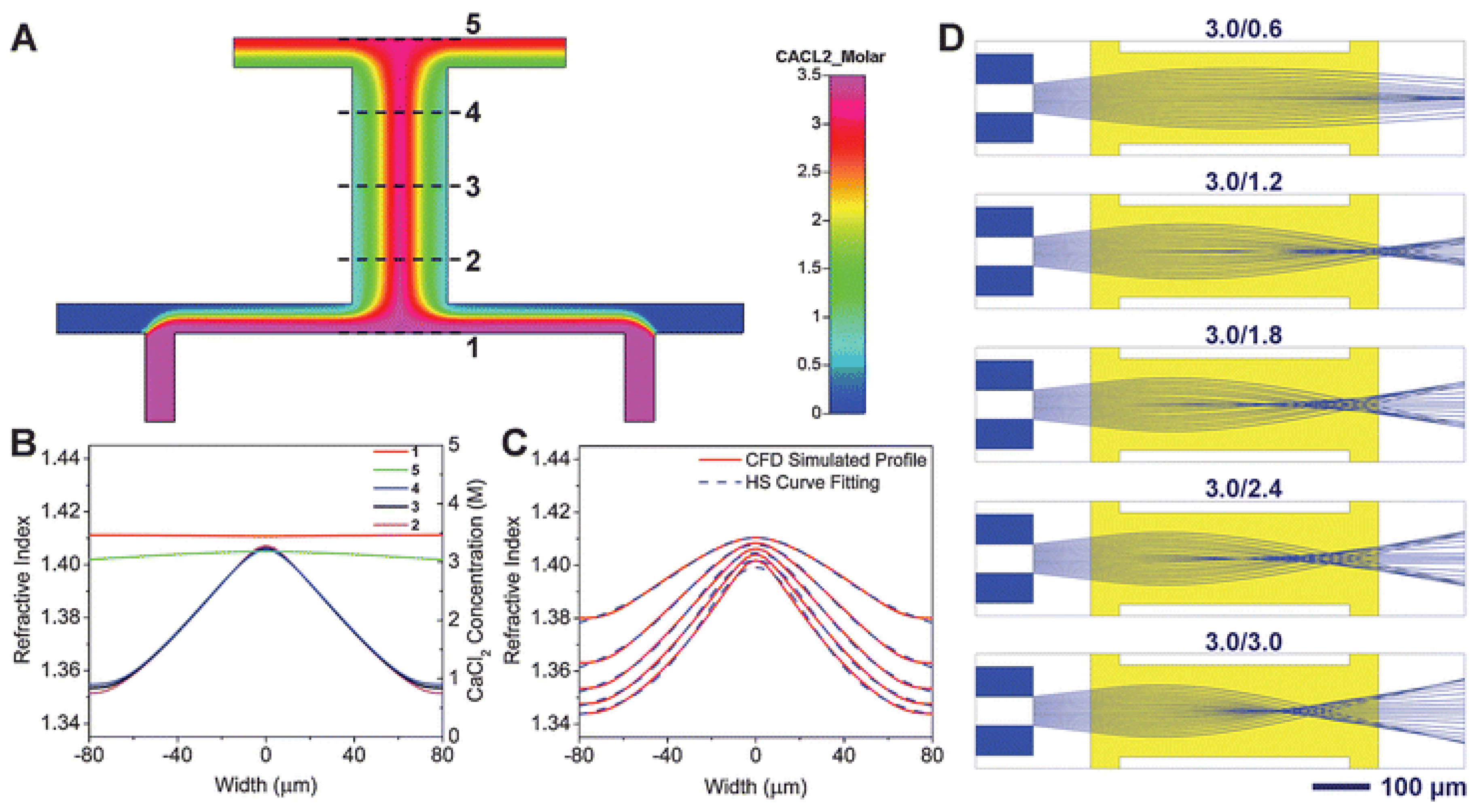
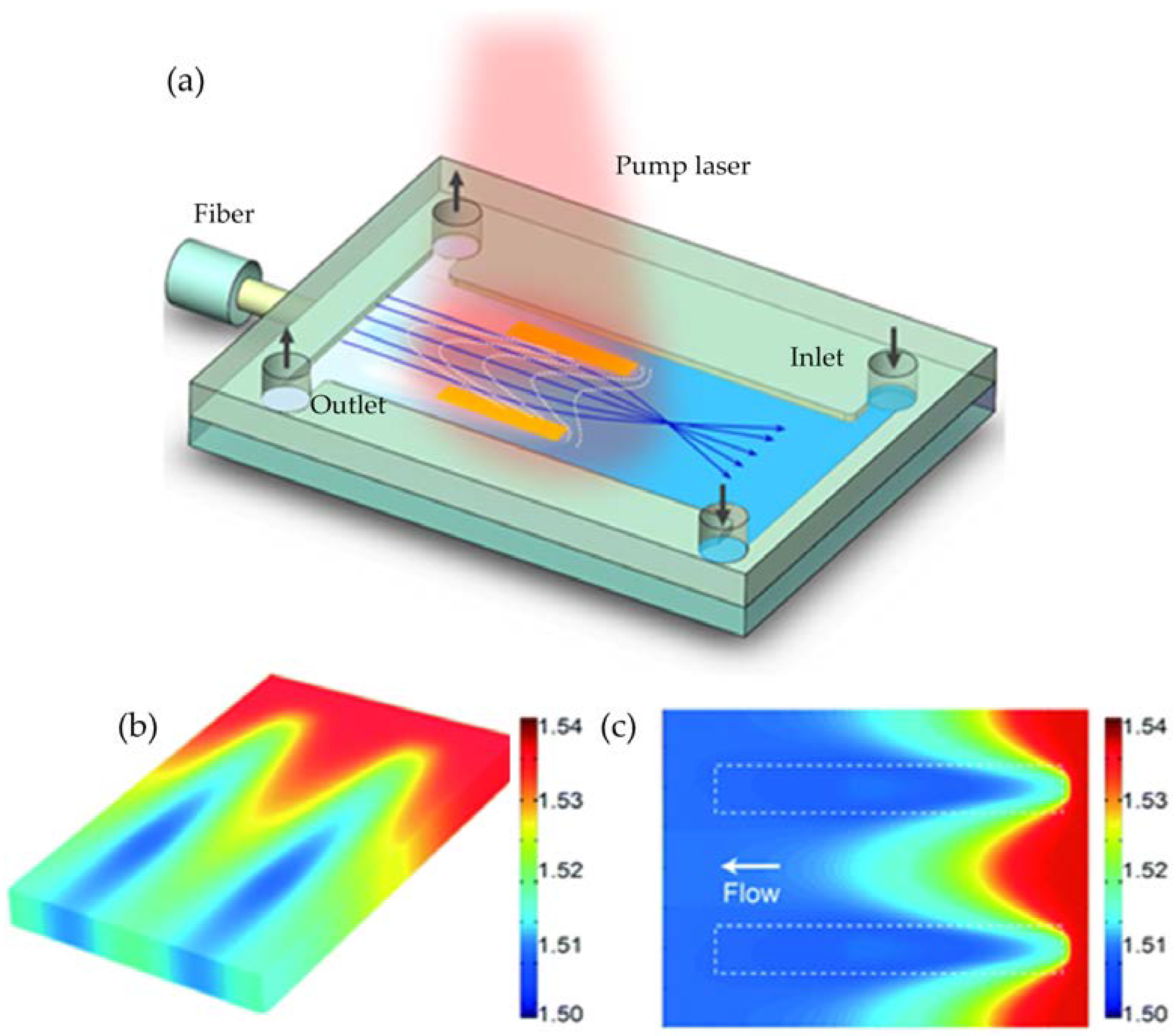
2.2. Refractive Index (RI) Modulation
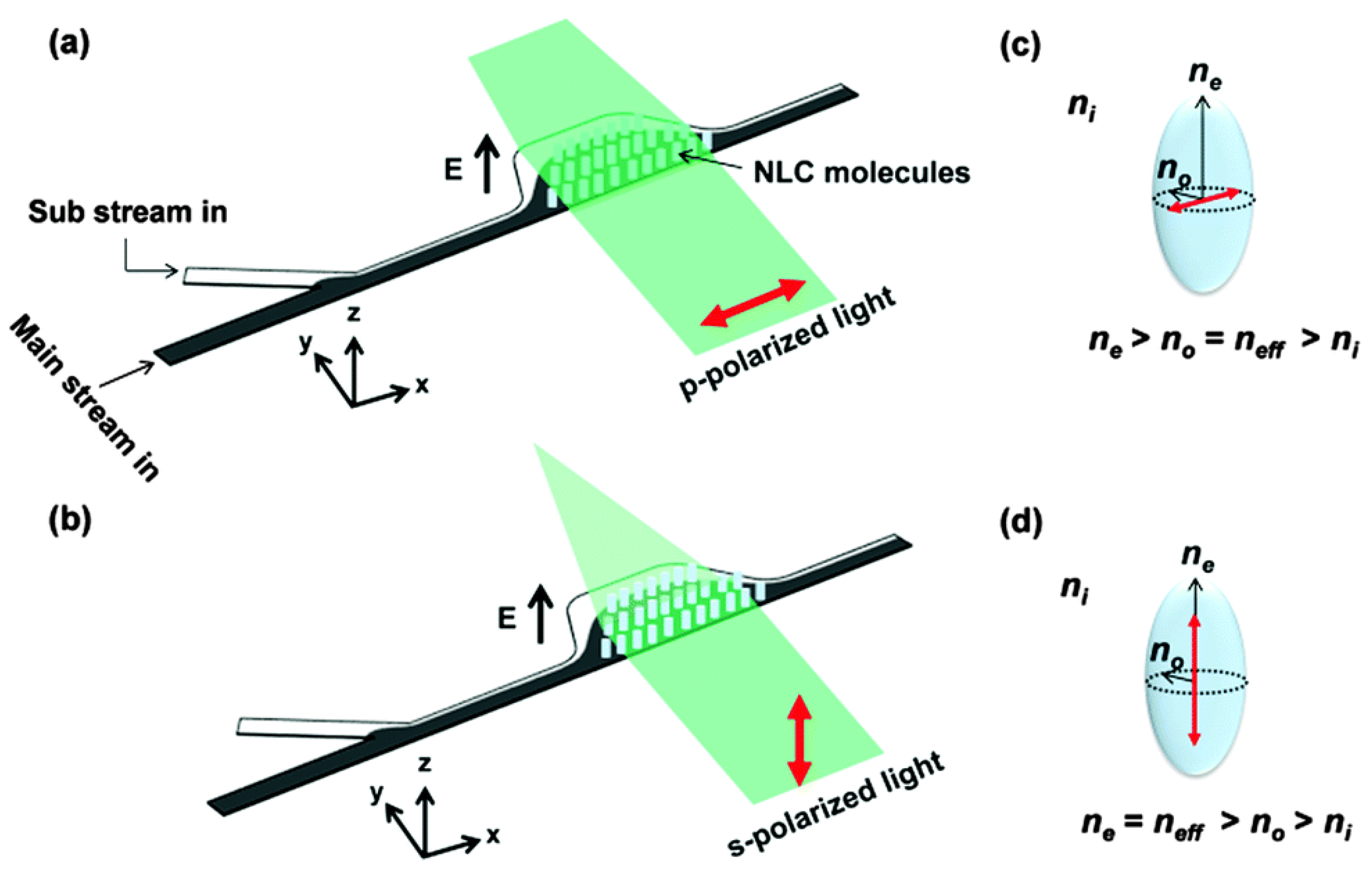
2.3. Others
3. Applications of In-Plane Liquid Lenses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Psaltis, D.; Quake, S.R.; Yang, C.H. Developing optofluidic technology through the fusion of microfluidics and optics. Nature 2006, 442, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Stratton, Z.S.; Guo, F.; Lapsley, M.I.; Chan, C.Y.; Lin, S.-C.S.; Huang, T.J. Optofluidic imaging: Now and beyond. Lab Chip 2013, 13, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Monat, C.; Domachuk, P.; Eggleton, B.J. Integrated optofluidics: A new river of light. Nat. Photon. 2007, 1, 106–114. [Google Scholar] [CrossRef]
- Fan, X.D.; White, I.M. Optofluidic Microsystems for Chemical and Biological Analysis. Nat. Photon. 2011, 5, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Chen, H.M.; Freeman, L.M.; Fainman, Y. Optofluidic devices and applications in photonics, sensing and imaging. Lab Chip 2012, 12, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Jiang, L.; Mancuso, M.; Jain, A.; Oncescu, V.; Erickson, D. Optofluidic opportunities in global health, food, water and energy. Nanoscale 2012, 4, 4839–4857. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Sinton, D.; Psaltis, D. Optofluidics for energy applications. Nat. Photon. 2011, 5, 583–590. [Google Scholar] [CrossRef]
- Levy, U.; Shamai, R. Tunable optofluidic devices. Microfluid. Nanofluidics 2007, 4, 97–105. [Google Scholar] [CrossRef]
- Monat, C.; Domachuk, P.; Grillet, C.; Collins, M.; Eggleton, B.J.; Cronin-Golomb, M.; Mutzenich, S.; Mahmud, T.; Rosengarten, G.; Mitchell, A. Optofluidics: A novel generation of reconfigurable and adaptive compact architectures. Microfluid. Nanofluidics 2007, 4, 81–95. [Google Scholar] [CrossRef]
- Erickson, D. Optofluidics. In Microfluidics Based Microsystems; Springer: Dordrecht, The Netherlands, 2010; pp. 529–551. [Google Scholar]
- Hunt, H.C.; Wilkinson, J.S. Optofluidic integration for microanalysis. Microfluid. Nanofluidics 2008, 4, 53–79. [Google Scholar] [CrossRef]
- Nguyen, N.-T. Micro-optofluidic Lenses: A review. Biomicrofluidics 2010, 4, 031501. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.D.; Yun, S.H. The potential of optofluidic biolasers. Nat. Methods 2014, 11, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Erickson, D. Nanoscale optofluidic sensor arrays. Opt. Express 2008, 16, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Measor, P.; Lunt, E.J.; Phillips, B.S.; Deamer, D.W.; Hawkins, A.R.; Schmidt, H. Loss-based optical trap for on-chip particle analysis. Lab Chip 2009, 9, 2212–2216. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.S.; Yang, A.H.; Erickson, D.; Lipson, M. Optofluidic trapping and transport on solid core waveguides within a microfluidic device. Opt. Express 2007, 15, 14322–14334. [Google Scholar] [CrossRef] [PubMed]
- Shopova, S.I.; Zhou, H.Y.; Fan, X.D.; Zhang, P. Optofluidic ring resonator based dye laser. Appl. Phys. Lett. 2007, 90, 221101. [Google Scholar] [CrossRef]
- Tang, S.K.Y.; Mayers, B.T.; Vezenov, D.V.; Whitesides, G.M. Optical waveguiding using thermal gradients across homogeneous liquids in microfluidic channels. Appl. Phys. Lett. 2006, 88, 061112. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, A.Q.; Chin, L.K.; Zhang, X.M.; Tsai, D.P.; Lin, C.L.; Lu, C.; Wang, G.P.; Zheludev, N.I. Optofluidic waveguide as a transformation optics device for lightwave bending and manipulation. Nat. Commun. 2012, 3, 651. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, S.; Hendriks, B.H.W. Variable-focus liquid lens for miniature cameras. Appl. Phys. Lett. 2004, 85, 1128–1130. [Google Scholar] [CrossRef]
- Chiu, C.P.; Chiang, T.J.; Chen, J.K.; Chang, F.C.; Ko, F.-H.; Chu, C.W.; Kuo, S.-W.; Fan, S.K. Liquid Lenses and Driving Mechanisms: A Review. J. Adhes. Sci. Technol. 2012, 26, 1773–1788. [Google Scholar] [CrossRef]
- Mishra, K.; van den Ende, D.; Mugele, F. Recent Developments in Optofluidic Lens Technology. Micromachines 2016, 7, 102. [Google Scholar] [CrossRef]
- Xu, S.; Ren, H.W.; Wu, S.T. Dielectrophoretically tunable optofluidic devices. J. Phys. D Appl. Phys. 2013, 46, 483001. [Google Scholar] [CrossRef]
- Krogmann, F.; Monch, W.; Zappe, H. Electrowetting for Tunable Microoptics. J. Microelectromech. Syst. 2008, 17, 1501–1512. [Google Scholar] [CrossRef]
- Shi, J.; Stratton, Z.; Lin, S.C.S.; Huang, H.; Huang, T.J. Tunable optofluidic microlens through active pressure control of an air–liquid interface. Microfluid. Nanofluidics 2009, 9, 313–318. [Google Scholar] [CrossRef]
- Mao, X.L.; Waldeisen, J.R.; Juluri, B.K.; Huang, T.J. Hydrodynamically tunable optofluidic cylindrical microlens. Lab Chip 2007, 7, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.L.; Dai, B.; Xu, Q.; Zhuo, R.; Wang, Q.; Wang, X.; Zhang, D.W. Hydrodynamically reconfigurable optofluidic microlens with continuous shape tuning from biconvex to biconcave. Opt. Express 2017, 25, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.L.; Lin, S.C.S.; Lapsley, M.I.; Shi, J.; Juluri, B.K.; Huang, T.J. Tunable Liquid Gradient Refractive Index (L-GRIN) lens with two degrees of freedom. Lab Chip 2009, 9, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M.; Jian, A.Q.; Li, Z.H.; Zhang, X.M. Optofluidic tunable lenses using laser-induced thermal gradient. Lab Chip 2016, 16, 104–111. [Google Scholar] [CrossRef] [PubMed]
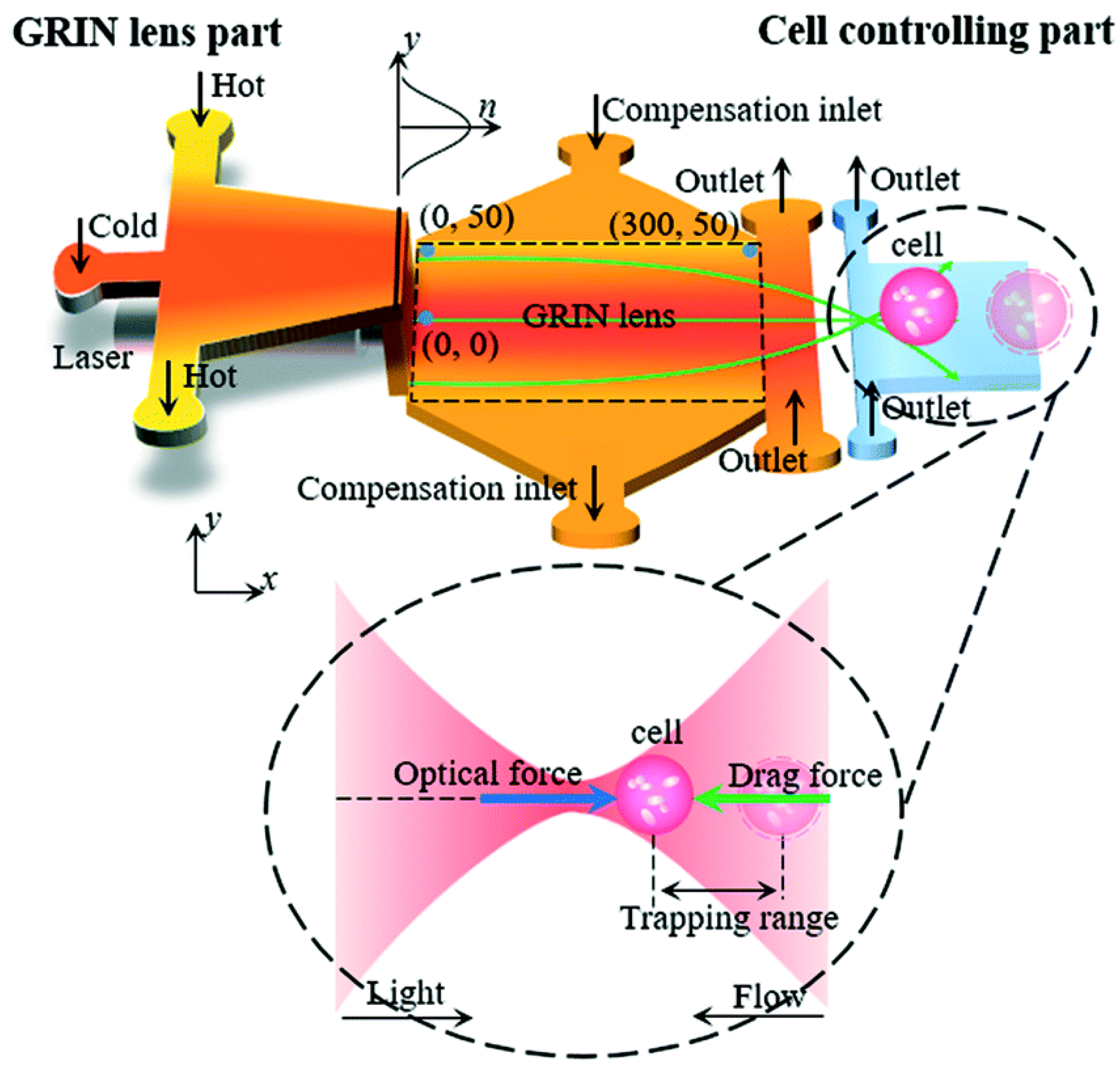
- Liu, H.L.; Shi, Y.; Liang, L.; Li, L.; Guo, S.S.; Yin, L.; Yang, Y. A liquid thermal gradient refractive index lens and using it to trap single living cell in flowing environments. Lab Chip 2017, 17, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Wee, D.; Hwang, S.H.; Song, Y.S.; Youn, J.R. Tunable optofluidic birefringent lens. Soft Matter 2016, 12, 3868–3876. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhu, X.Q.; Liang, L.; Yang, Y. Tunable focusing properties using optofluidic Fresnel zone plates. Lab Chip 2016, 16, 4554–4559. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.K.; Stan, C.A.; Whitesides, G.M. Dynamically reconfigurable liquid-core liquid-cladding lens in a microfluidic channel. Lab Chip 2008, 8, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Jiang, H.R. Selective Formation and Removal of Liquid Microlenses at Predetermined Locations Within Microfluidics through Pneumatic Control. J. Microelectromech. Syst. 2008, 17, 381–392. [Google Scholar] [CrossRef]
- Hsiung, S.K.; Lee, C.H.; Lee, G.B. Microcapillary electrophoresis chips utilizing controllable micro-lens structures and buried optical fibers for on-line optical detection. Electrophoresis 2008, 29, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.C.; Liu, A.Q.; Chin, L.K.; Li, X.C.; Huang, H.J.; Cheng, T.H.; Zhou, X.Q. Different curvatures of tunable liquid microlens via the control of laminar flow rate. Appl. Phys. Lett. 2008, 93, 084101. [Google Scholar] [CrossRef]
- Song, C.; Nguyen, N.T.; Tan, S.H.; Asundi, A.K. Modelling and optimization of micro optofluidic lenses. Lab Chip 2009, 9, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, C.; Luong, T.D.; Nguyen, N.T.; Wong, T.N. An electrokinetically tunable optofluidic bi-concave lens. Lab Chip 2012, 12, 3680–3687. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.S.; Lin, M.S.; Yang, R.J. An in-plane optofluidic microchip for focal point control. Lab Chip 2013, 13, 3886–3892. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.C.; Lim, S.P.; Lee, H.P. Optofluidic variable-focus lenses for light manipulation. Lab Chip 2012, 12, 3810–3815. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chin, L.K.; Tsai, J.M.; Tsai, D.P.; Zheludev, N.I.; Liu, A.Q. Transformation optofluidics for large-angle light bending and tuning. Lab Chip 2012, 12, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Zhu, X.Q.; Liang, L.; Zhang, X.M.; Yang, Y. Tunable transformation optical waveguide bends in liquid. Optica 2017, 4, 839–846. [Google Scholar] [CrossRef]
- Shi, Y.; Liang, L.; Zhu, X.Q.; Zhang, X.M.; Yang, Y. Tunable self-imaging effect using hybrid optofluidic waveguides. Lab Chip 2015, 15, 4398–4403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.T.; Yang, Y.; Chin, L.K.; Chen, H.F.; Zhu, W.M.; Zhang, J.B.; Yap, P.H.; Liedberg, B.; Wang, K.; Wang, G.; et al. Optofluidic lens with low spherical and low field curvature aberrations. Lab Chip 2016, 16, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D.B.; Vezenov, D.V.; Mayers, B.T.; Whitesides, G.M.; Conroy, R.S.; Prentiss, M.G. Diffusion-controlled optical elements for optofluidics. Appl. Phys. Lett. 2005, 87, 181105. [Google Scholar] [CrossRef]
- Lin, H.C.; Chen, M.S.; Lin, Y.H. A Review of Electrically Tunable Focusing Liquid Crystal Lenses. Trans. Electr. Electron. Mater. 2011, 12, 234–240. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Xiong, S.; Chin, L.K.; Yang, Y.; Zhang, J.B.; Ser, W.; Wu, J.H.; Chen, T.N.; Yang, Z.C.; Hao, Y.L.; et al. High-resolution and multi-range particle separation by microscopic vibration in an optofluidic chip. Lab Chip 2017, 17, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Z.; Xiong, S.; Chin, L.K.; Zhang, J.B.; Ser, W.; Wu, J.H.; Chen, T.N.; Yang, Z.C.; Hao, Y.L.; Liedberg, B.; et al. Nanometer-precision linear sorting with synchronized optofluidic dual barriers. Sci. Adv. 2018, 4, eaao0773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Watts, B.R.; Guo, T.Y.; Zhang, Z.Y.; Xu, C.Q.; Fang, Q.Y. Optofluidic Device Based Microflow Cytometers for Particle/Cell Detection: A Review. Micromachines 2016, 7, 70. [Google Scholar] [CrossRef]
- Watts, B.R.; Zhang, Z.Y.; Xu, C.Q.; Cao, X.D.; Lin, M. Scattering detection using a photonic-microfluidic integrated device with on-chip collection capabilities. Electrophoresis 2014, 35, 271–281. [Google Scholar] [CrossRef] [PubMed]
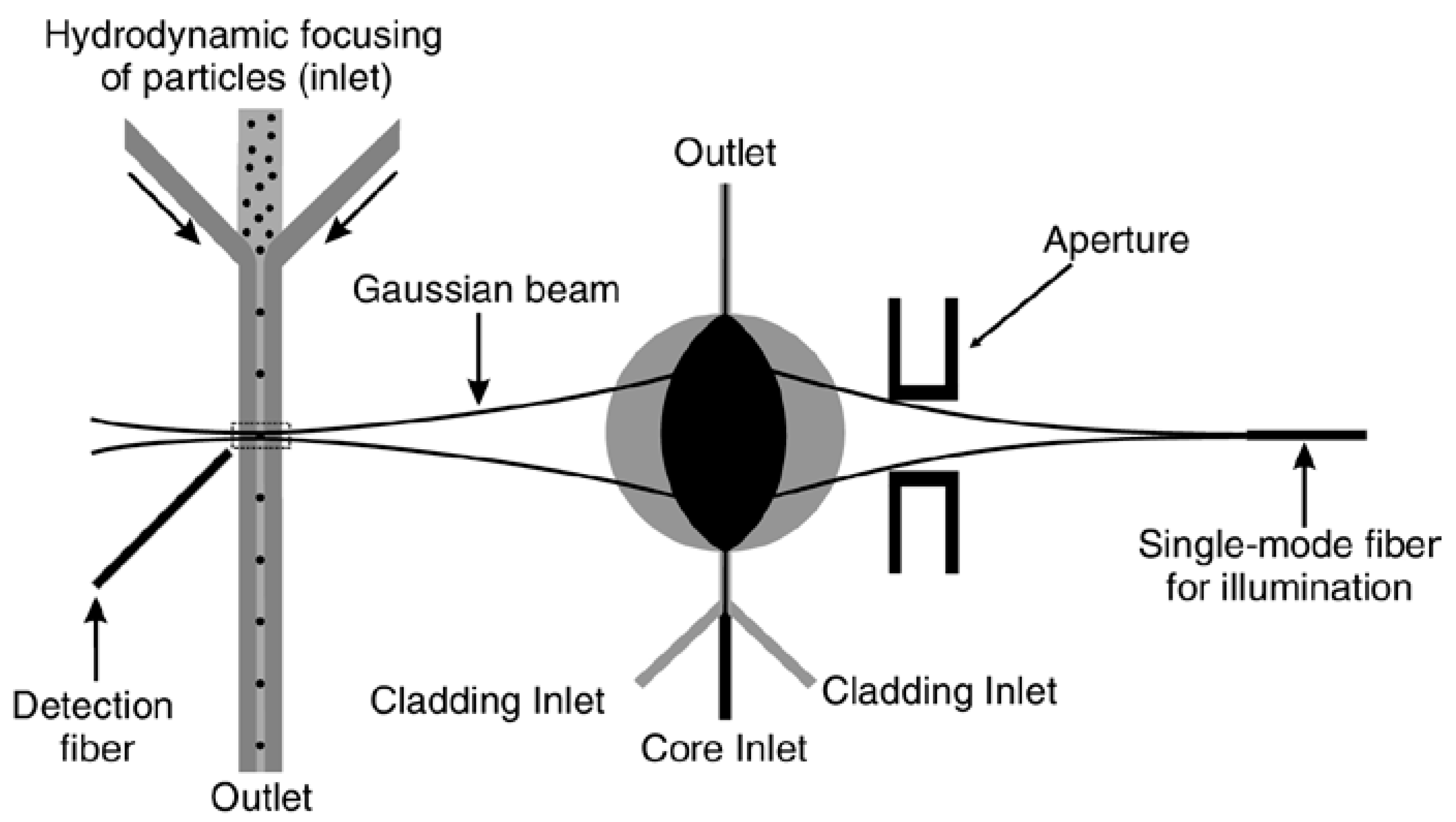
- Godin, J.; Lien, V.; Lo, Y.H. Demonstration of two-dimensional fluidic lens for integration into microfluidic flow cytometers. Appl. Phys. Lett. 2006, 89, 061106. [Google Scholar] [CrossRef]
- Song, C.; Luong, T.D.; Kong, T.F.; Nguyen, N.T.; Asundi, A.K. Disposable flow cytometer with high efficiency in particle counting and sizing using an optofluidic lens. Opt. Lett. 2011, 36, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gijs, M.A.M. Micro-optics for microfluidic analytical applications. Chem. Soc. Rev. 2018, in press. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Li, T.; Li, Z.; Long, J.; Zhang, X. Optofluidic Tunable Lenses for In-Plane Light Manipulation. Micromachines 2018, 9, 97. https://doi.org/10.3390/mi9030097
Chen Q, Li T, Li Z, Long J, Zhang X. Optofluidic Tunable Lenses for In-Plane Light Manipulation. Micromachines. 2018; 9(3):97. https://doi.org/10.3390/mi9030097
Chicago/Turabian StyleChen, Qingming, Tenghao Li, Zhaohui Li, Jinlin Long, and Xuming Zhang. 2018. "Optofluidic Tunable Lenses for In-Plane Light Manipulation" Micromachines 9, no. 3: 97. https://doi.org/10.3390/mi9030097



