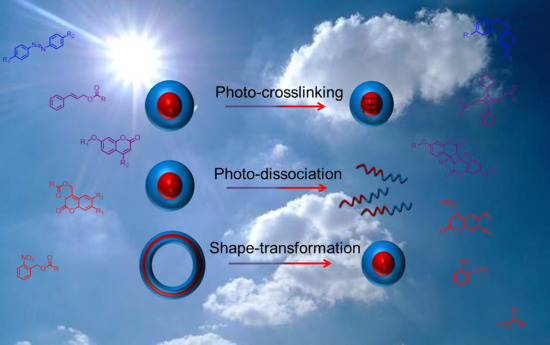
Progress in Photo-Responsive Polypeptide Derived Nano-Assemblies
Abstract
:1. Introduction
2. Photo-Chemistry of Light-Responsive Polypeptide Nanoparticles
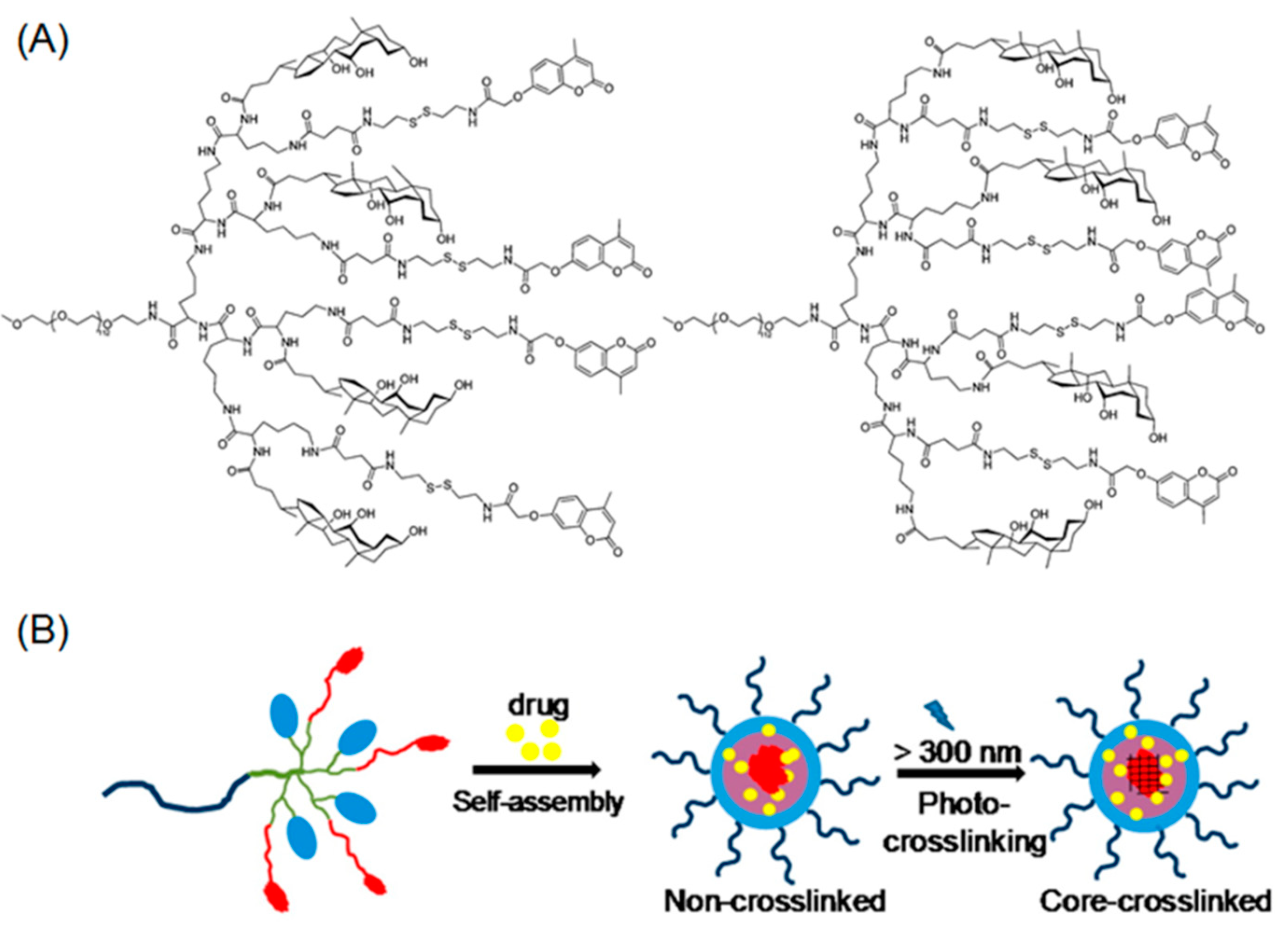
2.1. Photo-Crosslinkable Nanoparticles
2.2. Photo-Cleavable Nano-Objects
2.3. Photo-Isomerizable Nano-Assemblies
3. Properties and Applications
4. Current Challenges and Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Kabb, C.P.; Dai, Y.; Hill, M.R.; Ghiviriga, I.; Bapat, A.P.; Sumerlin, B.S. Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture. Nat. Chem. 2017, 9, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Herbert, K.M.; Schrettl, S.; Rowan, S.J.; Weder, C. 50th anniversary perspective: Solid-state multistimuli, multiresponsive polymeric materials. Macromolecules 2017, 50, 8845–8870. [Google Scholar] [CrossRef]
- Rowan, S.; Cantrill, S.; Cousins, G.; Sanders, J.; Stoddart, J. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar] [CrossRef]
- Stuart, M.; Huck, W.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.; Szleifer, I.; Tsukruk, V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Alfurhood, J.A.; Sun, H.; Kabb, C.P.; Tucker, B.S.; Matthews, J.H.; Luesch, H.; Sumerlin, B.S. Poly(n-(2-hydroxypropyl)-methacrylamide)-valproic acid conjugates as block copolymer nanocarriers. Polym. Chem. 2017, 8, 4983–4987. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Tsarevsky, N.V. Preparation and functionalization of linear and reductively degradable highly branched cyanoacrylate-based polymers. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3683–3693. [Google Scholar] [CrossRef]
- Tang, H.L.; Tsarevsky, N.V. Lipoates as building blocks of sulfur-containing branched macromolecules. Polym. Chem. 2015, 6, 6936–6945. [Google Scholar] [CrossRef]
- Wan, S.; Zhang, L.Q.; Wang, S.; Liu, Y.; Wu, C.C.; Cui, C.; Sun, H.; Shi, M.L.; Jiang, Y.; Li, L.; et al. Molecular recognition-based DNA nanoassemblies on the surfaces of nanosized exosomes. J. Am. Chem. Soc. 2017, 139, 5289–5292. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Dobbins, D.J.; Dai, Y.; Kabb, C.P.; Wu, S.; Alfurhood, J.A.; Rinaldi, C.; Sumerlin, B.S. Radical departure: Thermally-triggered degradation of azo-containing poly(β-thioester)s. ACS Macro Lett. 2016, 5, 688–693. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, H.; Pal, S.; Zhang, Y.; Park, S.; Kabb, C.; Wei, W.; Sumerlin, B. Near-ir-induced dissociation of thermally-sensitive star polymers. Chem. Sci. 2017, 8, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Alfurhood, J.A.; Sun, H.; Bachler, P.R.; Sumerlin, B.S. Hyperbranched poly(n-(2-hydroxypropyl)methacrylamide) via raft self-condensing vinyl polymerization. Polym. Chem. 2016, 7, 2099–2104. [Google Scholar] [CrossRef]
- Liu, G.; Liu, N.; Zhou, L.Z.; Su, Y.; Dong, C.M. Nir-responsive polypeptide copolymer upconversion composite nanoparticles for triggered drug release and enhanced cytotoxicity. Polym. Chem. 2015, 6, 4030–4039. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.J.; Sun, H.; Cui, C.; Zhang, L.Q.; Jiang, Y.; Wu, Y.X.; Wang, Y.Y.; Li, J.; Sumerlin, B.S.; et al. Thiol-ene click chemistry: A biocompatible way for orthogonal bioconjugation of colloidal nanoparticles. Chem. Sci. 2017, 8, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhong, M.; Johnson, J. Light-controlled radical polymerization: Mechanisms, methods, and applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.B.; Li, X.L.; Zeng, R.M.; Liu, D.D.; Xu, Q.; He, J.; Zhang, Y.X.; Dai, X.C.; Yu, L.L.; Zeng, Z.H.; et al. Expanding the scope of polymerization-induced self-assembly: Z-raft-mediated photoinitiated dispersion polymerization. ACS Macro Lett. 2018, 7, 255–262. [Google Scholar] [CrossRef]
- Yeow, J.; Boyer, C. Photoinitiated polymerization-induced self-assembly (photo-pisa): New insights and opportunities. Adv. Sci. 2017, 4, 1700137. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.B.; Huang, C.D.; Liu, D.D.; Zhang, X.C.; Bai, Y.H.; Zhang, L. Alcoholic photoinitiated polymerization-induced self-assembly (photo-pisa): A fast route toward poly(isobornyl acrylate)-based diblock copolymer nano-objects. ACS Macro Lett. 2016, 5, 894–899. [Google Scholar] [CrossRef]
- Tan, J.B.; Sun, H.; Yu, M.G.; Sumerlin, B.S.; Zhang, L. Photo-pisa: Shedding light on polymerization-induced self-assembly. ACS Macro Lett. 2015, 4, 1249–1253. [Google Scholar] [CrossRef]
- Niu, J.; Lunn, D.J.; Pusuluri, A.; Yoo, J.I.; O'Malley, M.A.; Mitragotri, S.; Soh, H.T.; Hawker, C.J. Engineering live cell surfaces with functional polymers via cytocompatible controlled radical polymerization. Nat. Chem. 2017, 9, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Treat, N.; Sprafke, H.; Kramer, J.; Clark, P.; Barton, B.; de Alaniz, J.; Fors, B.; Hawker, C. Metal-free atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 16096–16101. [Google Scholar] [CrossRef] [PubMed]
- Goetz, A.E.; Boydston, A.J. Metal-free preparation of linear and cross-linked polydicyclopentadiene. J. Am. Chem. Soc. 2015, 137, 7572–7575. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.A.; Goetz, A.E.; Boydston, A.J. Metal-free ring-opening metathesis polymerization. J. Am. Chem. Soc. 2015, 137, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Blasco, E.; Sims, M.B.; Goldmann, A.S.; Sumerlin, B.S.; Barner-Kowollik, C. 50th anniversary perspective: Polymer functionalization. Macromolecules 2017, 50, 5215–5252. [Google Scholar] [CrossRef]
- Xu, J.; Shanmugam, S.; Fu, C.; Aguey-Zinsou, K.; Boyer, C. Selective photoactivation: From a single unit monomer insertion reaction to controlled polymer architectures. J. Am. Chem. Soc. 2016, 138, 3094–3106. [Google Scholar] [CrossRef] [PubMed]
- Kottisch, V.; Michaudel, Q.; Fors, B.P. Photocontrolled interconversion of cationic and radical polymerizations. J. Am. Chem. Soc. 2017, 139, 10665–10668. [Google Scholar] [CrossRef] [PubMed]
- Michaudel, Q.; Chauvire, T.; Kottisch, V.; Supej, M.J.; Stawiasz, K.J.; Shen, L.X.; Zipfel, W.R.; Abruna, H.D.; Freed, J.H.; Fors, B.P. Mechanistic insight into the photocontrolled cationic polymerization of vinyl ethers. J. Am. Chem. Soc. 2017, 139, 15530–15538. [Google Scholar] [CrossRef] [PubMed]
- Rwei, A.Y.; Wang, W.P.; Kohane, D.S. Photoresponsive nanoparticles for drug delivery. Nano Today 2015, 10, 451–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Tremblay, L.; Zhao, Y. Doubly photoresponsive and water-soluble block copolymers: Synthesis and thermosensitivity. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4055–4066. [Google Scholar] [CrossRef]
- Fomina, N.; McFearin, C.L.; Sermsakdi, M.; Morachis, J.M.; Almutairi, A. Low power, biologically benign nir light triggers polymer disassembly. Macromolecules 2011, 44, 8590–8597. [Google Scholar] [CrossRef] [PubMed]
- Bauri, K.; Nandi, M.; De, P. Amino acid-derived stimuli-responsive polymers and their applications. Polym. Chem. 2018, 9, 1257–1287. [Google Scholar] [CrossRef]
- Gradisar, H.; Bozic, S.; Doles, T.; Vengust, D.; Hafner-Bratkovic, I.; Mertelj, A.; Webb, B.; Sali, A.; Klavzar, S.; Jerala, R. Design of a single-chain polypeptide tetrahedron assembled from coiled-coil segments. Nat. Chem. Biol. 2013, 9, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, L.; del Valle, L.J.; Puiggali, J. Smart systems related to polypeptide sequences. AIMS Mater. Sci. 2016, 3, 289–323. [Google Scholar] [CrossRef]
- Ashkenasy, N.; Schneider, J. Functional peptide and protein nanostructures. Isr. J. Chem. 2015, 55, 621. [Google Scholar] [CrossRef]
- Aemissegger, A.; Hilvert, D. Synthesis and application of an azobenzene amino acid as a light-switchable turn element in polypeptides. Nat. Protoc. 2007, 2, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ungerleider, J.L.; Kammeyer, J.K.; Braden, R.L.; Christman, K.L.; Gianneschi, N.C. Enzyme-targeted nanoparticles for delivery to ischemic skeletal muscle. Polym. Chem. 2017, 8, 5212–5219. [Google Scholar] [CrossRef] [PubMed]
- Carlini, A.S.; Adamiak, L.; Gianneschi, N.C. Biosynthetic polymers as functional materials. Macromolecules 2016, 49, 4379–4394. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Thang, S.H. Raft polymerization of a rgd peptide-based methacrylamide monomer for cell adhesion. Polym. Chem. 2018, 9, 1780–1786. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.W.; Huang, Y.R.; Thompson, M.P.; LeGuyader, C.L.M.; Sahu, S.; Gianneschi, N.C. Enzyme-regulated topology of a cyclic peptide brush polymer for tuning assembly. Chem. Commun. 2015, 51, 17108–17111. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Fu, X.H.; Wan, Y.M.; Sun, Y.L.; Li, Z.B.; Lu, H. Synthesis and multimodal responsiveness of poly(alpha-amino acid)s bearing oegylated azobenzene side-chains. Polym. Chem. 2016, 7, 6375–6382. [Google Scholar] [CrossRef]
- Deming, T.J. Synthesis of side-chain modified polypeptides. Chem. Rev. 2016, 116, 786–808. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Fan, J.W.; He, X.; Zhang, S.Y.; Wang, H.; Wooley, K.L. A facile glovebox-free strategy to significantly accelerate the syntheses of well-defined polypeptides by n-carboxyanhydride (nca) ring-opening polymerizations. Macromolecules 2013, 46, 4223–4226. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Han, Z.Y.; Lv, S.X.; Chen, C.Y.; Chen, L.; Yin, L.C.; Cheng, J.J. Synthetic polypeptides: From polymer design to supramolecular assembly and biomedical application. Chem. Soc. Rev. 2017, 46, 6570–6599. [Google Scholar] [CrossRef] [PubMed]
- Le Droumaguet, B.; Nicolas, J. Recent advances in the design of bioconjugates from controlled/living radical polymerization. Polym. Chem. 2010, 1, 563–598. [Google Scholar] [CrossRef]
- Ayres, L.; Koch, K.; Adams, P.H.H.M.; van Hest, J.C.M. Stimulus responsive behavior of elastin-based side chain polymers. Macromolecules 2005, 38, 1699–1704. [Google Scholar] [CrossRef] [Green Version]
- Blum, A.P.; Kammeyer, J.K.; Gianneschi, N.C. Activating peptides for cellular uptake via polymerization into high density brushes. Chem. Sci. 2016, 7, 989–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianneschi, N. Peptide-polymer amphiphiles as programmable synthons for biologically-responsive nanomaterials. Abstr. Pap. Am. Chem. Soc. 2015, 249, 317. [Google Scholar]
- Blum, A.P.; Kammeyer, J.K.; Rush, A.M.; Callmann, C.E.; Hahn, M.E.; Gianneschi, N.C. Stimuli-responsive nanomaterials for biomedical applications. J. Am. Chem. Soc. 2015, 137, 2140–2154. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.; Fu, H.L.; Song, Z.Y.; Lin, Y.; Cheng, J.J. Cooperative polymerization of alpha-helices induced by macromolecular architecture. Nat. Chem. 2017, 9, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.W.; Borguet, Y.P.; Su, L.; Nguyen, T.P.; Wang, H.; He, X.; Zou, J.; Wooley, K.L. Two-dimensional controlled syntheses of polypeptide molecular brushes via n-carboxyanhydride ring-opening polymerization and ring-opening metathesis polymerization. ACS Macro Lett. 2017, 6, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kabb, C.P.; Sumerlin, B.S. Thermally-labile segmented hyperbranched copolymers: Using reversible-covalent chemistry to investigate the mechanism of self-condensing vinyl copolymerization. Chem. Sci. 2014, 5, 4646–4655. [Google Scholar] [CrossRef]
- Dong, P.; Sun, H.; Quan, D.P. Synthesis of poly(l-lactide-co-5-amino-5-methyl-1,3-dioxan-2-ones) [p(l-la-co-tac)] containing amino groups via organocatalysis and post-polymerization functionalization. Polymer 2016, 97, 614–622. [Google Scholar] [CrossRef]
- Perrier, S. 50th anniversary perspective: Raft polymerization—A user guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Grubbs, R.B.; Grubbs, R.H. 50th anniversary perspective: Living polymerization-emphasizing the molecule in macromolecules. Macromolecules 2017, 50, 6979–6997. [Google Scholar] [CrossRef]
- Tan, J.B.; He, J.; Li, X.L.; Xu, Q.; Huang, C.D.; Liu, D.D.; Zhang, L. Rapid synthesis of well-defined all-acrylic diblock copolymer nano-objects via alcoholic photoinitiated polymerization-induced self-assembly (photo-pisa). Polym. Chem. 2017, 8, 6853–6864. [Google Scholar] [CrossRef]
- Ren, J.; McKenzie, T.; Fu, Q.; Wong, E.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.; Boyer, C.; Qiao, G. Star polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef] [PubMed]
- Kerr, A.; Hartlieb, M.; Sanchis, J.; Smith, T.; Perrier, S. Complex multiblock bottle-brush architectures by raft polymerization. Chem. Commun. 2017, 53, 11901–11904. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Atom transfer radical polymerization (atrp): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Architecturally complex polymers with controlled heterogeneity. Science 2011, 333, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Bapat, A.; Roy, D.; Ray, J.; Savin, D.; Sumerlin, B. Dynamic-covalent macromolecular stars with boronic ester linkages. J. Am. Chem. Soc. 2011, 133, 19832–19838. [Google Scholar] [CrossRef] [PubMed]
- Sumerlin, B.; Vogt, A. Macromolecular engineering through click chemistry and other efficient transformations. Macromolecules 2010, 43, 1–13. [Google Scholar] [CrossRef]
- Aoki, D.; Aibara, G.; Uchida, S.; Takata, T. A rational entry to cyclic polymers via selective cyclization by self-assembly and topology transformation of linear polymers. J. Am. Chem. Soc. 2017, 139, 6791–6794. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yagyu, S.; Tezuka, Y. Light- and heat-triggered reversible linear-cyclic topological conversion of telechelic polymers with anthryl end groups. J. Am. Chem. Soc. 2016, 138, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Bielawski, C.; Benitez, D.; Grubbs, R. An “endless” route to cyclic polymers. Science 2002, 297, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.B.; Lu, X.Y.; Wang, W.Y.; Kang, N.G.; Mays, J.W. Block copolymers: Synthesis, self-assembly, and applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef]
- Ding, J.X.; Zhuang, X.L.; Xiao, C.S.; Cheng, Y.L.; Zhao, L.; He, C.L.; Tang, Z.H.; Chen, X.S. Preparation of photo-cross-linked ph-responsive polypeptide nanogels as potential carriers for controlled drug delivery. J. Mater. Chem. 2011, 21, 11383–11391. [Google Scholar] [CrossRef]
- Yan, L.S.; Yang, L.X.; He, H.Y.; Hu, X.L.; Xie, Z.G.; Huang, Y.B.; Jing, X.B. Photo-cross-linked mpeg-poly(gamma-cinnamyl-l-glutamate) micelles as stable drug carriers. Polym. Chem. 2012, 3, 1300–1307. [Google Scholar] [CrossRef]
- Shao, Y.; Shi, C.Y.; Xu, G.F.; Guo, D.D.; Luo, J.T. Photo and redox dual responsive reversibly cross-linked nanocarrier for efficient tumor-targeted drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 10381–10392. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Dong, C.M. Photoresponsive poly(s-(o-nitrobenzyl)-l-cysteine)-b-peo from a l-cysteine n-carboxyanhydride monomer: Synthesis, self-assembly, and phototriggered drug release. Biomacromolecules 2012, 13, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhou, L.Z.; Guan, Y.F.; Su, Y.; Dong, C.M. Multi-responsive polypeptidosome: Characterization, morphology transformation, and triggered drug delivery. Macromol. Rapid Commun. 2014, 35, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Negri, G.E.; Deming, T.J. Triggered copolypeptide hydrogel degradation using photolabile lysine protecting groups. ACS Macro Lett. 2016, 5, 1253–1256. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.C.; Dong, C.M. Photosensitive poly(o-nitrobenzyloxycarbonyl-l-lysine)-b-peo polypeptide copolymers: Synthesis, multiple self-assembly behaviors, and the photo/ph-thermo-sensitive hydrogels. Polym. Chem. 2017, 8, 7033–7043. [Google Scholar] [CrossRef]
- Jana, S.; Biswas, Y.; Mandal, T.K. Methionine-based cationic polypeptide/polypeptide block copolymer with triple-stimuli responsiveness: DNA polyplexation and phototriggered release. Polym. Chem. 2018, 9, 1869–1884. [Google Scholar] [CrossRef]
- Kumar, S.; Allard, J.F.; Morris, D.; Dory, Y.L.; Lepage, M.; Zhao, Y. Near-infrared light sensitive polypeptide block copolymer micelles for drug delivery. J. Mater. Chem. 2012, 22, 7252–7257. [Google Scholar] [CrossRef]
- Mba, M.; Mazzier, D.; Silvestrini, S.; Toniolo, C.; Fatas, P.; Jimenez, A.I.; Cativiela, C.; Moretto, A. Photocontrolled self-assembly of a bis-azobenzene-containing alpha-amino acid. Chemistry 2013, 19, 15841–15846. [Google Scholar] [CrossRef] [PubMed]
- Mazzier, D.; Maran, M.; Perucchin, O.P.; Crisma, M.; Zerbetto, M.; Causin, V.; Toniolo, C.; Moretto, A. Photoresponsive supramolecular architectures based on polypeptide hybrids. Macromolecules 2014, 47, 7272–7283. [Google Scholar] [CrossRef]
- Zhao, Y.A.; Lei, B.Q.; Wang, M.F.; Wu, S.T.; Qi, W.; Su, R.X.; He, Z.M. A supramolecular approach to construct a hydrolase mimic with photo-switchable catalytic activity. J. Mater. Chem. B 2018, 6, 2444–2449. [Google Scholar] [CrossRef]
- Kotharangannagari, V.K.; Sanchez-Ferrer, A.; Ruokolainen, J.; Mezzenga, R. Photoresponsive reversible aggregation and dissolution of rod-coil polypeptide diblock copolymers. Macromolecules 2011, 44, 4569–4573. [Google Scholar] [CrossRef]
- Bhola, R.; Payamyar, P.; Murray, D.J.; Kumar, B.; Teator, A.J.; Schmidt, M.U.; Hammer, S.M.; Saha, A.; Sakamoto, J.; Schluter, A.D.; et al. A two-dimensional polymer from the anthracene dimer and triptycene motifs. J. Am. Chem. Soc. 2013, 135, 14134–14141. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tremblay, L.; Lacelle, S.; Zhao, Y. Preparation of polymer single chain nanoparticles using intramolecular photodimerization of coumarin. Soft Matter 2011, 7, 2380–2386. [Google Scholar] [CrossRef]
- Inkinen, J.; Niskanen, J.; Talka, T.; Sahle, C.J.; Muller, H.; Khriachtchev, L.; Hashemi, J.; Akbari, A.; Hakala, M.; Huotari, S. X-ray induced dimerization of cinnamic acid: Time-resolved inelastic X-ray scattering study. Sci. Rep. 2015, 5, 15851. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yu, Y.L. Photodeformable polymer gels and crosslinked liquid-crystalline polymers. Soft Matter 2012, 8, 8050–8059. [Google Scholar] [CrossRef]
- Jamroz-Piegza, M.; Walach, W.; Dworak, A.; Trzebicka, B. Polyether nanoparticles from covalently crosslinked copolymer micelles. J. Colloid Interface Sci. 2008, 325, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, H.; Jiang, J.Q.; Yan, X.Y.; Liu, Z.T.; Liu, Z.W. The photodimerization characteristics of anthracene pendants within amphiphilic polymer micelles in aqueous solution. RSC Adv. 2014, 4, 25912–25915. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Qi, B.; Lepage, M.; Zhao, Y. Polymer micelles stabilization on demand through reversible photo-cross-linking. Macromolecules 2007, 40, 790–792. [Google Scholar] [CrossRef]
- Tardy, A.; Nicolas, J.; Gigmes, D.; Lefay, C.; Guillaneuf, Y. Radical ring-opening polymerization: Scope, limitations, and application to (bio)degradable materials. Chem. Rev. 2017, 117, 1319–1406. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Jiang, X.S.; Yin, J. Responsive fluorescent core-crosslinked polymer particles based on the anthracene-containing hyperbranched poly(ether amine) (hPEA-An). Soft Matter 2011, 7, 6853–6862. [Google Scholar] [CrossRef]
- Xie, X.Y.; Yang, Y.F.; Yang, Y.; Zhang, H.; Li, Y.; Mei, X.G. A photo-responsive peptide- and asparagine-glycine-arginine (NGR) peptide-mediated liposomal delivery system. Drug Deliv. 2016, 23, 2445–2456. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chou, C.F. Reversible photodimerization of coumarin derivatives dispersed in poly(vinyl acetate). J. Polym. Sci. Part A Polym. Chem. 1995, 33, 2705–2714. [Google Scholar] [CrossRef]
- Flint, D.G.; Kumita, J.R.; Smart, O.S.; Woolley, G.A. Using an azobenzene cross-linker to either increase or decrease peptide helix content upon trans-to-cis photoisomerization. Chem. Biol. 2002, 9, 391–397. [Google Scholar] [CrossRef]
- Kinoshita, T.; Sato, M.; Takizawa, A.; Tsujita, Y. Photocontrol of polypeptide membrane functions by cis-trans isomerization in side-chain azobenzene groups. Macromolecules 1986, 19, 51–55. [Google Scholar] [CrossRef]
- Li, Y.F.; Niu, Y.L.; Hu, D.; Song, Y.W.; He, J.W.; Liu, X.Y.; Xia, X.N.; Lu, Y.B.; Xu, W.J. Preparation of light-responsive polyester micelles via ring-opening polymerization of o-carboxyanhydride and azide-alkyne click chemistry. Macromol. Chem. Phys. 2015, 216, 77–84. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciardelli, F.; Fabbri, D.; Pieroni, O.; Fissi, A. Photomodulation of polypeptide conformation by sunlight in spiropyran-containing poly(l-glutamic acid). J. Am. Chem. Soc. 1989, 111, 3470–3472. [Google Scholar] [CrossRef]
- Yin, R.; Dai, T.H.; Avci, P.; Jorge, A.E.S.; de Melo, W.C.M.A.; Vecchio, D.; Huang, Y.Y.; Gupta, A.; Hamblin, M.R. Light based anti-infectives: Ultraviolet c irradiation, photodynamic therapy, blue light, and beyond. Curr. Opin. Pharmacol. 2013, 13, 731–762. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, O.; Gohy, J. Photo-responsive polymers: Synthesis and applications. Polym. Chem. 2017, 8, 52–73. [Google Scholar] [CrossRef]
- Zhao, Y. Rational design of light-controllable polymer micelles. Chem. Rec. 2007, 7, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.; Chen, A.; Zhang, H.B.; Burt, H.; Chiao, M. Design and near-infrared actuation of a gold nanorod-polymer microelectromechanical device for on-demand drug delivery. Micromachines 2018, 9, 28. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.Y.; Liu, L.P.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.Y.; Li, Z.J.; Dong, B.Q.; et al. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Linsley, C.S.; Wu, B.M. Recent advances in light-responsive on-demand drug- delivery systems. Ther. Deliv. 2017, 8, 89–107. [Google Scholar] [CrossRef] [PubMed]











| Polypeptide | Synthetic Method | Photo-Responsive Moiety | Light-Triggered Events | Application | Ref. |
|---|---|---|---|---|---|
| PEG-b-P(LGA/CLG) | ROP a | Cinamyl | Micellar Core-Crosslink | Drug Delivery | [66] |
| PEG-b-PCLG | ROP | Cinamyl | Micellar Core-Crosslink | Drug Delivery | [67] |
| PEG-CA4LS4Co4 | Solution-PPS b | Coumarin | Micellar Core-Crosslink | Drug Delivery | [68] |
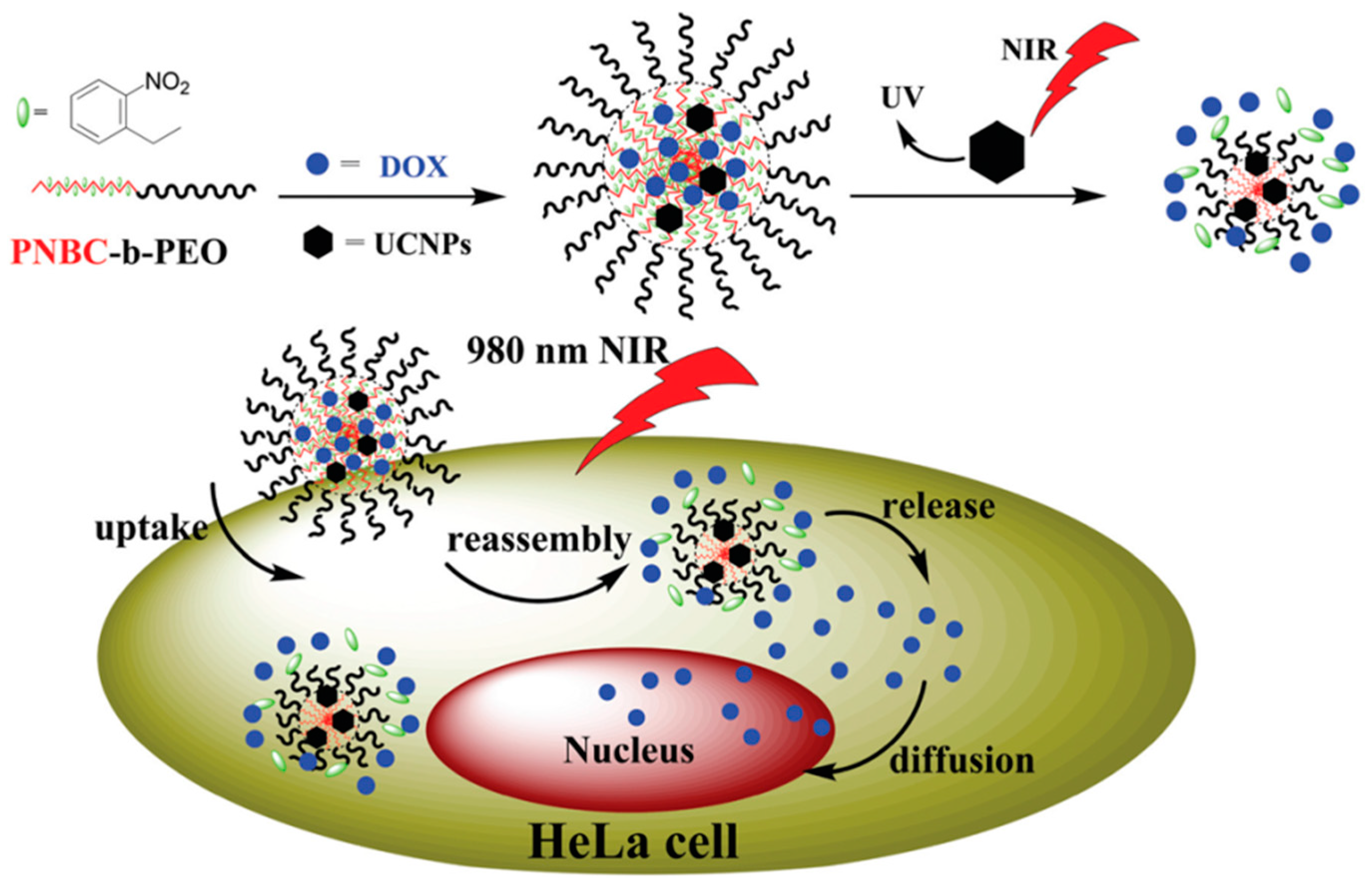
| PNBC-b-PEO | ROP | O-nitrobenzyl | Micellar Disassembly | Drug Delivery | [69] |
| PNBC-b-PEO | ROP | O-nitrobenzyl | Vesicle to Micelle | N/A | [70] |
| PNBC-b-PEO | ROP | O-nitrobenzyl | Composite Nanoparticle | Drug Delivery | [13] |
| PNBL-b-PLL | ROP | O-nitrobenzyl | Sol-Gel Transition | N/A | [71] |
| PNBL-b-PEO | ROP | O-nitrobenzyl | Sol-Gel Transition | N/A | [72] |
| PEtOx-b-P[MetNB][Br] | ROP | O-nitrobenzyl | Composite Nanoparticle | Gene Delivery | [73] |
| PEO-b-P(LGA-co-COU) | ROP | 6-Bromo-7-hydroxyl-coumarine | Micellar Disassembly | Drug Delivery | [74] |
| Fmoc-Phe-pazoDbg | Solution-PPS | Azobenzene | Micelle to Fiber | N/A | [75] |
| PBLG-azobenzene | ROP | Azobenzene | Vesicle Disruption | Cargo Release | [76] |
| P(OEG-Azo) | ROP | Azobenzene | Sol-Gel Transition | N/A | [40] |
| Azo-GFGH | Solid-PPS | Azobenzene | Nanofiber Assembly | Catalysis | [77] |
| PLGASP-b-PEO | ROP | Spiropyran | Micellar Disassembly | Drug Delivery | [78] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Tang, H.; Sun, H. Progress in Photo-Responsive Polypeptide Derived Nano-Assemblies. Micromachines 2018, 9, 296. https://doi.org/10.3390/mi9060296
Yang L, Tang H, Sun H. Progress in Photo-Responsive Polypeptide Derived Nano-Assemblies. Micromachines. 2018; 9(6):296. https://doi.org/10.3390/mi9060296
Chicago/Turabian StyleYang, Lu, Houliang Tang, and Hao Sun. 2018. "Progress in Photo-Responsive Polypeptide Derived Nano-Assemblies" Micromachines 9, no. 6: 296. https://doi.org/10.3390/mi9060296