Microfluidic Line-Free Mass Sensor Based on an Antibody-Modified Mechanical Resonator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
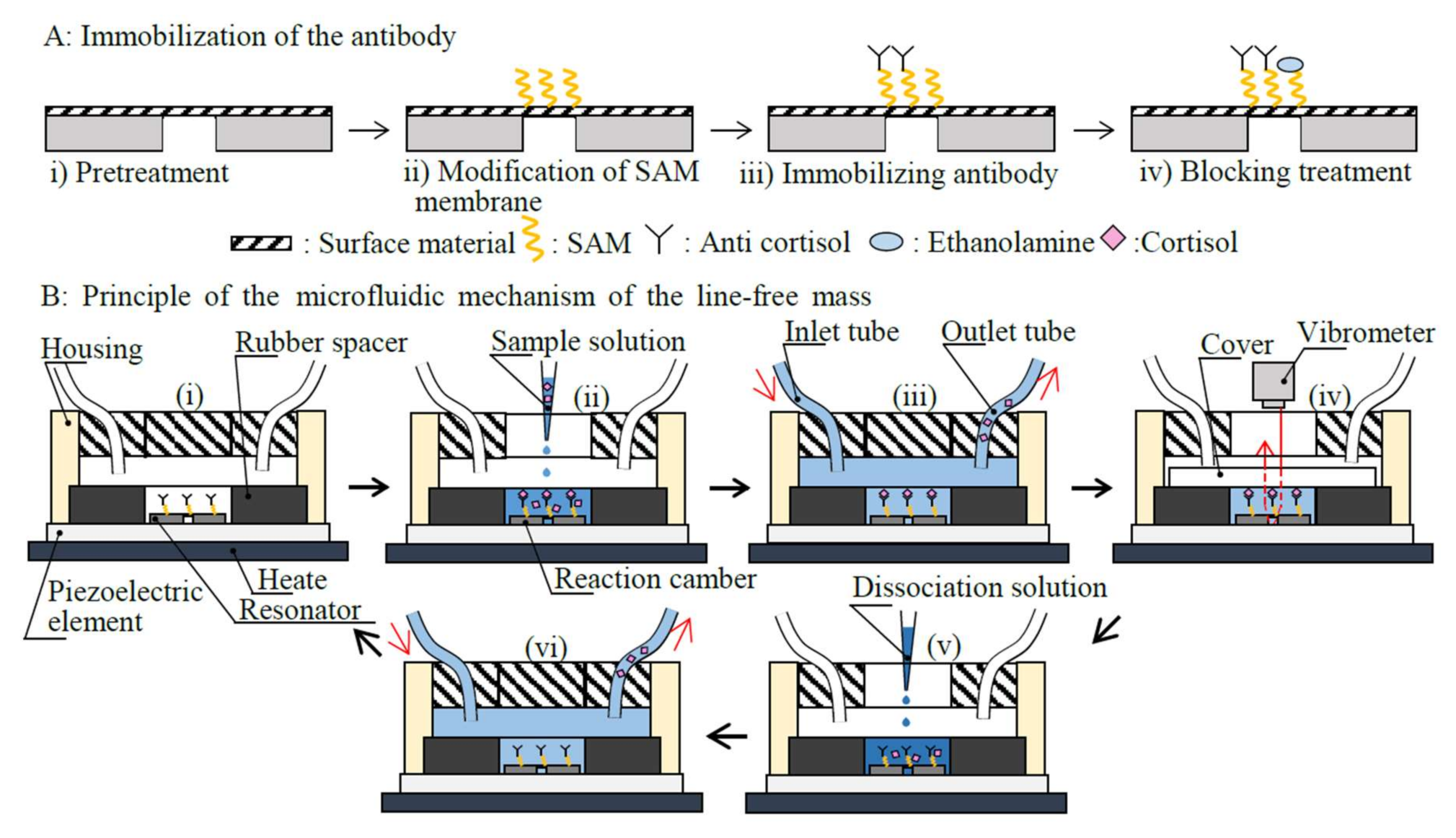
2.2. Structure and Principle
- Step i:
- As a pretreatment, the surface material (aluminum) of the disk-shaped resonator was cleaned of organic residue using piranha solution for 10 min. Nitrogen gas was sprayed onto the flat electrode to dry it.
- Step ii:
- A 10-carboxydecylphosphonic acid was used to form the SAM membrane on the disk-shaped resonator. The disk-shaped resonator was soaked in a solution consisting of 100 mmol/L N-hydroxysulfosuccinimide and 100 mmol/L 1-ethy1-3-carbodiimide at 25 °C for 10 min to activate the SAM membrane.
- Step iii:
- Subsequently, an anti-cortisol antibody (mass: x µg) was coupled to the SAM membrane using acetate buffer (10 mM, pH 5.5) for 30 min.
- Step iv:
- Un-coupled activated carboxymethyl dextran was blocked with ethanolamine.
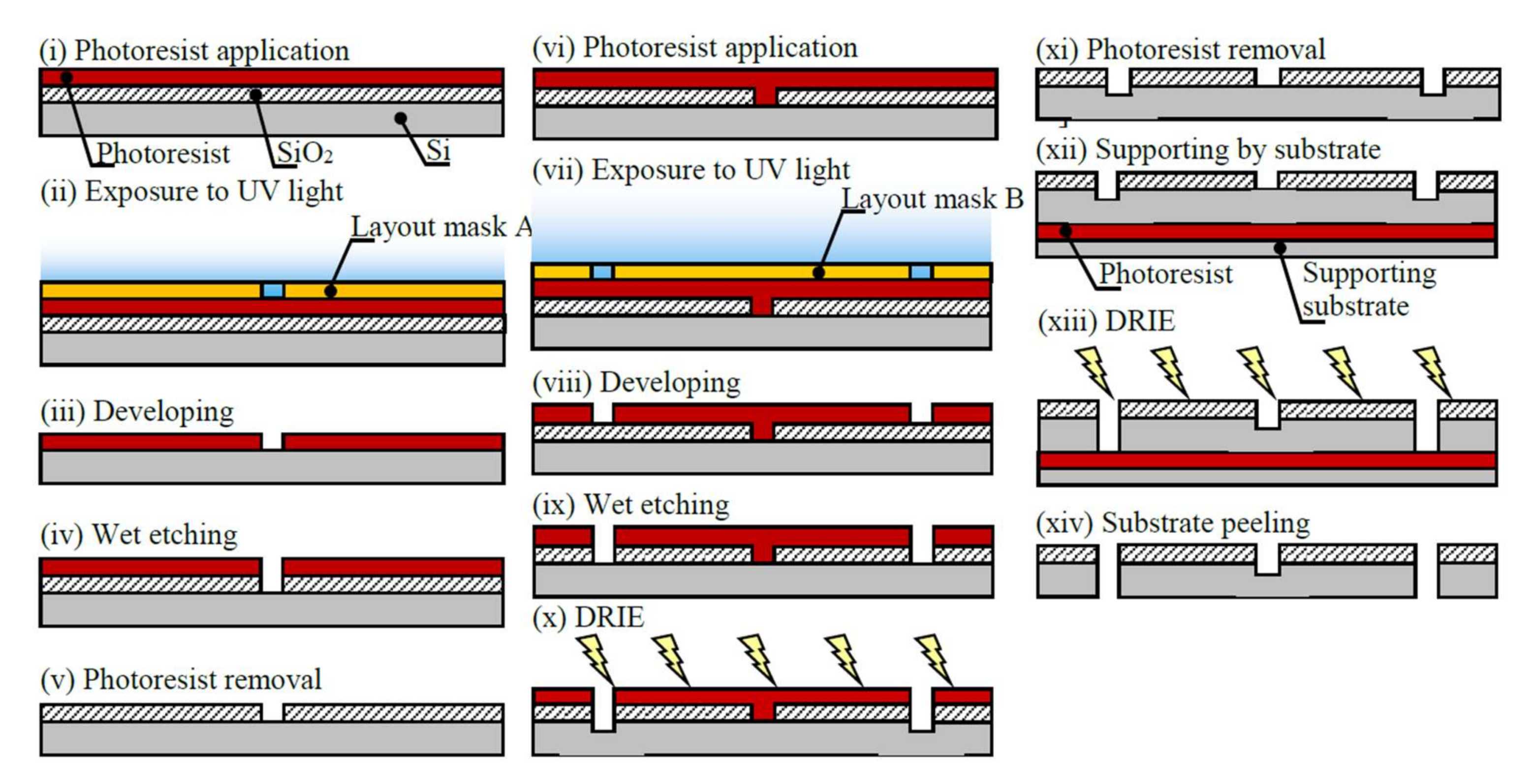
2.3. Design and Fabrication of Mechanical Resonator
2.4. Tuning of Sensor Parameters
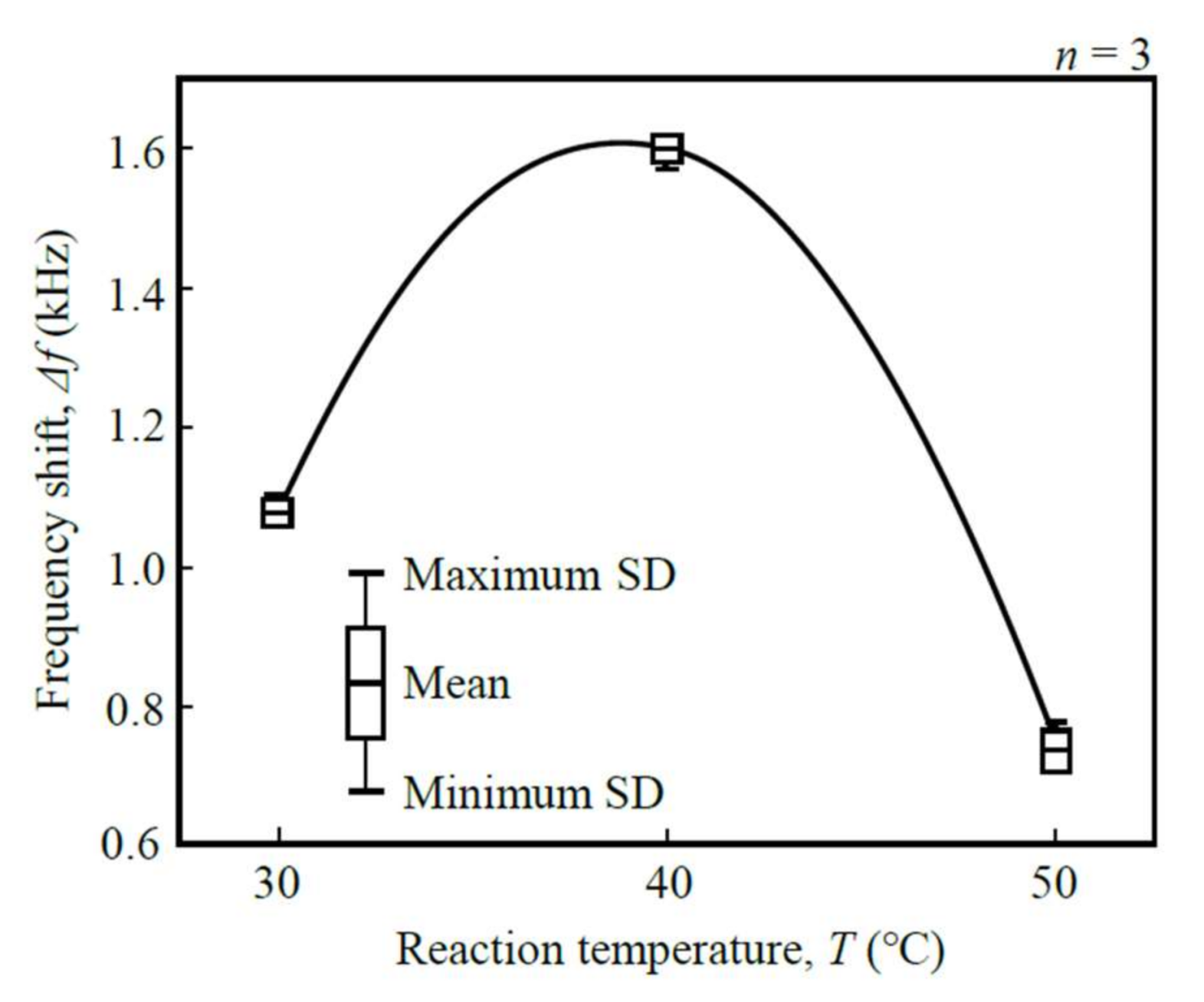
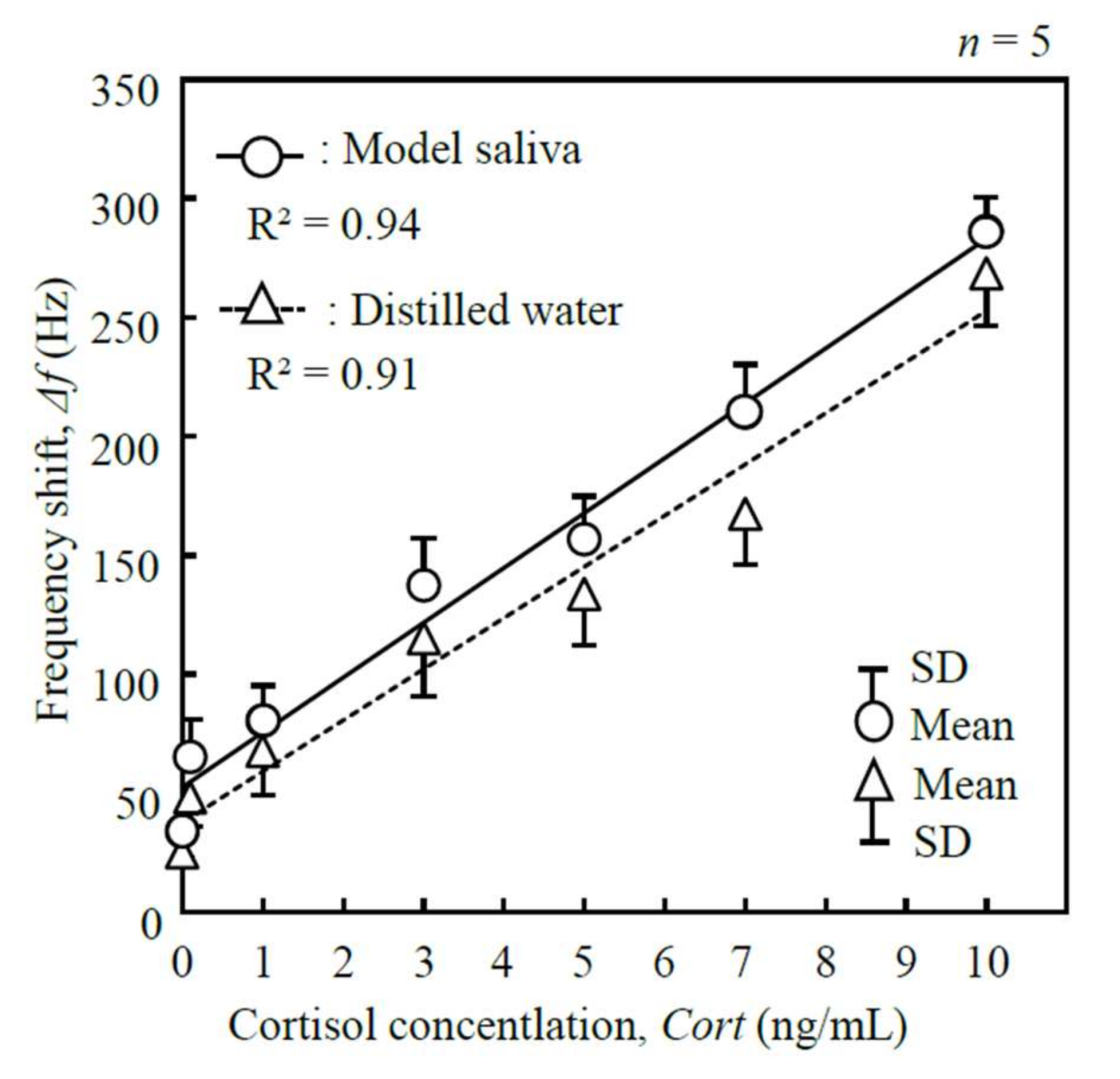
2.5. Calibration Curve
3. Results and Discussion
3.1. Design and Fabrication of Mechanical Resonator
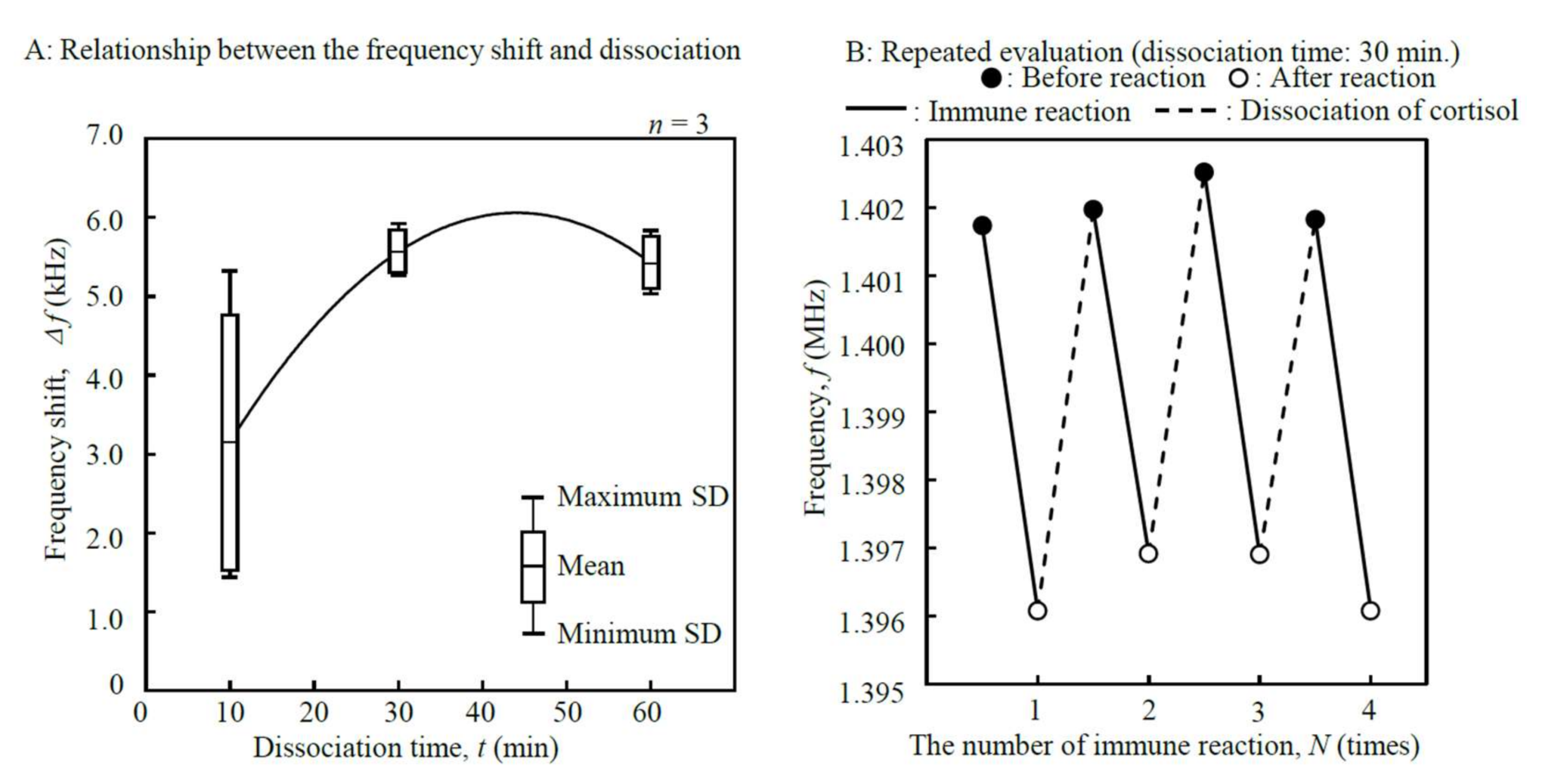
3.2. Tuning of Sensor Parameters
3.3. Calibration Curve
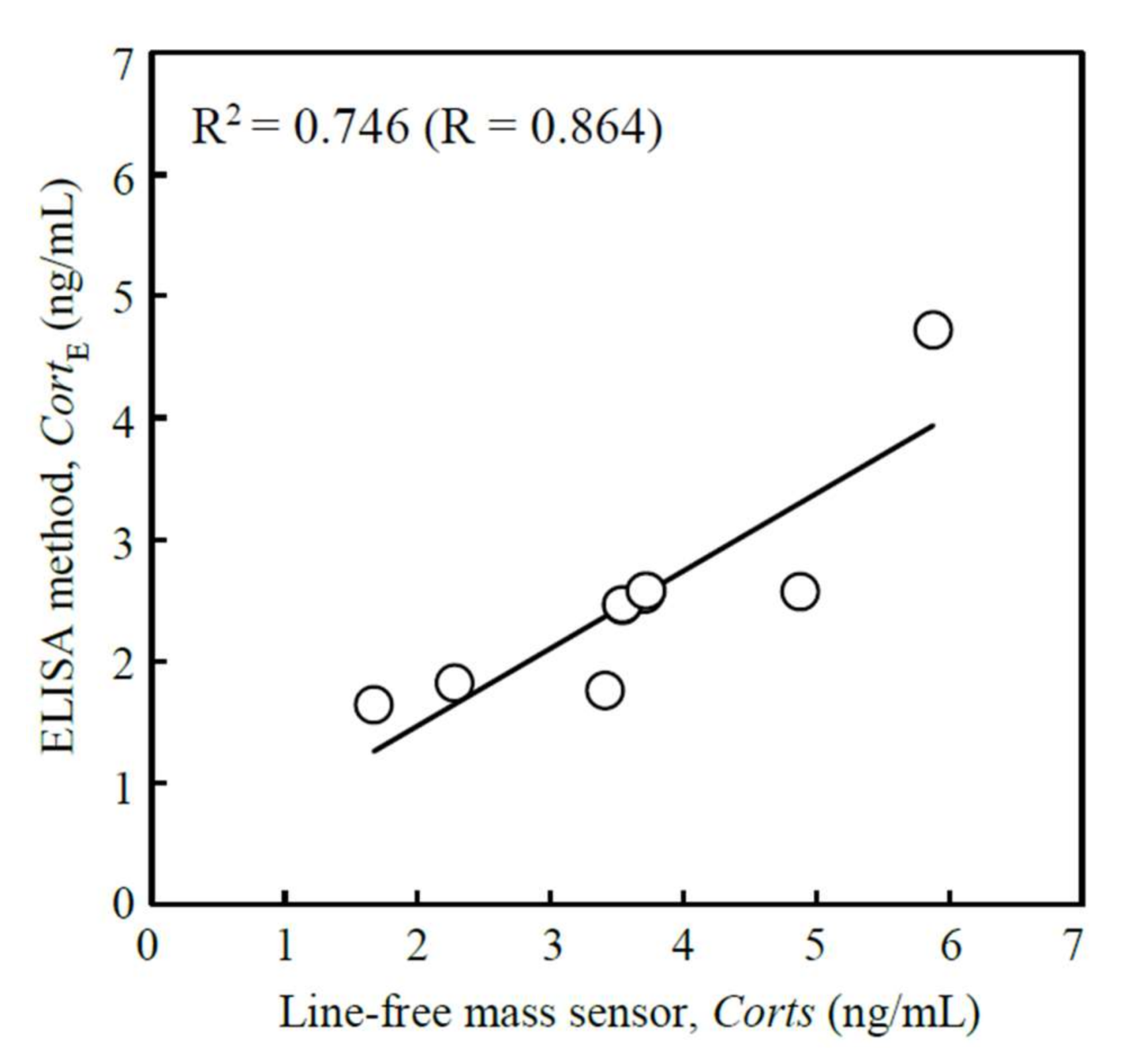
3.4. Analysis of Human Salivary Cortisol
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113. [Google Scholar] [CrossRef] [PubMed]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics integrated biosensors: A leading technology towards lab-on-a-chip and sensing applications. Sensors (Basel) 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in microfluidics for nanoparticle separation. Lab Chip 2016, 17, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Shetty, V. Centrifugal microfluidic control mechanisms for biosensors. Sens. Mater. 2016, 28, 1117–1127. [Google Scholar] [CrossRef]
- Holm-Hansen, C.; Tong, G.; Davis, C.; Abrams, W.R.; Malamud, D. Comparison of oral fluid collectors for use in a rapid point-of-care diagnostic device. Clin. Diagn. Lab. Immunol. 2004, 11, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Charney, D.S. Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. Am. J. Psychiatry 2004, 161, 195. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Pasha, S.K.; Bhansali, S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014, 53, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, M.; Santos, S.; Rigatto, C.; Liu, Y.; Lin, F. Lab-on-a-chip platforms for detection of cardiovascular disease and cancer biomarkers. Sensors (Basel) 2017, 17, 2934. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Mohd-Naim, N.F.; Ahmed, M.U. Trends and advances in electrochemiluminescence nanobiosensors. Sensors (Basel) 2018, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.E.; Rohani, A.; Farmehini, V.; Swami, N.S. Microbial analysis in dielectrophoretic microfluidic systems. Anal. Chim. Acta 2017, 966, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Okuno, J.; Maehashi, K.; Kerman, K.; Takamura, Y.; Matsumoto, K.; Tamiya, E. Label-free immunosensor for prostate-specific antigen based on single-walled carbon nanotube array-modified microelectrodes. Biosens. Bioelectron. 2007, 22, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, K.S.; Meyer, N.L.; Yuan, J.; Hirst, L.S.; Chase, P.B.; Xiong, P. Functionalized SnO2 nanobelt field-effect transistor sensors for label-free detection of cardiac troponin. Biosens. Bioelectron. 2011, 26, 4538–4544. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sharma, A.; Hong, S.; Jang, J. Electrical immunosensor based on dielectrophoretically-deposited carbon nanotubes for detection of influenza virus H1N1. Analyst 2014, 139, 5415–5421. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.M.; Thundat, T. Microcantilever biosensors. Methods 2005, 37, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J. Cantilever biosensors. Analyst 2008, 133, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Verwendung von Schwingquarzen zur wägung dünner schichten und zur mikrowägung. Zeitschrift für Physik 1959, 155, 206–222. (In Germany) [Google Scholar] [CrossRef]
- Buttry, D.A.; Ward, M.D. Measurement of interfacial process at electrode surfaces with the electrochemical quartz crystal microbalance. Chem. Rev. 1992, 92, 1355–1379. [Google Scholar] [CrossRef]
- Lavrik, N.V.; Sepaniak, M.J.; Datskos, P.G. Cantilever transducers as a platform for chemical and biological sensors. Rev. Sci. Instrum. 2004, 75, 2229–2253. [Google Scholar] [CrossRef]
- Ricciardi, C.; Canavese, G.; Castagna, R.; Ferrante, I.; Ricci, A.; Marasso, S.L.; Napione, L.; Bussolino, F. Integration of microfluidic and cantilever technology for biosensing application in liquid environment. Biosens. Bioelectron. 2010, 26, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bai, X.; Wang, N.; Chen, X.; Li, J.; Zhang, Z.; Tang, J. Aptamer-based microcantilever biosensor for ultrasensitive detection of tumor marker nucleolin. Talanta 2016, 146, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhu, Y.; Zhang, J.; Liao, J.; Wang, S.; Yang, J.; Yang, F. A high accuracy cantilever array sensor for early liver cancer diagnosis. Biomed. Microdevices 2016, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Teva, J.; Abadal, G.; Torres, F.; Verd, J.; Pérez-Murano, F.; Barniol, N. A femtogram resolution mass sensor platform, based on SOI electrostatically driven resonant cantilever. Part I: Electromechanical model and parameter extraction. Ultramicroscopy 2006, 106, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Resonant Type Mass Sensor. U.S. Patent No. 9,023,283, 5 May 2015. [Google Scholar]
- Yamaguchi, M.; Kimura, Y. Proposal for a power supply line-free mass sensor for measuring total protein in human saliva. Sens. Lett. 2014, 12, 1186–1189. [Google Scholar] [CrossRef]
- Yonekura, T.; Takeda, K.; Shetty, V.; Yamaguchi, M. Relationship between salivary cortisol and depression in adolescent survivors of a major natural disaster. J. Physiol. Sci. 2014, 64, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawes, C. The effects of exercise on protein and electrolyte secretion in parotid saliva. J. Physiol. 1981, 320, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, S.; Woinowsky-Krieger, S. Theory of Plates and Shells, 2nd ed.; McGraw-Hill Book Company: New York, NY, USA, 1987. [Google Scholar]
- Zhang, C.; Cocking, A.; Freeman, E.; Liu, Z.; Tadigadapa, S. On-chip glass microspherical shell whispering gallery mode resonators. Sci. Rep. 2017, 7, 14965. [Google Scholar] [CrossRef] [PubMed]
- Canavese, G.; Ricci, A.; Gazzadi, G.C.; Ferrante, I.; Mura, A.; Marasso, S.L.; Ricciardi, C. Resonating behaviour of nanomachined holed microcantilevers. Sci. Rep. 2015, 5, 17837. [Google Scholar] [CrossRef] [PubMed]
- Körner, J.; Reiche, C.F.; Gemming, T.; Büchner, B.; Gerlach, G.; Mühl, T. Signal enhancement in cantilever magnetometry based on a co-resonantly coupled sensor. Beilstein J. Nanotechnol. 2016, 7, 1033. [Google Scholar] [CrossRef] [PubMed]
- DiGiacomo, R.A.; Xie, L.; Cullen, C.; Indelicato, S.R. Development and validation of a kinetic assay for analysis of anti-human interleukin-5 monoclonal antibody (SCH 55700) and human interleukin-5 interactions using surface plasmon resonance. Anal. Biochem. 2004, 327, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, B.; Löfås, S.; Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal. Biochem. 1991, 198, 268–277. [Google Scholar] [CrossRef]
- Kaur, J.; Jacobs, R.; Huang, Y.; Salvo, N.; Politis, C. Salivary biomarkers for oral cancer and pre-cancer screening: A review. Clin. Oral Investig. 2018. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Khurshid, Z.; Yahya Ibrahim Asiri, F. Advancing point-of-care (PoC) testing using human saliva as liquid biopsy. Diagnostics (Basel) 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Ngamchuea, K.; Chaisiwamongkhol, K.; Batchelor-McAuley, C.; Compton, R.G. Chemical analysis in saliva and the search for salivary biomarkers—A tutorial review. Analyst 2017, 143, 81. [Google Scholar] [CrossRef] [PubMed]



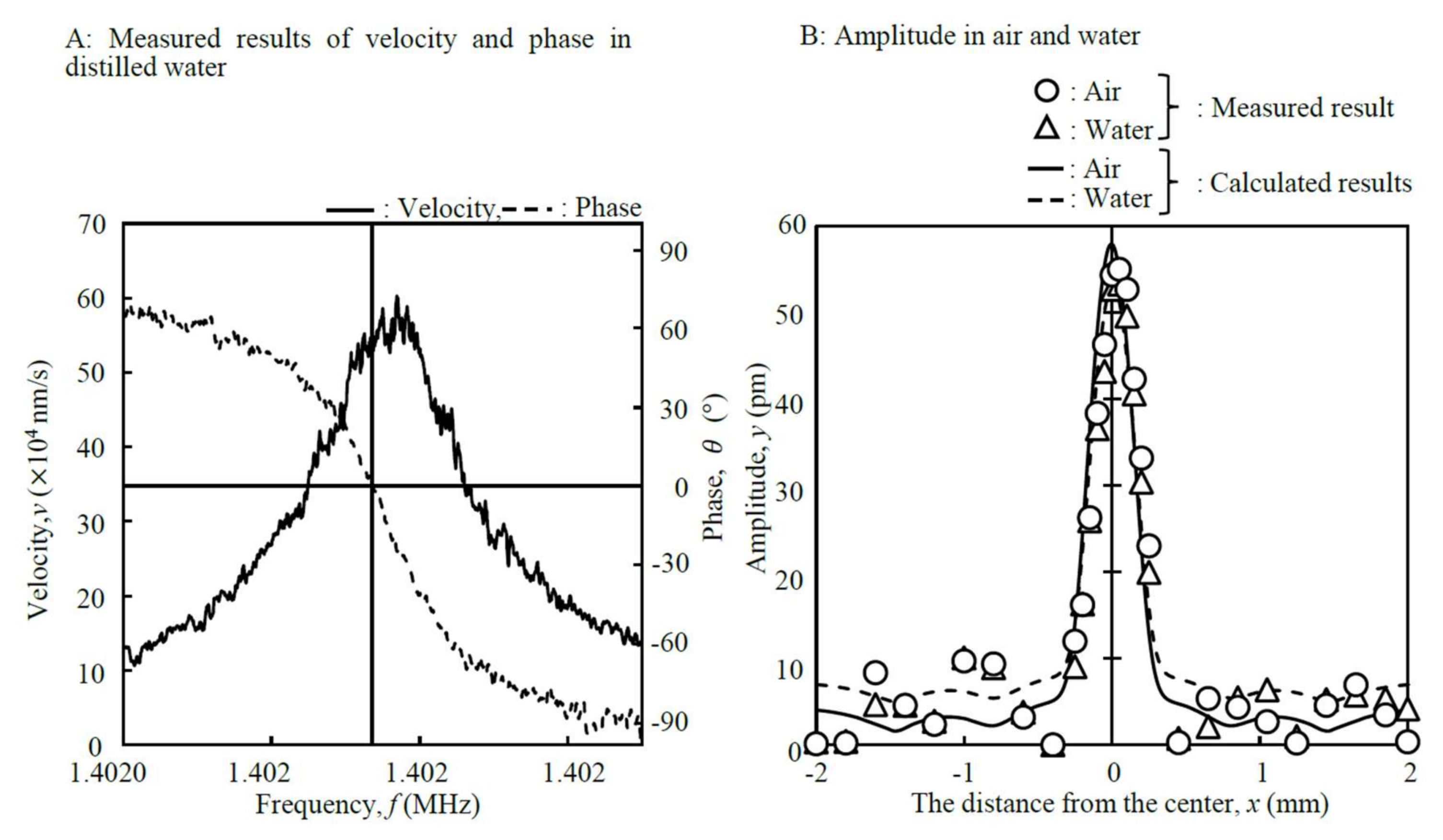


| Medium | Frequency (MHz) | Maximum Amplitude (pm) | Mesh Model | |
|---|---|---|---|---|
| Calculated | Measured | |||
| Air | 3.862 | 3.841 | 58 |  |
| Distilled water | 2.025 | 1.402 | 55 | |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, M. Microfluidic Line-Free Mass Sensor Based on an Antibody-Modified Mechanical Resonator. Micromachines 2018, 9, 177. https://doi.org/10.3390/mi9040177
Yamaguchi M. Microfluidic Line-Free Mass Sensor Based on an Antibody-Modified Mechanical Resonator. Micromachines. 2018; 9(4):177. https://doi.org/10.3390/mi9040177
Chicago/Turabian StyleYamaguchi, Masaki. 2018. "Microfluidic Line-Free Mass Sensor Based on an Antibody-Modified Mechanical Resonator" Micromachines 9, no. 4: 177. https://doi.org/10.3390/mi9040177





