Clinical Significance of CK19 Negative Breast Cancer
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Histopathological Examination
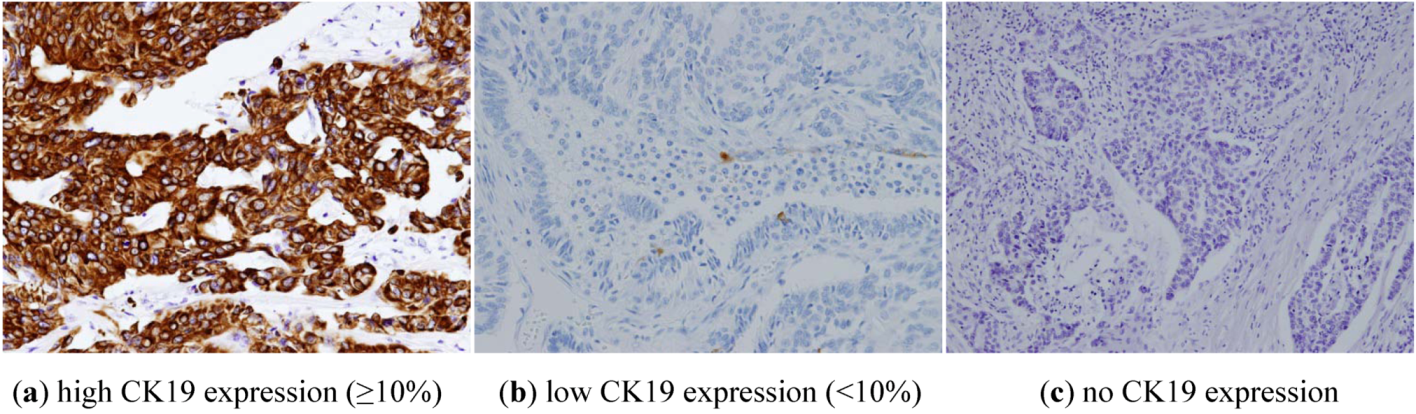
2.3. OSNA
2.4. Evaluation of Lymph Node Metastasis Using Imprint Smear Cytology
2.5. Statistical Analysis
3. Results
3.1. Evaluation of Lymph Node Metastasis
| Characteristics | No. of cases (percentage) |
|---|---|
| Age (years) Mean ± SD (range) | 58.6 ± 13.4 (23–90) |
| Menopause | |
| Pre | 64 (29.2%) |
| Post | 155 (70.8%) |
| Nodal status/All cases | |
| Negative | 135 (61.6%) |
| Positive | 84 (38.4%) |
| OSNA/Cases with SLNB | |
| Negative | 135 (75.4%) |
| Positive | 44 (24.6%) |
| -Micrometastasis (1+) | 30 |
| -Macrometastasis (2+, i+) | 14 |
| Number of SLN | |
| Mean (range) | 1.8 (1–5) |
| Non-SLN metastasis with OSNA positive | |
| Negative | 28 (61.4%) |
| Positive | 16 (38.6%) |
| imprint cytology/Cases with SLNB | |
| Negative | 150 (83.8%) |
| Positive | 29 (16.2%) |
| Tumor size (cm) | |
| Mean (range) | 1.64 (0.1–10) |
| Histological type | |
| Ductal | 188 (85.8%) |
| Lobular | 11 (5.0%) |
| Others (special type) | 20 (9.2%) |
| -metaplastic carcinoma | 3 |
| Nuclear Grade | |
| Grade 1 | 46 (22.3%) |
| Grade 2 | 146 (66.7%) |
| Grade 3 | 27 (11%) |
| ER status | |
| Positive | 170 (77.6%) |
| Negative | 49 (22.4%) |
| PgR status | |
| Positive | 157 (71.7%) |
| Negative | 62 (28.3%) |
| HER2 status | |
| Positive | 34 (15.5%) |
| Negative | 185 (84.5%) |
| Ki 67 | |
| Median | 20.0 |
| Low (<20%) | 103 (47.0%) |
| Intermediate (20%≤, <50%) | 86 (39.3%) |
| High (≥50%) | 30 (13.7%) |
| p53 | |
| Positive (overexpression) | 31 (14.2%) |
| Negative | 188 (85.8%) |
| Subtype | |
| Total Luminal | 170 (77.6%) |
| -Luminal A | 98 (44.7%) |
| -Luminal B (HER2 negative) | 62 (28.3%) |
| -Luminal B (HER2 positive) | 10 (4.6%) |
| HER2 positive | 24 (11.0%) |
| Triple-negative | 25 (11.4%) |
| Cytokeratin 19 status | |
| Positive (≥10%) | 192 (87.7%) |
| Negative | 27 (12.3%) |
| -absent | 3 |
| -low expression | 24 |
3.2. CK19 Status and Clinico-Pathological Characteristics
| CK19 negative | CK19 positive | Total | p-value | |
|---|---|---|---|---|
| n = 27 | n = 192 | |||
| Age (years) | ||||
| Mean (range) | 58.8 (23–87) | 58.4 (30–90) | 0.81 | |
| Menopause | ||||
| Pre | 8 (29.6%) | 56 (29.2%) | 64 | |
| Post | 19 | 136 | 155 | 0.96 |
| Tumor size (cm) | ||||
| Mean (range) | 1.32 (0–40) | 1.69 (0–10) | 0.36 | |
| Nodal status | ||||
| Negative | 19 (70.4%) | 128 (66.7%) | 147 | 0.70 |
| Positive | 8 | 64 | 72 | |
| Histology | ||||
| Ductal | 21 (77.8%) | 167 (87.0%) | 188 | |
| Lobular | 1 | 10 | 11 | 0.84 |
| Others | 5 1 | 15 2 | 20 | |
| Nuclear Grade | ||||
| Grade 1 | 2 |  | 46 | |
| Grade 2 | 18 | 146 | 0.06 | |
| Grade 3 | 7 (25.9%) | 27 | ||
| ER status | ||||
| Positive | 15 (55.6%) | 155 (80.7%) | 170 | 0.003 |
| Negative | 12 | 37 | 49 | |
| PgR | ||||
| Positive | 11 (40.7%) | 146 (76.0%) | 157 | 0.0001 |
| Negative | 16 | 46 | 62 | |
| HER2 status | ||||
| Positive | 5 (18.5%) | 29 (15.1%) | 34 | 0.64 |
| Negative | 22 | 163 | 185 | |
| Ki 67 | ||||
| Low (<20%) | 13 |  | 103 | |
| Intermediate (20–50%) | 6 | 86 | ||
| High (≥50%) | 8 (29.6%) | 30 | 0.01 | |
| p53 | ||||
| Positive | 7 (25.9%) | 24 (12.5) | 31 | |
| Negative | 20 | 168 | 188 | 0.06 |
3.3. Subtypes and CK19 Expression
| CK19 negative | CK19 positive | p-Value | ||
|---|---|---|---|---|
| n = 27 | n = 192 | vs. Luminal | vs. TN | |
| Total Luminal | 15 (55.6%) | 155 (80.7%) | 0.005 | |
| -Luminal A | 11 (40.7%) | 87 (45.3%) | 0.03 | |
| -Luminal B (HER2 negative) | 4 (14.8%) | 58 (30.2%) | 0.002 | |
| -Luminal B (HER2 positive) | 0 | 10 (5.2%) | 0.06 | |
| HER-2 positive | 5 (18.5%) | 19 (9.9%) | 0.07 | 0.56 |
| Triple-negative | 7 (25.9%) | 18 (9.4%) | 0.005 | |
3.4. Relationship between OSNA and Imprint Smear Cytology of SLN According to CK19 Status
| CK19 negative | Imprint smear cytology | Total | |||
| Positive | Negative | ||||
| OSNA | Positive | Macro | 2 | 0 | 3 1 |
| Micro | 1 | ||||
| Negative | 1 2 | 16 | 17 | ||
| 4 | 16 | 20 | |||
| CK19 positive | Imprint smear cytology | Total | |||
| Positive | Negative | ||||
| OSNA | Positive | Macro | 25 | 3 | 41 |
| Micro | 2 | 11 | |||
| Negative | 0 | 118 | 118 | ||
| 27 | 132 | 159 | |||
4. Discussion
5. Conclusions
Acknowledgments
References
- Abd El-Rehim, D.M.; Pinder, S.E.; Paish, C.E.; Bell, J.; Blamey, R.W.; Robertson, J.F.; Nicholson, R.H.; Ellis, I.O. Expression of luminal and basal cytokeratins in human breast carcinoma. J. Pathol. 2004, 203, 661–671. [Google Scholar] [CrossRef]
- Shao, M.M.; Chan, S.K.; Yu, A.M.; Lam, C.C.; Tsang, J.Y.; Lui, P.C.; Law, B.K.; Tan, P.H.; Tse, G.M. Keratin expression in breast cancers. Virchows Arch. 2012, 461, 313–322. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Nakabayashi, K.; Yoshidome, K.; Kaneko, T.; Iwase, T.; Akiyama, F.; Kato, Y.; Tsuda, H.; Ueda, S.; Sato, K.; et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin. Cancer Res. 2007, 13, 4807–4816. [Google Scholar] [CrossRef]
- Tamaki, Y. One-step nucleic acid amplification assay (OSNA) for sentinel lymph node biopsy. Breast Cancer 2012. [Google Scholar] [CrossRef]
- Kawano, K.; Miyayama, H.; Arima, N.; Nishimura, R.; Baba, T.; Shimamoto, K.; Matsumoto, R.; Umeda, K. Intraoperative cytologic diagnosis of sentinel lymph node metastasis in breast cancer-Combination of imprint cytology and immunocytochemistry. J. Jpn. Soc. Clin. Cytol. 2004, 43, 363–369. (in Japanese). [Google Scholar]
- Parikh, R.R.; Yang, Q.; Higgins, S.A.; Haffty, B.G. Outcomes in young women with breast cancer of triple-negative phenotype: The prognostic significance of CK19 expression. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 35–42. [Google Scholar] [CrossRef]
- Jeong, H.; Ryu, Y.J.; An, J.; Lee, Y.; Kim, A. Epithelial-mesenchymal transition in breast cancer correlates with high histological grade and triple-negative phenotype. Histopathology 2012, 60, E87–E95. [Google Scholar] [CrossRef]
- Alvarenga, C.A.; Paravidino, P.I.; Alvarenga, M.; Dufloth, R.; Gomes, M.; Zeferino, L.C.; Schmitt, F. Expression of CK19 in invasive breast carcinomas of special histological types: Implications for the use of one-step nucleic acid amplification. J. Clin. Pathol. 2011, 64, 493–497. [Google Scholar] [CrossRef]
- Kai, K.; Nishimura, R.; Arima, N.; Miyayama, H.; Iwase, H. p53 expression status is a significant molecular marker in predicting the time to endocrine therapy failure in recurrent breast cancer: A cohort study. Int. J. Clin. Oncol. 2006, 11, 426–433. [Google Scholar] [CrossRef]
- Nishimura, R.; Osako, T.; Okumura, Y.; Hayashi, M.; Toyozumi, Y.; Arima, N. Ki-67 as a prognosticmarkeraccording to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp. Ther. Med. 2010, 1, 747–754. [Google Scholar]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J.; Panel members. Strategies for subtypes-dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar]
- Osako, T.; Iwase, T.; Kimura, K.; Yamashita, K.; Horii, R.; Yanagisawa, A.; Akiyama, F. Intraoperative molecular assay for sentinel lymph node metastases in early stage breast cancer: A comparative analysis between one-step nucleic acid amplification whole node assay and routine frozen section histology. Cancer 2011, 117, 4365–4374. [Google Scholar] [CrossRef]
- Zhang, D.H.; Tai, L.Z.K.; Wong, L.L.; Sethi, S.K.; Koay, E.S. Proteomics of breast cancer: Enhanced expression of cytokeratin 19 in human epidermal growth factor receptor type 2 positive breast tumors. Proteomics 2005, 5, 1797–1805. [Google Scholar] [CrossRef]
- Vilardell, F.; Novell, A.; Martin, J.; Santacana, M.; Velasco, A.; Díez-Castro, M.J.; Cuevas, D.; Panadés, M.J.; González, S.; Llombart, A.; et al. Importance of assessing CK19 immunostaining in core biopsies in patients subjected to sentinel node study by OSNA. Virchows Arch. 2012, 460, 569–575. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhang, J.X.; Chen, J.J.; Liu, Z.B.; Huang, X.Y.; Cheng, J.Y.; Yang, W.T.; Shao, Z.M.; Shen, Z.Z.; Wu, J. Factors associated with the misdiagnosis of sentinel lymph nodes using touch imprint cytology for early stage breast cancer. Oncol. Lett. 2011, 2, 277–281. [Google Scholar]
- Eetvelde, E.; Vanhoeij, M.; Verfaillie, G.; Bourgain, C.; Lamote, J. Role of intra-operative touch imprint cytology in the treatment of breast cancer. Acta Chir. Belg. 2011, 111, 130–135. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fujisue, M.; Nishimura, R.; Okumura, Y.; Tashima, R.; Nishiyama, Y.; Osako, T.; Toyozumi, Y.; Arima, N. Clinical Significance of CK19 Negative Breast Cancer. Cancers 2013, 5, 1-11. https://doi.org/10.3390/cancers5010001
Fujisue M, Nishimura R, Okumura Y, Tashima R, Nishiyama Y, Osako T, Toyozumi Y, Arima N. Clinical Significance of CK19 Negative Breast Cancer. Cancers. 2013; 5(1):1-11. https://doi.org/10.3390/cancers5010001
Chicago/Turabian StyleFujisue, Mamiko, Reiki Nishimura, Yasuhiro Okumura, Rumiko Tashima, Yasuyuki Nishiyama, Tomofumi Osako, Yasuo Toyozumi, and Nobuyuki Arima. 2013. "Clinical Significance of CK19 Negative Breast Cancer" Cancers 5, no. 1: 1-11. https://doi.org/10.3390/cancers5010001




