Proton Beam Therapy without Fiducial Markers Using Four-Dimensional CT Planning for Large Hepatocellular Carcinomas
Abstract
:1. Introduction
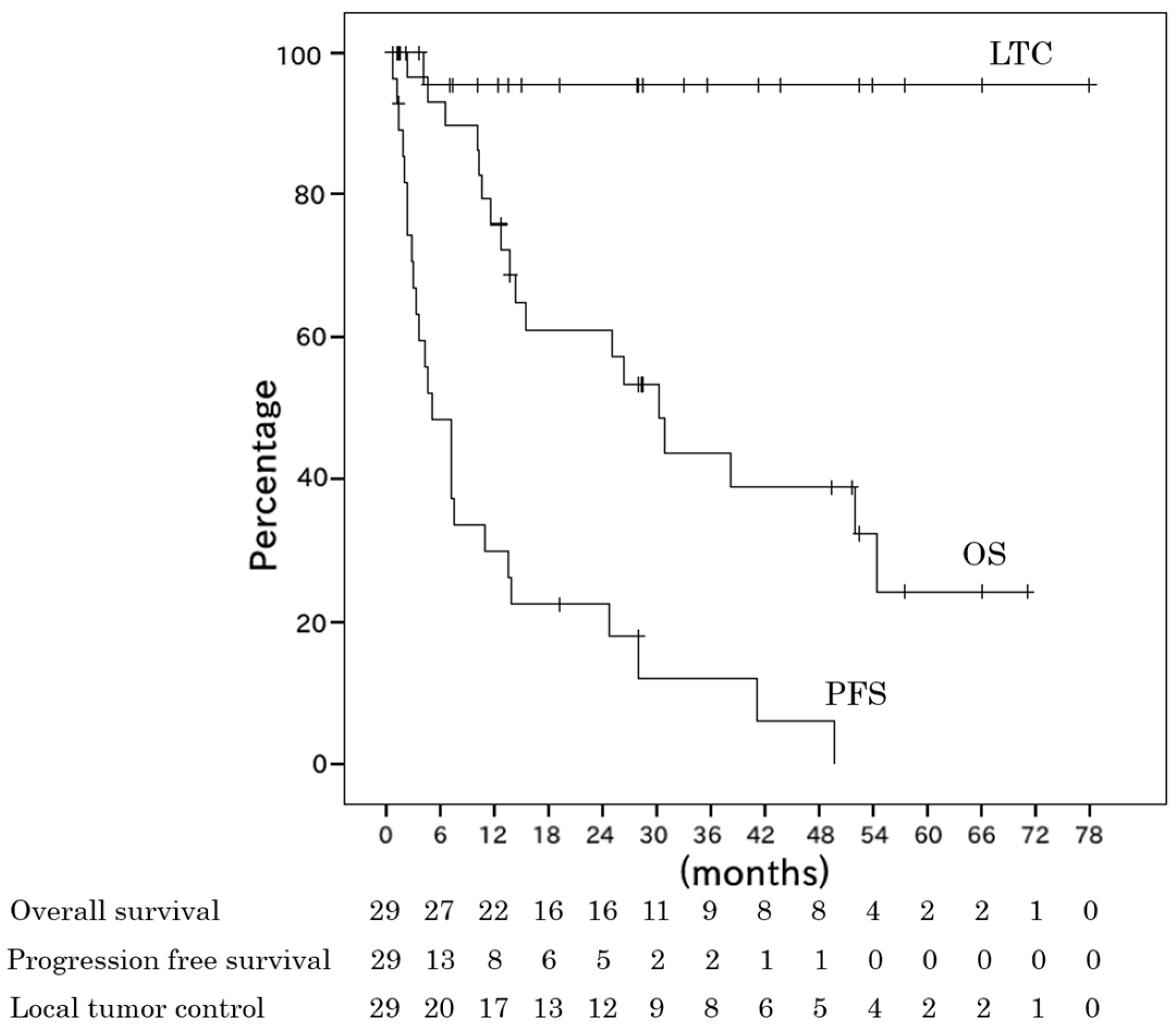
2. Results
2.1. Toxicities
2.2. Survival
3. Discussion
4. Patients and Methods
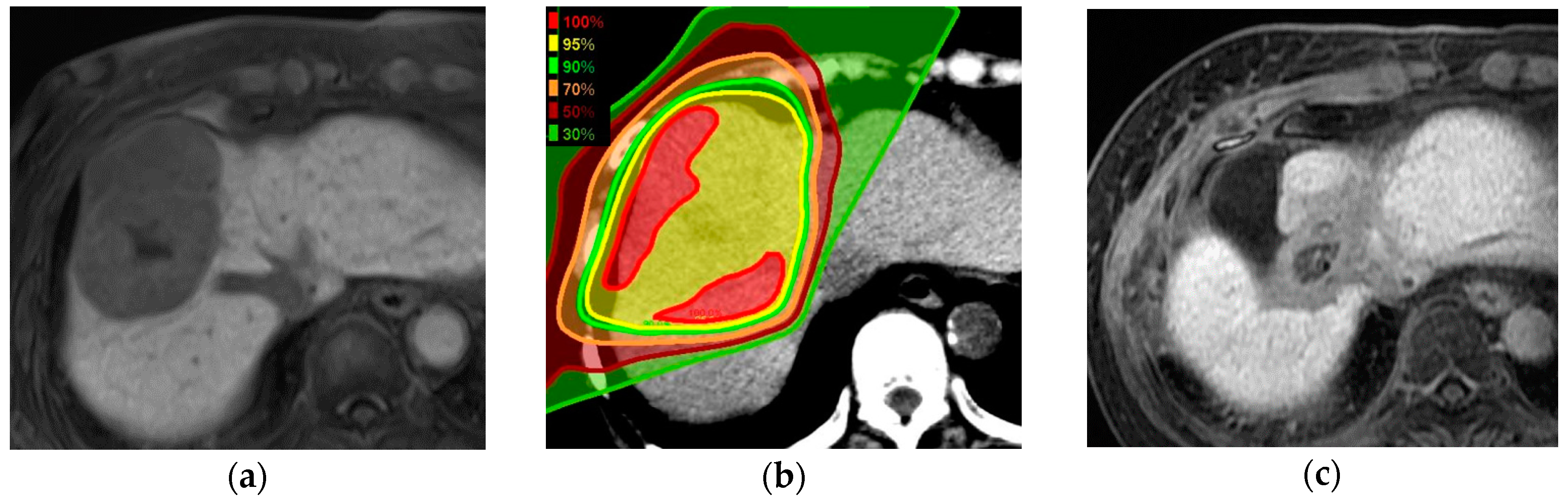
4.1. Proton Beam Therapy Planning
4.2. Proton Beam Treatment
4.3. Follow-Up and Toxicity Evaluation
4.4. Statistical Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tanaka, K.; Hirohata, T.; Koga, S.; Sugimachi, K.; Kanematsu, T.; Ohryohji, F.; Nawata, H.; Ishibashi, H.; Maeda, Y.; Kiyokawa, H.; et al. Hepatitis C and hepatitis B in the etiology of hepatocellular carcinoma in the Japanese population. Cancer Res. 1991, 51, 2842–2847. [Google Scholar] [PubMed]
- Bruix, J.; Sherman, M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Kokudo, N.; Makuuchi, M.; Izumi, N.; Ichida, T.; Kudo, M.; Ku, Y.; Sakamoto, M.; Nakashima, O.; Matsui, O.; et al. Comparison of resection and ablation for hepatocellular carcinoma: A cohort study based on a Japanese nationwide survey. J. Hepatol. 2013, 58, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, W.; Liang, X.; Li, D.; Lou, H.; Chen, R.; Wang, K.; Pan, H. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2014, 29, 193–200. [Google Scholar] [CrossRef] [PubMed]
- The Japan Society of Hepatology. Clinical Practice Guidelines for Hepatocellular Carcinoma 2017; Kanehara Shuppan: Tokyo, Japan, 2017. [Google Scholar]
- Teraoka, Y.; Kimura, T.; Aikata, H.; Daijo, K.; Osawa, M.; Honda, F.; Nakamura, Y.; Morio, K.; Morio, R.; Hatooka, M.; et al. Clinical outcomes of stereotactic body radiotherapy for elderly patients with hepatocellular carcinoma. Hepatol. Res. 2018, 48, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Sanuki, N.; Tsurugai, Y.; Iwabuchi, S.; Matsunaga, K.; Ebinuma, H.; Imajo, K.; Aoki, Y.; Saito, H.; Kunieda, E. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 2016, 122, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Toramatsu, C.; Katoh, N.; Shimizu, S.; Nihongi, H.; Matsuura, T.; Takao, S.; Miyamoto, N.; Suzuki, R.; Sutherland, K.; Kinoshita, R.; et al. What is the appropriate size criterion for proton radiotherapy for hepatocellular carcinoma? A dosimetric comparison of spot-scanning proton therapy versus intensity-modulated radiation therapy. Radiat. Oncol. 2013, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, M.; Okumura, T.; Hashimoto, T.; Fukuda, K.; Oshiro, Y.; Fukumitsu, N.; Abei, M.; Kawaguchi, A.; Hayashi, Y.; Ookawa, A.; et al. Proton beam therapy for hepatocellular carcinoma: A comparison of three treatment protocols. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, N.; Sugahara, S.; Nakayama, H.; Fukuda, K.; Mizumoto, M.; Abei, M.; Shoda, J.; Thono, E.; Tsuboi, K.; Tokuuye, K. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Pham, D.; Gill, S.; Bressel, M.; Dang, K.; Devereux, T.; Kron, T.; Foroudi, F. An analysis of respiratory induced kidney motion on four-dimensional computed tomography and its implications for stereotactic kidney radiotherapy. Radiat. Oncol. 2013, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Omari, E.A.; Erickson, B.; Ehlers, C.; Quiroz, F.; Noid, G.; Cooper, D.T.; Lachaine, M.; Li, X.A. Preliminary results on the feasibility of using ultrasound to monitor intrafractional motion during radiation therapy for pancreatic cancer. Med. Phys. 2016, 43, 5252. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.T.; Apisarnthanarax, S.; Yin, L.; Zou, W.; Rosen, M.; Plastaras, J.P.; Ben-Josef, E.; Metz, J.M.; Teo, B.K. Comparative assessment of liver tumor motion using cine-magnetic resonance imaging versus 4-dimensional computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Oldrini, G.; Taste-George, H.; Renard-Oldrini, S.; Baumann, A.S.; Marchesi, V.; Troufléau, P.; Peiffert, D.; Didot-Moisei, A.; Boyer, B.; Grignon, B.; et al. Implantation of fiducial markers in the liver for stereotactic body radiation therapy: Feasibility and results. Diagn. Interv. Imaging 2015, 96, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, Y.K.; Chen, Y.X.; Shi, S.M.; Zeng, Z.C. 4D-CT scans reveal reduced magnitude of respiratory liver motion achieved by different abdominal compression plate positions in patients with intrahepatic tumors undergoing helical tomotherapy. Med. Phys. 2016, 43, 4335. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, S.; Takanaka, T.; Kumano, T.; Mizuno, E.; Shibata, S.; Ohashi, S. Reproducibility of diaphragm position assessed with a voluntary breath-holding device. Jpn. J. Radiol. 2013, 31, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.M.; Hong, T.S.; Kambadakone, A.; Arellano, R.S. CT-guided implantation of intrahepatic fiducial markers for proton beam therapy of liver lesions: Assessment of success rate and complications. AJR Am. J. Roentgenol. 2015, 204, W207–W213. [Google Scholar] [CrossRef] [PubMed]
- Kothary, N.; Heit, J.J.; Louie, J.D.; Kuo, W.T.; Loo, B.W., Jr.; Koong, A.; Chang, D.T.; Hovsepian, D.; Sze, D.Y.; Hofmann, L.V. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J. Vasc. Interv. Radiol. 2009, 20, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Heinz, C.; Gerum, S.; Freislederer, P.; Ganswindt, U.; Roeder, F.; Corradini, S.; Belka, C.; Niyazi, M. Feasibility study on image guided patient positioning for stereotactic body radiation therapy of liver malignancies guided by liver motion. Radiat. Oncol. 2016, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Yoon, S.M.; Park, S.H.; Cho, B.; Park, J.W.; Jung, J.; Park, J.H.; Kim, J.H.; Ahn, S.D. Four-dimensional dose evaluation using deformable image registration in radiotherapy for liver cancer. Med. Phys. 2013, 40, 011706. [Google Scholar] [CrossRef] [PubMed]
- Taddei, P.J.; Howell, R.M.; Krishnan, S.; Scarboro, S.B.; Mirkovic, D.; Newhauser, W.D. Risk of second malignant neoplasm following proton versus intensity-modulated photon radiotherapies for hepatocellular carcinoma. Phys. Med. Biol. 2010, 55, 7055–7065. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, S.; Oshiro, Y.; Nakayama, H.; Fukuda, K.; Mizumoto, M.; Abei, M.; Shoda, J.; Matsuzaki, Y.; Thono, E.; Tokita, M.; et al. Proton beam therapy for large hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Nakamura, T.; Ono, T.; Azami, Y.; Suzuki, M.; Wada, H.; Takayama, K.; Endo, H.; Takeyama, T.; Hirose, K.; et al. Clinical results of proton beam therapy for hepatocellular carcinoma over 5 cm. Hepatol. Res. 2017, 47, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Fukumoto, T.; Demizu, Y.; Miyawaki, D.; Terashima, K.; Sasaki, R.; Hori, Y.; Hishikawa, Y.; Ku, Y.; Murakami, M. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer 2011, 117, 4890–4904. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 2003, 38, 200–207. [Google Scholar] [CrossRef]
- Poon, R.T.; Ng, I.O.; Fan, S.T.; Lai, E.C.; Lo, C.M.; Liu, C.L.; Wong, J. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: A study of a prospective cohort. J. Clin. Oncol. 2001, 19, 3037–3044. [Google Scholar] [CrossRef] [PubMed]
- Arii, S.; Tanaka, J.; Yamazoe, Y.; Minematsu, S.; Morino, T.; Fujita, K.; Maetani, S.; Tobe, T. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992, 69, 913–919. [Google Scholar] [CrossRef]
- Nakayama, H.; Sugahara, S.; Fukuda, K.; Abei, M.; Shoda, J.; Sakurai, H.; Tsuboi, K.; Matsuzaki, Y.; Tokuuye, K. Proton beam therapy for hepatocellular carcinoma located adjacent to the alimentary tract. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.A.; Smith, J.C.; Slater, J.D.; Volk, M.L.; Reeves, M.E.; Cheng, J.; Grove, R.; de Vera, M.E. Randomized Clinical Trial Comparing Proton Beam Radiation Therapy with Transarterial Chemoembolization for Hepatocellular Carcinoma: Results of an Interim Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Won, H.J.; Kim, S.Y.; Shin, Y.M.; Kim, P.N.; Yoon, S.M.; Park, J.H.; Kim, J.H. Efficacy and safety of ultrasound-guided implantation of fiducial markers in the liver for stereotactic body radiation therapy. PLoS ONE 2017, 12, e0179676. [Google Scholar] [CrossRef] [PubMed]
- Balter, J.M.; Dawson, L.A.; Kazanjian, S.; McGinn, C.; Brock, K.K.; Lawrence, T.; Ten Haken, R. Determination of ventilatory liver movement via radiographic evaluation of diaphragm position. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 267–270. [Google Scholar] [CrossRef]
- Yang, J.; Cai, J.; Wang, H.; Chang, Z.; Czito, B.G.; Bashir, M.R.; Palta, M.; Yin, F.F. Is diaphragm motion a good surrogate for liver tumor motion? Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Kuriyama, K.; Komiyama, T.; Tanaka, S.; Ueki, J.; Sano, N.; Araki, T.; Ikenaga, S.; Tateda, Y.; Aikawa, Y. CT evaluation of patient deep inspiration self-breath-holding: How precisely can patients reproduce the tumor position in the absence of respiratory monitoring devices? Med. Phys. 2003, 30, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.P.; Cosse, J.P.; Ninane, N.; Raes, M.; Michiels, C. Hypoxia protects HepG2 cells against etoposide-induced apoptosis via a HIF-1-independent pathway. Exp. Cell Res. 2006, 312, 2908–2920. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, S.; Yamamoto, K.; Maeda, Y.; Kawamura, M.; Shibata, S.; Sato, Y.; Terashima, K.; Shimizu, Y.; Tameshige, Y.; Sasaki, M.; et al. Evaluation of Focal Liver Reaction after Proton Beam Therapy for Hepatocellular Carcinoma Examined Using Gd-EOB-DTPA Enhanced Hepatic Magnetic Resonance Imaging. PLoS ONE 2016, 11, e0167155. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Okumura, T.; Akisada, M.; Inada, T.; Mori, T.; Yokota, H.; Calaguas, M.J. Irradiation synchronized with respiration gate. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 853–857. [Google Scholar] [CrossRef]
- Tsunashima, Y.; Vedam, S.; Dong, L.; Umezawa, M.; Sakae, T.; Bues, M.; Balter, P.; Smith, A.; Mohan, R. Efficiency of respiratory-gated delivery of synchrotron-based pulsed proton irradiation. Phys. Med. Biol. 2008, 53, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, M.; Okumura, T.; Hashimoto, T.; Fukuda, K.; Oshiro, Y.; Fukumitsu, N.; Abei, M.; Kawaguchi, A.; Hayashi, Y.; Ohkawa, A.; et al. Evaluation of liver function after proton beam therapy for hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e529–e535. [Google Scholar] [CrossRef] [PubMed]
- Gabryś, D.; Kulik, R.; Trela, K.; Ślosarek, K. Dosimetric comparison of liver tumour radiotherapy in all respiratory phases and in one phase using 4DCT. Radiother. Oncol. 2011, 100, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Underberg, R.W.; Lagerwaard, F.J.; Slotman, B.U.; Cuijpers, J.P.; Senan, S. Benefit of respiration-taged stereotactic radiotherapy for stage I lung cancer: An analysis of 4DCT datasets. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Sasaki, R.; Miyawaki, D.; Nishimura, H.; Demizu, Y.; Akagi, T.; Suga, D.; Sakamoto, H.; Murakami, M.; Sugimura, K.; et al. Physiologic reactions after proton beam therapy in patients with prostate cancer: Significance of urinary autoactivation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Mizuhata, M.; Takamatsu, S.; Shibata, S.; Bou, S.; Sato, Y.; Kawamura, M.; Asahi, S.; Tameshige, Y.; Maeda, Y.; Sasaki, M.; et al. Respiratory-gated Proton Beam Therapy for Hepatocellular Carcinoma Adjacent to the Gastrointestinal Tract without Fiducial Markers. Cancers 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 2nd ed.; Kanehara Shuppan: Tokyo, Japan, 2003. [Google Scholar]
| Characteristics | n |
|---|---|
| Number of patients | 29 |
| Gender, male/female | 22/7 |
| Age (years); median (range) | 71 (38–87) |
| PS 0/1/2 | 21/7/1 |
| Tumor size; median (range) | 69 (50–139) |
| 50–100 mm/>100 mm | 22/7 |
| CH HCV/HBV/alcoholic/others | 5/11/4/9 |
| Child Pugh A/B | 24/5 |
| Tumor thrombus PV/HV/bile duct | 9/4/1 |
| Prior treatment TACE/RFA/surgery | 13/4/3 |
| Operable/inoperable | 7/22 |
| Solitary/multiple (two or more) | 14/15 |
| Single nodular type/non single nodular type | 5/24 |
| T stage 1/2/3a/3b/4 | 4/8/9/7/1 |
| GTV (cm3); median (range) | 107 (23–1056) |
| PTV (cm3); median (range) | 293 (138–1566) |
| Liver volume (cm3); median (range) | 1310 (810–2259) |
| Variables | HR | 95% CI | p-Value |
|---|---|---|---|
| T stage (T1-2/T3-4) | 0.28 | 0.09–0.87 | 0.03 |
| Tumor size (≤100 mm/>100 mm) | 0.24 | 0.06–1.00 | 0.049 |
| Volume of PTV (≤300 mL/>300 mL) | 1.04 | 0.27–4.02 | 0.95 |
| Operable/inoperable | 0.63 | 0.14–2.74 | 0.54 |
| History of previous treatment | 0.95 | 0.33–2.77 | 0.92 |
| Author | Number | Median Tumor Size (Range) | Median Treatment Dose | OS 2 Years | LTC 2 Years |
|---|---|---|---|---|---|
| Mizumoto et al. [9] | 266 | 34 mm (6–130 mm) | 72.6 CGE/22 Fr | 61% (3 years) | 87% (3 years) |
| Fukumitsu et al. [10] | 51 | 28 mm (8–93 mm) | 66.0 CGE/10 Fr | 49% (3 years) | 95% (3 years) |
| Sugahara et al. [22] | 22 | 110 mm (100–140 mm) | 72.6 CGE/22 Fr | 36% | 87% |
| Kimura et al. [23] | 24 | 90 mm (50–180 mm) | 72.6 CGE/22 Fr | 52% | 87% |
| This study | 29 | 69 mm (50–139 mm) | 76.0 CGE/20 Fr | 61% | 95% |
| Total Dose (CGE) | Number of Fractions | Cases (n = 29) | The Number of Re-Plan | Fraction at Re-Plan (Cases) |
|---|---|---|---|---|
| 66 | 10 | 4 | - | - |
| 76 | 20 | 13 | 1 | 13 (1) |
| 80.5 | 23 | 1 | - | - |
| 80 | 25 | 1 | - | - |
| 67.5 | 25 | 1 | - | - |
| 70.4 | 32 | 5 | 5 | 20 (1), 22 (4) |
| 76 | 38 | 4 | 4 | 20 (2), 30 (2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, S.; Takamatsu, S.; Yamamoto, K.; Mizuhata, M.; Bou, S.; Sato, Y.; Kawamura, M.; Asahi, S.; Tameshige, Y.; Maeda, Y.; et al. Proton Beam Therapy without Fiducial Markers Using Four-Dimensional CT Planning for Large Hepatocellular Carcinomas. Cancers 2018, 10, 71. https://doi.org/10.3390/cancers10030071
Shibata S, Takamatsu S, Yamamoto K, Mizuhata M, Bou S, Sato Y, Kawamura M, Asahi S, Tameshige Y, Maeda Y, et al. Proton Beam Therapy without Fiducial Markers Using Four-Dimensional CT Planning for Large Hepatocellular Carcinomas. Cancers. 2018; 10(3):71. https://doi.org/10.3390/cancers10030071
Chicago/Turabian StyleShibata, Satoshi, Shigeyuki Takamatsu, Kazutaka Yamamoto, Miu Mizuhata, Sayuri Bou, Yoshitaka Sato, Mariko Kawamura, Satoko Asahi, Yuji Tameshige, Yoshikazu Maeda, and et al. 2018. "Proton Beam Therapy without Fiducial Markers Using Four-Dimensional CT Planning for Large Hepatocellular Carcinomas" Cancers 10, no. 3: 71. https://doi.org/10.3390/cancers10030071





