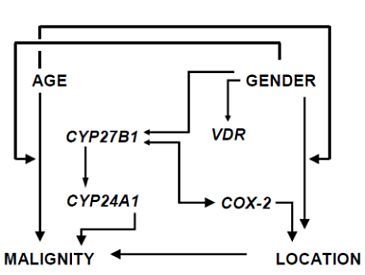
Relative Expression of Vitamin D Hydroxylases, CYP27B1 and CYP24A1, and of Cyclooxygenase-2 and Heterogeneity of Human Colorectal Cancer in Relation to Age, Gender, Tumor Location, and Malignancy: Results from Factor and Cluster Analysis
Abstract
:1. Introduction
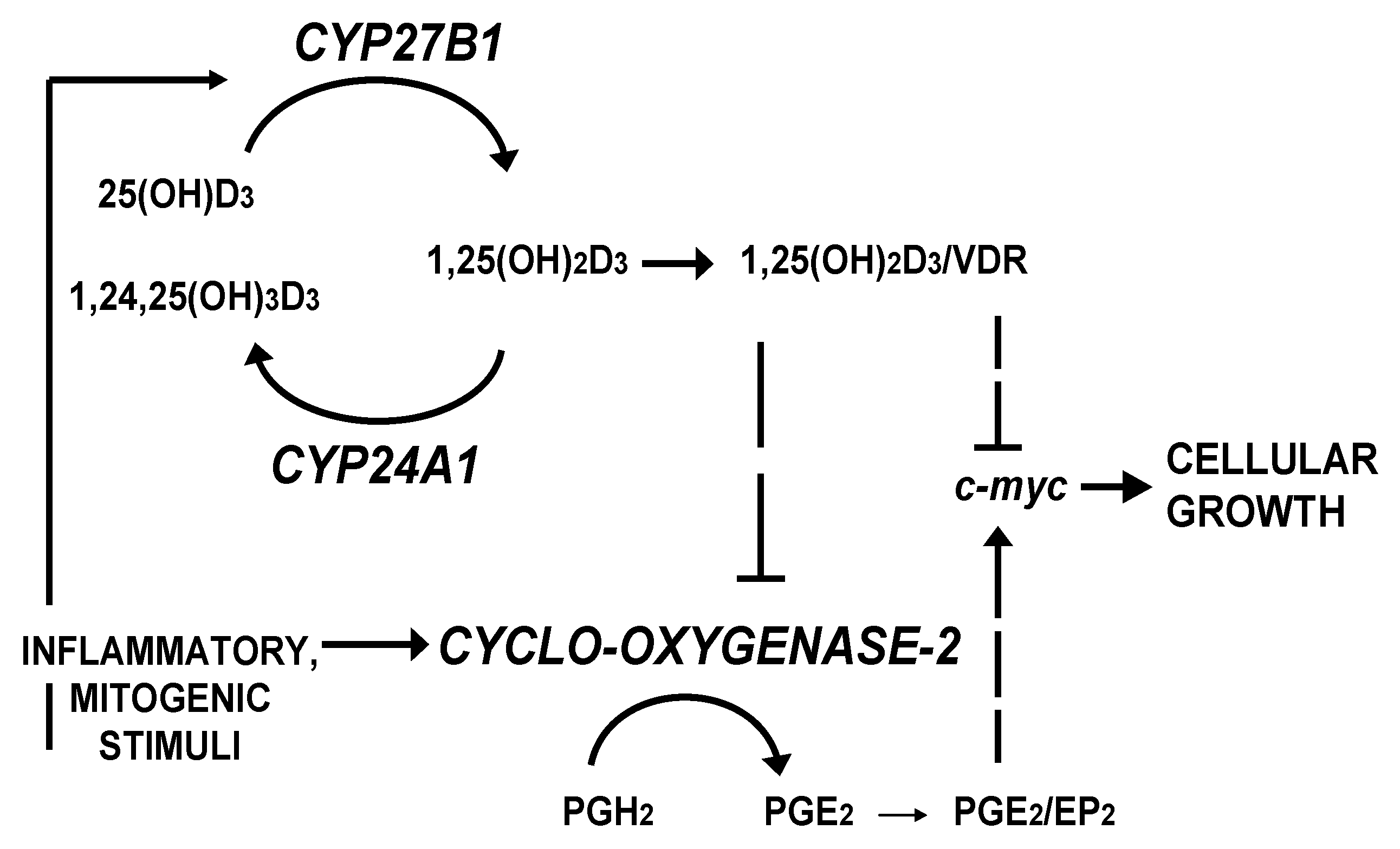
2. Patients and Methods
2.1. Patient Data and Analysis of Tissue Samples
2.2. Expression Analysis
2.3. Statistical Methods
3. Results
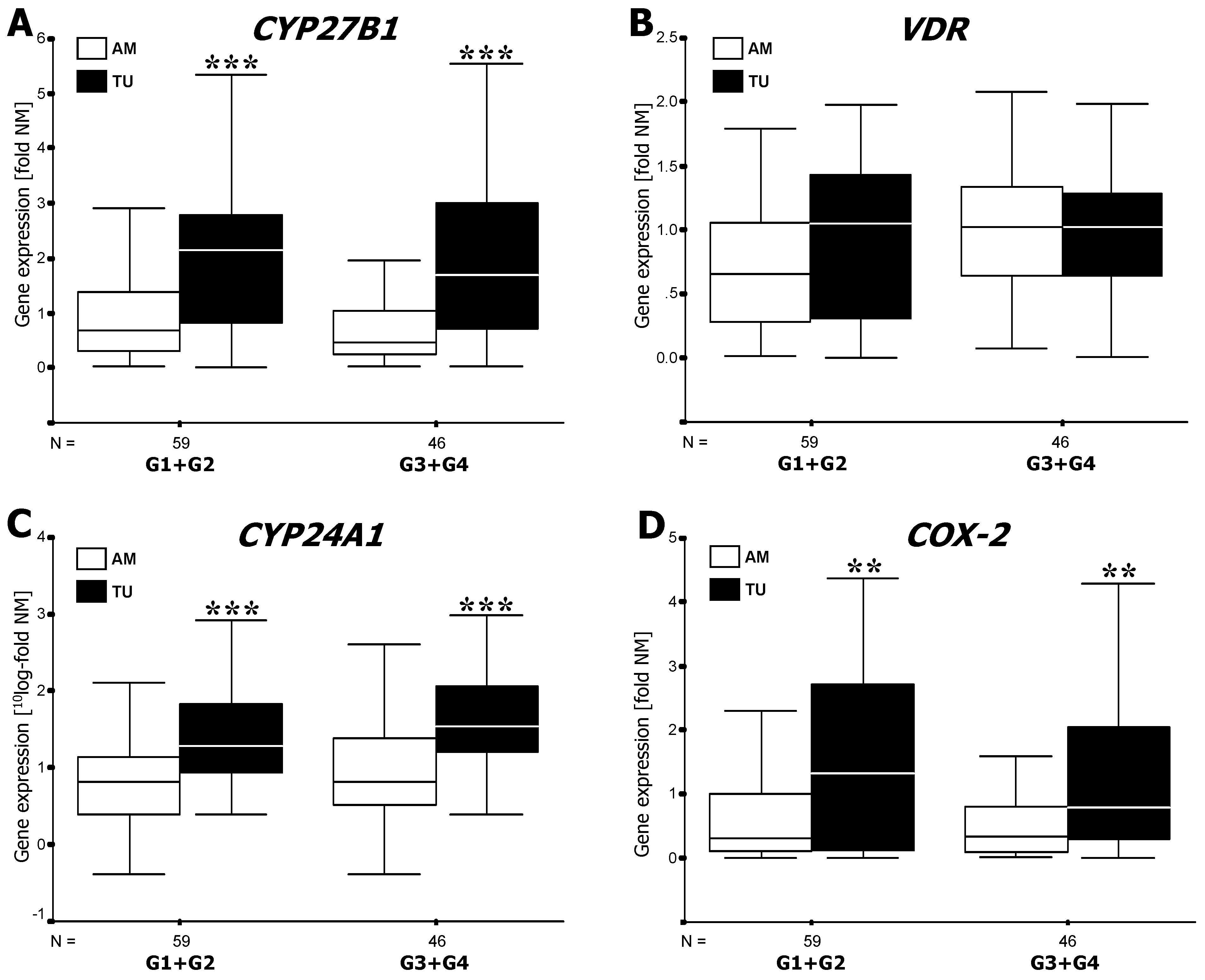
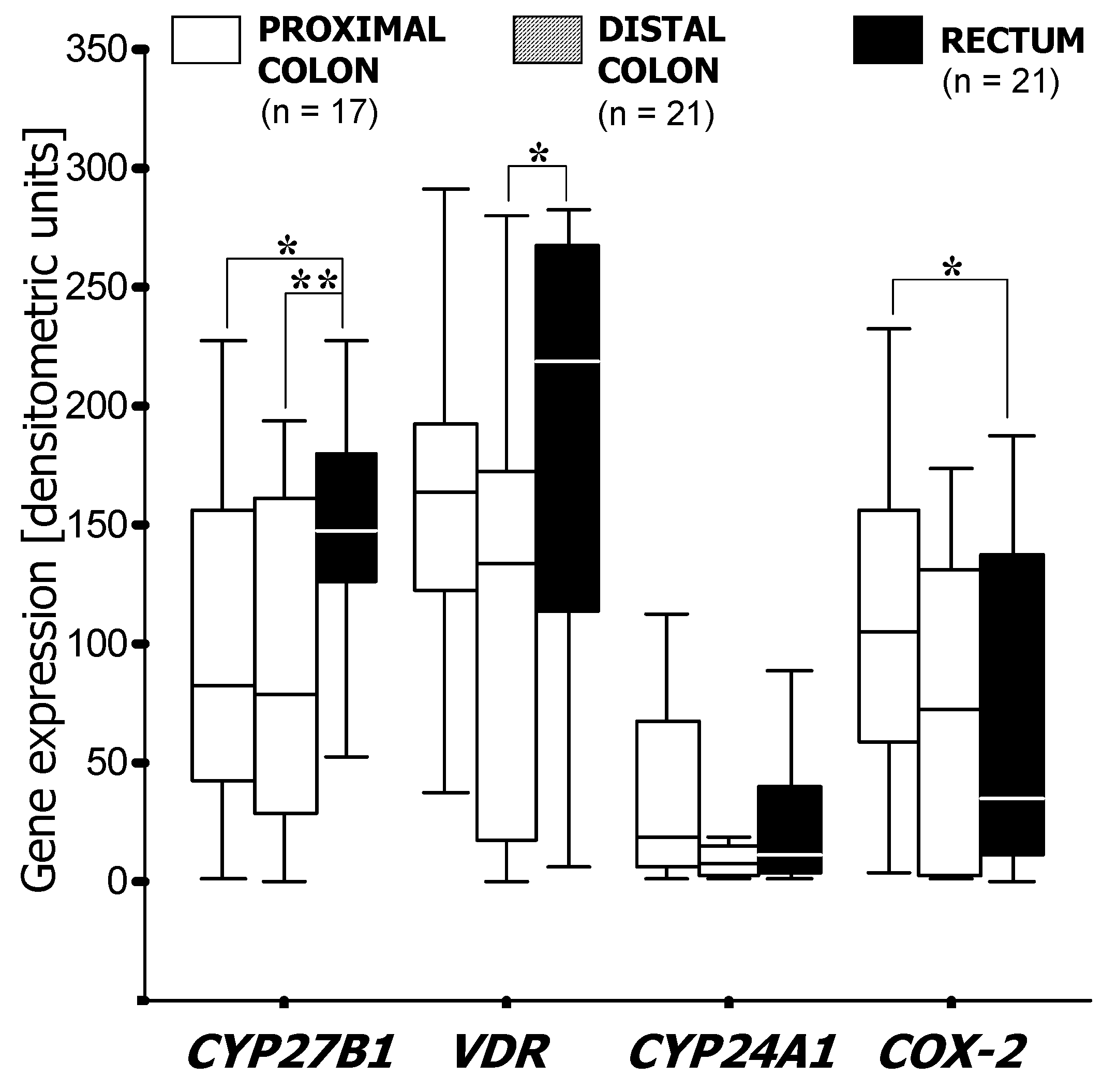
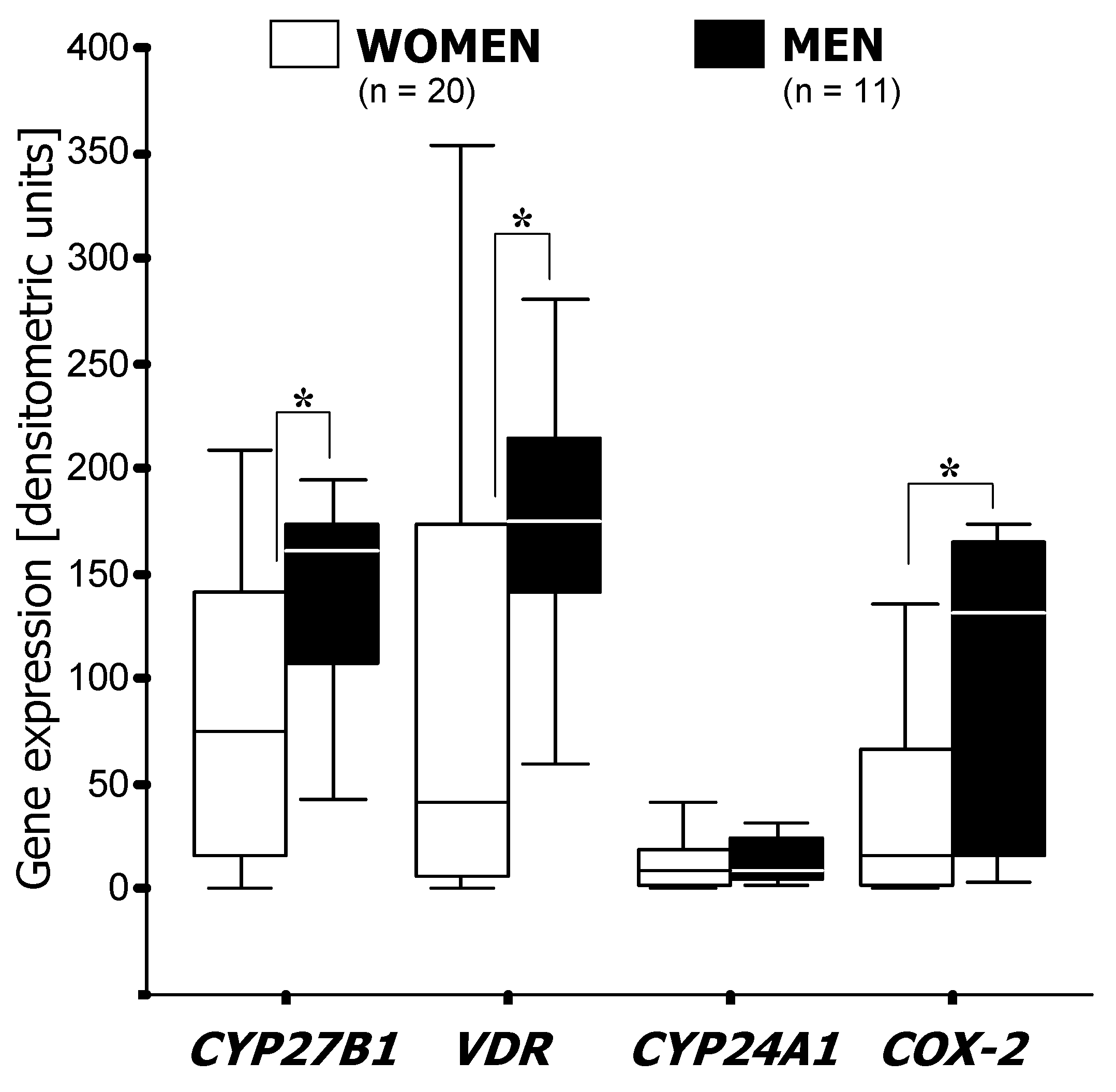
3.1. RT-PCR Analysis of mRNA Expression
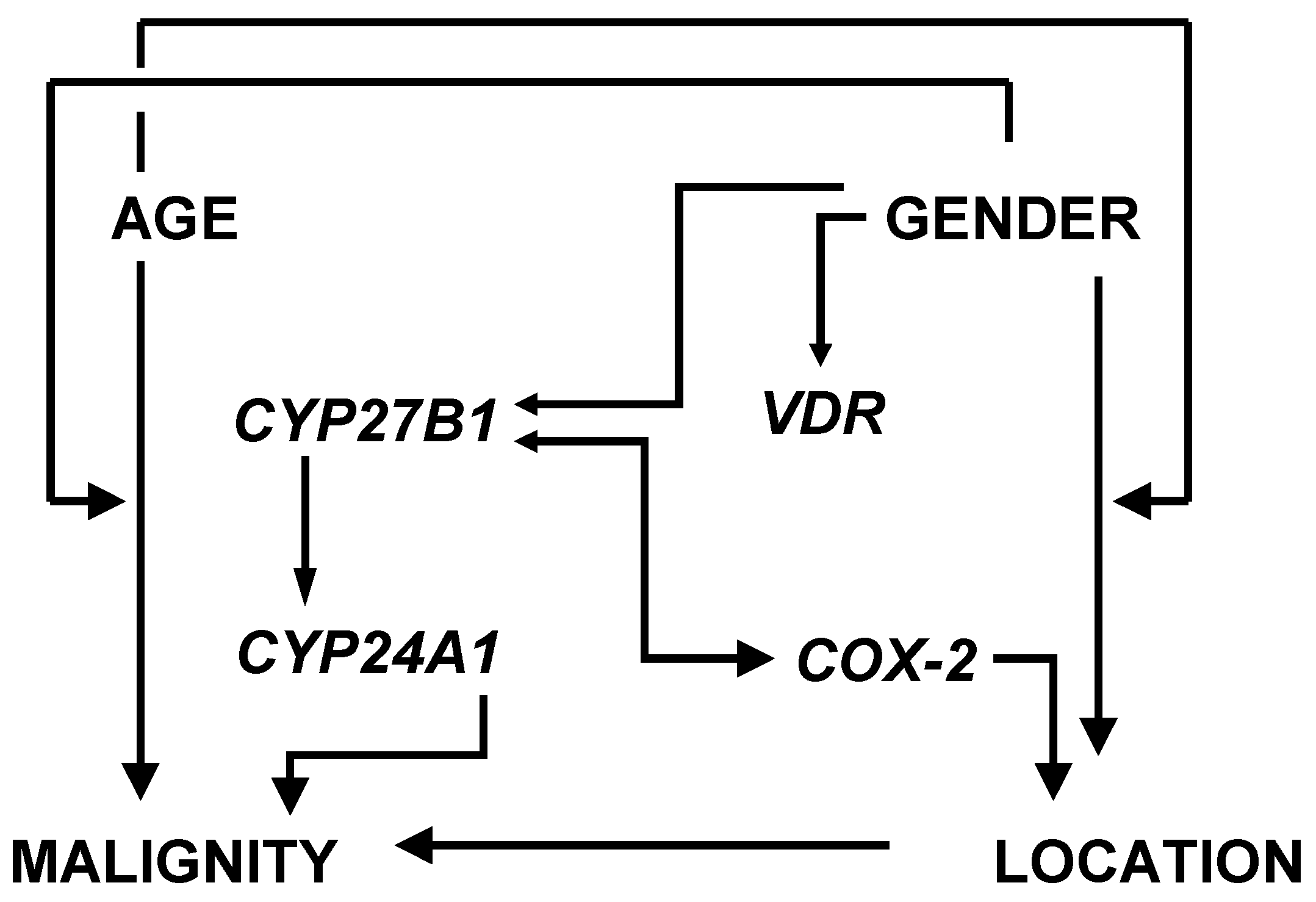
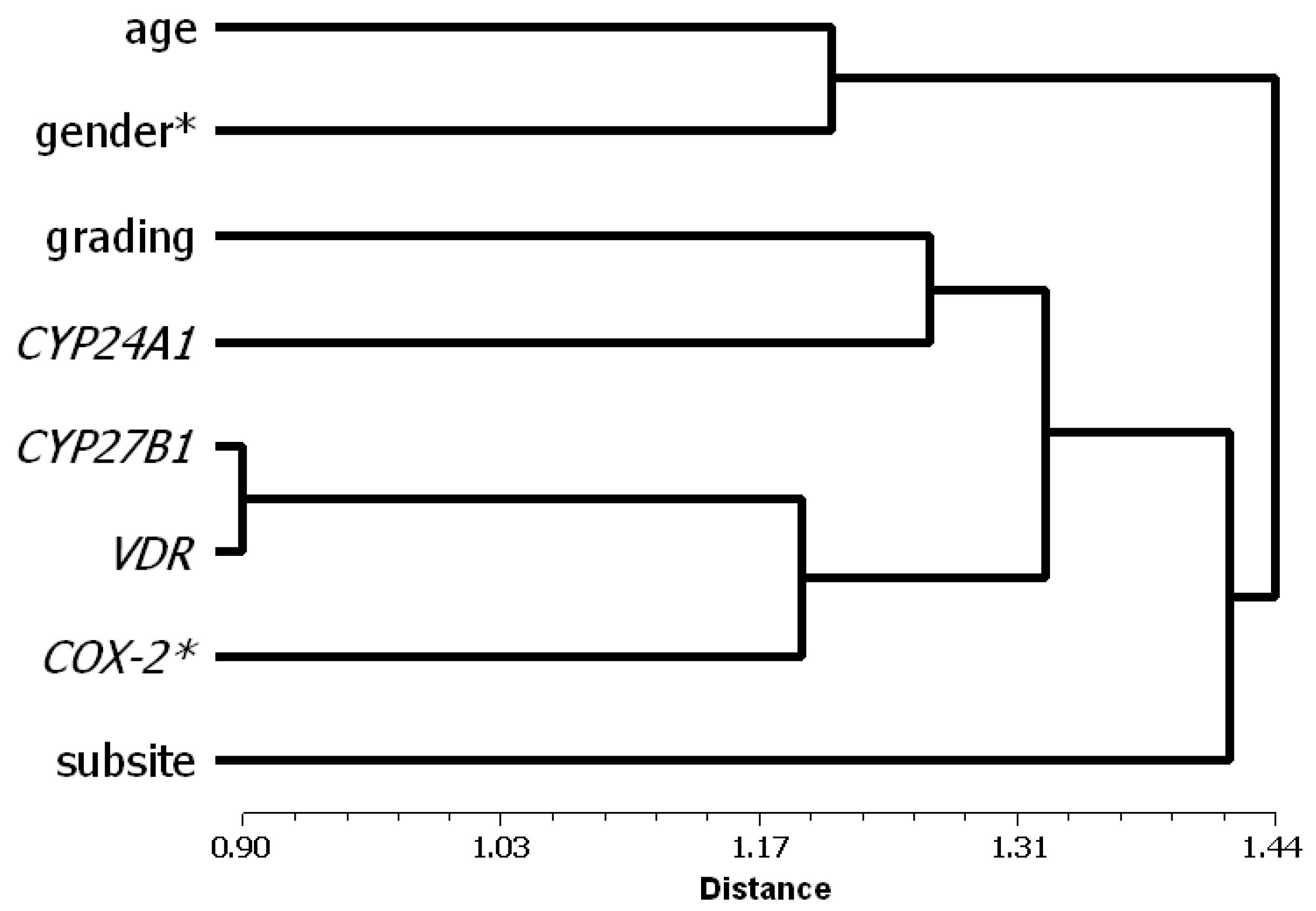
3.2. Factor and Cluster Analysis
| Colorectal cancer tissue | ||||||||
| F1TU | F2TU | F3TU | F4TU | |||||
| PF | ML | PF | ML | PF | ML | PF | ML | |
| CYP27B1 | 0.73 | 0.61 | 0.07 | 0.15 | −0.08 | −0.11 | 0.05 | 0.15 |
| VDR | 0.81 | 0.92 | −0.11 | 0.01 | −0.03 | −0.01 | 0.2 | 0.19 |
| COX-2 | 0.34 | 0.38 | −0.50 | -0.3 | −0.10 | −0.14 | −0.02 | 0.01 |
| subsite | 0.2 | 0.1 | 0.62 | 0.9 | −0.12 | −0.10 | −0.16 | −0.13 |
| gender | −0.18 | −0.17 | 0.33 | 0.24 | 0.45 | 0.95 | 0.18 | 0.14 |
| age | 0 | −0.02 | −0.12 | -0.13 | 0.7 | 0.31 | −0.07 | 0 |
| grading | −0.01 | 0.04 | −0.03 | -0.05 | 0 | 0.05 | 0.4 | 0.25 |
| CYP24A1 | 0.24 | 0.16 | −0.03 | 0.01 | 0.02 | −0.04 | 0.5 | 0.77 |
| Adjacent Mucosa | ||||||||
| CYP27B1 | 1 | 0.99 | 0.03 | 0.08 | 0.06 | 0.06 | −0.03 | 0.13 |
| VDR | 0.42 | 0.34 | −0.06 | −0.04 | 0.11 | 0.07 | 0.6 | 0.69 |
| COX-2 | 0.25 | 0.18 | −0.05 | −0.03 | 0.1 | 0.07 | 0.3 | 0.34 |
| subsite | 0.06 | 0.08 | 0.16 | 0.19 | −0.25 | −0.20 | −0.16 | -0.15 |
| gender | −0.04 | −0.08 | 0.77 | 0.63 | 0.14 | 0.18 | 0.01 | 0 |
| age | 0.12 | 0.11 | 0.2 | 0.15 | 0.83 | 0.98 | −0.02 | 0.02 |
| grading | −0.11 | −0.20 | 0.09 | 0.1 | −0.03 | −0.03 | 0.52 | 0.49 |
| CYP24A1 | 0.32 | 0.29 | 0.31 | 0.41 | −0.17 | −0.14 | 0.1 | 0.11 |
4. Discussion
5. Conclusions
Conflict of Interest
References
- Jass, J.R. Molecular heterogeneity of colorectal cancer: Implications for cancer control. Surg. Oncol. 2007, 16, S7–S9. [Google Scholar] [CrossRef]
- Brozek, W.; Kriwanek, S.; Bonner, E.; Peterlik, M.; Cross, H.S. Mutual associations between malignancy, age, gender, and subsite incidence of colorectal cancer. Anticancer Res. 2009, 29, 3721–3726. [Google Scholar]
- Cross, H.S.; Nittke, T.; Kallay, E. Colonic vitamin D metabolism: Implications for the pathogenesis of inflammatory bowel disease and colorectal cancer. Mol. Cell. Endocrinol. 2011, 347, 70–79. [Google Scholar]
- Krishnan, A.V.; Moreno, J.; Nonn, L.; Malloy, P.; Swami, S.; Peng, L.; Peehl, D.M.; Feldman, D. Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in prostate cancer. J. Steroid Biochem. Mol. Biol. 2007, 103, 694–702. [Google Scholar] [CrossRef]
- Cross, H.S.; Kallay, E. Regulation of the colonic vitamin D system for prevention of tumor progression: An update. Future Oncol. 2009, 5, 493–507. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Lechner, D.; Kallay, E.; Cross, H.S. 1alpha,25-Dihydroxyvitamin D3 downregulates CYP27B1 and induces CYP24A1 in colon cells. Mol. Cell. Endocrinol. 2007, 263, 55–64. [Google Scholar]
- Horvath, H.C.; Lakatos, P.; Kosa, J.P.; Bacsi, K.; Borka, K.; Bises, G.; Nittke, T.; Hershberger, P.A.; Speer, G.; Kallay, E. The candidate oncogene CYP24A1: A potential biomarker for colorectal tumorigenesis. J. Histochem. Cytochem. 2010, 58, 277–285. [Google Scholar] [CrossRef]
- Nittke, T.; Selig, S.; Kallay, E.; Cross, H.S. Nutritional calcium modulates colonic expression of vitamin D receptor and pregnane X receptor target genes. Mol. Nutr. Food Res. 2008, 52, 45–51. [Google Scholar]
- Eberhart, C.E.; Coffey, R.J.; Radhika, A.; Giardiello, F.M.; Ferrenbach, S.; DuBois, R.N. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994, 107, 1183–1188. [Google Scholar]
- Fosslien, E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann. Clin. Lab. Sci. 2000, 30, 3–21. [Google Scholar]
- Leedham, S.J.; Graham, T.A.; Oukrif, D.; McDonald, S.A.; Rodriguez-Justo, M.; Harrison, R.F.; Shepherd, N.A.; Novelli, M.R.; Jankowski, J.A.; Wright, N.A. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology 2009, 136, 542–550. [Google Scholar]
- Wang, D.; Mann, J.R.; DuBois, R.N. WNT and cyclooxygenase-2 cross-talk accelerates adenoma growth. Cell Cycle 2004, 3, 1512–1515. [Google Scholar] [CrossRef]
- Sheehan, K.M.; Sheahan, K.; O’Donoghue, D.P.; MacSweeney, F.; Conroy, R.M.; Fitzgerald, D.J.; Murray, F.E. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA 1999, 282, 1254–1257. [Google Scholar] [CrossRef]
- Baron, J.A.; Cole, B.F.; Sandler, R.S.; Haile, R.W.; Ahnen, D.; Bresalier, R.; McKeown-Eyssen, G.; Summers, R.W.; Rothstein, R.; Burke, C.A.; et al. A randomized trial of aspirin to prevent colorectal adenomas. N. Engl. J. Med. 2003, 348, 891–899. [Google Scholar]
- Jass, J.R.; Sobin, L.H.; Watanabe, H. The World Health Organization’s histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer 1990, 66, 2162–2167. [Google Scholar] [CrossRef]
- Fabrigar, L.R.; Wegener, D.T.; MacCallum, R.C.; Strahan, E.J. Evaluating the use of exploratory factor analysis in psychological research. Psychol. Methods 1999, 4, 272–299. [Google Scholar] [CrossRef]
- Gorsuch, R.L. Factor Analysis, 2nd ed; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1983. [Google Scholar]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy; W.H. Freeman and Company: San Francisco, CA, USA, 1973; pp. 230–234. [Google Scholar]
- Bareis, P.; Bises, G.; Bischof, M.G.; Cross, H.S.; Peterlik, M. 25-Hydroxyvitamin D metabolism in human colon cancer cells during tumor progression. Biochem. Biophys. Res. Commun. 2001, 285, 1012–1017. [Google Scholar] [CrossRef]
- Cross, H.S.; Bareis, P.; Hofer, H.; Bischof, M.G.; Bajna, E.; Kriwanek, S.; Bonner, E.; Peterlik, M. 25-Hydroxyvitamin D(3)-1alpha-hydroxylase and vitamin D receptor gene expression in human colonic mucosa is elevated during early cancerogenesis. Steroids 2001, 66, 287–292. [Google Scholar] [CrossRef]
- Gustafsson Asting, A.; Caren, H.; Andersson, M.; Lonnroth, C.; Lagerstedt, K.; Lundholm, K. COX-2 gene expression in colon cancer tissue related to regulating factors and promoter methylation status. BMC Cancer 2011, 11, 238. [Google Scholar] [CrossRef]
- Peterlik, M. Role of bile acid secretion in human colorectal cancer. Wien. Med. Wochenschr. 2008, 158, 539–541. [Google Scholar] [CrossRef]
- Miyaki, A.; Yang, P.; Tai, H.H.; Subbaramaiah, K.; Dannenberg, A.J. Bile acids inhibit NAD+-dependent 15-hydroxyprostaglandin dehydrogenase transcription in colonocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G559–G566. [Google Scholar] [CrossRef]
- Nehring, J.A.; Zierold, C.; DeLuca, H.F. Lithocholic acid can carry out in vivo functions of vitamin D. Proc. Natl. Acad. Sci. USA 2007, 104, 10006–10009. [Google Scholar]
- Lai, T.Y.; Chen, L.M.; Lin, J.Y.; Tzang, B.S.; Lin, J.A.; Tsai, C.H.; Lin, Y.M.; Huang, C.Y.; Liu, C.J.; Hsu, H.H. 17beta-Estradiol inhibits prostaglandin E2-induced COX-2 expressions and cell migration by suppressing Akt and ERK1/2 signaling pathways in human LoVo colon cancer cells. Mol. Cell Biochem. 2010, 342, 63–70. [Google Scholar] [CrossRef]
- Lechner, D.; Cross, H.S. Phytoestrogens and 17beta-estradiol influence vitamin D metabolism and receptor expression-relevance for colon cancer prevention. Recent Results Cancer Res. 2003, 164, 379–391. [Google Scholar]
- Lechner, D.; Bajna, E.; Adlercreutz, H.; Cross, H.S. Genistein and 17beta-estradiol, but not equol, regulate vitamin D synthesis in human colon and breast cancer cells. Anticancer Res. 2006, 26, 2597–2603. [Google Scholar]
- Lechner, D.; Kallay, E.; Cross, H.S. Phytoestrogens and colorectal cancer prevention. Vitam Horm. 2005, 70, 169–198. [Google Scholar] [CrossRef]
- Protiva, P.; Cross, H.S.; Hopkins, M.E.; Kallay, E.; Bises, G.; Dreyhaupt, E.; Augenlicht, L.; Lipkin, M.; Lesser, M.; Livote, E.; et al. Chemoprevention of colorectal neoplasia by estrogen: Potential role of vitamin D activity. Cancer Prev. Res. (Phila) 2009, 2, 43–51. [Google Scholar] [CrossRef]
- Hendifar, A.; Yang, D.; Lenz, F.; Lurje, G.; Pohl, A.; Lenz, C.; Ning, Y.; Zhang, W.; Lenz, H.J. Gender disparities in metastatic colorectal cancer survival. Clin. Cancer Res. 2009, 15, 6391–6397. [Google Scholar]
- Oates, J.A. Cardiovascular risk markers and mechanisms in targeting the COX pathway for colorectal cancer prevention. Cancer Prev. Res. (Phila) 2011, 4, 1145–1148. [Google Scholar] [CrossRef]
- Aparna, R.; Subhashini, J.; Roy, K.R.; Reddy, G.S.; Robinson, M.; Uskokovic, M.R.; Venkateswara Reddy, G.; Reddanna, P. Selective inhibition of cyclooxygenase-2 (COX-2) by 1alpha,25-dihydroxy-16-ene-23-yne-vitamin D3, a less calcemic vitamin D analog. J. Cell Biochem. 2008, 104, 1832–1842. [Google Scholar]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Cross, H.S.; Bises, G.; Lechner, D.; Manhardt, T.; Kallay, E. The vitamin d endocrine system of the gut—Its possible role in colorectal cancer prevention. J. Steroid Biochem. Mol. Biol. 2005, 97, 121–128. [Google Scholar] [CrossRef]
- Dobnig, H.; Pilz, S.; Scharnagl, H.; Renner, W.; Seelhorst, U.; Wellnitz, B.; Kinkeldei, J.; Boehm, B.O.; Weihrauch, G.; Maerz, W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch. Intern. Med. 2008, 168, 1340–1349. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brozek, W.; Manhardt, T.; Kállay, E.; Peterlik, M.; Cross, H.S. Relative Expression of Vitamin D Hydroxylases, CYP27B1 and CYP24A1, and of Cyclooxygenase-2 and Heterogeneity of Human Colorectal Cancer in Relation to Age, Gender, Tumor Location, and Malignancy: Results from Factor and Cluster Analysis. Cancers 2012, 4, 763-776. https://doi.org/10.3390/cancers4030763
Brozek W, Manhardt T, Kállay E, Peterlik M, Cross HS. Relative Expression of Vitamin D Hydroxylases, CYP27B1 and CYP24A1, and of Cyclooxygenase-2 and Heterogeneity of Human Colorectal Cancer in Relation to Age, Gender, Tumor Location, and Malignancy: Results from Factor and Cluster Analysis. Cancers. 2012; 4(3):763-776. https://doi.org/10.3390/cancers4030763
Chicago/Turabian StyleBrozek, Wolfgang, Teresa Manhardt, Enikö Kállay, Meinrad Peterlik, and Heide S. Cross. 2012. "Relative Expression of Vitamin D Hydroxylases, CYP27B1 and CYP24A1, and of Cyclooxygenase-2 and Heterogeneity of Human Colorectal Cancer in Relation to Age, Gender, Tumor Location, and Malignancy: Results from Factor and Cluster Analysis" Cancers 4, no. 3: 763-776. https://doi.org/10.3390/cancers4030763