A Facile Interfacial Self-Assembly of Crystalline Colloidal Monolayers by Tension Gradient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Monolayer Preparation
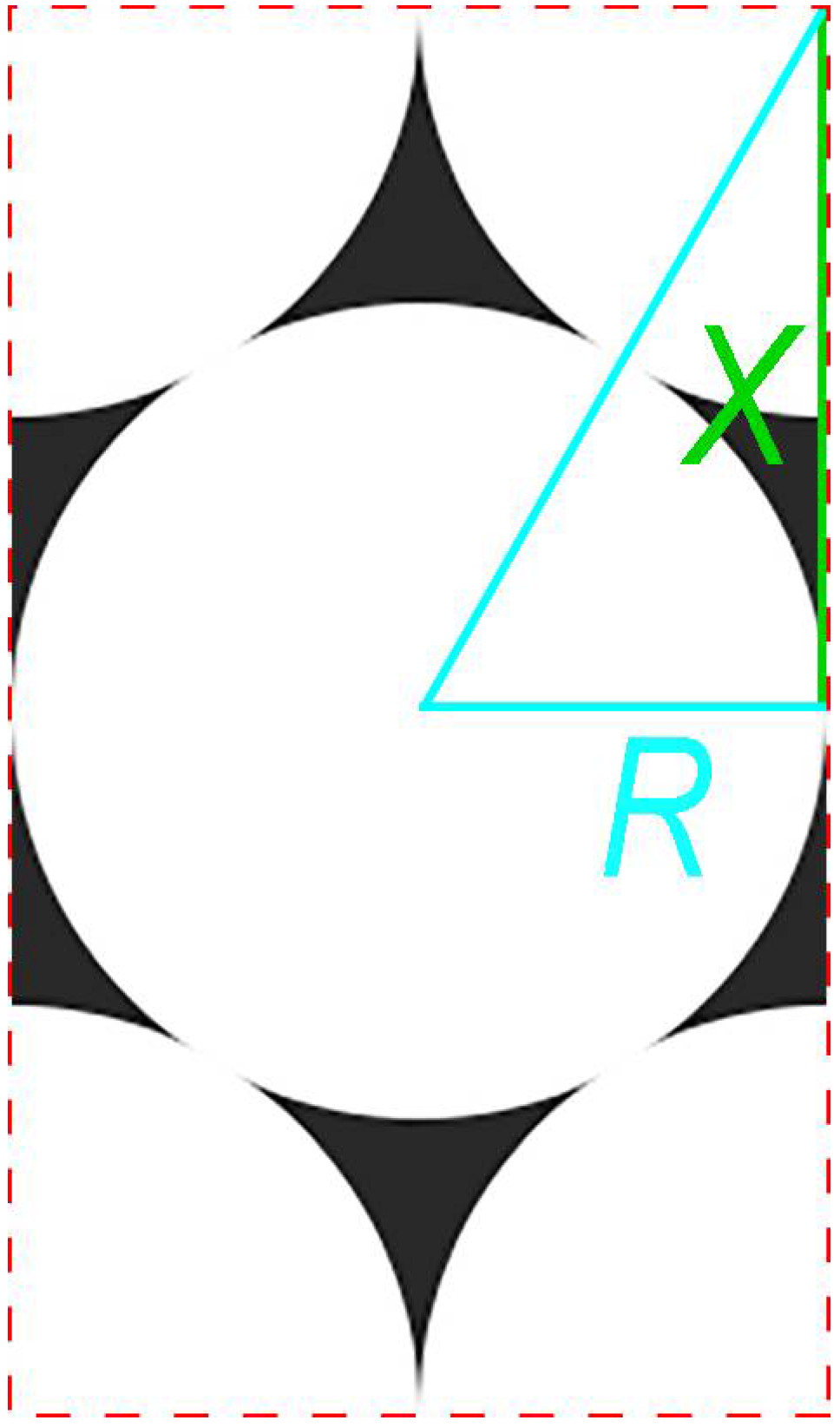
2.3. Determination of the Contact Angle of Nanoparticles at the Interface
2.4. Characterization
3. Results and Discussion
3.1. Self-Assembly Process of the Colloidal Monolayer at the Air-Water Interface
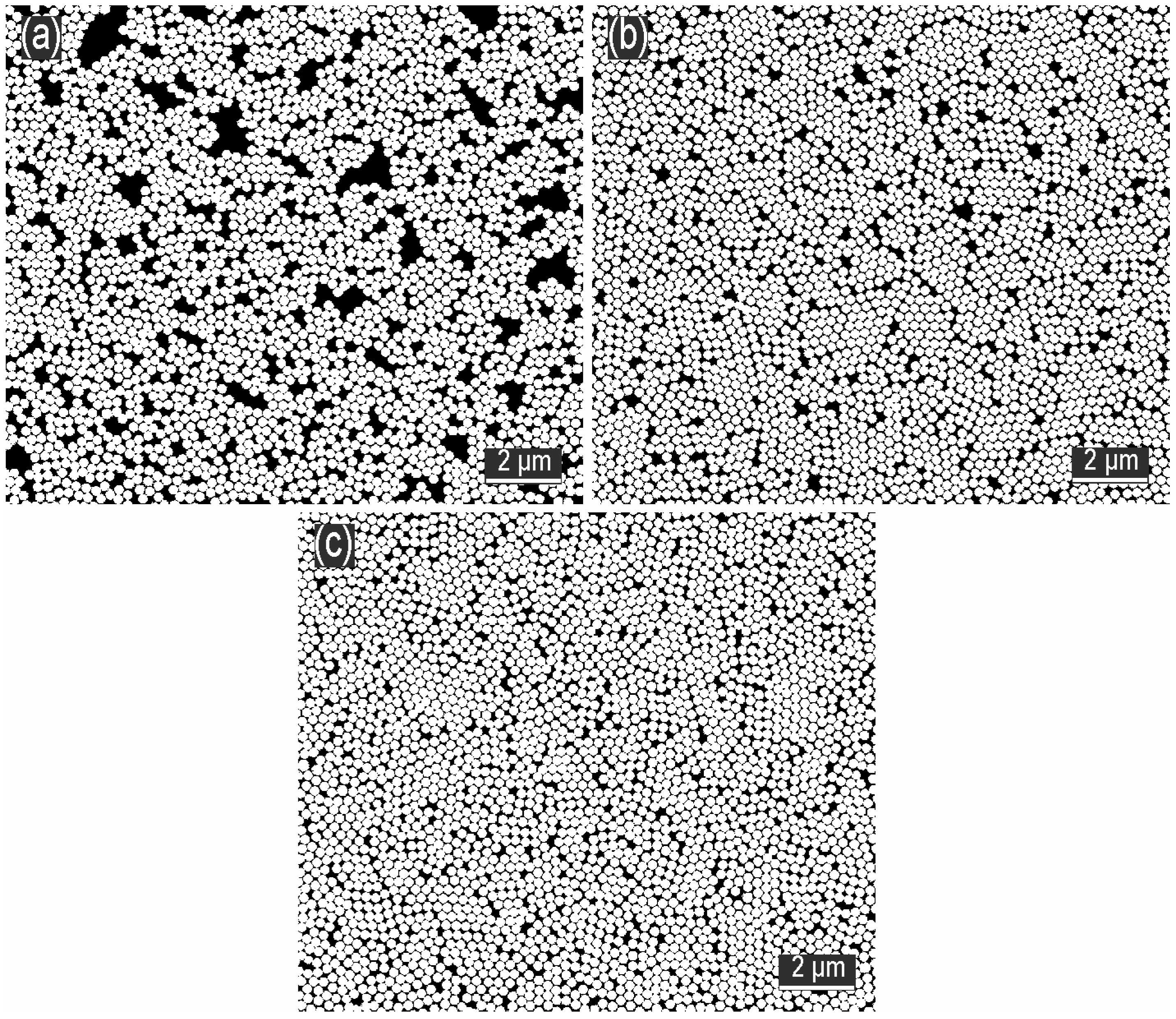
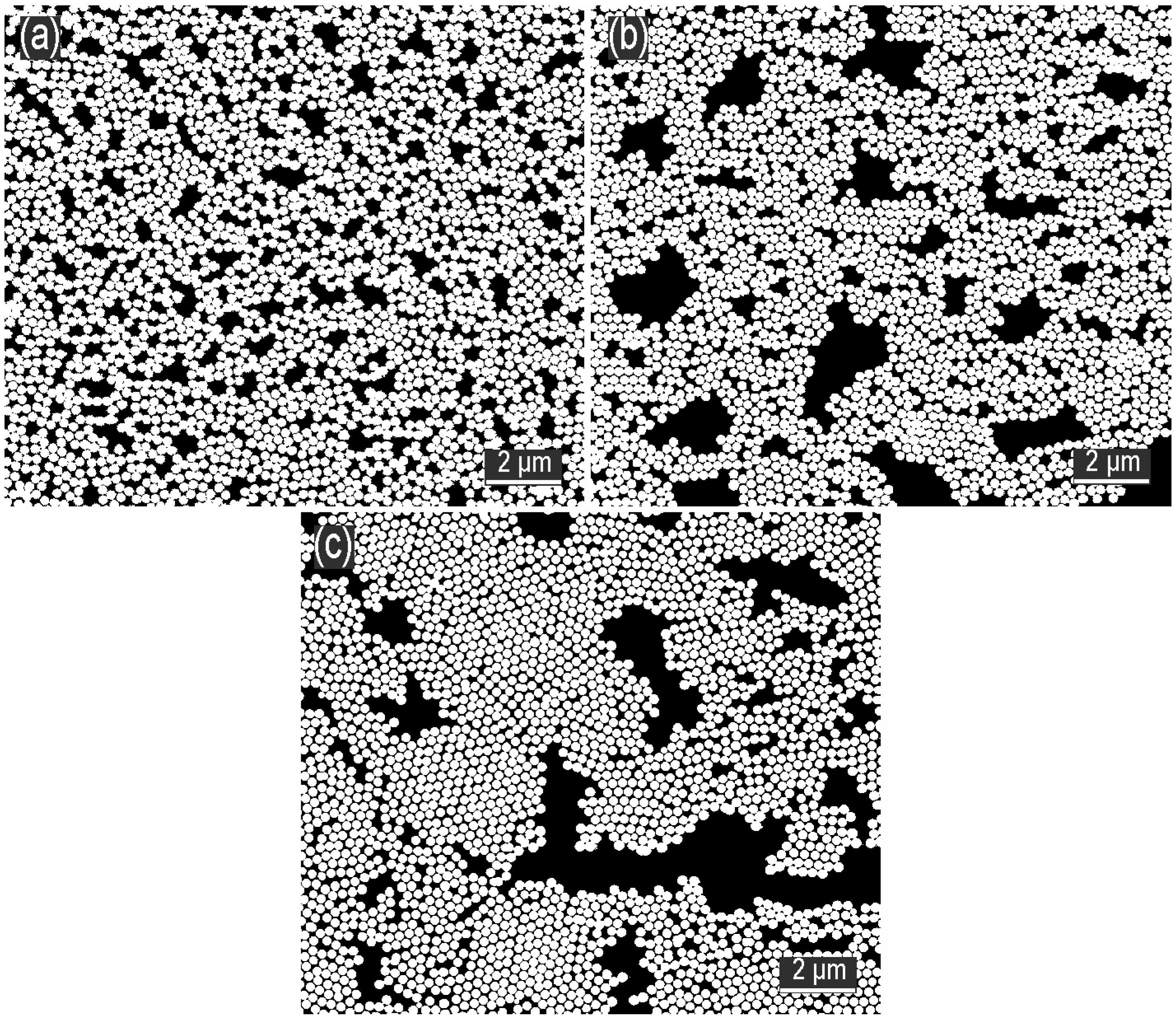
3.2. Influence of SDS Concentration in the Subphase on the Formation of the Monolayer
3.3. Influence of Ethanol Concentration in the Subphase on the Formation of the Monolayer
3.4. Relationship Between the Particle Wettability and the Formation of the Monolayer
3.5. Influence of Ethanol Concentration in the Suspension on the Formation of the Monolayer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A

Appendix B
References
- Si, S.; Liang, W.; Sun, Y.; Huang, J.; Ma, W.; Liang, Z.; Bao, Q.; Jiang, L. Facile fabrication of high-density sub-1-nm gaps from Au nanoparticle monolayers as reproducible SERS substrates. Adv. Funct. Mater. 2016, 26, 8137–8145. [Google Scholar] [CrossRef]
- Feng, D.; Weng, D.; Wang, B.; Wang, J. Laser pulse number dependent nanostructure evolution by illuminating self-assembled microsphere array. J. Appl. Phys. 2017, 122, 243102. [Google Scholar] [CrossRef]
- Armstrong, E.; Khunsin, W.; Osiak, M.; Blömker, M.; Torres, C.M.S.; O’Dwyer, C. Ordered 2D colloidal photonic crystals on gold substrates by surfactant-assisted fast-rate dip coating. Small 2014, 10, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Hansson, P.M.; Skedung, L.; Claesson, P.M.; Swerin, A.; Schoelkopf, J.; Gane, P.A.; Rutland, M.W.; Thormann, E. Robust hydrophobic surfaces displaying different surface roughness scales while maintaining the same wettability. Langmuir 2011, 27, 8153–8159. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, P.; Di, D.; Wang, C.; Wang, H.; Wang, J.; Wu, X. Controllable fabrication of 2D colloidal-crystal films with polystyrene nanospheres of various diameters by spin-coating. Appl. Surf. Sci. 2013, 270, 6–15. [Google Scholar] [CrossRef]
- Ko, H.-Y.; Lee, H.-W.; Moon, J. Fabrication of colloidal self-assembled monolayer (SAM) using monodisperse silica and its use as a lithographic mask. Thin Solid Films 2004, 447, 638–644. [Google Scholar] [CrossRef]
- Prevo, B.G.; Hon, E.W.; Velev, O.D. Assembly and characterization of colloid-based antireflective coatings on multicrystalline silicon solar cells. J. Mater. Chem. 2007, 17, 791–799. [Google Scholar] [CrossRef]
- Lotito, V.; Zambelli, T. Approaches to self-assembly of colloidal monolayers: A guide for nanotechnologists. Adv. Colloid Interface Sci. 2017, 246, 217–274. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; Goerres, S.; Landfester, K.; Weiss, C.K. A convenient method to produce close-and non-close-packed monolayers using direct assembly at the air–water interface and subsequent plasma-induced size reduction. Macromol. Chem. Phys. 2011, 212, 1719–1734. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, M. Fabrication of large scale two-dimensional colloidal crystal of polystyrene particles by an interfacial self-ordering process. J. Colloid Interface Sci. 2011, 361, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gu, B. Self-assembly of two-and three-dimensional particle arrays by manipulating the hydrophobicity of silica nanospheres. J. Phys. Chem. B 2005, 109, 22175–22180. [Google Scholar] [CrossRef] [PubMed]
- Retsch, M.; Zhou, Z.; Rivera, S.; Kappl, M.; Zhao, X.S.; Jonas, U.; Li, Q. Fabrication of large-area, transferable colloidal monolayers utilizing self-assembly at the air/water interface. Macromol. Chem. Phys. 2009, 210, 230–241. [Google Scholar] [CrossRef]
- Zhang, J.T.; Wang, L.; Lamont, D.N.; Velankar, S.S.; Asher, S.A. Fabrication of large-area two-dimensional colloidal crystals. Angew. Chem. Int. Ed. 2012, 51, 6117–6120. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hong, G.; Wang, P.; Yu, D.; Qi, L. Wet chemical approaches to patterned arrays of well-aligned ZnO nanopillars assisted by monolayer colloidal crystals. Chem. Mater. 2009, 21, 891–897. [Google Scholar] [CrossRef]
- Gao, P.; He, J.; Zhou, S.; Yang, X.; Li, S.; Sheng, J.; Wang, D.; Yu, T.; Ye, J.; Cui, Y. Large-area nanosphere self-assembly by a micro-propulsive injection method for high throughput periodic surface nanotexturing. Nano Lett. 2015, 15, 4591–4598. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yan, Q.; Shen, D. Co-self-assembly of binary colloidal crystals at the air−water interface. ACS Appl. Mater. Interfaces 2010, 2, 1922–1926. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; de Viguerie, L.; Jonas, U.; Weiss, C.K.; Landfester, K. Wafer-scale fabrication of ordered binary colloidal monolayers with adjustable stoichiometries. Adv. Funct. Mater. 2011, 21, 3064–3073. [Google Scholar] [CrossRef]
- Vogel, N.; Ally, J.; Bley, K.; Kappl, M.; Landfester, K.; Weiss, C.K. Direct visualization of the interfacial position of colloidal particles and their assemblies. Nanoscale 2014, 6, 6879–6885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comyn, J. Moisture cure of adhesives and sealants. Int. J. Adhes. Adhes. 1998, 18, 247–253. [Google Scholar] [CrossRef]
- Lotito, V.; Zambelli, T. Self-assembly of single-sized and binary colloidal particles at air/water interface by surface confinement and water discharge. Langmuir 2016, 32, 9582–9590. [Google Scholar] [CrossRef] [PubMed]
- Weekes, S.M.; Ogrin, F.Y.; Murray, W.A.; Keatley, P.S. Macroscopic arrays of magnetic nanostructures from self-assembled nanosphere templates. Langmuir 2007, 23, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-H.; Yang, Y.-M.; Maa, J.-R. Effect of artificially provoked marangoni convection at a gas/liquid interface on absorption. Ind. Eng. Chem. Res. 1996, 35, 1921–1928. [Google Scholar] [CrossRef]
- Sha, Y.; Chen, H.; Yin, Y.; Tu, S.; Ye, L.; Zheng, Y. Characteristics of the marangoni convection induced in initial quiescent water. Ind. Eng. Chem. Res. 2010, 49, 8770–8777. [Google Scholar] [CrossRef]
- Young, N.O.; Goldstein, J.S.; Block, M.J. The motion of bubbles in a vertical temperature gradient. J. Fluid Mech. 1959, 6, 350–356. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W. A review on inorganic nanostructure self-assembly. J. Nanosci. Nanotechnol. 2010, 10, 1563–1583. [Google Scholar] [CrossRef] [PubMed]
- Ahualli, S.; Iglesias, G.; Wachter, W.; Dulle, M.; Minami, D.; Glatter, O. Adsorption of anionic and cationic surfactants on anionic colloids: Supercharging and destabilization. Langmuir 2011, 27, 9182–9192. [Google Scholar] [CrossRef] [PubMed]
- Kralchevsky, P.A.; Denkov, N.D. Capillary forces and structuring in layers of colloid particles. Curr. Opin. Colloid Interface Sci. 2001, 6, 383–401. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Yoon, R.-H.; Eriksson, J.C. Surface forces in thin liquid films of n-alcohols and of water–ethanol mixtures confined between hydrophobic surfaces. J. Colloid Interface Sci. 2012, 379, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Nalaskowski, J.; Miller, J.D.; Butt, H.-J. Attraction between hydrophobic surfaces studied by atomic force microscopy. Int. J. Miner. Process. 2003, 72, 215–225. [Google Scholar] [CrossRef]
- Stavroulakis, P.I.; Christou, N.; Bagnall, D. Improved deposition of large scale ordered nanosphere monolayers via liquid surface self-assembly. Mater. Sci. Eng. B 2009, 165, 186–189. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Du, Z.-C.; Lin, W.-X.; Yang, Y.-M. Monolayer behavior of silica particles at air/water interface: A comparison between chemical and physical modifications of surface. J. Colloid Interface Sci. 2006, 296, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.W.; David, R.; Zuo, Y. Applied Surface Thermodynamics; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Zhang, R.; Somasundaran, P. Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv. Colloid Interface Sci. 2006, 123, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.; Lumsdon, S. Influence of particle wettability on the type and stability of surfactant-free emulsions. Langmuir 2000, 16, 8622–8631. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, M.A.; Binks, B.P.; Rodriguez-Valverde, M.A.; Cabrerizo-Vilchez, M.A.; Hidalgo-Alvarez, R. Particles adsorbed at various non-aqueous liquid-liquid interfaces. Adv. Colloid Interface Sci. 2017, 247, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Bresme, F.; Oettel, M. Nanoparticles at fluid interfaces. J. Phys. Condens. Matter 2007, 19, 413101. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Mi, J.; Zhong, C. Wetting behavior of spherical nanoparticles at a vapor–liquid interface: A density functional theory study. Phys. Chem. Chem. Phys. 2011, 13, 3932–3941. [Google Scholar] [CrossRef] [PubMed]
- Snoeyink, C.; Barman, S.; Christopher, G.F. Contact angle distribution of particles at fluid interfaces. Langmuir 2015, 31, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Shishido, M.; Kitagawa, D. Preparation of ordered mono-particulate film from colloidal solutions on the surface of water and continuous transcription of film to substrate. Colloids Surf. A 2007, 311, 32–41. [Google Scholar] [CrossRef]



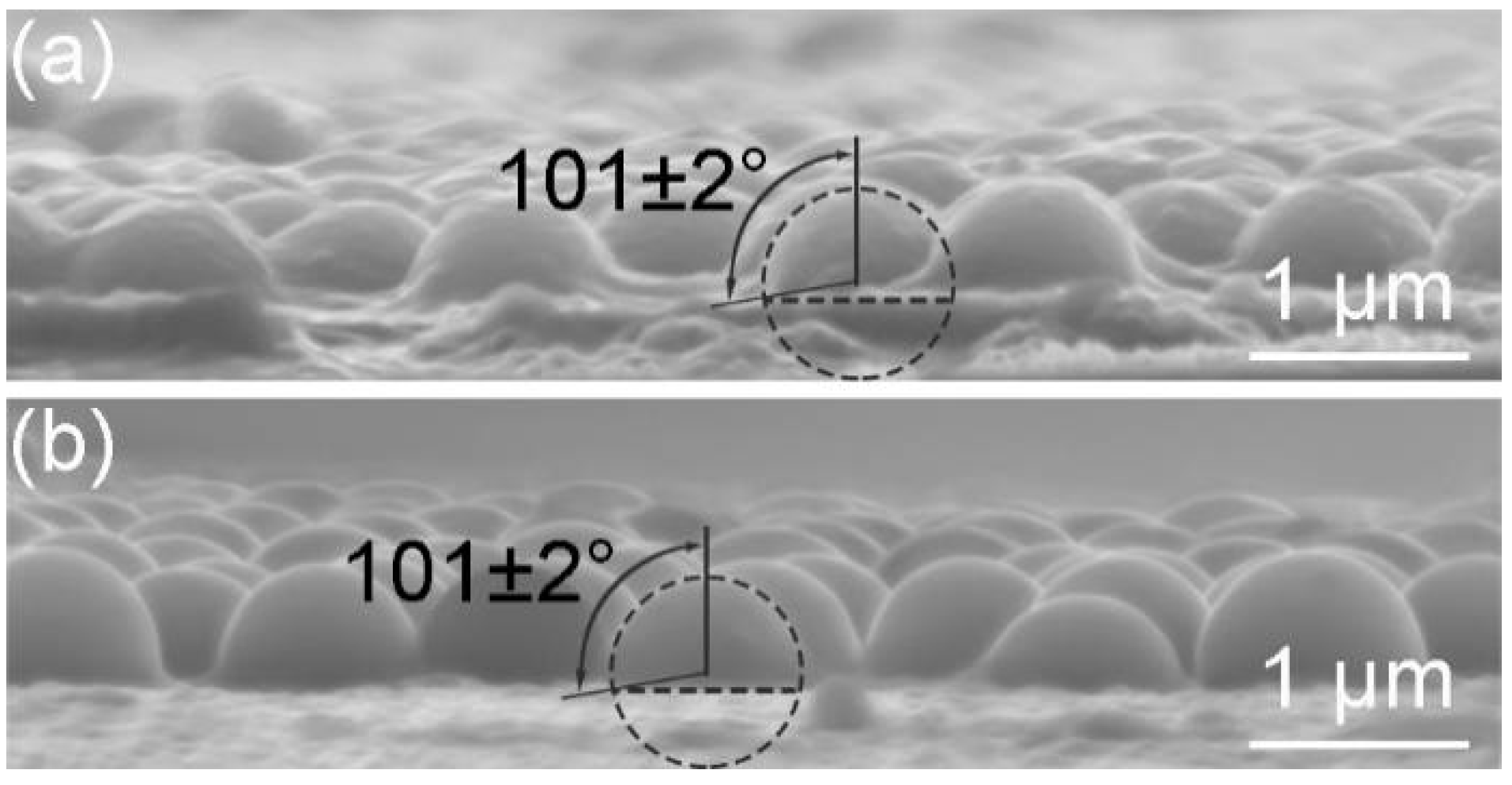










| Suphase | Surface Tension (mN/m) | Contact Angle (deg) | Detachment Energy () |
|---|---|---|---|
| deionized water | 72.98 ± 0.01 | 101 ± 2 | 160 |
| 0.1 mmol/L SDS | 67.77 ± 0.03 | 73 ± 2 | 52.4 |
| 0.4 mmol/L SDS | 60.24 ± 0.07 | 70 ± 1 | 40.3 |
| 0.7 mmol/L SDS | 48.05 ± 0.01 | 65 ± 1 | 24.8 |
| 10 vol.% ethanol | 51.19 ± 0.04 | 71 ± 1 | 36.0 |
| 20 vol.% ethanol | 40.80 ± 0.02 | 64 ± 2 | 19.9 |
| 30 vol.% ethanol | 35.30 ± 0.07 | 44 ± 2 | 4.30 |
| 40 vol.% ethanol | 31.30 ± 0.05 | 40 ± 2 | 2.65 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, D.; Weng, D.; Wang, J. A Facile Interfacial Self-Assembly of Crystalline Colloidal Monolayers by Tension Gradient. Micromachines 2018, 9, 297. https://doi.org/10.3390/mi9060297
Feng D, Weng D, Wang J. A Facile Interfacial Self-Assembly of Crystalline Colloidal Monolayers by Tension Gradient. Micromachines. 2018; 9(6):297. https://doi.org/10.3390/mi9060297
Chicago/Turabian StyleFeng, Dong, Ding Weng, and Jiadao Wang. 2018. "A Facile Interfacial Self-Assembly of Crystalline Colloidal Monolayers by Tension Gradient" Micromachines 9, no. 6: 297. https://doi.org/10.3390/mi9060297





