A Microfluidic Platform for Investigating Transmembrane Pressure-Induced Glomerular Leakage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Podocyte Culture
2.2. Podocyte Spreading and Proliferation on Porous Membrane
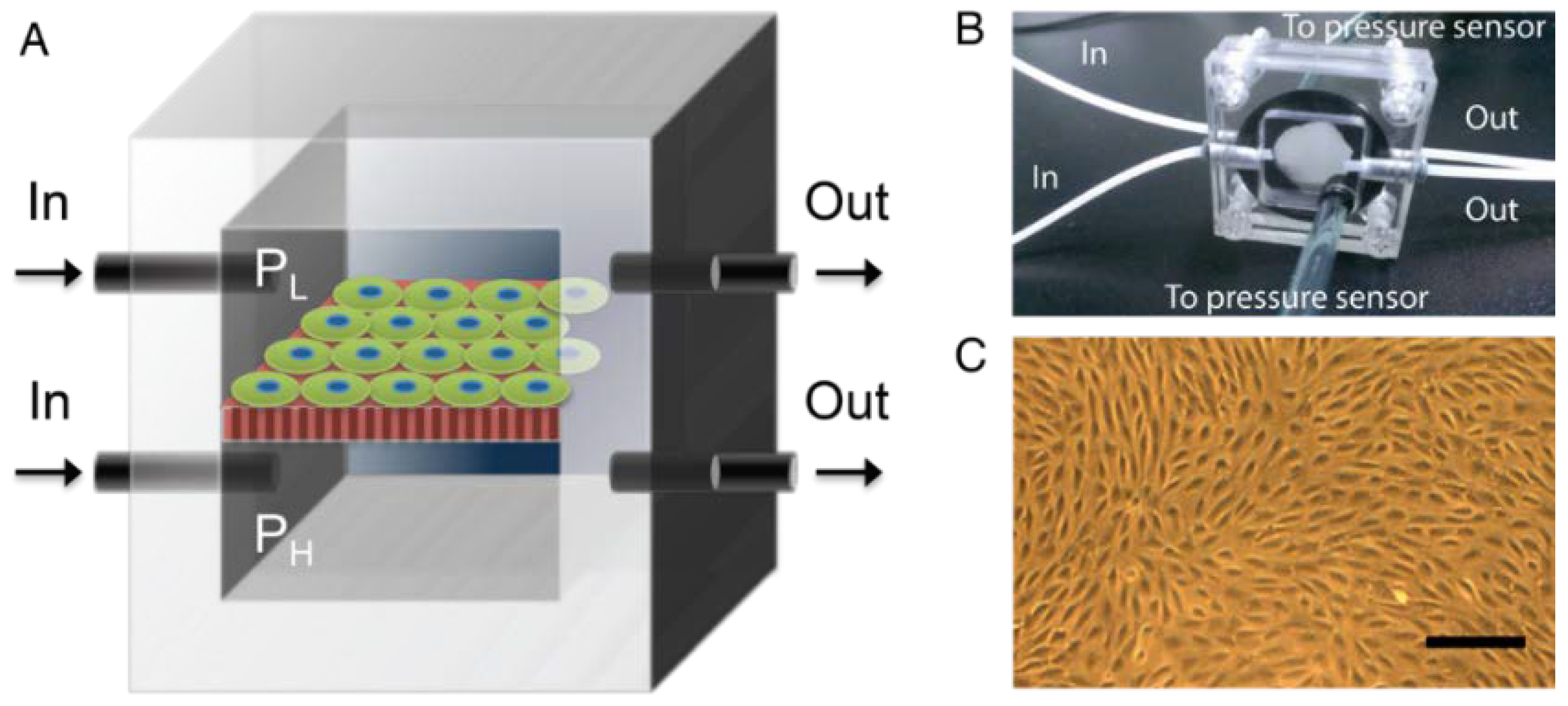
2.3. Pressure-Supplying Device
2.4. Dextran Filtration
2.5. Quantitative Real-Time Polymerase Chain Reaction (q-PCR)
2.6. Fluorescence Staining of Actin
3. Results
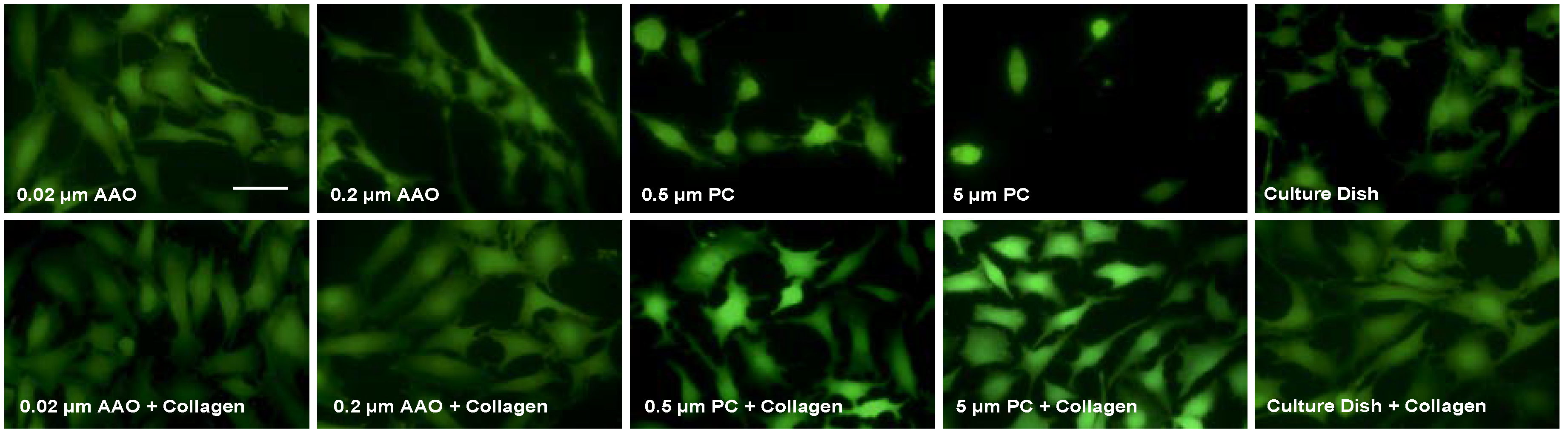
3.1. Cell Proliferation on Porous Membranes
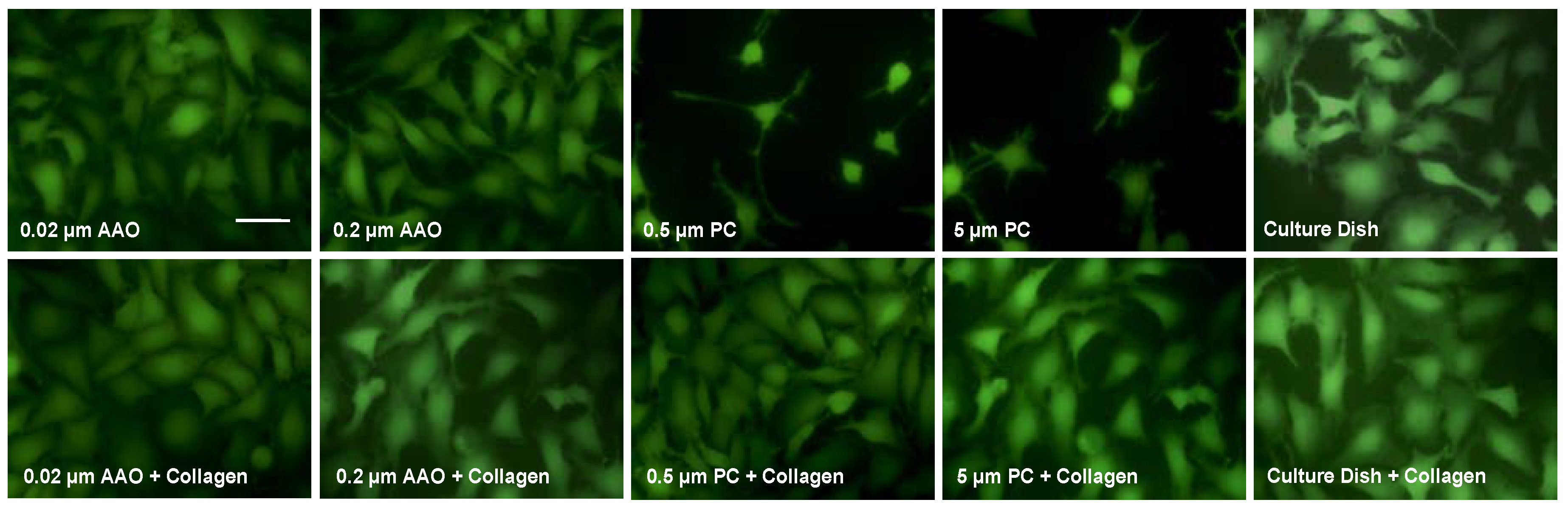
3.2. Cell Spreading on Porous Membranes
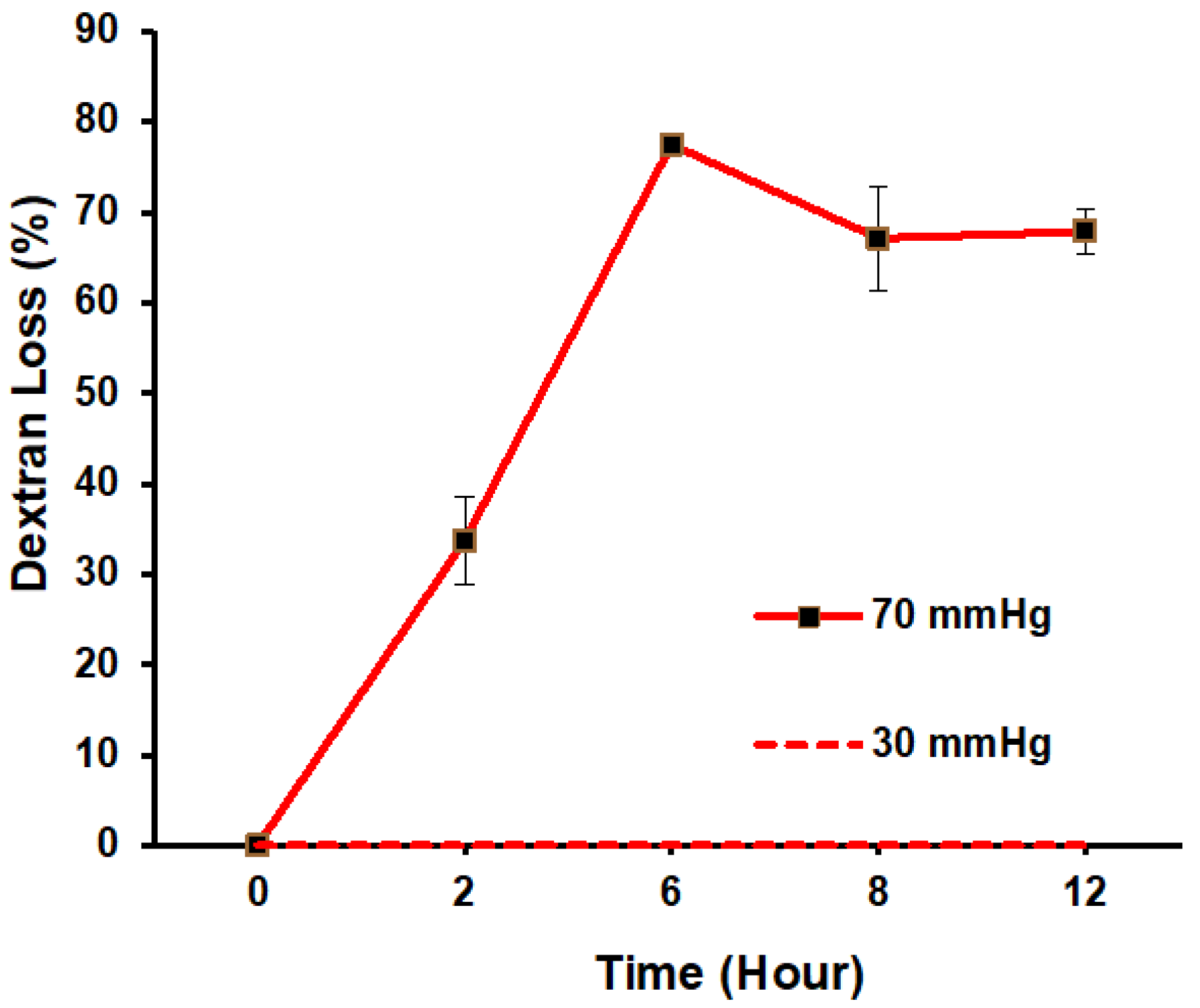
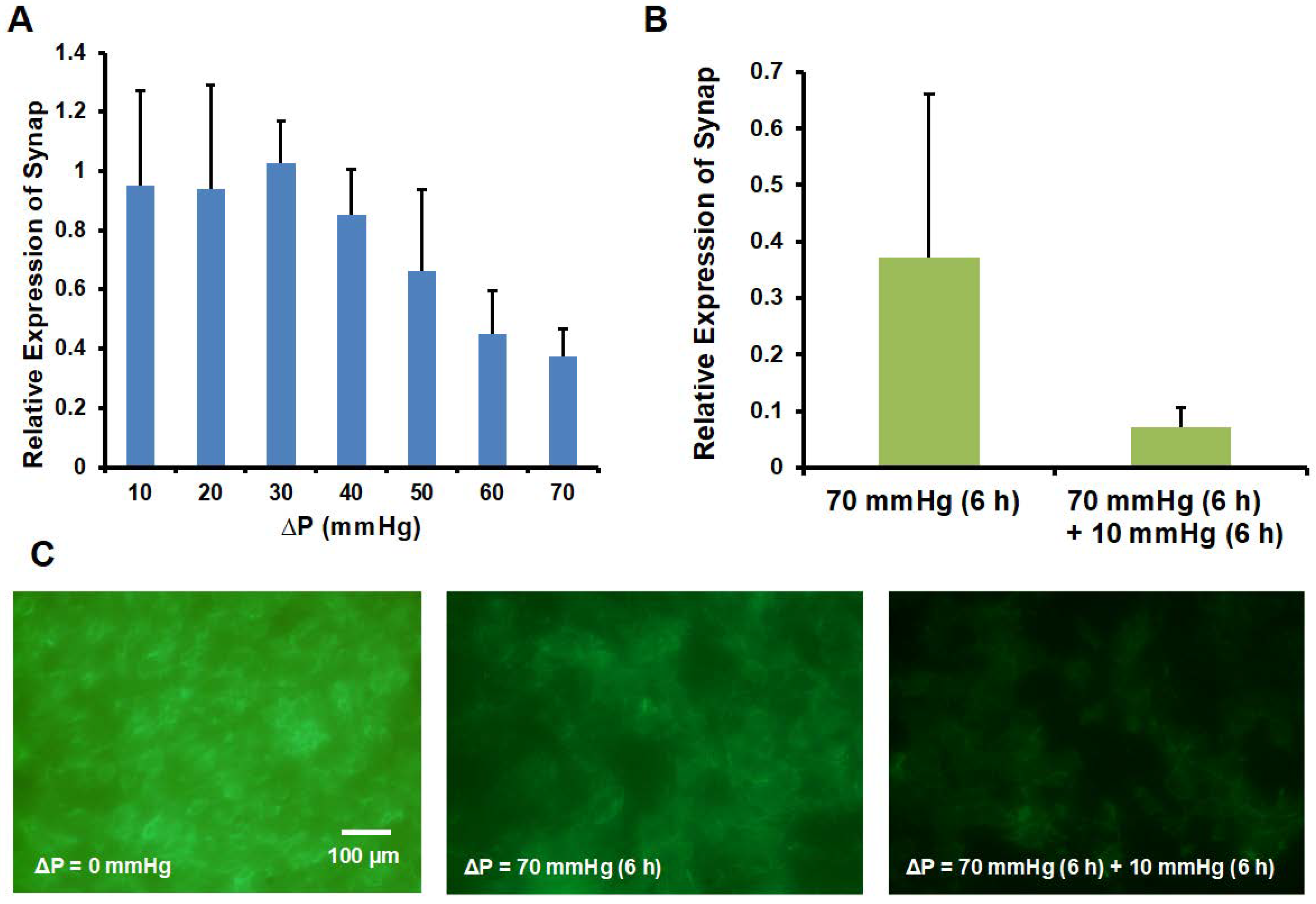
3.3. Filtration Function in Response to ΔP
3.4. Synaptopodin and Actin Expression
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Greka, A.; Mundel, P. Cell biology and pathology of podocytes. Annu. Rev. Physiol. 2012, 74, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Pavenstadt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef] [PubMed]
- Wennmann, D.O.; Hsu, H.H.; Pavenstadt, H. The renin-angiotensin-aldosterone system in podocytes. Semin. Nephrol. 2012, 32, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D. Caught flat-footed: Podocyte damage and the molecular bases of focal glomerulosclerosis. J. Clin. Investig. 2001, 108, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.H.; Hoffmann, S.; Di Marco, G.S.; Endlich, N.; Peter-Katalinic, J.; Weide, T.; Pavenstadt, H. Downregulation of the antioxidant protein peroxiredoxin 2 contributes to angiotensin ii-mediated podocyte apoptosis. Kidney Int. 2011, 80, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.H.; Hoffmann, S.; Endlich, N.; Velic, A.; Schwab, A.; Weide, T.; Schlatter, E.; Pavenstadt, H. Mechanisms of angiotensin ii signaling on cytoskeleton of podocytes. J. Mol. Med. 2008, 86, 1379–1394. [Google Scholar] [CrossRef] [PubMed]
- Shankland, S.J. The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int. 2006, 69, 2131–2147. [Google Scholar] [CrossRef] [PubMed]
- Babelova, A.; Jansen, F.; Sander, K.; Lohn, M.; Schafer, L.; Fork, C.; Ruetten, H.; Plettenburg, O.; Stark, H.; Daniel, C.; et al. Activation of Rac-1 and RhoA contributes to podocyte injury in chronic kidney disease. PLoS ONE 2013, 8, e80328. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Troy, J.L.; Daugharty, T.M. The dynamics of glomerular ultrafiltration in the rat. J. Clin. Investig. 1971, 50, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M. Nephron adaptation to renal injury or ablation. Am. J. Physiol. 1985, 249, F324–F337. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Cheng, H.C.; Lin, Y.; Wu, C.W.; Shen, Y.K. Study on cellar behaviors on different nanostructures by nanoporous alumina template. Int. J. Precis. Eng. Manuf. 2014, 15, 689–693. [Google Scholar] [CrossRef]
- Endlich, N.; Kress, K.R.; Reiser, J.; Uttenweiler, D.; Kriz, W.; Mundel, P.; Endlich, K. Podocytes respond to mechanical stress in vitro. J. Am. Soc. Nephrol. 2001, 12, 413–422. [Google Scholar] [PubMed]
- Ravera, M.; Re, M.; Deferrari, L.; Vettoretti, S.; Deferrari, G. Importance of blood pressure control in chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, S98–S103. [Google Scholar] [CrossRef] [PubMed]
- Wojciak-Stothard, B.; Ridley, A.J. Shear stress–induced endothelial cell polarization is mediated by Rho and Rac but not cdc42 or PI 3-kinases. J. Cell Biol. 2003, 161, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Butcher, J.T.; Penrod, A.M.; García, A.J.; Nerem, R.M. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Hsiai, T.K.; Cho, S.K.; Reddy, S.; Hama, S.; Navab, M.; Demer, L.L.; Honda, H.M.; Ho, C.M. Pulsatile flow regulates monocyte adhesion to oxidized lipid-induced endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Yoshigi, M.; Hoffman, L.M.; Jensen, C.C.; Yost, H.J.; Beckerle, M.C. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 2005, 171, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.M.; Cheng, G.; Tyrrell, J.A.; Wilcox-Adelman, S.A.; Boucher, Y.; Jain, R.K.; Munn, L.L. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl. Acad. Sci. USA 2012, 109, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Beningo, K.A. Cancer cell invasion is enhanced by applied mechanical stimulation. PLoS ONE 2011, 6, e17277. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bonnetnann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.G.; Gojgini, S.; Lam, J.; Segura, T. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. Biomaterials 2011, 32, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Shankland, S.J.; Pippin, J.W.; Reiser, J.; Mundel, P. Podocytes in culture: Past, present, and future. Kidney Int. 2007, 72, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Schiwek, D.; Endlich, N.; Holzman, L.; Holthofer, H.; Kriz, W.; Endlich, K. Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int. 2004, 66, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Jat, P.S.; Noble, M.D.; Ataliotis, P.; Tanaka, Y.; Yannoutsos, N.; Larsen, L.; Kioussis, D. Direct derivation of conditionally immortal cell-lines from an h-2kb-tsa58 transgenic mouse. Proc. Natl. Acad. Sci. USA. 1991, 88, 5096–5100. [Google Scholar] [CrossRef] [PubMed]
- Haraldsson, B.; Nystrom, J. The glomerular endothelium: New insights on function and structure. Curr. Opin. Nephrol. Hypertens. 2012, 21, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, J.A.; Alpers, C.E.; Shankland, S.J. Podocyte biology for the bedside. Am. J. Kidney Dis. 2011, 58, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, P.W. The podocyte as a target for therapies-new and old. Nat. Rev. Nephrol. 2012, 8, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.B.Y.; Qu, X.L.; Zhang, X.M.; Caruana, G.; Bertram, J.F.; Li, J.H. Glomerular endothelial cell injury and damage precedes that of podocytes in adriamycin-induced nephropathy. PLoS ONE 2013, 8, e55027. [Google Scholar] [CrossRef] [PubMed]
- Mundel, P.; Heid, H.W.; Mundel, T.M.; Kruger, M.; Reiser, J.; Kriz, W. Synaptopodin: An actin-associated protein in telencephalic dendrites and renal podocytes. J. Cell Biol. 1997, 139, 193–204. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-H.; Chen, J.-S.; Ko, Y.-C.; Chen, J.-W.; Chu, H.-Y.; Lu, C.-S.; Chu, C.-W.; Hsu, H.-H.; Tseng, F.-G. A Microfluidic Platform for Investigating Transmembrane Pressure-Induced Glomerular Leakage. Micromachines 2018, 9, 228. https://doi.org/10.3390/mi9050228
Chen T-H, Chen J-S, Ko Y-C, Chen J-W, Chu H-Y, Lu C-S, Chu C-W, Hsu H-H, Tseng F-G. A Microfluidic Platform for Investigating Transmembrane Pressure-Induced Glomerular Leakage. Micromachines. 2018; 9(5):228. https://doi.org/10.3390/mi9050228
Chicago/Turabian StyleChen, Ting-Hsuan, Jie-Sheng Chen, Yi-Ching Ko, Jyun-Wei Chen, Hsueh-Yao Chu, Chih-Shuan Lu, Chiao-Wen Chu, Hsiang-Hao Hsu, and Fan-Gang Tseng. 2018. "A Microfluidic Platform for Investigating Transmembrane Pressure-Induced Glomerular Leakage" Micromachines 9, no. 5: 228. https://doi.org/10.3390/mi9050228



