JNK, p38, ERK, and SGK1 Inhibitors in Cancer
Abstract
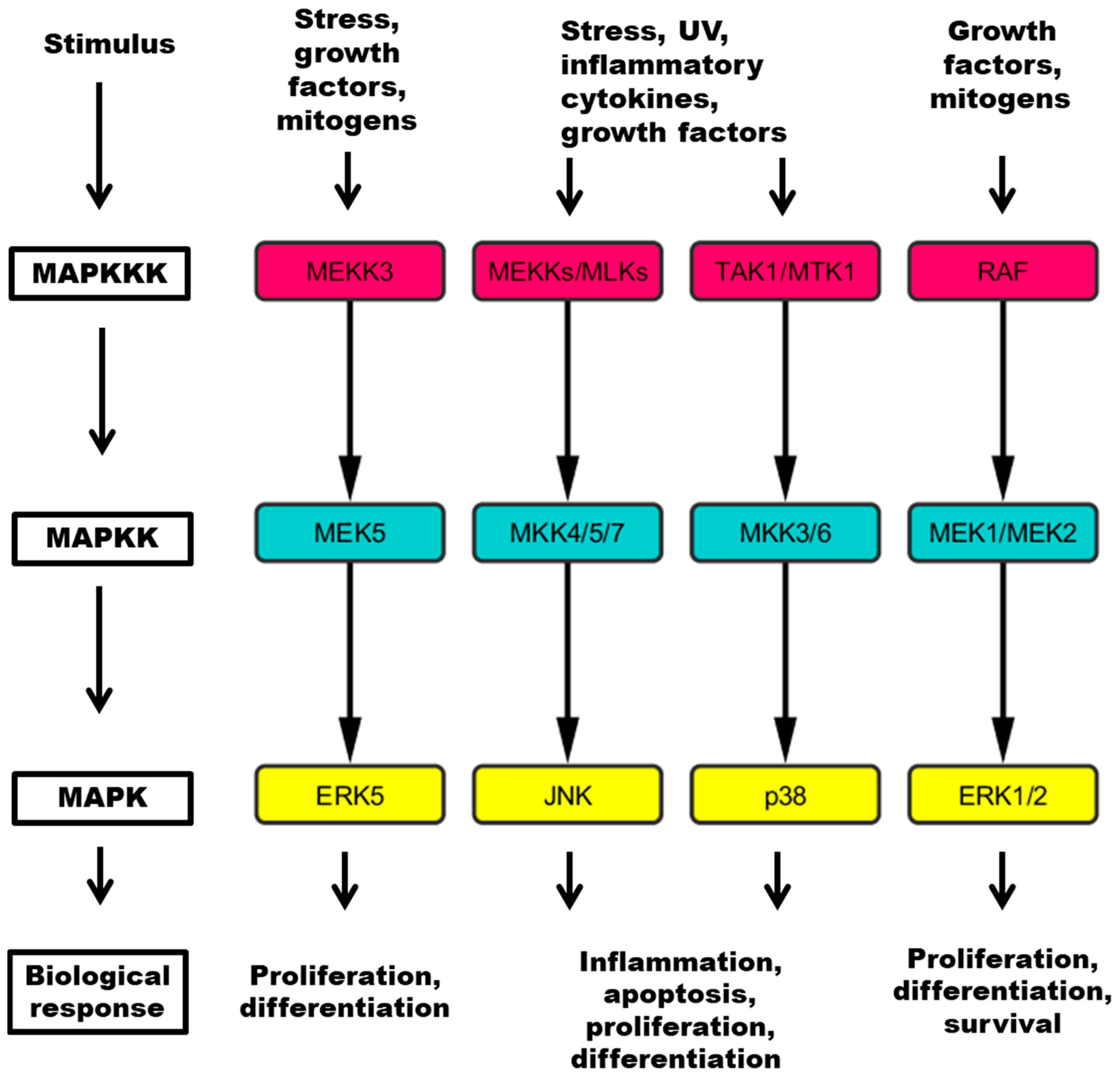
:1. Introduction
2. MAP Kinase Inhibitors in Cancer Research
3. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Cicenas, J. The potential role of Akt phosphorylation in human cancers. Int. J. Biol. Markers 2008, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Urban, P.; Vuaroqueaux, V.; Labuhn, M.; Kung, W.; Wight, E.; Mayhew, M.; Eppenberger, U.; Eppenberger-Castori, S. Increased level of phosphorylated akt measured by chemiluminescence-linked immunosorbent assay is a predictor of poor prognosis in primary breast cancer overexpressing erbb-2. Breast Cancer Res. 2005, 7, R394–R401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyndall, C.; Watt, F.; Molloy, P.L.; Vincent, P.C.; Frommer, M. Binding of proteins from embryonic and differentiated cells to a bidirectional promoter contained within a CpG island. J. Mol. Biol. 1992, 226, 289–299. [Google Scholar] [CrossRef]
- Cicenas, J.; Urban, P.; Kung, W.; Vuaroqueaux, V.; Labuhn, M.; Wight, E.; Eppenberger, U.; Eppenberger-Castori, S. Phosphorylation of tyrosine 1248-erbb2 measured by chemiluminescence-linked immunoassay is an independent predictor of poor prognosis in primary breast cancer patients. Eur. J. Cancer 2006, 42, 636–645. [Google Scholar] [CrossRef] [PubMed]
- DiGiovanna, M.P.; Stern, D.F.; Edgerton, S.M.; Whalen, S.G.; Moore, D., 2nd; Thor, A.D. Relationship of epidermal growth factor receptor expression to erbb-2 signaling activity and prognosis in breast cancer patients. J. Clin. Oncol. 2005, 23, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Milde-Langosch, K.; Bamberger, A.M.; Rieck, G.; Grund, D.; Hemminger, G.; Muller, V.; Loning, T. Expression and prognostic relevance of activated extracellular-regulated kinases (erk1/2) in breast cancer. Br. J. Cancer 2005, 92, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, J.; Elmberger, G.; Ohd, J.; Linderholm, B.; Bjohle, J.; Hellborg, H.; Nordgren, H.; Borg, A.L.; Skoog, L.; Bergh, J. Activated erk1/2 and phosphorylated oestrogen receptor alpha are associated with improved breast cancer survival in women treated with tamoxifen. Eur. J. Cancer 2006, 42, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.J.; Wan, X.B.; Fu, X.H.; Wu, P.H.; Chen, D.K.; Wang, P.N.; Jiang, L.; Wang, D.H.; Chen, Z.T.; Huang, Y.; et al. Phosphorylated p38, a negative prognostic biomarker, complements TNM staging prognostication in colorectal cancer. Tumour Biol. 2014, 35, 10487–10495. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Kung, W.; Eppenberger, U.; Eppenberger-Castori, S. Increased level of phosphorylated ShcA measured by chemiluminescence-linked immunoassay is a predictor of good prognosis in primary breast cancer expressing low levels of estrogen receptor. Cancers 2010, 2, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [PubMed]
- Campbell, J.S.; Seger, R.; Graves, J.D.; Graves, L.M.; Jensen, A.M.; Krebs, E.G. The map kinase cascade. Recent Prog. Horm. Res. 1995, 50, 131–159. [Google Scholar] [PubMed]
- Cicenas, J.; Tamosaitis, L.; Kvederaviciute, K.; Tarvydas, R.; Staniute, G.; Kalyan, K.; Meskinyte-Kausiliene, E.; Stankevicius, V.; Valius, M. KRAS, NRAS and BRAF mutations in colorectal cancer and melanoma. Med. Oncol. 2017, 34, 26. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Kvederaviciute, K.; Meskinyte, I.; Meskinyte-Kausiliene, E.; Skeberdyte, A.; Cicenas, J. KRAS, TP53, CDKN2A, SMAD4, BRCA1, and BRCA2 mutations in pancreatic cancer. Cancers 2017, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Derijard, B.; Hibi, M.; Wu, I.H.; Barrett, T.; Su, B.; Deng, T.; Karin, M.; Davis, R.J. Jnk1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 1994, 76, 1025–1037. [Google Scholar] [CrossRef]
- Zarubin, T.; Han, J. Activation and signaling of the p38 map kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.A.; Burow, M.E.; Beckman, B.S. MEK5/ERK5 pathway: The first fifteen years. Biochim. Biophys. Acta 2012, 1825, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Gharwan, H.; Groninger, H. Kinase inhibitors and monoclonal antibodies in oncology: Clinical implications. Nat. Rev. Clin. Oncol. 2016, 13, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Valius, M. The cdk inhibitors in cancer research and therapy. J. Cancer Res. Clin. Oncol. 2011, 137, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Kalyan, K.; Sorokinas, A.; Jatulyte, A.; Valiunas, D.; Kaupinis, A.; Valius, M. Highlights of the latest advances in research on CDK inhibitors. Cancers 2014, 6, 2224–2242. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Kalyan, K.; Sorokinas, A.; Stankunas, E.; Levy, J.; Meskinyte, I.; Stankevicius, V.; Kaupinis, A.; Valius, M. Roscovitine in cancer and other diseases. Ann. Transl. Med. 2015, 3, 135. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J. The aurora kinase inhibitors in cancer research and therapy. J. Cancer Res. Clin. Oncol. 2016, 142, 1995–2012. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Cicenas, E. Multi-kinase inhibitors, AURKs and cancer. Med. Oncol. 2016, 33, 43. [Google Scholar] [CrossRef] [PubMed]
- Grassi, E.S.; Vezzoli, V.; Negri, I.; Labadi, A.; Fugazzola, L.; Vitale, G.; Persani, L. Sp600125 has a remarkable anticancer potential against undifferentiated thyroid cancer through selective action on rock and p53 pathways. Oncotarget 2015, 6, 36383–36399. [Google Scholar] [PubMed]
- Kim, J.H.; Kim, T.H.; Kang, H.S.; Ro, J.; Kim, H.S.; Yoon, S. Sp600125, an inhibitor of Jnk pathway, reduces viability of relatively resistant cancer cells to doxorubicin. Biochem. Biophys. Res. Commun. 2009, 387, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chae, M.; Choi, A.R.; Sik Kim, H.; Yoon, S. Sp600125 overcomes antimitotic drug-resistance in cancer cells by increasing apoptosis with independence of P-gp inhibition. Eur. J. Pharmacol. 2014, 723, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Chen, T.S.; Wang, X.P.; Qu, J.L.; Chen, M. The JNK inhibitor sp600125 enhances dihydroartemisinin-induced apoptosis by accelerating Bax translocation into mitochondria in human lung adenocarcinoma cells. FEBS Lett. 2010, 584, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, B.; Liang, H.; Lu, Y.; Ai, X.; Zhang, B.; Chen, X. JNK inhibitor SP600125 enhances TGF-beta-induced apoptosis of RBE human cholangiocarcinoma cells in a sSmad-dependent manner. Mol. Med. Rep. 2013, 8, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Jemaa, M.; Vitale, I.; Kepp, O.; Berardinelli, F.; Galluzzi, L.; Senovilla, L.; Marino, G.; Malik, S.A.; Rello-Varona, S.; Lissa, D.; et al. Selective killing of p53-deficient cancer cells by sp600125. EMBO Mol. Med. 2012, 4, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Ninomiya, T.; Kohno, T.; Kikuchi, S.; Sawada, N.; Kojima, T. c-Jun n-terminal kinase inhibitor sp600125 enhances barrier function and elongation of human pancreatic cancer cell line HPAC in a Ca-switch model. Histochem. Cell Biol. 2015, 143, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Huang, J.Y.; Xing, B.; Ren, K.W.; Li, M.; Wei, D.; Gu, P.Y.; Chen, G.; Gu, B.; Zhang, G.F.; et al. Sp600125, a JNK inhibitor, suppresses growth of JNK-inactive glioblastoma cells through cell-cycle g2/m phase arrest. Die Pharm. 2012, 67, 942–946. [Google Scholar] [PubMed]
- Wu, H.M.; Fang, L.; Shen, Q.Y.; Liu, R.Y. Sp600125 promotes resolution of allergic airway inflammation via tlr9 in an ova-induced murine acute asthma model. Mol. Immunol. 2015, 67, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Altavilla, D.; Marini, H.; Passaniti, M.; Bitto, A.; Seminara, P.; Venuti, F.S.; Famulari, C.; Macri, A.; Versaci, A.; et al. Protective effects of sp600125 a new inhibitor of c-Jun n-terminal kinase (JNK) and extracellular-regulated kinase (erk1/2) in an experimental model of cerulein-induced pancreatitis. Life Sci. 2004, 75, 2853–2866. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Maruthi, M.; Babu, P.P. The specific, reversible JNK inhibitor sp600125 improves survivability and attenuates neuronal cell death in experimental cerebral malaria (ECM). Parasitol. Res. 2013, 112, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, L.; Xie, Y.; Ma, C.; Li, W.; Su, X.; Huang, S.; Chen, R.; Zhu, Z.; Mao, Z.; et al. Sp600125, a new JNK inhibitor, protects dopaminergic neurons in the MPTP model of parkinson’s disease. Neurosci. Res. 2004, 48, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Cerbone, A.; Toaldo, C.; Pizzimenti, S.; Pettazzoni, P.; Dianzani, C.; Minelli, R.; Ciamporcero, E.; Roma, G.; Dianzani, M.U.; Canaparo, R.; et al. As601245, an anti-inflammatory jnk inhibitor, and clofibrate have a synergistic effect in inducing cell responses and in affecting the gene expression profile in caco-2 colon cancer cells. PPAR Res. 2012, 2012, 269751. [Google Scholar] [CrossRef] [PubMed]
- Cerbone, A.; Toaldo, C.; Minelli, R.; Ciamporcero, E.; Pizzimenti, S.; Pettazzoni, P.; Roma, G.; Dianzani, M.U.; Ullio, C.; Ferretti, C.; et al. Rosiglitazone and as601245 decrease cell adhesion and migration through modulation of specific gene expression in human colon cancer cells. PLoS ONE 2012, 7, e40149. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, Q.; Wang, J.; Lv, M.; Zhu, N.; Li, Y.; Feng, J.; Shen, B.; Zhang, J. Basal c-jun nh2-terminal protein kinase activity is essential for survival and proliferation of t-cell acute lymphoblastic leukemia cells. Mol. Cancer Ther. 2009, 8, 3214–3222. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.M.; Fernandez, C.; Amran, D.; Esteban, D.; de Blas, E.; Palacios, M.A.; Aller, P. Pharmacologic inhibitors of extracellular signal-regulated kinase (ERKs) and c-Jun NH(2)-terminal kinase (JNK) decrease glutathione content and sensitize human promonocytic leukemia cells to arsenic trioxide-induced apoptosis. J. Cell. Physiol. 2006, 209, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, P.; Jeanclaude-Etter, I.; Ardissone, V.; Arkinstall, S.; Cambet, Y.; Camps, M.; Chabert, C.; Church, D.; Cirillo, R.; Gretener, D.; et al. Design and synthesis of the first generation of novel potent, selective, and in vivo active (benzothiazol-2-yl)acetonitrile inhibitors of the c-Jun n-terminal kinase. J. Med. Chem. 2005, 48, 4596–4607. [Google Scholar] [CrossRef] [PubMed]
- Wolschendorf, F.; Bosque, A.; Shishido, T.; Duverger, A.; Jones, J.; Planelles, V.; Kutsch, O. Kinase control prevents hiv-1 reactivation in spite of high levels of induced NF-κB activity. J. Virol. 2012, 86, 4548–4558. [Google Scholar] [CrossRef] [PubMed]
- Nacken, W.; Ehrhardt, C.; Ludwig, S. Small molecule inhibitors of the c-Jun n-terminal kinase (JNK) possess antiviral activity against highly pathogenic avian and human pandemic influenza a viruses. Biol. Chem. 2012, 393, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Vasilevskaya, I.A.; Selvakumaran, M.; Hierro, L.C.; Goldstein, S.R.; Winkler, J.D.; O’Dwyer, P.J. Inhibition of JNK sensitizes hypoxic colon cancer cells to DNA-damaging agents. Clin. Cancer Res. 2015, 21, 4143–4152. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.Y.; Flanc, R.S.; Tesch, G.H.; Bennett, B.L.; Friedman, G.C.; Nikolic-Paterson, D.J. Blockade of the c-Jun amino terminal kinase prevents crescent formation and halts established anti-GBM glomerulonephritis in the rat. Lab. Investig. 2009, 89, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Flanc, R.S.; Ma, F.Y.; Tesch, G.H.; Han, Y.; Atkins, R.C.; Bennett, B.L.; Friedman, G.C.; Fan, J.H.; Nikolic-Paterson, D.J. A pathogenic role for JNK signaling in experimental anti-GBM glomerulonephritis. Kidney Int. 2007, 72, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Bennett, B.; Sakata, S.T.; Satoh, Y.; Bilter, G.K.; Westwick, J.K.; Brenner, D.A. Jnk mediates hepatic ischemia reperfusion injury. J. Hepatol. 2005, 42, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Kanellis, J.; Ma, F.Y.; Kandane-Rathnayake, R.; Dowling, J.P.; Polkinghorne, K.R.; Bennett, B.L.; Friedman, G.C.; Nikolic-Paterson, D.J. Jnk signalling in human and experimental renal ischaemia/reperfusion injury. Nephrol. Dial. Transplant. 2010, 25, 2898–2908. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kuramoto, K.; Takeda, H.; Watarai, H.; Sakaki, H.; Seino, S.; Seino, M.; Suzuki, S.; Kitanaka, C. The novel jnk inhibitor as602801 inhibits cancer stem cells in vitro and in vivo. Oncotarget 2016, 7, 27021–27032. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Cheng, J.K.; Zeng, Q.; Xu, Z.Z.; Decosterd, I.; Xu, X.; Ji, R.R. Selective inhibition of jnk with a peptide inhibitor attenuates pain hypersensitivity and tumor growth in a mouse skin cancer pain model. Exp. Neurol. 2009, 219, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Posthumadeboer, J.; van Egmond, P.W.; Helder, M.N.; de Menezes, R.X.; Cleton-Jansen, A.M.; Belien, J.A.; Verheul, H.M.; van Royen, B.J.; Kaspers, G.J.; van Beusechem, V.W. Targeting jnk-interacting-protein-1 (jip1) sensitises osteosarcoma to doxorubicin. Oncotarget 2012, 3, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Podar, K.; Chauhan, D.; Ishitsuka, K.; Mitsiades, C.; Tai, Y.T.; Hamasaki, M.; Raje, N.; Hideshima, H.; Schreiner, G.; et al. P38 MAPK inhibition enhances PS-341 (bortezomib)-induced cytotoxicity against multiple myeloma cells. Oncogene 2004, 23, 8766–8776. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.N.; Stebbins, E.G.; Henson, M.; O’Young, G.; Choi, S.J.; Quon, D.; Damm, D.; Reddy, M.; Ma, J.Y.; Haghnazari, E.; et al. Normalizing the bone marrow microenvironment with p38 inhibitor reduces multiple myeloma cell proliferation and adhesion and suppresses osteoclast formation. Exp. Cell Res. 2006, 312, 1909–1923. [Google Scholar] [CrossRef] [PubMed]
- Navas, T.A.; Nguyen, A.N.; Hideshima, T.; Reddy, M.; Ma, J.Y.; Haghnazari, E.; Henson, M.; Stebbins, E.G.; Kerr, I.; O’Young, G.; et al. Inhibition of p38alpha MAPK enhances proteasome inhibitor-induced apoptosis of myeloma cells by modulating hsp27, Bcl-X(L), Mcl-1 and p53 levels in vitro and inhibits tumor growth in vivo. Leukemia 2006, 20, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Vanderkerken, K.; Medicherla, S.; Coulton, L.; De Raeve, H.; Willems, A.; Lawson, M.; Van Camp, B.; Protter, A.A.; Higgins, L.S.; Menu, E.; et al. Inhibition of p38alpha mitogen-activated protein kinase prevents the development of osteolytic bone disease, reduces tumor burden, and increases survival in murine models of multiple myeloma. Cancer Res. 2007, 67, 4572–4577. [Google Scholar] [CrossRef] [PubMed]
- Medicherla, S.; Reddy, M.; Ying, J.; Navas, T.A.; Li, L.; Nguyen, A.N.; Kerr, I.; Hanjarappa, N.; Protter, A.A.; Higgins, L.S. P38alpha-selective map kinase inhibitor reduces tumor growth in mouse xenograft models of multiple myeloma. Anticancer Res. 2008, 28, 3827–3833. [Google Scholar] [PubMed]
- Giafis, N.; Katsoulidis, E.; Sassano, A.; Tallman, M.S.; Higgins, L.S.; Nebreda, A.R.; Davis, R.J.; Platanias, L.C. Role of the p38 mitogen-activated protein kinase pathway in the generation of arsenic trioxide-dependent cellular responses. Cancer Res. 2006, 66, 6763–6771. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Cohen, S.B.; Wofsy, D.; Weinblatt, M.E.; Firestein, G.S.; Brahn, E.; Strand, V.; Baker, D.G.; Tong, S.E. A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral scio-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. J. Rheumatol. 2011, 38, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Sokol, L.; Cripe, L.; Kantarjian, H.; Sekeres, M.A.; Parmar, S.; Greenberg, P.; Goldberg, S.L.; Bhushan, V.; Shammo, J.; Hohl, R.; et al. Randomized, dose-escalation study of the p38alpha mapk inhibitor scio-469 in patients with myelodysplastic syndrome. Leukemia 2013, 27, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.E.; Daniels, S.E.; Black, P.; Chang, S.; Protter, A.; Desjardins, P.J. Novel p38alpha mitogen-activated protein kinase inhibitor shows analgesic efficacy in acute postsurgical dental pain. J. Clin. Pharmacol. 2012, 52, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Hideshima, T.; Ikeda, H.; Jin, J.; Ocio, E.M.; Kiziltepe, T.; Okawa, Y.; Vallet, S.; Podar, K.; Ishitsuka, K.; et al. Birb 796 enhances cytotoxicity triggered by bortezomib, heat shock protein (Hsp) 90 inhibitor, and dexamethasone via inhibition of p38 mitogen-activated protein kinase/hsp27 pathway in multiple myeloma cell lines and inhibits paracrine tumour growth. Br. J. Haematol. 2007, 136, 414–423. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhao, X.Q.; Chen, X.G.; Fang, Y.; Singh, S.; Talele, T.T.; Qiu, H.J.; Liang, Y.J.; Wang, X.K.; Zhang, G.Q.; et al. Birb796, the inhibitor of p38 mitogen-activated protein kinase, enhances the efficacy of chemotherapeutic agents in abcb1 overexpression cells. PLoS ONE 2013, 8, e54181. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Mo, Q.; Zhang, Y.; Gao, Y.; Wu, Y.; Li, J.; Hao, X.; Ma, D.; Gao, Q.; Chen, P. The p38 MAPK inhibitor birb796 enhances the antitumor effects of vx680 in cervical cancer. Cancer Biol. Ther. 2016, 17, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.; Choi, J.; Kim, J.; Bae, S.; Hong, J.; Jo, S.; Kim, S.; Lee, Y. Birb 796 has distinctive anti-inflammatory effects on different cell types. Immune Netw. 2013, 13, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Feagan, B.; D’Haens, G.; Colombel, J.F.; Geboes, K.; Yurcov, M.; Isakov, V.; Golovenko, O.; Bernstein, C.N.; Ludwig, D.; et al. Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active crohn’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006, 4, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, K.; Hideshima, T.; Neri, P.; Vallet, S.; Shiraishi, N.; Okawa, Y.; Shen, Z.; Raje, N.; Kiziltepe, T.; Ocio, E.M.; et al. P38 mitogen-activated protein kinase inhibitor ly2228820 enhances bortezomib-induced cytotoxicity and inhibits osteoclastogenesis in multiple myeloma; therapeutic implications. Br. J. Haematol. 2008, 141, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.M.; Anderson, B.D.; Brooks, N.A.; Brooks, H.B.; Chan, E.M.; De Dios, A.; Gilmour, R.; Graff, J.R.; Jambrina, E.; Mader, M.; et al. Characterization of ly2228820 dimesylate, a potent and selective inhibitor of p38 MAPK with antitumor activity. Mol. Cancer Ther. 2014, 13, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, T.; Wang, G.; Wang, H.; Che, X.; Gao, X.; Liu, H. Rac3 regulates cell invasion, migration and EMT in lung adenocarcinoma through p38 MAPK pathway. J. Cancer 2017, 8, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Haluska, P.; Tolcher, A.W.; Erlichman, C.; Papadopoulos, K.P.; Lensing, J.L.; Beeram, M.; Molina, J.R.; Rasco, D.W.; Arcos, R.R.; et al. A first-in-human phase i study of the oral p38 mapk inhibitor, ralimetinib (ly2228820 dimesylate), in patients with advanced cancer. Clin. Cancer Res. 2016, 22, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- A Study of LY2228820 for Recurrent Ovarian Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01663857 (accessed on 8 September 2017).
- Hideshima, T.; Akiyama, M.; Hayashi, T.; Richardson, P.; Schlossman, R.; Chauhan, D.; Anderson, K.C. Targeting p38 MAPK inhibits multiple myeloma cell growth in the bone marrow milieu. Blood 2003, 101, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Igea, A.; Papaioannou, M.; Lopez-Casas, P.P.; Llonch, E.; Hidalgo, M.; Gorgoulis, V.G.; Nebreda, A.R. Pharmacological inhibition of p38 MAPK reduces tumor growth in patient-derived xenografts from colon tumors. Oncotarget 2015, 6, 8539–8551. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kanno, T.; Fujita, Y.; Gotoh, A.; Nakano, T.; Nishizaki, T. Mesothelioma cell proliferation through autocrine activation of pdgf-betabeta receptor. Cell. Physiol. Biochem. 2012, 29, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, M.; Statello, L.; Maugeri, M.; Majorana, A.; Barbagallo, D.; Salito, L.; Sammito, M.; Santonocito, M.; Angelica, R.; Cavallaro, A.; et al. Specific alterations of the microRNA transcriptome and global network structure in colorectal cancer after treatment with MAPK/ERK inhibitors. J. Mol. Med. 2012, 90, 1421–1438. [Google Scholar] [CrossRef] [PubMed]
- Saglam, A.S.; Alp, E.; Elmazoglu, Z.; Menevse, E.S. Effect of api-1 and fr180204 on cell proliferation and apoptosis in human dld-1 and lovo colorectal cancer cells. Oncol. Lett. 2016, 12, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Saotome, T.; Usui, T.; Ohama, T.; Sato, K. Regulation of intestinal myofibroblasts by KRas-mutated colorectal cancer cells through heparin-binding epidermal growth factor-like growth factor. Oncol. Rep. 2017, 37, 3128–3136. [Google Scholar] [CrossRef] [PubMed]
- Ohori, M.; Takeuchi, M.; Maruki, R.; Nakajima, H.; Miyake, H. Fr180204, a novel and selective inhibitor of extracellular signal-regulated kinase, ameliorates collagen-induced arthritis in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 374, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Deng, X.; Lu, B.; Cameron, M.; Fearns, C.; Patricelli, M.P.; Yates, J.R., 3rd; Gray, N.S.; Lee, J.D. Pharmacological inhibition of bmk1 suppresses tumor growth through promyelocytic leukemia protein. Cancer Cell 2010, 18, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pesakhov, S.; Weng, A.; Kafka, M.; Gocek, E.; Nguyen, M.; Harrison, J.S.; Danilenko, M.; Studzinski, G.P. ERK 5/mapk pathway has a major role in 1alpha,25-(oh)2 vitamin d3-induced terminal differentiation of myeloid leukemia cells. J. Steroid Biochem. Mol. Biol. 2014, 144, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Sureban, S.M.; May, R.; Weygant, N.; Qu, D.; Chandrakesan, P.; Bannerman-Menson, E.; Ali, N.; Pantazis, P.; Westphalen, C.B.; Wang, T.C.; et al. Xmd8-92 inhibits pancreatic tumor xenograft growth via a dclk1-dependent mechanism. Cancer Lett. 2014, 351, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Rovida, E.; Di Maira, G.; Tusa, I.; Cannito, S.; Paternostro, C.; Navari, N.; Vivoli, E.; Deng, X.; Gray, N.S.; Esparis-Ogando, A.; et al. The mitogen-activated protein kinase erk5 regulates the development and growth of hepatocellular carcinoma. Gut 2015, 64, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Simoes, A.E.; Gomes, S.E.; Castro, R.E.; Carvalho, T.; Rodrigues, C.M.; Borralho, P.M. Mek5/erk5 signaling inhibition increases colon cancer cell sensitivity to 5-fluorouracil through a p53-dependent mechanism. Oncotarget 2016, 7, 34322–34340. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, L.; Xu, Q.; Wang, K.; Xie, D.; Yu, Z.; Jiang, K.; Liao, L.; Yates, J.R.; Lee, J.D.; et al. Targeting bmk1 impairs the drug resistance to combined inhibition of BRAF and MEK1/2 in melanoma. Sci. Rep. 2017, 7, 46244. [Google Scholar] [CrossRef] [PubMed]
- Conza, D.; Mirra, P.; Cali, G.; Tortora, T.; Insabato, L.; Fiory, F.; Schenone, S.; Amato, R.; Beguinot, F.; Perrotti, N.; et al. The sgk1 inhibitor si113 induces autophagy, apoptosis, and endoplasmic reticulum stress in endometrial cancer cells. J. Cell. Physiol. 2017, 232, 3735–3743. [Google Scholar] [CrossRef] [PubMed]
- Talarico, C.; Dattilo, V.; D’Antona, L.; Barone, A.; Amodio, N.; Belviso, S.; Musumeci, F.; Abbruzzese, C.; Bianco, C.; Trapasso, F.; et al. Si113, a sgk1 inhibitor, potentiates the effects of radiotherapy, modulates the response to oxidative stress and induces cytotoxic autophagy in human glioblastoma multiforme cells. Oncotarget 2016, 7, 15868–15884. [Google Scholar] [CrossRef] [PubMed]
- Talarico, C.; D’Antona, L.; Scumaci, D.; Barone, A.; Gigliotti, F.; Fiumara, C.V.; Dattilo, V.; Gallo, E.; Visca, P.; Ortuso, F.; et al. Preclinical model in HCC: The sgk1 kinase inhibitor si113 blocks tumor progression in vitro and in vivo and synergizes with radiotherapy. Oncotarget 2015, 6, 37511–37525. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, S.D.; Stines Nahreini, T.; Aigner, S.; Ahn, N.G.; Uhlenbeck, O.C. Rna aptamers as pathway-specific map kinase inhibitors. Chem. Biol. 2000, 7, 833–843. [Google Scholar] [CrossRef]
- Suckfuell, M.; Lisowska, G.; Domka, W.; Kabacinska, A.; Morawski, K.; Bodlaj, R.; Klimak, P.; Kostrica, R.; Meyer, T. Efficacy and safety of am-111 in the treatment of acute sensorineural hearing loss: A double-blind, randomized, placebo-controlled phase ii study. Otol. Neurotol. 2014, 35, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.P.; Bastikar, V.A.; Kuciauskas, D.; Kothari, S.L.; Cicenas, J.; Valius, M. Molecular modeling and structure-based drug discovery approach reveals protein kinases as off-targets for novel anticancer drug rh1. Med. Oncol. 2017, 34, 176. [Google Scholar] [CrossRef] [PubMed]
- Duchowicz, P.R.; Castro, E.A. Qsar studies for the pharmacological inhibition of glycogen synthase kinase-3. Med. Chem. 2007, 3, 393–417. [Google Scholar] [CrossRef] [PubMed]
- Selness, S.R.; Devraj, R.V.; Devadas, B.; Walker, J.K.; Boehm, T.L.; Durley, R.C.; Shieh, H.; Xing, L.; Rucker, P.V.; Jerome, K.D.; et al. Discovery of ph-797804, a highly selective and potent inhibitor of p38 map kinase. Bioorg. Med. Chem. Lett. 2011, 21, 4066–4071. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Iqbal, S.; Hernandez, P.; Park, H.; LoGrasso, P.V.; Feng, Y. Design and synthesis of highly potent and isoform selective jnk3 inhibitors: SAR studies on aminopyrazole derivatives. J. Med. Chem. 2014, 57, 10013–10030. [Google Scholar] [CrossRef] [PubMed]
- Ortuso, F.; Amato, R.; Artese, A.; D’Antona, L.; Costa, G.; Talarico, C.; Gigliotti, F.; Bianco, C.; Trapasso, F.; Schenone, S.; et al. In silico identification and biological evaluation of novel selective serum/glucocorticoid-inducible kinase 1 inhibitors based on the pyrazolo-pyrimidine scaffold. J. Chem. Inf. Model. 2014, 54, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kaushik, G.; Subramaniam, D.; Dandawate, P.; Neville, K.; Chastain, K.; Anant, S. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J. Hematol. Oncol. 2017, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Xie, H.; Yang, F.; Shan, Q.; Dai, H.; Zhuo, J.; Wei, X.; Song, P.; Zhou, L.; Xu, X.; et al. Metformin potentiates the effect of arsenic trioxide suppressing intrahepatic cholangiocarcinoma: Roles of p38 MAPK, erk3, and mtorc1. J. Hematol. Oncol. 2017, 10, 59. [Google Scholar] [CrossRef] [PubMed]
| Ihibitor | Target | Potential Usages in Cancer |
|---|---|---|
| SP600125 | JNK | stomach cancer [24], oral squamous carcinoma [25], lung adenocarcinoma [26], cholangiocarcinoma [27], colon carcinoma [28], pancreatic cancer [29], glioblastoma [30] |
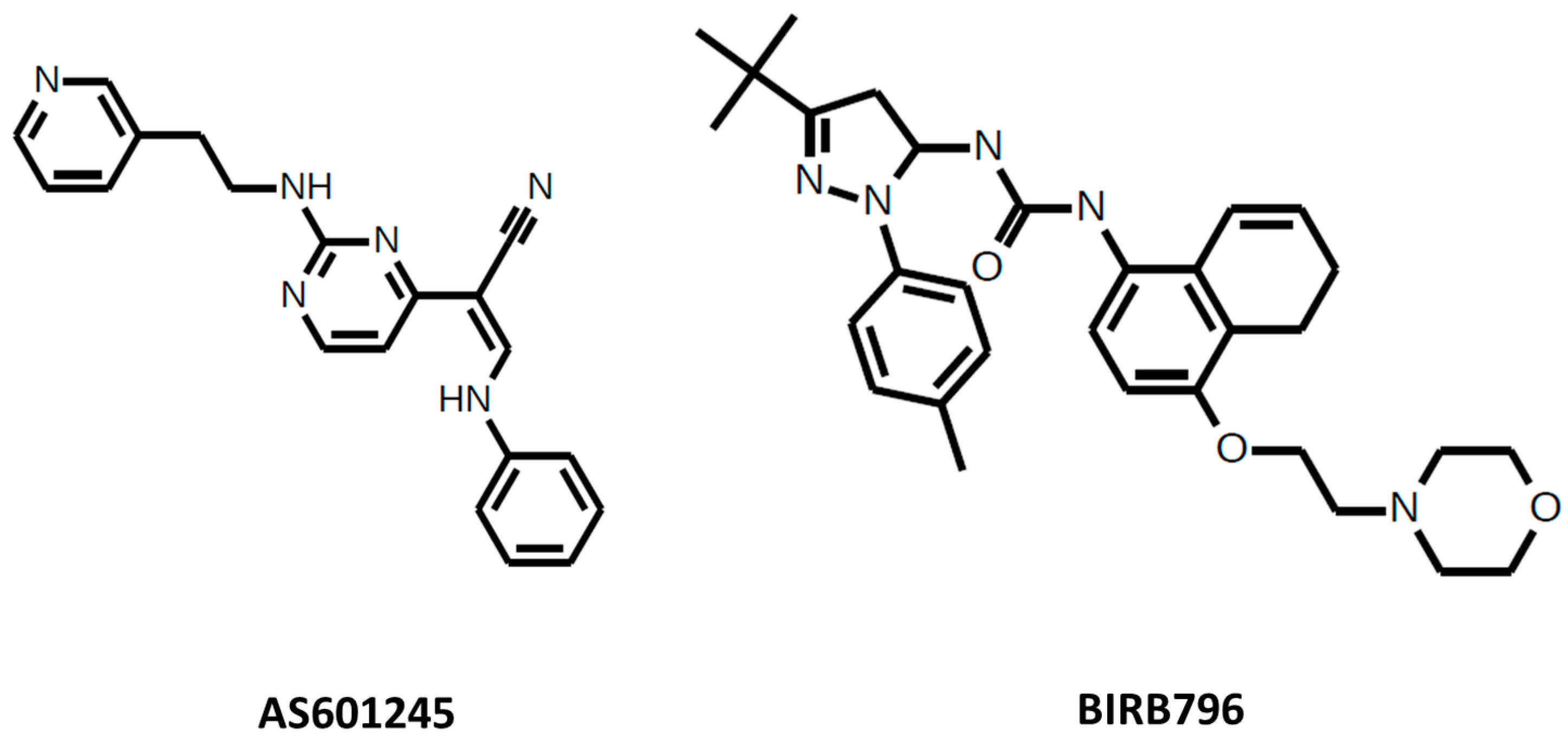
| AS601245 | JNK | colon cancer [35], leukemia [37,38] |
| CC-401 | JNK | colon cancer [42] |
| SCIO-469 | p38 | multiple myeloma [50], leukemia [55] |
| BIRB-796 | p38 | multiple myeloma [59], oral epidermoid carcinoma [60], cervical cancer [61] |
| LY2228820 | p38 | melanoma, non-small cell lung cancer, ovarian cancer, glioma, myeloma, breast cancer [65], lung adenocarcinoma [66], phase I clinical trial in colorectal, breast, sarcoma, NSCLC, renal, pancreatic, melanoma and ovarian [67], phase I/II trial [68] |
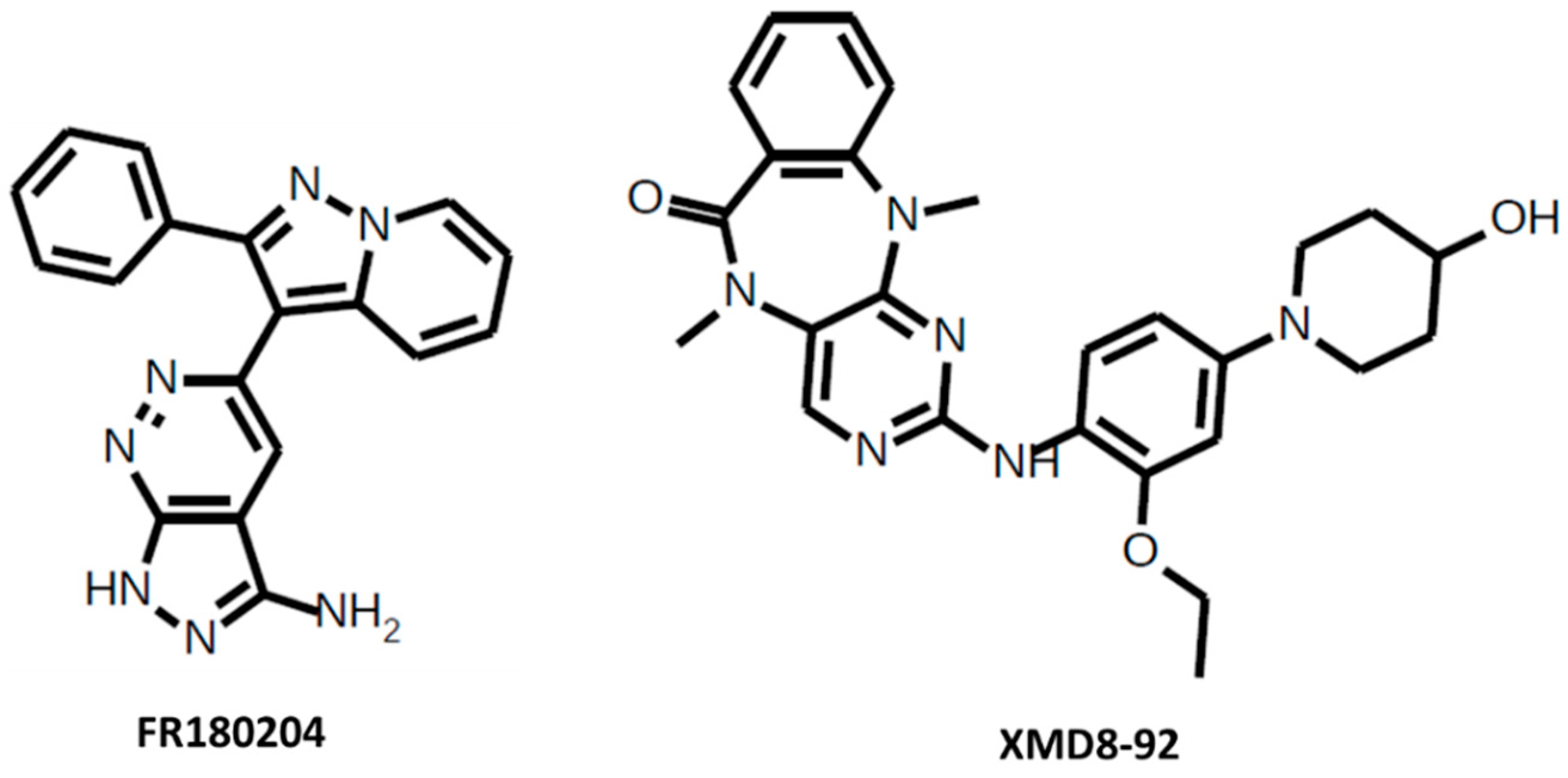
| FR180204 | ERK1/2 | colorectal cancer [72] |
| XMD8-92 | ERK5 | lung cancer, cervical cancer [76], acute myeloid leukemia [77], pancreatic cancer [78], hepatocellular carcinoma [79], colon cancer [80] |
| SI113 | SGK1 | endometrial cancer [82], glioblastoma [83], hepatocellular carcinoma [84] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicenas, J.; Zalyte, E.; Rimkus, A.; Dapkus, D.; Noreika, R.; Urbonavicius, S. JNK, p38, ERK, and SGK1 Inhibitors in Cancer. Cancers 2018, 10, 1. https://doi.org/10.3390/cancers10010001
Cicenas J, Zalyte E, Rimkus A, Dapkus D, Noreika R, Urbonavicius S. JNK, p38, ERK, and SGK1 Inhibitors in Cancer. Cancers. 2018; 10(1):1. https://doi.org/10.3390/cancers10010001
Chicago/Turabian StyleCicenas, Jonas, Egle Zalyte, Arnas Rimkus, Dalius Dapkus, Remigijus Noreika, and Sigitas Urbonavicius. 2018. "JNK, p38, ERK, and SGK1 Inhibitors in Cancer" Cancers 10, no. 1: 1. https://doi.org/10.3390/cancers10010001






