Immunohistochemistry for Diagnosis of Metastatic Carcinomas of Unknown Primary Site
Abstract
:1. Introduction
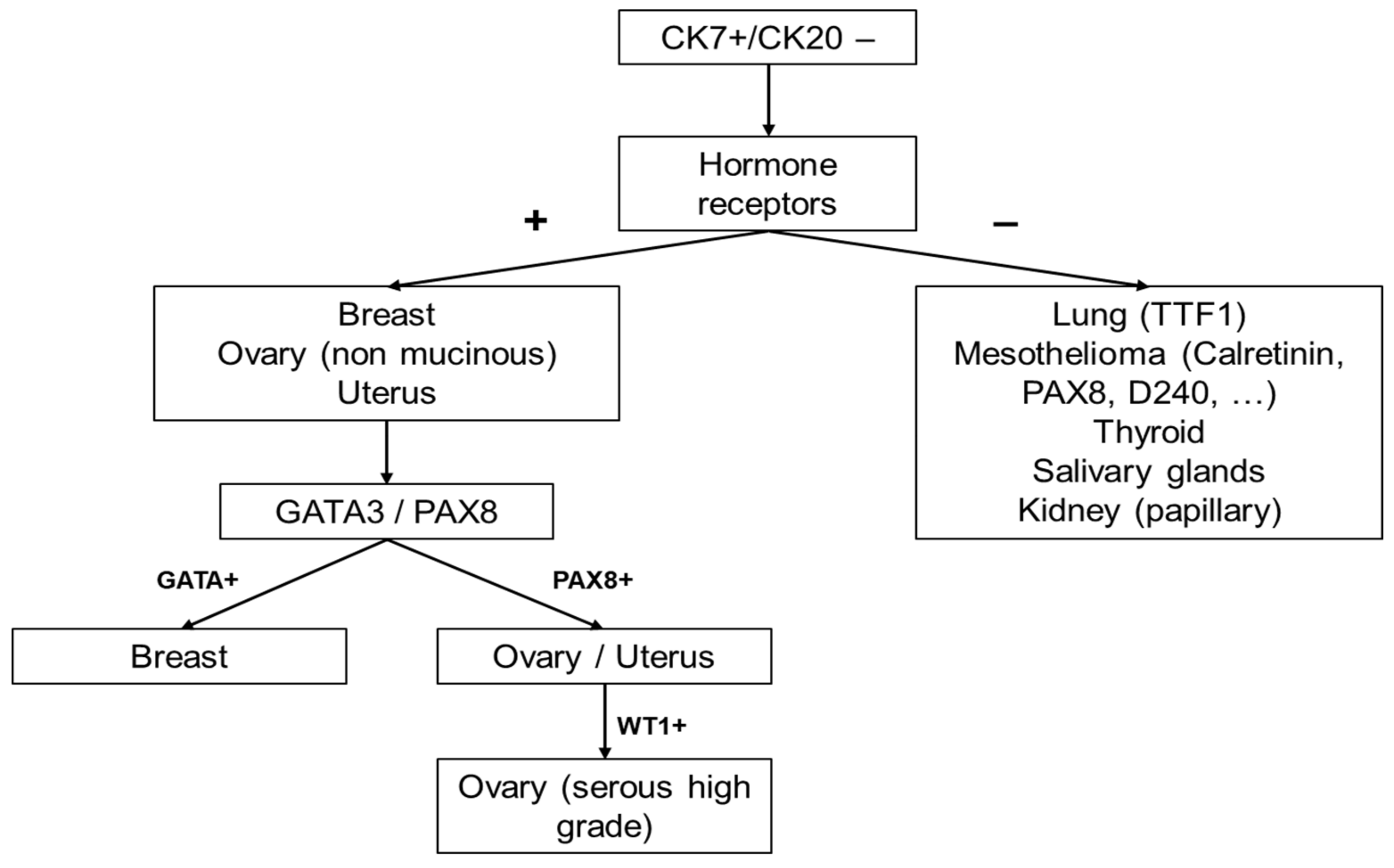
2. Diagnostic Workflow of CK7+/CK20− CUPs
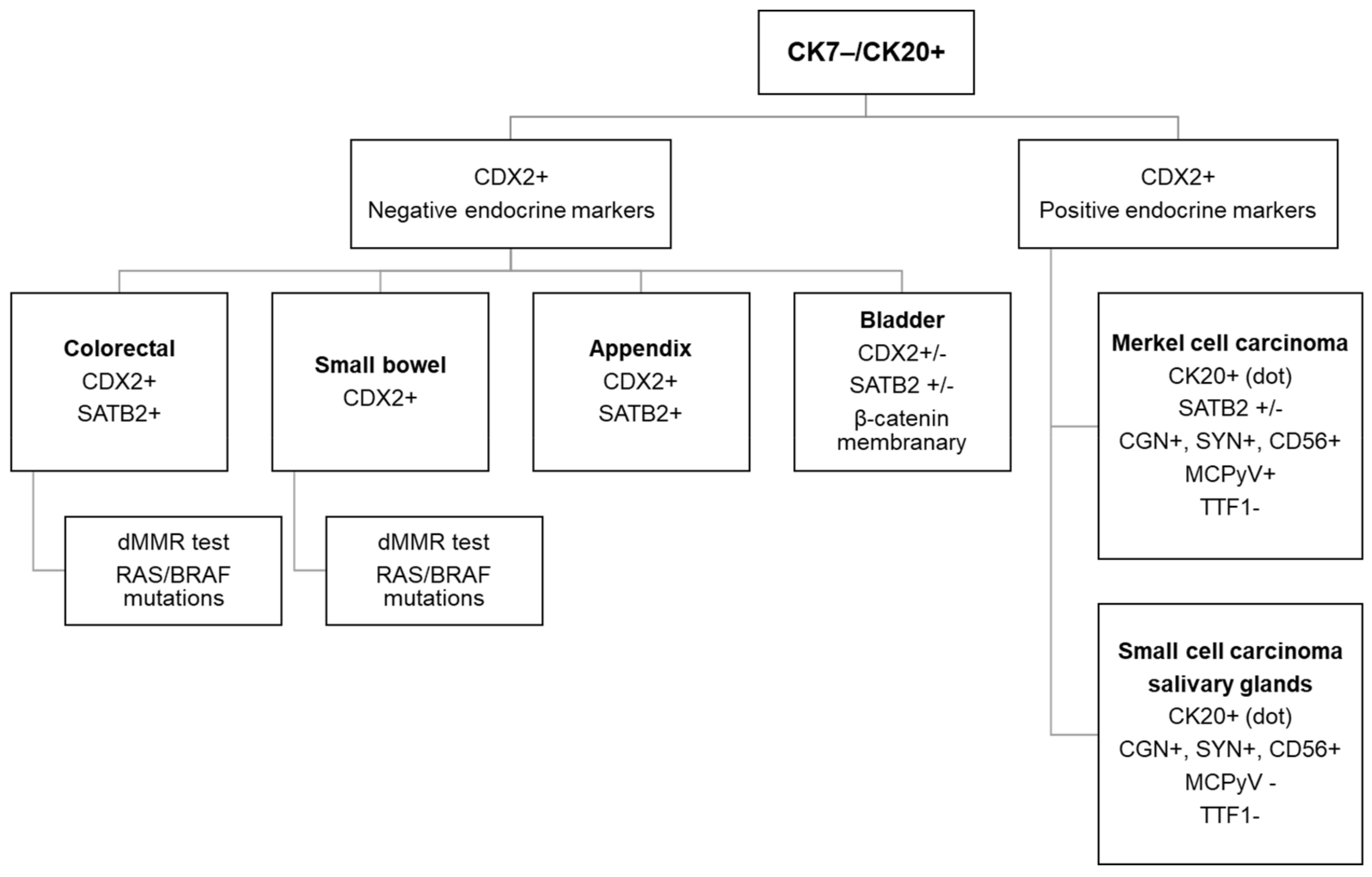
3. Diagnostic Workflow of CK7−/CK20+ CUPs
4. Diagnostic Workflow of CK7+/CK20+ CUPs
5. Diagnostic Workflow of CK7−/CK20− CUPs
6. Complementary Tools for Diagnosis and Prediction of Treatment Response in CUPs
7. Selected IHC Pitfalls
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Benderra, M.A.; Ilie, M.; Hofman, P.; Massard, C. Standard of care of carcinomas on cancer of unknown primary site in 2016. Bull. Cancer 2016, 103, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Wang, K.; Gay, L.; Otto, G.A.; White, E.; Iwanik, K.; Palmer, G.; Yelensky, R.; Lipson, D.M.; Chmielecki, J.; et al. Comprehensive genomic profiling of carcinoma of unknown primary site: New routes to targeted therapies. JAMA Oncol. 2015, 1, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, N.; Pentheroudakis, G. Cancer of unknown primary site. Lancet 2012, 379, 1428–1435. [Google Scholar] [CrossRef]
- Bahrami, A.; Truong, L.D.; Ro, J.Y. Undifferentiated tumor: True identity by immunohistochemistry. Arch. Pathol. Lab. Med. 2008, 132, 326–348. [Google Scholar] [PubMed]
- Greco, F.A. Cancer of unknown primary site: Evolving understanding and management of patients. Clin. Adv. Hematol. Oncol. 2012, 10, 518–524. [Google Scholar] [CrossRef]
- Lesimple, T.; Voigt, J.J.; Bataillard, A.; Coindre, J.M.; Culine, S.; Lortholary, A.; Merrouche, Y.; Ganem, G.; Kaminsky, M.C.; Negrier, S.; et al. Clinical practice guidelines: Standards, options and recommendations for the diagnosis of carcinomas of unknown primary site. Bull. Cancer 2003, 90, 1071–1096. [Google Scholar] [PubMed]
- Lin, F.; Liu, H. Immunohistochemistry in undifferentiated neoplasm/tumor of uncertain origin. Arch. Pathol. Lab. Med. 2014, 138, 1583–1610. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.G.; Weiss, L.M. Keratin expression in human tissues and neoplasms. Histopathology 2002, 40, 403–439. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, N.G. Broad-spectrum immunohistochemical epithelial markers: A review. Hum. Pathol. 2013, 44, 1195–1215. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.R.; Hornick, J.L. Metastatic carcinoma of unknown primary: Diagnostic approach using immunohistochemistry. Adv. Anat. Pathol. 2015, 22, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Rubin, M.S.; Spigel, D.R.; Boccia, R.V.; Raby, S.; Quinn, R.; Greco, F.A. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: A prospective trial of the Sarah Cannon research institute. J. Clin. Oncol. 2013, 31, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Vallicelli, C.; Cavaliere, D.; Catena, F.; Coccolini, F.; Ansaloni, L.; Poiasina, E.; Abongwa, H.K.; De Simone, B.; Alberici, L.; Framarini, M.; et al. Management of peritoneal carcinomatosis from colorectal cancer: Review of the literature. Int. J. Colorectal Dis. 2014, 29, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Mhawech-Fauceglia, P.; Wang, D.; Menesses, T.; Chandavarkar, U.; Ough, F.; Lin, Y.; Liu, S. PAX-8 is a reliable marker in making the diagnosis in advanced stage epithelial ovarian carcinoma and primary peritoneal carcinoma for neoadjuvant chemotherapy on cell block and biopsy specimens. Histopathology 2012, 60, 1019–1020. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, J.; Wilkerson, M.L.; Lin, F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: A useful immunomarker for breast and urothelial carcinomas. Am. J. Clin. Pathol. 2012, 138, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.G.; Siddiqui, M.T.; Oprea-Ilies, G.; Stevens, K.; Osunkoya, A.O.; Cohen, C.; Li, X.B. GATA-3 and FOXA1 expression is useful to differentiate breast carcinoma from other carcinomas. Hum. Pathol. 2016, 47, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.Z.; Beriwal, S.; Dabbs, D.J.; Bhargava, R. Semiquantitative GATA-3 immunoreactivity in breast, bladder, gynecologic tract, and other cytokeratin 7-positive carcinomas. Am. J. Clin. Pathol. 2014, 142, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Rys, J.; Czapiewski, P.; Wazny, K.; Langfort, R.; Waloszczyk, P.; Biernat, W.; Lasota, J.; et al. GATA3: A multispecific but potentially useful marker in surgical pathology: A systematic analysis of 2500 epithelial and nonepithelial tumors. Am. J. Surg. Pathol. 2014, 38, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, D.; Chiriboga, L.; Soslow, R.A. Expression of PAX8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am. J. Surg. Pathol. 2008, 32, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Laury, A.R.; Perets, R.; Piao, H.; Krane, J.F.; Barletta, J.A.; French, C.; Chirieac, L.R.; Lis, R.; Loda, M.; Hornick, J.L.; et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am. J. Surg. Pathol. 2011, 35, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Tacha, D.; Zhou, D.; Cheng, L. Expression of PAX8 in normal and neoplastic tissues: A comprehensive immunohistochemical study. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.; Shen, S.S.; Hamilton, C.; Anjana, K.; Coffey, D.; Krishnan, B.; Truong, L.D. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: A comprehensive immunohistochemical study. Mod. Pathol. 2011, 24, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L. Histopathologic classification of lung cancer: Relevance of cytokeratin and TTF-1 immunophenotyping. Ann. Diagn. Pathol. 2004, 8, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Miyagi, E.; Murata, S.; Kawaoi, A.; Katoh, R. Expression of thyroid transcription factor-1 in normal and neoplastic lung tissues. Mod. Pathol. 2002, 15, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Chernock, R.D.; El-Mofty, S.K.; Becker, N.; Lewis, J.S., Jr. Napsin A expression in anaplastic, poorly differentiated, and micropapillary pattern thyroid carcinomas. Am. J. Surg. Pathol. 2013, 37, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- El-Maqsoud, N.M.; Tawfiek, E.R.; Abdelmeged, A.; Rahman, M.F.; Moustafa, A.A. The diagnostic utility of the triple markers napsin a, TTF-1, and PAX8 in differentiating between primary and metastatic lung carcinomas. Tumour Biol. 2016, 37, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, R.; Haltas, H.; Yenidunya, S. The value of CDX2 and cytokeratins 7 and 20 expression in differentiating colorectal adenocarcinomas from extraintestinal gastrointestinal adenocarcinomas: Cytokeratin 7−/20+ phenotype is more specific than CDX2 antibody. Diagn. Pathol. 2012, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Salari, K.; Spulak, M.E.; Cuff, J.; Forster, A.D.; Giacomini, C.P.; Huang, S.; Ko, M.E.; Lin, A.Y.; van de Rijn, M.; Pollack, J.R. CDX2 is an amplified lineage-survival oncogene in colorectal cancer. Proc. Natl. Acad. Sci. USA 2012, 109, E3196–E3205. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Eiholm, S.; Kirkeby, L.T.; Espersen, M.L.; Jess, P.; Gogenur, I.; Troelsen, J.T. CDX2 downregulation is associated with poor differentiation and MMR deficiency in colon cancer. Exp. Mol. Pathol. 2016, 100, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Kim, C.J.; Koo, J.; Zhou, W.; Choi, E.K.; Arcega, R.; Chen, Z.E.; Wang, H.; Zhang, L.; Lin, F. Practical immunohistochemistry in neoplastic pathology of the gastrointestinal tract, liver, biliary tract, and pancreas. Arch. Pathol. Lab. Med. 2017, 141, 1155–1180. [Google Scholar] [CrossRef] [PubMed]
- Downes, M.R.; Torlakovic, E.E.; Aldaoud, N.; Zlotta, A.R.; Evans, A.J.; van der Kwast, T.H. Diagnostic utility of androgen receptor expression in discriminating poorly differentiated urothelial and prostate carcinoma. J. Clin. Pathol. 2013, 66, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.N.; Colby, T.; Ordonez, N.; Krausz, T.; Attanoos, R.; Beasley, M.B.; Borczuk, A.C.; Butnor, K.; Cagle, P.T.; Chirieac, L.R.; et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the international mesothelioma interest group. Arch. Pathol. Lab. Med. 2013, 137, 647–667. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.N.; Colby, T.V.; Ordonez, N.G.; Krausz, T.; Borczuk, A.; Cagle, P.T.; Chirieac, L.R.; Churg, A.; Galateau-Salle, F.; Gibbs, A.R.; et al. Guidelines for pathologic diagnosis of malignant mesothelioma: A consensus statement from the international mesothelioma interest group. Arch. Pathol. Lab. Med. 2009, 133, 1317–1331. [Google Scholar] [PubMed]
- Mery, E.; Hommell-Fontaine, J.; Capovilla, M.; Chevallier, A.; Bibeau, F.; Croce, S.; Dartigues, P.; Kaci, R.; Lang-Averous, G.; Laverriere, M.H.; et al. Peritoneal malignant mesothelioma: Review and recent data. Ann. Pathol. 2014, 34, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, S.; Oji, Y.; Horiuchi, T.; Kanda, T.; Kitagawa, M.; Takeuchi, T.; Kawano, K.; Kuwae, Y.; Yamauchi, A.; Okumura, M.; et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod. Pathol. 2006, 19, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, A.; de Wit, M.; Johansson, C.; Uhlen, M.; Ponten, F. The role of SATB2 as a diagnostic marker for tumors of colorectal origin: Results of a pathology-based clinical prospective study. Am. J. Clin. Pathol. 2014, 141, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Shi, J.; Zhu, S.; Chen, Z.; Li, A.; Chen, T.; Wang, H.L.; Liu, H. Cadherin-17 and SATB2 are sensitive and specific immunomarkers for medullary carcinoma of the large intestine. Arch. Pathol. Lab. Med. 2014, 138, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Modos, O.; Niedworok, C.; Reis, H.; Szendroi, A.; Szasz, M.A.; Nyirady, P. Clinical, prognostic, and therapeutic aspects of urachal carcinoma-a comprehensive review with meta-analysis of 1010 cases. Urol. Oncol. 2016, 34, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, A.; Sharp, D.S.; Fine, S.W.; Tickoo, S.K.; Herr, H.W.; Reuter, V.E.; Olgac, S. Urachal carcinoma: A clinicopathologic analysis of 24 cases with outcome correlation. Am. J. Surg. Pathol. 2009, 33, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Williamson, S.R.; Lopez-Beltran, A.; Montironi, R.; Huang, W.; Eble, J.N.; Grignon, D.J.; Koch, M.O.; Idrees, M.T.; Emerson, R.E.; et al. Distinguishing primary adenocarcinoma of the urinary bladder from secondary involvement by colorectal adenocarcinoma: Extended immunohistochemical profiles emphasizing novel markers. Mod. Pathol. 2013, 26, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Trpkov, K.; Lopez-Beltran, A.; Grignon, D. Best practices recommendations in the application of immunohistochemistry in the bladder lesions: Report from the international society of urologic pathology consensus conference. Am. J. Surg. Pathol. 2014, 38, e20–e34. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, B.; Mridha, A.R.; Yadav, S.; Kumar, R.; Gamanagatti, S. Fine needle aspiration cytology diagnosis of an urachal adenocarcinoma. J. Clin. Diagn. Res. 2016, 10, ED10–ED12. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Smith, M.A.; Cieply, K.M.; Acquafondata, M.B.; Parwani, A.V. Primary bladder adenocarcinoma versus metastatic colorectal adenocarcinoma: A persisting diagnostic challenge. Diagn. Pathol. 2012, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Min, B.S.; Lee, K.Y.; Kim, N.K.; Kim, S.N.; Choi, J.; Kim, H. Loss of e-cadherin and MUC2 expressions correlated with poor survival in patients with stages II and III colorectal carcinoma. Ann. Surg. Oncol. 2011, 18, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.L.; Chang, A.G.; Cimino-Mathews, A.; Argani, P.; Youssef, R.F.; Kapur, P.; Montgomery, E.A.; Epstein, J.I. GATA-3 immunohistochemistry in the differential diagnosis of adenocarcinoma of the urinary bladder. Am. J. Surg. Pathol. 2013, 37, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, M.; Agnarsdottir, M.; Edqvist, P.H.; Coter, A.; Ponten, F. SATB2 is expressed in merkel cell carcinoma. Arch. Dermatol. Res. 2016, 308, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Lugli, A.; Tzankov, A.; Zlobec, I.; Terracciano, L.M. Differential diagnostic and functional role of the multi-marker phenotype CDX2/CK20/CK7 in colorectal cancer stratified by mismatch repair status. Mod. Pathol. 2008, 21, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Yamamoto, H.; Fujiwara, M.; Koga, Y.; Tsuruta, S.; Ihara, E.; Oki, E.; Nakamura, M.; Ogawa, Y.; Oda, Y. Clinicopathologic and molecular characteristics of synchronous colorectal carcinoma with mismatch repair deficiency. Am. J. Surg. Pathol. 2018, 42, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; Van Schil, P.E.; et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Chen, Z.E.; Wang, H.L. Utility of immunohistochemistry in the pancreatobiliary tract. Arch. Pathol. Lab. Med. 2015, 139, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, M.; Sanz-Ortega, J.; Sirohi, D.; Divatia, M.K.; Ohe, C.; Zampini, C.; Luthringer, D.; Smith, S.C.; Amin, M.B. S100P as a marker for urothelial histogenesis: A critical review and comparison with novel and traditional urothelial immunohistochemical markers. Adv. Anat. Pathol. 2017, 24, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kandalaft, P.L.; Gown, A.M. Practical applications in immunohistochemistry: Carcinomas of unknown primary site. Arch. Pathol. Lab. Med. 2016, 140, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.G.; French, C.A.; Cameron, M.J.; Fletcher, C.D.; Jackman, D.M.; Lathan, C.S.; Sholl, L.M. Pathologic characteristics of nut midline carcinoma arising in the mediastinum. Am. J. Surg. Pathol. 2012, 36, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Haack, H.; Johnson, L.A.; Fry, C.J.; Crosby, K.; Polakiewicz, R.D.; Stelow, E.B.; Hong, S.M.; Schwartz, B.E.; Cameron, M.J.; Rubin, M.A.; et al. Diagnosis of nut midline carcinoma using a nut-specific monoclonal antibody. Am. J. Surg. Pathol. 2009, 33, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.A.; French, C.A.; Costello, B.A.; Marks, R.S.; Dronca, R.S.; Nerby, C.L.; Roden, A.C.; Peddareddigari, V.G.; Hilton, J.; Shapiro, G.I.; et al. Nut midline carcinoma: An aggressive intrathoracic neoplasm. J. Thorac. Oncol. 2013, 8, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.M.; Wu, J.; et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnsen, J.A.; Hellsten, S. Lymphatogenous spread of renal cell carcinoma: An autopsy study. J. Urol. 1997, 157, 450–453. [Google Scholar] [CrossRef]
- Sheridan, T.; Herawi, M.; Epstein, J.I.; Illei, P.B. The role of P501S and PSA in the diagnosis of metastatic adenocarcinoma of the prostate. Am. J. Surg. Pathol. 2007, 31, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Carder, P.J.; Speirs, V.; Ramsdale, J.; Lansdown, M.R. Expression of prostate specific antigen in male breast cancer. J. Clin. Pathol. 2005, 58, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Graddis, T.J.; McMahan, C.J.; Tamman, J.; Page, K.J.; Trager, J.B. Prostatic acid phosphatase expression in human tissues. Int. J. Clin. Exp. Pathol. 2011, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.H.; Walter, B.A.; Moreno, V.; Pack, S.D.; Pinto, P.; Merino, M.J. Correlation between erg fusion protein and androgen receptor expression by immunohistochemistry in prostate, possible role in diagnosis and therapy. J. Cancer 2017, 8, 2604–2613. [Google Scholar] [CrossRef] [PubMed]
- Queisser, A.; Hagedorn, S.A.; Braun, M.; Vogel, W.; Duensing, S.; Perner, S. Comparison of different prostatic markers in lymph node and distant metastases of prostate cancer. Mod. Pathol. 2015, 28, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Skinnider, B.F.; Amin, M.B. An immunohistochemical approach to the differential diagnosis of renal tumors. Semin. Diagn. Pathol. 2005, 22, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.T.; Ramachandran, R.; Kakar, S. Immunohistochemical approach for the diagnosis of a liver mass on small biopsy specimens. Hum. Pathol. 2017, 63, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Varadhachary, G.R.; Talantov, D.; Raber, M.N.; Meng, C.; Hess, K.R.; Jatkoe, T.; Lenzi, R.; Spigel, D.R.; Wang, Y.; Greco, F.A.; et al. Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J. Clin. Oncol. 2008, 26, 4442–4448. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.A.; Spigel, D.R.; Yardley, D.A.; Erlander, M.G.; Ma, X.J.; Hainsworth, J.D. Molecular profiling in unknown primary cancer: Accuracy of tissue of origin prediction. Oncologist 2010, 15, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Su, A.I.; Welsh, J.B.; Sapinoso, L.M.; Kern, S.G.; Dimitrov, P.; Lapp, H.; Schultz, P.G.; Powell, S.M.; Moskaluk, C.A.; Frierson, H.F., Jr.; et al. Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res. 2001, 61, 7388–7393. [Google Scholar] [PubMed]
- Hainsworth, J.D.; Greco, F.A. Gene expression profiling in patients with carcinoma of unknown primary site: From translational research to standard of care. Virchows Arch. 2014, 464, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Monzon, F.A.; Lyons-Weiler, M.; Buturovic, L.J.; Rigl, C.T.; Henner, W.D.; Sciulli, C.; Dumur, C.I.; Medeiros, F.; Anderson, G.G. Multicenter validation of a 1550-gene expression profile for identification of tumor tissue of origin. J. Clin. Oncol. 2009, 27, 2503–2508. [Google Scholar] [CrossRef] [PubMed]
- Erlander, M.G.; Ma, X.J.; Kesty, N.C.; Bao, L.; Salunga, R.; Schnabel, C.A. Performance and clinical evaluation of the 92-gene real-time pcr assay for tumor classification. J. Mol. Diagn. 2011, 13, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.J.; Patel, R.; Wang, X.; Salunga, R.; Murage, J.; Desai, R.; Tuggle, J.T.; Wang, W.; Chu, S.; Stecker, K.; et al. Molecular classification of human cancers using a 92-gene real-time quantitative polymerase chain reaction assay. Arch. Pathol. Lab. Med. 2006, 130, 465–473. [Google Scholar] [PubMed]
- Culine, S.; Lortholary, A.; Voigt, J.J.; Bugat, R.; Theodore, C.; Priou, F.; Kaminsky, M.C.; Lesimple, T.; Pivot, X.; Coudert, B.; et al. Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: Results of a randomized phase II study—Trial for the french study group on carcinomas of unknown primary (GEFCAPI 01). J. Clin. Oncol. 2003, 21, 3479–3482. [Google Scholar] [CrossRef] [PubMed]
- Gross-Goupil, M.; Fourcade, A.; Blot, E.; Penel, N.; Negrier, S.; Culine, S.; Chaigneau, L.; Lesimple, T.; Priou, F.; Lortholary, A.; et al. Cisplatin alone or combined with gemcitabine in carcinomas of unknown primary: Results of the randomised GEFCAPI 02 trial. Eur. J. Cancer 2012, 48, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Moran, S.; Martinez-Cardus, A.; Sayols, S.; Musulen, E.; Balana, C.; Estival-Gonzalez, A.; Moutinho, C.; Heyn, H.; Diaz-Lagares, A.; de Moura, M.C.; et al. Epigenetic profiling to classify cancer of unknown primary: A multicentre, retrospective analysis. Lancet Oncol. 2016, 17, 1386–1395. [Google Scholar] [CrossRef]
- Greco, F.A. Diagnosis: Improved diagnosis, therapy and outcomes for patients with cup. Nat. Rev. Clin. Oncol. 2017, 14, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Anthony Greco, F. Lung adenocarcinoma with anaplastic lymphoma kinase (ALK) rearrangement presenting as carcinoma of unknown primary site: Recognition and treatment implications. Drugs Real World Outcomes 2016, 3, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Ilie, M.; Hofman, V.; Lassalle, S.; Butori, C.; Alsubaie, S.; Hofman, P. Role of the surgical pathologist for tissue management in oncology. Bull. Cancer 2013, 100, 837–845. [Google Scholar] [PubMed]
- Ilie, M.; Hofman, P. The potential value of immunohistochemistry as a screening tool for oncogenic targets of personalized lung cancer therapy. J. Oncopathol. 2013, 1, 82. [Google Scholar] [CrossRef]
- Hofman, V.; Lassalle, S.; Bence, C.; Long-Mira, E.; Nahon-Esteve, S.; Heeke, S.; Lespinet-Fabre, V.; Butori, C.; Ilie, M.; Hofman, P. Any place for immunohistochemistry within the predictive biomarkers of treatment in lung cancer patients? Cancers 2018, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Gatalica, Z.; Millis, S.Z.; Vranic, S.; Bender, R.; Basu, G.D.; Voss, A.; Von Hoff, D.D. Comprehensive tumor profiling identifies numerous biomarkers of drug response in cancers of unknown primary site: Analysis of 1806 cases. Oncotarget 2014, 5, 12440–12447. [Google Scholar] [CrossRef] [PubMed]
- Groschel, S.; Bommer, M.; Hutter, B.; Budczies, J.; Bonekamp, D.; Heining, C.; Horak, P.; Frohlich, M.; Uhrig, S.; Hubschmann, D.; et al. Integration of genomics and histology revises diagnosis and enables effective therapy of refractory cancer of unknown primary with pdl1 amplification. Cold Spring Harb. Mol. Case Stud. 2016, 2, a001180. [Google Scholar] [CrossRef] [PubMed]
- Kandukuri, S.R.; Lin, F.; Gui, L.; Gong, Y.; Fan, F.; Chen, L.; Cai, G.; Liu, H. Application of immunohistochemistry in undifferentiated neoplasms: A practical approach. Arch. Pathol. Lab. Med. 2017, 141, 1014–1032. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Rossi, S.; Acosta Marin, M.; Canal, F.; Sbaraglia, M.; Laurino, L.; Mazzoleni, G.; Montesco, M.C.; Valori, L.; Campo Dell’Orto, M.; et al. Primary synovial sarcoma (SS) of the digestive system: A molecular and clinicopathological study of fifteen cases. Clin. Sarcoma Res. 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Van Tine, B.A. Synovial sarcoma: Current concepts and future perspectives. J. Clin. Oncol. 2018, 36, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Binh, M.B.; Sastre-Garau, X.; Guillou, L.; de Pinieux, G.; Terrier, P.; Lagace, R.; Aurias, A.; Hostein, I.; Coindre, J.M. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: A comparative analysis of 559 soft tissue neoplasms with genetic data. Am. J. Surg. Pathol. 2005, 29, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, S.; Coindre, J.M.; Michels, J.J.; Terrier, P.; Bertrand, G.; Trassard, M.; Taylor, S.; Chateau, M.C.; Marques, B.; Picot, V.; et al. Pleomorphic liposarcoma: Clinicopathologic, immunohistochemical, and follow-up analysis of 63 cases: A study from the french federation of cancer centers sarcoma group. Am. J. Surg. Pathol. 2002, 26, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Hornick, J.L.; Bosenberg, M.W.; Mentzel, T.; McMenamin, M.E.; Oliveira, A.M.; Fletcher, C.D. Pleomorphic liposarcoma: Clinicopathologic analysis of 57 cases. Am. J. Surg. Pathol. 2004, 28, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Kohashi, K.; Oda, Y.; Yamamoto, H.; Tamiya, S.; Matono, H.; Iwamoto, Y.; Taguchi, T.; Tsuneyoshi, M. Reduced expression of SMARCB1/INI1 protein in synovial sarcoma. Mod. Pathol. 2010, 23, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Kravtsov, O.; Chang, J.; Hackbarth, D.; Giorgadze, T. Myoepithelioma of soft tissue: A cytological-pathological correlation with literature review. Ann. Diagn. Pathol. 2017, 27, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Thway, K. Malignant peripheral nerve sheath tumor with divergent glandular differentiation. Int. J. Surg. Pathol. 2017, 25, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Thway, K.; Hamarneh, W.; Miah, A.B.; Fisher, C. Malignant peripheral nerve sheath tumor with rhabdomyosarcomatous and glandular elements: Rare epithelial differentiation in a triton tumor. Int. J. Surg. Pathol. 2015, 23, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R.; Delahunt, B.; Magi-Galluzzi, C.; Algaba, F.; Egevad, L.; Ulbright, T.M.; Tickoo, S.K.; Srigley, J.R.; Epstein, J.I.; Berney, D.M. The world health organization 2016 classification of testicular germ cell tumours: A review and update from the international society of urological pathology testis consultation panel. Histopathology 2017, 70, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Berton-Rigaud, D.; Devouassoux-Shisheboran, M.; Ledermann, J.A.; Leitao, M.M.; Powell, M.A.; Poveda, A.; Beale, P.; Glasspool, R.M.; Creutzberg, C.L.; Harter, P.; et al. Gynecologic cancer intergroup (GCIG) consensus review for uterine and ovarian carcinosarcoma. Int. J. Gynecol. Cancer 2014, 24, S55–S60. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Zhang, L.; Chang, N.E.; Pawel, B.R.; Travis, W.; Katabi, N.; Edelman, M.; Rosenberg, A.E.; Nielsen, G.P.; Dal Cin, P.; et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer 2010, 49, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Fetsch, J.F.; Laskin, W.B.; Michal, M.; Remotti, F.; Heffner, D.; Ellis, G.; Furlong, M.; Miettinen, M. Ectopic hamartomatous thymoma: A clinicopathologic and immunohistochemical analysis of 21 cases with data supporting reclassification as a branchial anlage mixed tumor. Am. J. Surg. Pathol. 2004, 28, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
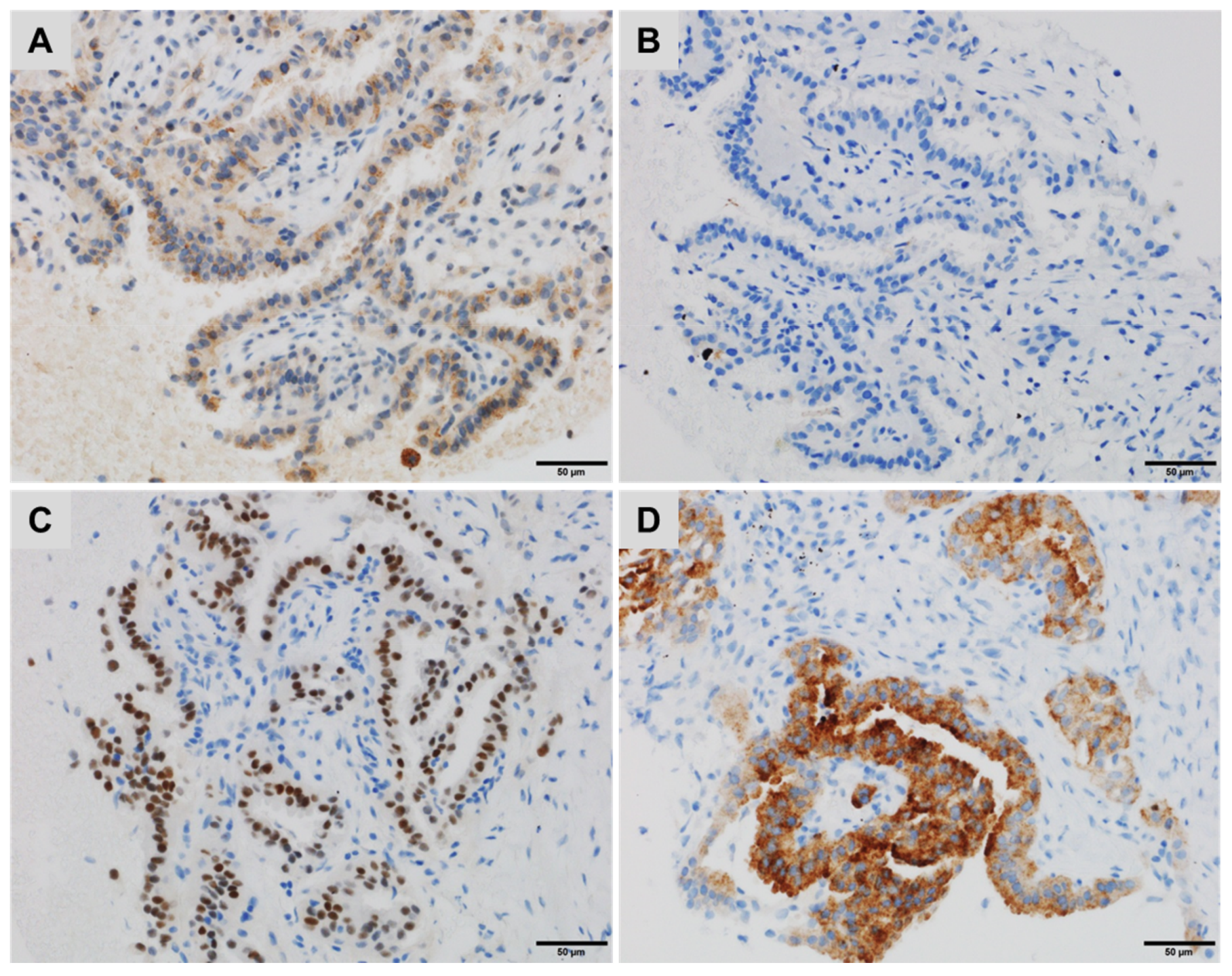
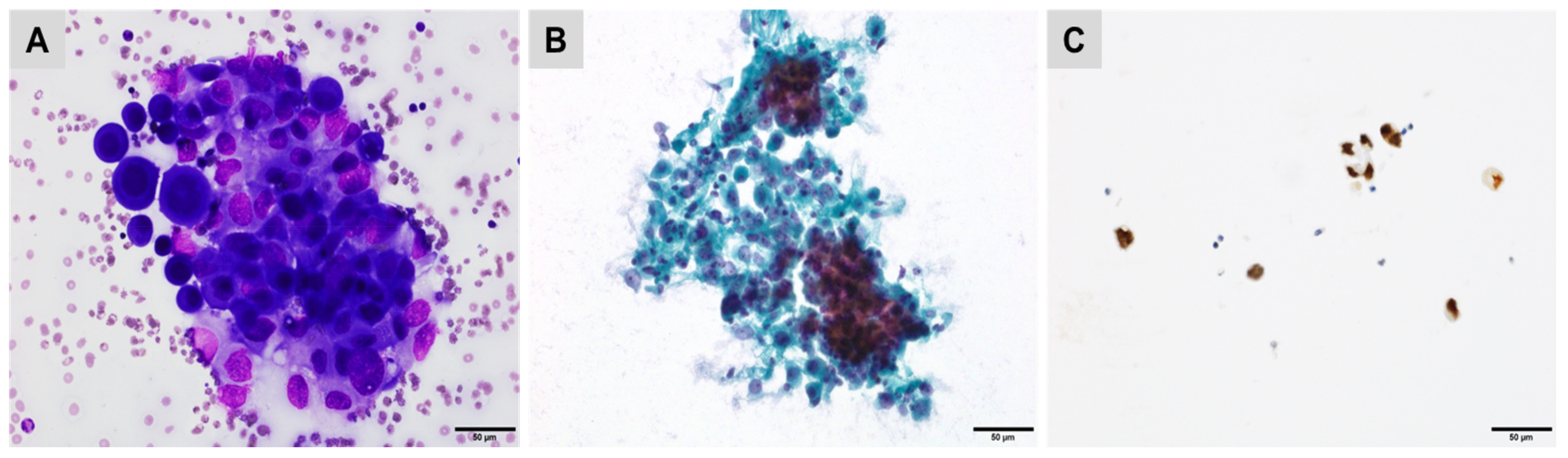
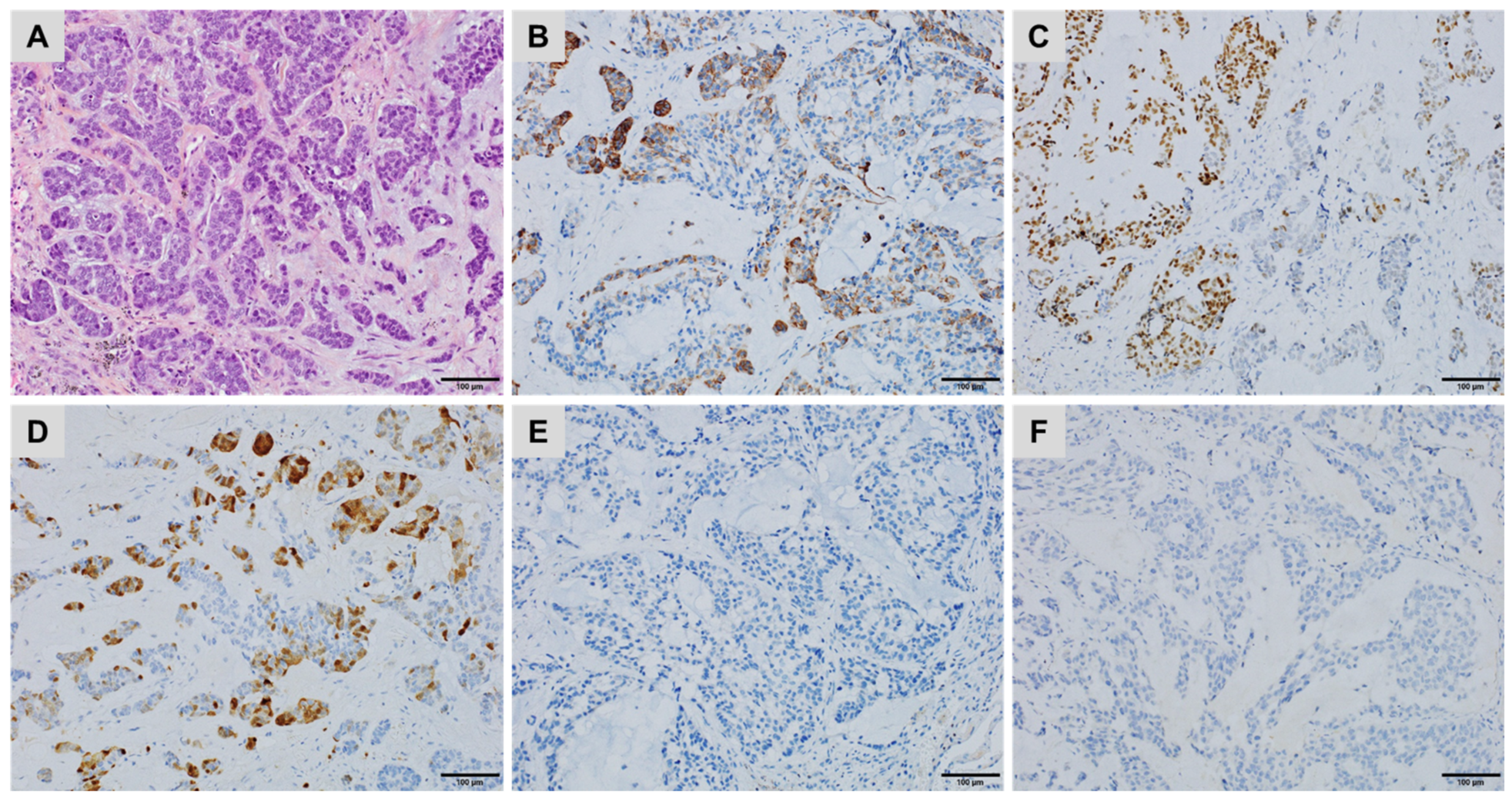
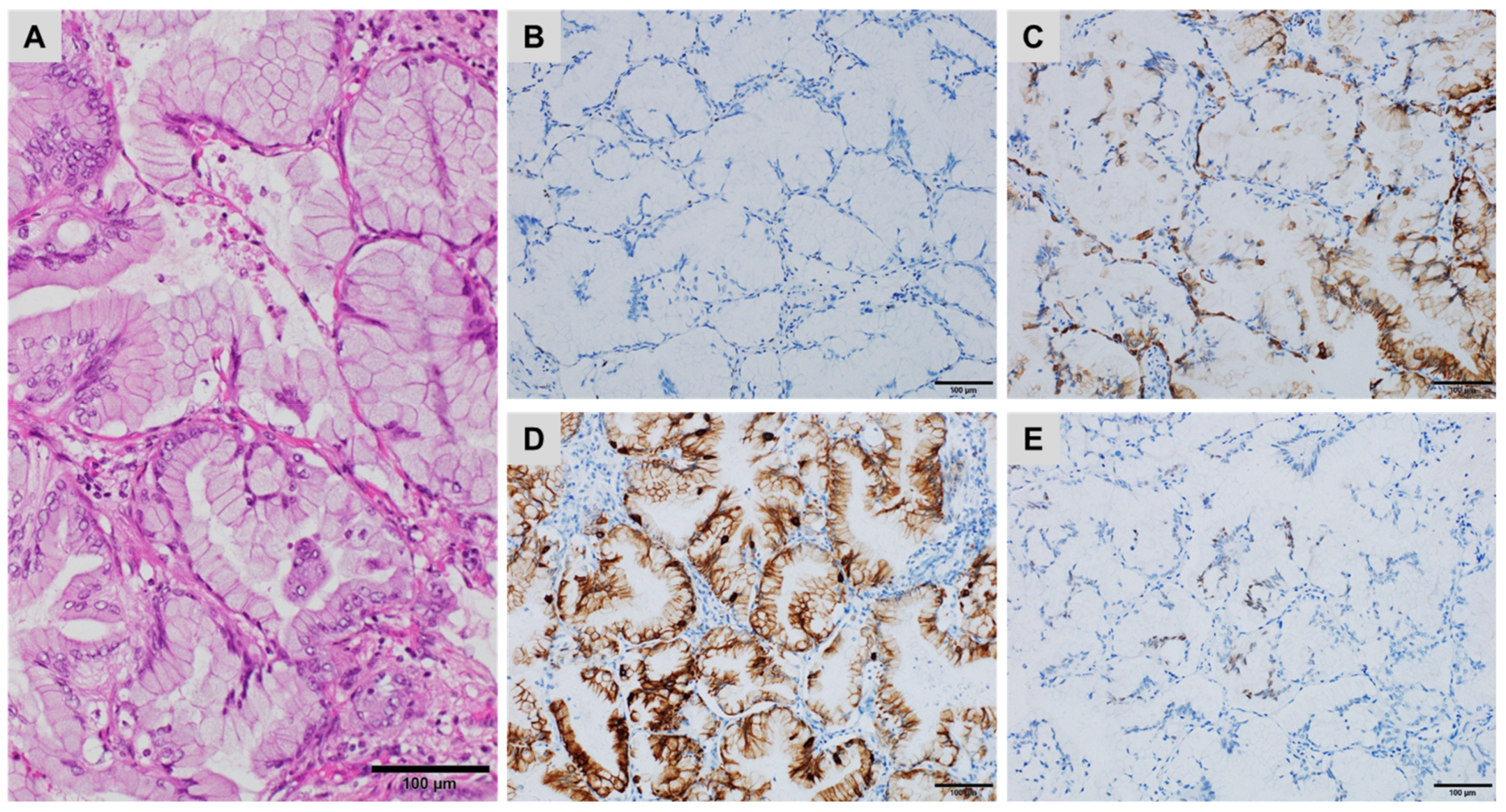
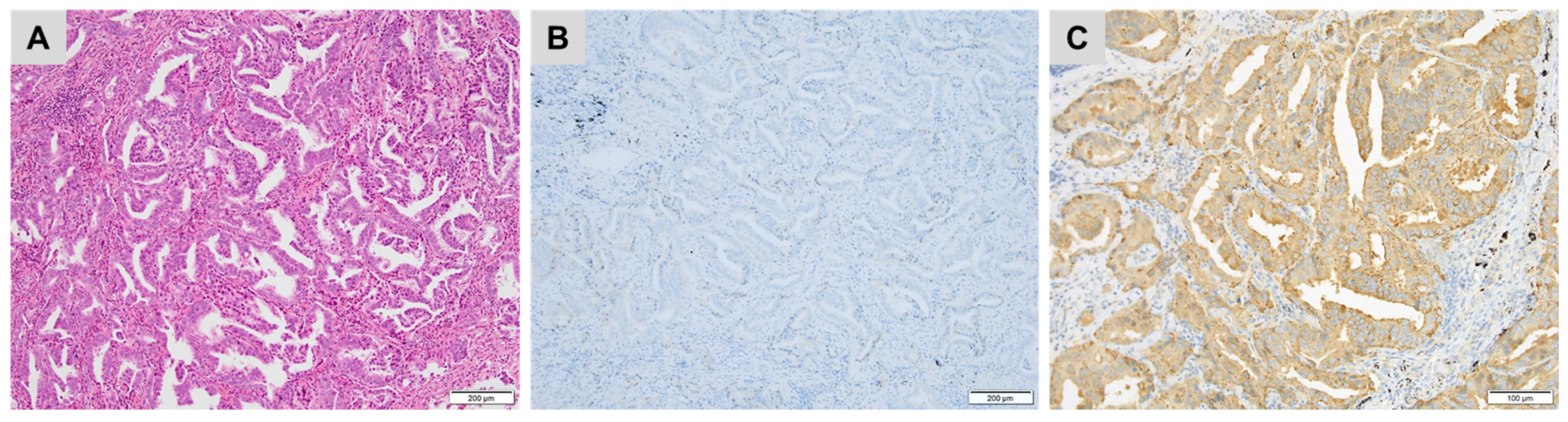
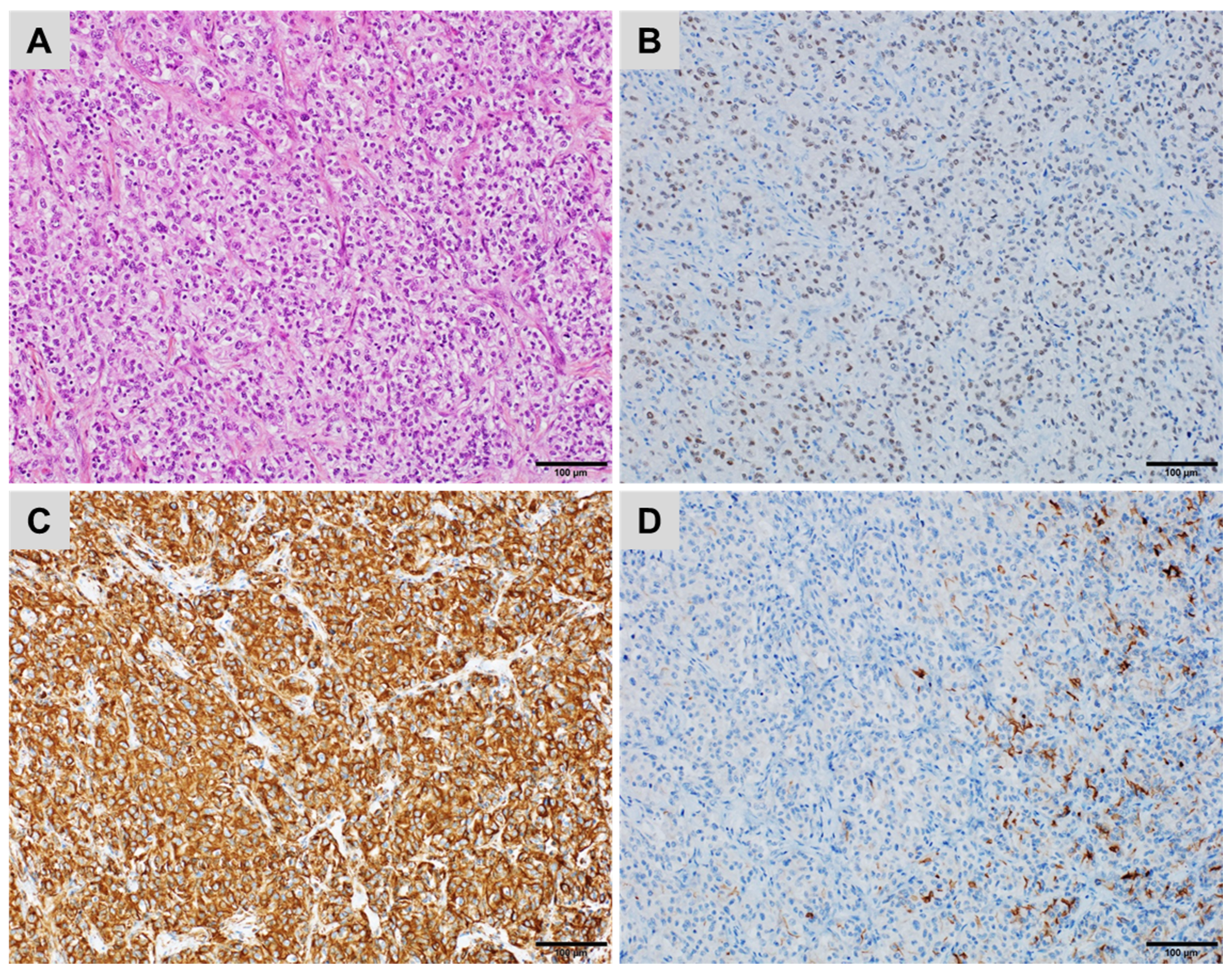
| CK7+/CK20− | CK7+/CK20+ | CK7−/CK20+ | CK7−/CK20− |
|---|---|---|---|
| Breast carcinoma Lung adenocarcinoma Endometrial adenocarcinoma Endocervical adenocarcinoma Ovarian (serous) carcinoma Cholangiocarcinoma Small cell lung carcinoma Mesothelioma Thyroid carcinoma Salivary gland tumours Kidney (papillary) Urothelial carcinoma (subset) Pancreatic adenocarcinoma Gastric adenocarcinoma | Urothelial carcinoma Pancreatic adenocarcinoma Ovarian mucinous carcinoma Bladder adenocarcinoma Gastric adenocarcinoma Cholangiocarcinoma | Colorectal adenocarcinoma Merkel cell carcinoma Gastric adenocarcinoma | Prostate adenocarcinoma Renal (clear cells) Hepatocellular carcinoma Adrenocortical carcinoma Non-seminoma germ cell tumours Mesothelioma Small cell lung carcinoma Gastric adenocarcinoma |
| Primary Site of Origin | Immunostaining Profile |
|---|---|
| Breast [8,14,15,16,17] | ER+/PgR+, GATA3+, GCDFP15−/+, MGB+/−, TFF1− |
| Ovary (serous) [17,18,19,20,21] | PAX8+, ER+, WT1+, TTF1−, TFF3−, GATA3− |
| Ovary (clear cell) [17,18,19,20,21] | pVHL+, HNF-1β+, Napsin A+, AFP−, WT1−, ER−, GPC3− |
| Endometrium [17,18,19,20,21] | ER+, PAX8+, Vimentin+ |
| Uterine cervix [17,18,19,20,21] | p16+, HPV+, CEA+, PR−, PAX2−, PAX8+/− |
| Lung [22,23,24] | TTF1+, Napsin A+, GATA3− |
| Thyroid (papillary/follicular) [23,24,25] | TTF1+, Thyroglobulin+, PAX8+ |
| Thyroid (medullary) [23,24,25] | TTF1+, Calcitonin+, CEA+ |
| Stomach [26,27,28,29] | CEA+, CDX2−/+, MUC1−/+, MUC5AC−/+, CDH17+/−, TTF1− |
| Oesophagus [26,27,28,29] | CDX2+/−, CEA+, CDH17+, MUC1−/+, MUC5AC−/+, SATB2− |
| Pancreas [26,27,28,29] | DPC4−/+, CK17+/−, pVHL−, Maspin+, S100P+, MUC5AC+ |
| Urinary bladder [17,18,19,20,21] | GATA3+, p63+, CK5/6+, p40+, S100P+, CK903+, UPII+/− |
| Thymus [19,20,21] | CD5+/−, p63+/−, PAX8+/−, CD117+/−, Glut1+/− |
| Salivary (ductal) [16,17,30] | GATA3+, AR+, GCDFP-15+ |
| Mesothelioma [30,31,32,33,34] | Calretinin+, WT1+, CK5/6+, TTF1−, CEA−, BerP4− |
| Mesothelial Markers | |
| Calretinin | Useful. Positive in 85–100% of MPMs. Positivity in 0–38% of PSPCs prevents its use as a single differential marker. |
| D2-40 | Potentially useful. Positive in 93–96% of MPMs but also focal positivity in 13–65% of PSPCs; additional data are needed. |
| CK5/6 | Limited use. Positive in 53–100% of MPMs but also focal positivity in 22–35% of PSPCs. |
| WT1 | Not useful. Positive in 43–93% of MPMs and positive in 89–93% of PSPCs. |
| PSPCs Markers | |
| MOC-31 | Very useful. Positive in 98% of PSPCs and 5% of MPMs. |
| PAX8 | Very useful. Positive in most Mullerian carcinomas and negative in MPMs. |
| BG8 | Very useful. Positive in 73% of PSPCs and 3–9% of MPMs. |
| BerEP4 | Useful. Positive in 83–100% of PSPCs and in 9–13% of MPMs. |
| B72.3 | Limited use. Positive in 65–100% of PSPCs and focal expression in 0–3% of MPMs. |
| CEA | Not useful. Positive in only 0–45% of PSPCs and negative in MPMs but sensitivity too low compared to other markers. |
| Oestrogen receptor | Useful. Positive in 60–93% of PSPCs and 0–8% of MPMs. |
| Progesterone receptor | Limited use. Lower sensitivity than oestrogen receptors in PSPCs, negative in MPMs. Can be useful when positive. |
| Mesothelioma vs. Non-Gynaecological Adenocarcinoma (Biliary, Pancreas, Stomach, Colon) | |
| Calretinin | Very useful. Positive in 85–100% of MPMs but also positive in 10% of pancreatic ADCs, limited value as single marker. |
| WT1 | Very useful. Positive in 43–93% of MPMs, 3% of gastric ADCs and 0% of pancreatic ADCs. |
| D2-40 | Potentially useful. Positive in 93–96% of MPMs, negative in gastric and pancreatic ADCs (limited data). |
| CK5/6 | Not useful. Positive in 53–100% of MPMs and 38% of pancreatic ADCs. |
| MOC-31 | Very useful. Positive in 5% of MPMs and 87% of ADCs. |
| BG8 | Very useful. Positive in 3–9% of MPMs and 89% of ADCs. |
| CEA | Very useful. Negative in MPMs and positive in 81% of ADCs. |
| B72.3 | Very useful. Positive in 0–3% of MPMs, 84% of pancreatic ADCs, 89% of biliary ADCs, 98% colon ADCs. |
| BerEP4 | Useful. Positive in 9–13% of MPMs et >98% pancreatic and gastric ADCs. |
| CDX2 | Useful. Positive in 90–100% of colon ADCs, 80% small intestine ADCs, 70% of gastric ADCs and negative in MPMs. |
| Primary Origin Site | Immunostaining Profile |
|---|---|
| Lung (mucinous) [49] | TTF1−/+, CK7−/+, CDX2−/+ |
| Pancreas [26,27,50,51] | Maspin A+, S100P+, IMP-3+, pVHL−, SMAD4−/+, MUC5AC+, CDX2−/+ |
| Stomach [26,27,50,51] | CEA+, CDX2−/+, MUC1−/+, MUC5AC−/+, CDH17+/−, TTF1− |
| Oesophagus [26,27,50,51] | CEA+, MUC5AC+/−, CDH17+, MUC1−/+, CDX2−/+ |
| Ovary (mucinous) [7,52] | DPC4+, CA-12.5+, CDX2+/− |
| Urinary bladder [17,18,19,20,21] | GATA3+, p63+, p40+, CK5/6+, CK20+/−, S100P+, CK903+, UPIII+/− |
| Small intestine [39] | CDX2+, CDH17+, Villin+/−, MUC5AC+/− |
| NUT midline carcinoma [53,54,55] | CK7+/−, CK20+/−, p40+/−, NUT |
| Primary Site | IHC Profile |
|---|---|
| Prostate [58,59,60,61,62] | PSA+, NKX3.1+/−, PSAP+, P504S+, ERG+/− |
| Colon (medullary) [29,35,36] | SATB2+, CDH17+, TFF3+/−, Calretinin+/−, CDX2−/+ |
| Renal [21,63] | CD10+, PAX8+, Vimentin+, pVHL+, RCCMa+, Inhibin−, TTF1−, CEA− |
| Liver [64] | HepPar1+, CD10+, pCEA+, mCEA−, AFP+, Glypican-3+, Arginase-1+, CK19− |
| Adrenal (cortical) [4,7,10] | Melan A+, Calretinin+, Inhibin A+, Synaptophysin+, Chromograni−, CEA− |
| Germ cell tumours [4,7,10] | CD117+, OCT4+, CD30+, Glypican-3+, PLAP+, SALL4+, NANOG+ |
| Type of Tumour | IHC Markers |
|---|---|
| Synovial sarcoma [83,84] | AE1/AE3+/−, EMA+/− (epithelial cells), S100+ (spindle cells), CD34+ |
| Dedifferentiated liposarcoma [85,86,87] | MDM2+, CDK4+, HMGA2+ |
| Mixed tumour/myoepithelioma of soft tissue [88,89] | AE1/AE3+, EMA+, S100+, SMA+/−, p63+/−, GFAP+/−, INI-1− |
| MPNST [90,91] | S100+, AE1/AE3−, EMA− (spindle cells) |
| Hamartomatous ectopic thymoma [92] | AE1/AE3+, CK5/6+, CK14+, SMA+, CD34+ (spindle cells), Desmin−, S100− |
| Biphasic mesothelioma(epithelial component) [31,32] | AE1/AE3+, CK5/6+, Calretinin+, WT1+ |
| Germ cell tumours [92] | AE1/AE3+, SALL-4+, CD117+, OCT-4+ |
| Malignant mixed Mullerian tumours (“carcinosarcoma”) [93] | AE1/AE3+, PAX8+, WT1+Sarcomatoid components: Desmin+, Myogenin, Caldesmon+, S100+ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selves, J.; Long-Mira, E.; Mathieu, M.-C.; Rochaix, P.; Ilié, M. Immunohistochemistry for Diagnosis of Metastatic Carcinomas of Unknown Primary Site. Cancers 2018, 10, 108. https://doi.org/10.3390/cancers10040108
Selves J, Long-Mira E, Mathieu M-C, Rochaix P, Ilié M. Immunohistochemistry for Diagnosis of Metastatic Carcinomas of Unknown Primary Site. Cancers. 2018; 10(4):108. https://doi.org/10.3390/cancers10040108
Chicago/Turabian StyleSelves, Janick, Elodie Long-Mira, Marie-Christine Mathieu, Philippe Rochaix, and Marius Ilié. 2018. "Immunohistochemistry for Diagnosis of Metastatic Carcinomas of Unknown Primary Site" Cancers 10, no. 4: 108. https://doi.org/10.3390/cancers10040108





