Miniaturized Optical Resolution Photoacoustic Microscope Based on a Microelectromechanical Systems Scanning Mirror
Abstract
:1. Introduction
2. Materials and Methods
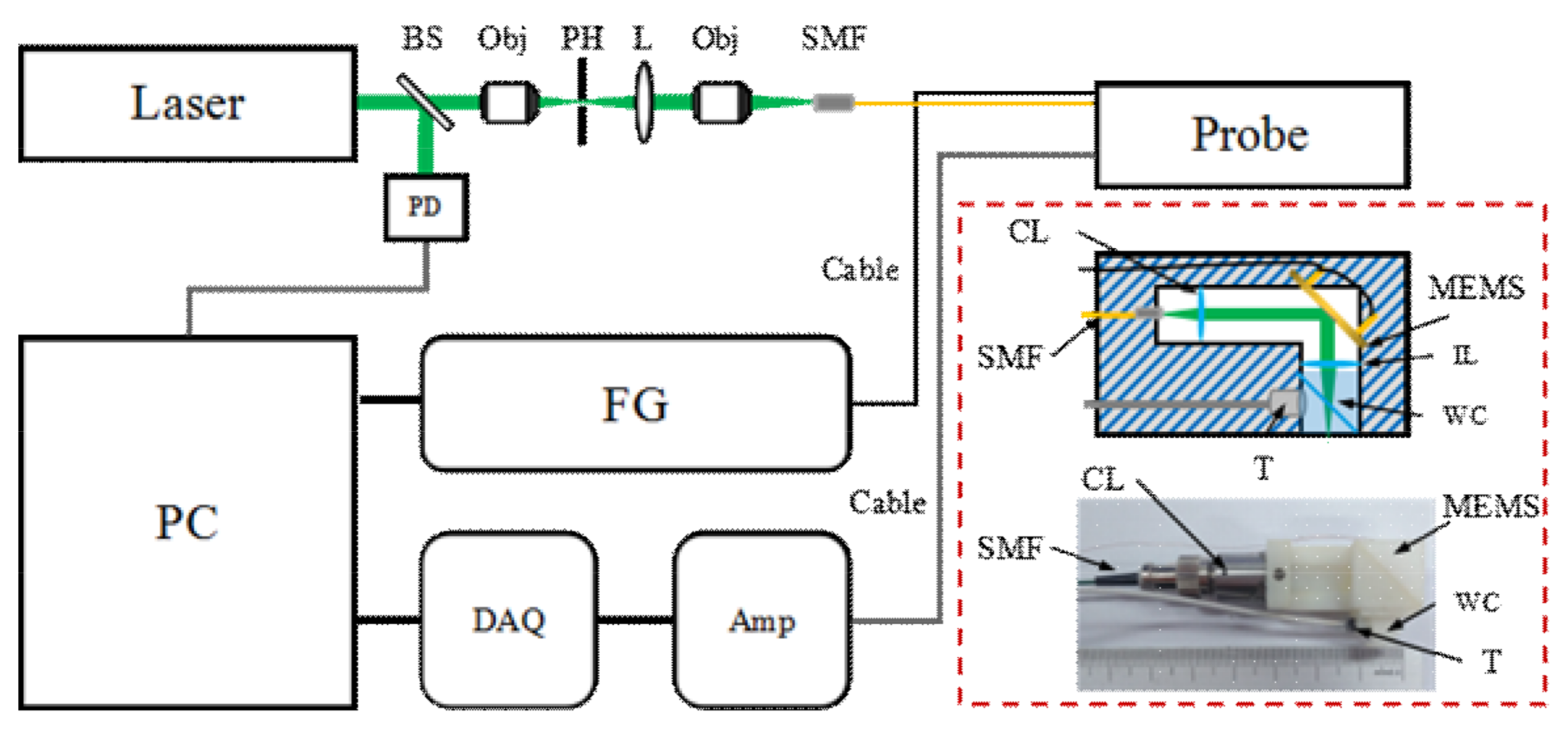
2.1. Configurations of the Imaging System and Probe
2.2. Phantom and Animal Experiments
2.3. In Vivo Human Experiments
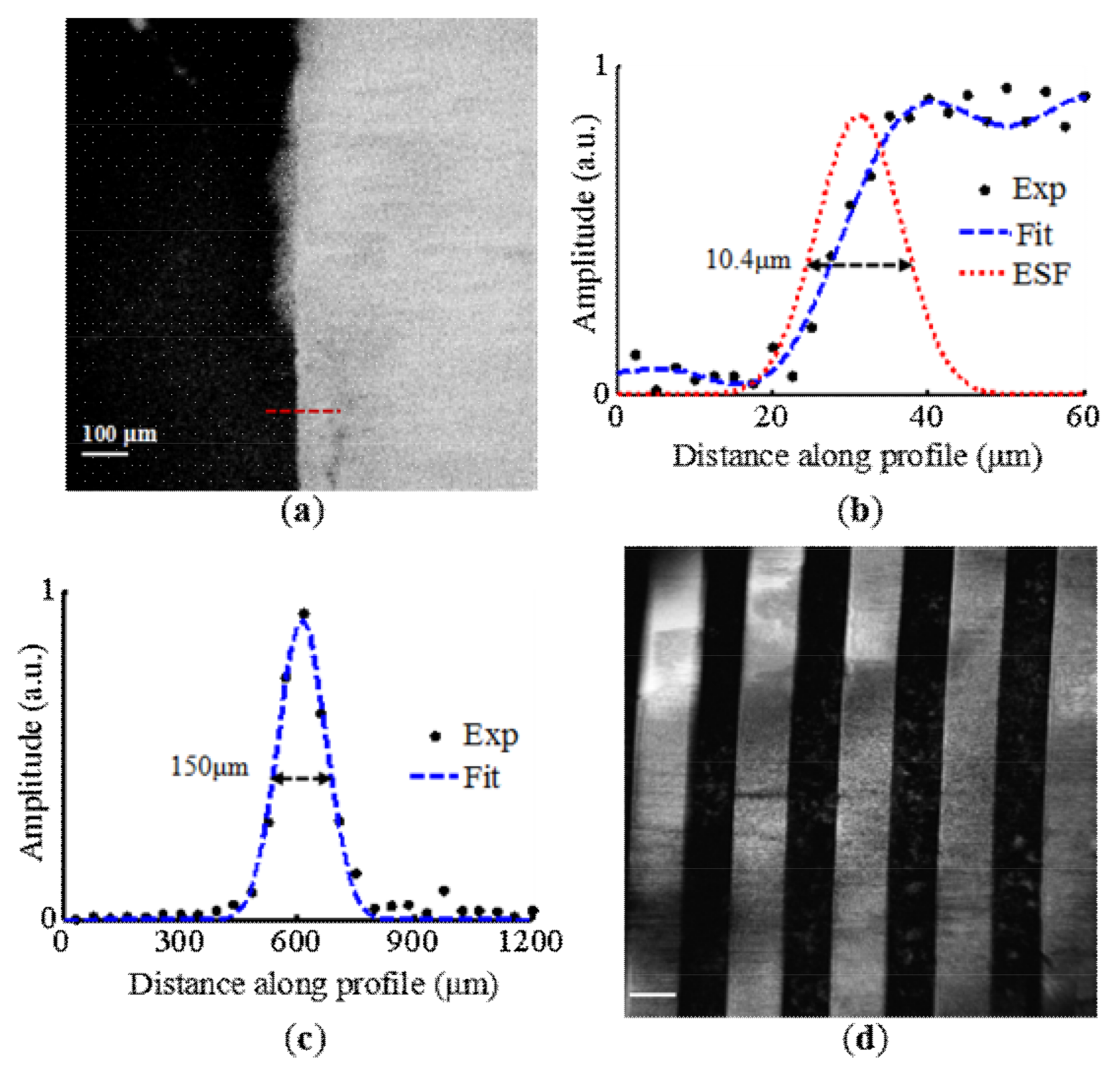
3. Results
4. Discussion and Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kim, C.; Song, K.H.; Gao, F.; Wang, L.V. Sentinel Lymph Nodes in the Rat: Noninvasive Photoacoustic and US Imaging with a Clinical US System. Radiology 2010, 25, 102–110. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, T.; Qi, W.; Mo, X.; Xi, L. Label-free photoacoustic imaging of the carido-cerebrovascular development in the embryonic zebrafish. Biomed. Opt. Exp. 2017, 8, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, N.; Cao, R.; Ning, B.; Chen, R.; Zhou, Q.; Hu, S. Multiparametric photoacoustic microscopy of the mouse brain with 300-kHz A-line rate. Neurophotonics 2016, 3, 045006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.T.W.; Zhang, R.; Zhang, C.; Hsu, H.; Maslov, K.I.; Wang, L.; Shi, J.; Chen, R.; Shung, K.K.; Zhou, Q.; et al. Label-free automated three-dimensional imaging of whole organs by microtomy-assisted photoacoustic microscopy. Nat. Commun. 2017, 8, 1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslov, K.; Zhang, H.F.; Hu, S.; Wang, L.V. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt. Lett. 2008, 33, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 2009, 3, 503–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Maslov, K.; Wang, L.V. Second-generation optical-resolution photoacoustic microscopy with improved sensitivity and speed. Opt. Lett. 2011, 36, 1134–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, L.; Gao, L.; Wang, L.V. Multiview optical resolution photoacoustic microscopy. Optica 2014, 1, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Maslov, K.; Yao, J.; Rao, B.; Wang, L.V. Fast voice-coil scanning optical-resolution photoacoustic microscopy. Opt. Lett. 2011, 36, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Sun, J.; Zhu, Y.; Wu, L.; Xie, H.; Jiang, H. Photoacoustic imaging based on MEMS mirror scanning. Biomed. Opt. Exp. 2010, 1, 1278–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, W.; Jin, T.; Rong, J.; Jiang, H.; Xi, L. Inverted multiscale optical resolution photoacoustic microscopy. J. Biophotonics 2017, 10, 1580–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, T.; Guo, H.; Jiang, H.; Ke, B.; Xi, L. Portable optical resolution photoacoustic microscopy (pORPAM) for human oral imaging. Opt. Lett. 2017, 42, 4434–4437. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, L.; Yang, J.; Maslov, K.I.; Wong, T.T.W.; Li, L.; Huang, C.; Zou, J.; Wang, L.V. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods 2015, 12, 407–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Wang, L.; Shi, J.; Yao, J.; Li, L.; Zhang, R.; Huang, C.H.; Zou, J.; Wang, L.V. In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating melanoma cells. Sci. Rep. 2016, 6, 39616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Wang, L.; Yang, J.M.; Gao, L.S.; Maslov, K.I.; Wang, L.V.; Huang, C.H.; Zou, J. Wide-field fast-scanning photoacoustic microscopy based on a water-immersible MEMS scanning mirror. J. Biomed. Opt. 2012, 17, 080505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Zhang, P.; Xu, S.; Shi, J.; Li, L.; Yao, J.; Wang, L.; Zou, J.; Wang, L.V. Handheld optical-resolution photoacoustic microscopy. J. Biomed. Opt. 2016, 22, 041002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.L.; Xie, Z.; Ling, T.; Guo, L.J.; Wei, X.; Wang, X. Miniaturized all-optical photoacoustic microscopy based on microelectromechanical systems mirror scanning. Opt. Lett. 2012, 37, 4263–4265. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. Fast optical-resolution photoacoustic microscopy using a 2-axis water-proofing MEMS scanner. Sci. Rep. 2015, 5, 7932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. A PDMS-Based 2-Axis Waterproof Scanner for Photoacoustic Microscopy. Sensors 2015, 15, 9815–9826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Lee, C.; Park, K.; Han, S.; Kim, C. High-speed and high-SNR photoacoustic microscopy based on a galvanometer mirror in nonconducting liquid. Sci. Rep. 2016, 6, 34803. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, J.Y.; Lee, C.; Jeon, S.; Lim, G.; Kim, C. Handheld Photoacoustic Microscopy Probe. Sci. Rep. 2017, 7, 13359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajireza, P.; Shi, W.; Zemp, R.J. Real-time handheld optical-resolution photoacoustic microscopy. Opt. Exp. 2011, 19, 20097–20102. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Song, C.; Xie, H.; Xi, L. Photoacoustic endomicroscopy (PAEM) based on a MEMS scanning mirror. Opt. Lett. 2017, 42, 4615–4618. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, W.; Chen, Q.; Guo, H.; Xie, H.; Xi, L. Miniaturized Optical Resolution Photoacoustic Microscope Based on a Microelectromechanical Systems Scanning Mirror. Micromachines 2018, 9, 288. https://doi.org/10.3390/mi9060288
Qi W, Chen Q, Guo H, Xie H, Xi L. Miniaturized Optical Resolution Photoacoustic Microscope Based on a Microelectromechanical Systems Scanning Mirror. Micromachines. 2018; 9(6):288. https://doi.org/10.3390/mi9060288
Chicago/Turabian StyleQi, Weizhi, Qian Chen, Heng Guo, Huikai Xie, and Lei Xi. 2018. "Miniaturized Optical Resolution Photoacoustic Microscope Based on a Microelectromechanical Systems Scanning Mirror" Micromachines 9, no. 6: 288. https://doi.org/10.3390/mi9060288




