Integrin Inhibitors in Prostate Cancer
Abstract
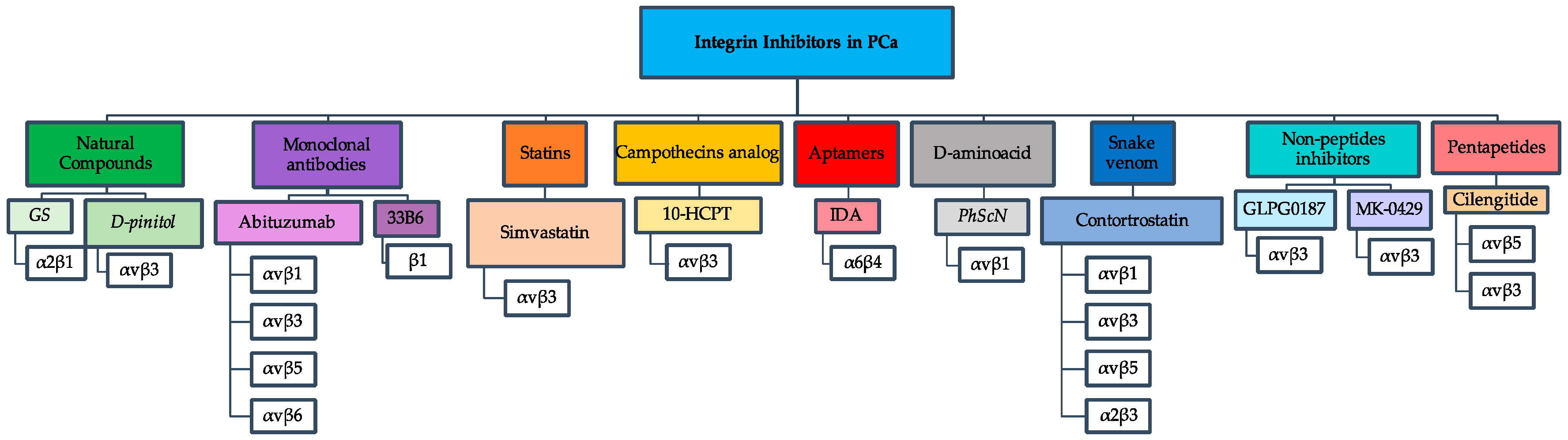
:1. Introduction
Integrin Alterations and Prostate Cancer
2. Natural Herbs Inhibit α2β1 and αvβ3 Integrins
2.1. Gleditsia Sinensis (GS)
2.2. d-Pinitol
3. Monoclonal Antibodies Inhibit αv and β1 Integrin
3.1. mAb Abituzumab (DI17E6, EMD, 525797)
3.2. mAb 33B6
4. Statins and Campothecins Analogs Inhibits αvβ3 Integrins
4.1. Simvastatin
4.2. 10-Hydroxycamptothecin (10-HCPT)
5. Aptamers Inhibits α6β4 Integrins
Integrin α6β4-Specific DNA Aptamer
6. d-Amino Acid Inhibits α5β1 Integrin
Ac-PhScN-NH2 (PhScN)
7. Snake Venom Inhibits Various Integrins
Contortrostatin
8. Non-Peptides and Peptides Inhibitors
8.1. GLPG0187
8.2. MK-0429
8.3. Cilengitide (EMD121974, NSC, 707544)
9. Summary
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goel, H.; Li, J. Integrins in Prostate Cancer Progression. Endocr. Relat. Cancer 2008, 15, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Park, K.M.; Lee, S.H. Gleditsia Sinensis Thorn Attenuates the Collagen-Based Migration of PC3 Prostate Cancer Cells through the Suppression of Integrin Expression. Int. J. Mol. Sci. 2016, 17, 328. [Google Scholar] [CrossRef] [PubMed]
- Winograd-Katz, S.E.; Fässler, R.; Geiger, B.; Legate, K.R. The Integrin Adhesome: From Genes and Proteins to Human Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Horton, E.R.; Byron, A.; Askari, J.A.; Ng, D.H.J.; Millon-Frémillon, A.; Robertson, J.; Koper, E.J.; Paul, N.R.; Warwood, S.; Knight, D.; et al. Definition of a Consensus Integrin Adhesome and Its Dynamics during Adhesion Complex Assembly and Disassembly. Nat. Cell Biol. 2015, 17, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Cancer Stat Facts: Prostate Cancer. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 30 August 2017).
- Yu, T.; Wang, C.; Yang, J.; Guo, Y.; Wu, Y.; Li, X. Metformin Inhibits SUV39H1-Mediated Migration of Prostate Cancer Cells. Oncogenesis 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Baribault, H. Vinculin Knockout Results in Heart and Brain Defects during Embryonic Development. Development 1998, 125, 327–337. [Google Scholar] [PubMed]
- Gilmore, A.P.; Romer, L.H. Inhibition of Focal Adhesion Kinase (FAK) Signaling in Focal Adhesions Decreases Cell Motility and Proliferation. Mol. Biol. Cell 1996, 7, 1209–1224. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Tan, T.W.; Tsai, T.H.; Chen, C.C.; Hsieh, T.F.; Lee, S.; Liu, H.H.; Chen, W.C.; Tang, C.H. D-Pinitol Inhibits Prostate Cancer Metastasis through Inhibition of αVβ3 Integrin by Modulating FAK, c-Src and NF-κB Pathways. Int. J. Mol. Sci. 2013, 14, 9790–9802. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J.G. Carbohydrates in Soybean Nodules: II. Distribution of compounds in seedlings during the onset of nitrogen fixation. Plant Physiol. 1980, 66, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Numata, A.; Takahashi, C.; Fujiki, R.; Kitano, E.; Kitajima, A.; Takemura, T. Plant Constituents Biologically Active to Insects. VI. Antifeedants for Larvae of the Yellow Butterfly, Eurema Hecabe Mandarina, in Osmunda Japonica. (2). Chem. Pharm. Bull. 1990, 38, 2862–2865. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Pandey, B.L.; Tripathi, M.; Pandey, V.B. Anti-Inflammatory Effect of (+)-Pinitol. Fitoterapia 2001, 72, 168–170. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, J.; Yao, Z.; Shelley, G.; Keller, E.T. Abituzumab Targeting of AlphaV-Class Integrins Inhibits Prostate Cancer Progression. Mol. Cancer Res. 2017, 15, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Jin, J.K.; Cheng, C.J.; Huang, C.F.; Song, J.H.; Huang, M.; Brown, W.S.; Zhang, S.; Yu-Lee, L.Y.; Yeh, E.T.; et al. Targeting Constitutively Activated β1 Integrin Inhibits Prostate Cancer Metastasis. Mol. Cancer Res. 2013, 11, 405–418. [Google Scholar] [CrossRef] [PubMed]
- De Franciscis, V. A Theranostic “SMART” Aptamer for Targeted Therapy of Prostate Cancer. Mol. Ther. 2014, 22, 1886–1888. [Google Scholar] [CrossRef] [PubMed]
- Al-Husein, B.; Goc, A.; Somanath, P.R. Suppression of Interactions between Prostate Tumor Cell-Surface Integrin and Endothelial ICAM-1 by Simvastatin Inhibits Micrometastasis. J. Cell. Physiol. 2013, 228, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Hoque, A.; Chen, H.; Xu, X. Statin Induces Apoptosis and Cell Growth Arrest in Prostate Cancer Cells. Cancer Epidemiol. Biomark. Prev. 2008, 17, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, G.; Delakas, D.; Nakopoulou, L.; Kassimatis, T. Statins and Prostate Cancer: Molecular and Clinical Aspects. Eur. J. Cancer 2011, 47, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Kochuparambil, S.T.; Al-Husein, B.; Goc, A.; Soliman, S.; Somanath, P.R. Anticancer Efficacy of Simvastatin on Prostate Cancer Cells and Tumor Xenografts Is Associated with Inhibition of Akt and Reduced Prostate-Specific Antigen Expression. J. Pharmacol. Exp. Ther. 2011, 336, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin Use and Reduced Cancer-Related Mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yu, K.; Yu, M.; Feng, Y.; Wang, J.; Li, M.; Chen, Z.; He, M.; Guo, R.; Tian, R.; et al. A Novel Multifunctional Poly(amidoamine) Dendrimeric Delivery System with Superior Encapsulation Capacity for Targeted Delivery of the Chemotherapy Drug 10-Hydroxycamptothecin. Int. J. Pharm. 2014, 465, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.E.; Peppas, N.A. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Lange, T.; Mittelberger, F.; Schumacher, U.; Hahn, U. Selection and Characterization of an α6β4 Integrin Blocking DNA Aptamer. Mol. Ther. Acids 2016, 5, e294. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Anderson, T.A.; Gard, J.M.C.; Sroka, I.C.; Strautman, S.R.; Nagle, R.B.; Morrissey, C.; Knudsen, B.S.; Cress, A.E. Characterization of Laminin Binding Integrin Internalization in Prostate Cancer Cells. J. Cell. Biochem. 2017, 118, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, S. Cell-SELEX Technology. Biores. Open Access 2012, 1, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Kruspe, S.; Meyer, C.; Hahn, U. Chlorin e6 Conjugated Interleukin-6 Receptor Aptamers Selectively Kill Target Cells Upon Irradiation. Mol. Ther. Nucleic Acids 2014, 3, e143. [Google Scholar] [CrossRef] [PubMed]
- Kruspe, S.; Mittelberger, F.; Szameit, K.; Hahn, U. Aptamers as Drug Delivery Vehicles. ChemMedChem 2014, 9, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J.J. Cell-Type-Specific, Aptamer-Functionalized Agents for Targeted Disease Therapy. Mol. Ther. Nucleic Acids 2014, 3, e169. [Google Scholar] [CrossRef] [PubMed]
- Dassie, J.P.; Hernandez, L.I.; Thomas, G.S.; Long, M.E.; Rockey, W.M.; Howell, C.A.; Chen, Y.; Hernandez, F.J.; Liu, X.Y.; Wilson, M.E.; et al. Targeted Inhibition of Prostate Cancer Metastases with an RNA Aptamer to Prostate-Specific Membrane Antigen. Mol. Ther. 2014, 22, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Veine, D.M.; Yao, H.; Stafford, D.R.; Fay, K.S.; Livant, D.L. A d-Amino Acid Containing Peptide as a Potent, Noncovalent Inhibitor of α5β1 Integrin in Human Prostate Cancer Invasion and Lung Colonization. Clin. Exp. Metastasis 2014, 31, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Rosca, E.V.; Koskimaki, J.E.; Rivera, C.G.; Pandey, N.B.; Tamiz, A.P.; Popel, A.S. Anti-Angiogenic Peptides for Cancer Therapeutics. Curr. Pharm. Biotechnol. 2011, 12, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.Z.; Jia, Y.; Hahn, N.J.; Markwart, S.M.; Rockwood, K.F.; Livant, D.L. Role of Focal Adhesion Kinase and Phosphatidylinositol 3 Kinase in Integrin Fibronectin Receptor-Mediated, Matrix Metalloproteinase-1-Dependent Invasion by Metastatic Prostate Cancer Cells. Cancer Res. 2006, 66, 8091–8099. [Google Scholar] [CrossRef] [PubMed]
- Livant, D.L.; Brabec, R.K.; Pienta, K.J.; Allen, D.L.; Kurachi, K.; Markwart, S.; Upadhyaya, A. Anti-Invasive, Antitumorigenic, and Antimetastatic Activities of the PHSCN Sequence in Prostate Carcinoma. Cancer Res. 2000, 60, 309–320. [Google Scholar] [PubMed]
- Zeng, Z.; Yao, H.; Staszewski, E.D.; Rockwood, K.F.; Markwart, S.M.; Fay, K.S.; Spalding, A.C.; Livant, D.L. alpha(5)beta(1) Integrin Ligand PHSRN Induces Invasion and alpha(5) mRNA in Endothelial Cells to Stimulate Angiogenesis. Transl. Oncol. 2009, 2, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, A.; Fedele, C.; Trerotola, M.; Ganguly, K.K.; Languino, L.R. IGF-IR Promotes Prostate Cancer Growth by Stabilizing α5β1 Integrin Protein Levels. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Wang, Q.; Swenson, S.; Jadvar, H.; Groshen, S.; Ye, W.; Markland, F.S.; Pinski, J. The Disintegrin Contortrostatin in Combination with Docetaxel Is a Potent Inhibitor of Prostate Cancer in Vitro and in Vivo. Prostate 2010, 70, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Swenson, S.; Costa, F.; Ernst, W.; Fujii, G.; Markland, F.S. Contortrostatin, a Snake Venom Disintegrin with Anti-Angiogenic and Anti-Tumor Activity. Pathophysiol. Haemost. Thromb. 2006, 34, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Trikha, M.; De Clerck, Y.A.; Markland, F.S. Contortrostatin, a Snake Venom Disintegrin, Inhibits β1 Integrin-Mediated Human Metastatic Melanoma Cell Adhesion and Blocks Experimental Metastasis. Cancer Res. 1994, 54, 4993–4998. [Google Scholar] [PubMed]
- Swenson, S.; Costa, F.; Minea, R.; Sherwin, R.P.; Ernst, W.; Fujii, G.; Yang, D.; Markland, F.S. Intravenous Liposomal Delivery of the Snake Venom Disintegrin Contortrostatin Limits Breast Cancer Progression. Mol. Cancer Ther. 2004, 3, 499–511. [Google Scholar] [PubMed]
- Nemeth, J.A.; Cher, M.L.; Zhou, Z.; Mullins, C.; Bhagat, S.; Trikha, M. Inhibition of alpha(v)beta3 Integrin Reduces Angiogenesis, Bone Turnover, and Tumor Cell Proliferation in Experimental Prostate Cancer Bone Metastases. Clin. Exp. Metastasis 2003, 20, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, S.; Choudhary, S.; Maus, E.; Shukla, D.; Swenson, S.; Markland, F.S.; Tiwari, V. Contortrostatin, a Homodimeric Disintegrin Isolated from Snake Venom Inhibits Herpes Simplex Virus Entry and Cell Fusion. Antivir. Ther. 2012, 17, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Cancer, H.P.; Heckmann, B.; Feyen, J.; Pujuguet, P.; Blanque, R. Targeting of α v-Integrins in Stem/Progenitor Cells and Supportive Microenvironment Impairs Bone Metastasis in. Neoplasia 2011, 13, 516–525. [Google Scholar]
- Abdollahi, A.; Griggs, D.W.; Zieher, H.; Roth, A.; Lipson, K.E.; Saffrich, R.; Gro, H.; Hallahan, D.E.; Reisfeld, R.A.; Debus, J.; et al. Inhibition of αvβ3 Integrin Survival Signaling Enhances Antiangiogenic and Antitumor Effects of Radiotherapy. Clin. Cancer Res. 2005, 11, 6270–6280. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Zhang, H.; Pallavicini, M.; Gray, J.W.; Baehner, F.; Park, J.; Bissell, M.J. β1 Integrin Inhibitory Antibody Induces Apoptosis of Breast Cancer Cells, Inhibits Growth, and Distinguishes Malignant from Normal Phenotype in Three Dimensional Cultures and In Vivo. Cancer Res. 2010, 66, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Van Aarsen, L.A.K.; Leone, D.R.; Ho, S.; Dolinski, B.M.; Mccoon, P.E.; Lepage, D.J.; Kelly, R.; Heaney, G.; Rayhorn, P.; Reid, C.; et al. Antibody-Mediated Blockade of Integrin A v B 6 Inhibits Tumor Progression In Vivo by a Transforming Growth Factor-B–Regulated Mechanism. Cancer Res. 2008, 68, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, K.; Yu, J.; Edlund, M.; Spohn, B.; Hung, M.; Chung, L.W.K.; Hsieh, C. Targeting ECM—Integrin Interaction with Liposome-Encapsulated Small Interfering RNAs Inhibits the Growth of Human Prostate Cancer in a Bone Xenograft Imaging Model. Mol. Ther. 2005, 12, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.A.; Davidson, P.; Rolland, F.; Campone, M.; Xue, L.; Han, T.H.; Mehta, A.; Berd, Y.; He, W.; Lombardi, A. Evaluation of the Safety, Pharmacokinetics and Treatment Effects of an alpha(nu)beta(3) Integrin Inhibitor on Bone Turnover and Disease Activity in Men with Hormone-Refractory Prostate Cancer and Bone Metastases. Asia Pac. J. Clin. Oncol. 2010, 6, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Alva, A.; Slovin, S.; Daignault, S.; Carducci, M.; Dipaola, R. Phase II study of Cilengitide (EMD 121974, NSC 707544) in patients with non-metastatic castration resistant prostate cancer, NCI-6735. A study by the DOD/PCF prostate cancer clinical trials consortium. Invest. New Drugs 2012, 30, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.A.; Daignault, S.; Ryan, C.J.; Robert, S.; Smith, D.C.; Small, E.; Gross, M.E.; Stein, M.N.; Chen, A.; Hussain, M. Cilengitide (EMD 121974, NSC 707544) in asymptomatic metastatic castration resistant prostate cancer patients: A randomized phase II trial by the prostate cancer clinical trials consortium. Investig. New Drugs 2012, 29, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Beekman, K.W.; Colevas, A.D.; Cooney, K.; Dipaola, R.; Dunn, R.L.; Gross, M.; Keller, E.T.; Pienta, K.J.; Ryan, C.J.; Smith, D.; et al. Current Trial Phase II Evaluations of Cilengitide in Asymptomatic Patients with Androgen-Independent Prostate Cancer : Scientific Rationale and Study Design. Clin. Genitourin. Cancer 2006, 4, 299–302. [Google Scholar] [CrossRef] [PubMed]
| Ligand | Integrin | Effect | Cell Line Tested |
|---|---|---|---|
| Collagen | αvβ1 | Upregulated | PC3, CWR22 |
| α2β1 | Upregulated | PC3 | |
| Fibrinogen | αIIβ3 | Upregulated | PC3, CWR22 |
| αvβ3 | Upregulated | PC3, 22RV1, CWR22 | |
| Vitronectin | αvβ3 | Upregulated | PC3, 22RV1, CWR22 |
| αvβ5 | Upregulated | PC3, 22RV1, CWR22 | |
| Fibronectin | αvβ1 | Upregulated | PC3, DU145 |
| α2β1 | Upregulated | PC3, CWR22 | |
| Laminin | α6β4 | Upregulated | PC3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juan-Rivera, M.C.; Martínez-Ferrer, M. Integrin Inhibitors in Prostate Cancer. Cancers 2018, 10, 44. https://doi.org/10.3390/cancers10020044
Juan-Rivera MC, Martínez-Ferrer M. Integrin Inhibitors in Prostate Cancer. Cancers. 2018; 10(2):44. https://doi.org/10.3390/cancers10020044
Chicago/Turabian StyleJuan-Rivera, Maylein C., and Magaly Martínez-Ferrer. 2018. "Integrin Inhibitors in Prostate Cancer" Cancers 10, no. 2: 44. https://doi.org/10.3390/cancers10020044




