Blood Outgrowth Endothelial Cells Increase Tumor Growth Rates and Modify Tumor Physiology: Relevance for Therapeutic Targeting
Abstract
:1. Introduction
2. Experimental Section
2.1. Tumor Cell Lines and Tumor Growth
2.2. Culture of Endothelial Cells and Injection
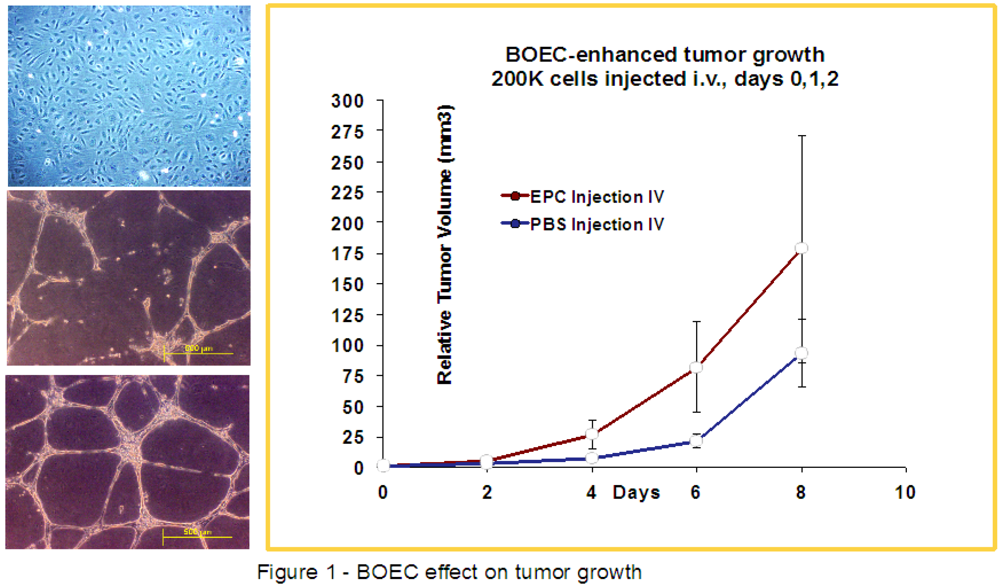
2.3. HIFU-Ablation of FSaII Tumors Followed by BOEC Injection
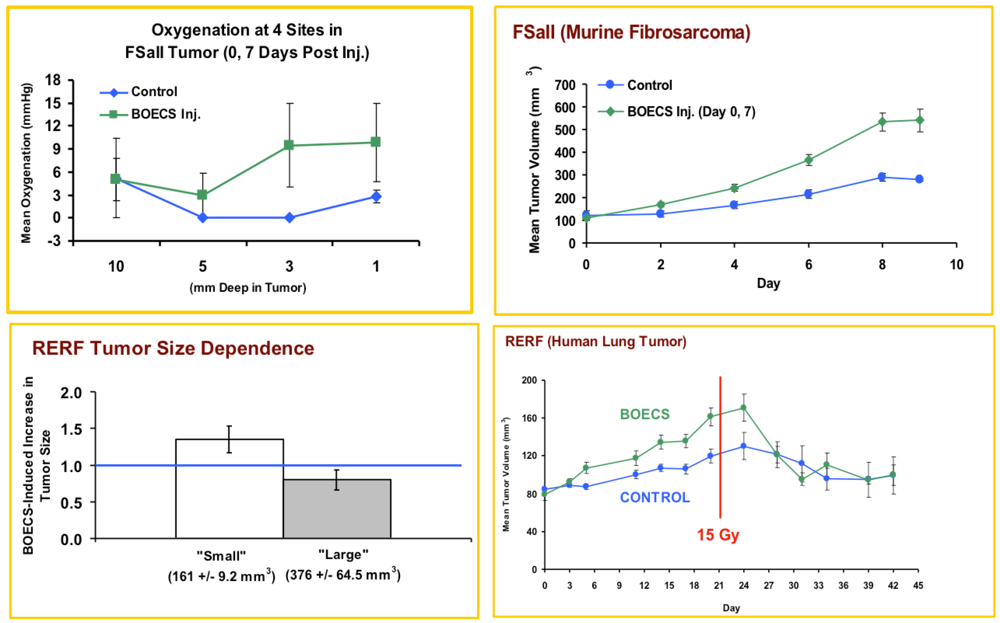
2.4. Oxygenation Measurement
2.5. Radiation Therapy
2.6. Statistical Analysis
3. Results
3.1. Injected BOECs Alter Tumor Oxygenation and Increase Tumors Response to Radiation
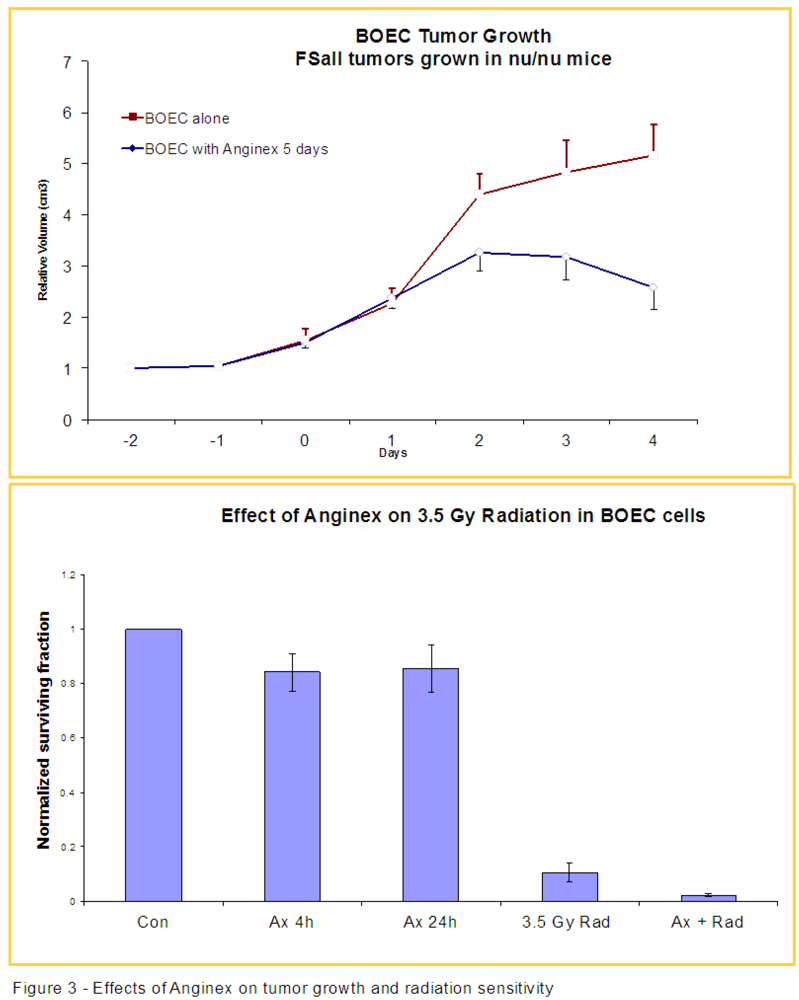
3.2. Anginex Antagonizes Vasculogenesis Stimulated by BOECs and Increases BOEC Susceptibility to Radiation
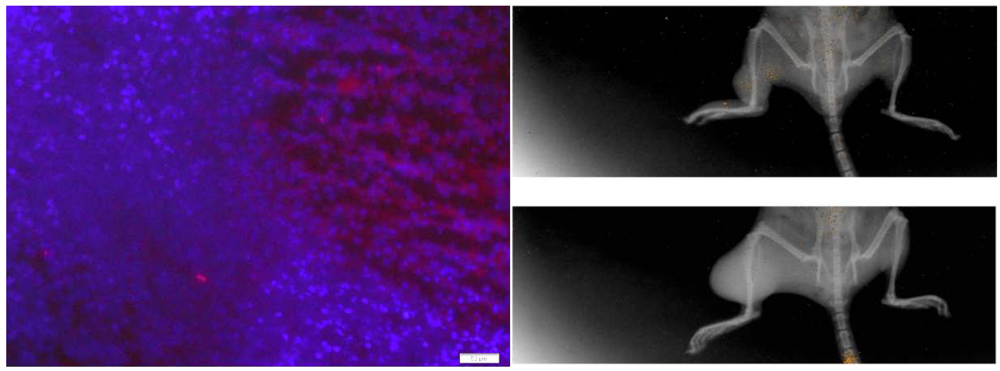
3.3. Injected BOECs Migrate to Tumors after Thermal Ablation
4. Discussion
5. Conclusions
Acknowledgments
References
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Crosby, J.R.; Kaminski, W.E.; Schatteman, G.; Martin, P.J.; Raines, E.W.; Seifert, R.A.; Bowen-Pope, D.F. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ. Res. 2000, 87, 728–730. [Google Scholar] [CrossRef]
- Asahara, T.; Kalka, C.; Isner, J.M. Stem cell therapy and gene transfer for regeneration. Gene Ther. 2000, 7, 451–457. [Google Scholar] [CrossRef]
- Rafii, S. Circulating endothelial precursors: Mystery, reality, and promise. J. Clin. Invest. 2000, 105, 17–19. [Google Scholar] [CrossRef]
- Bodempudi, V.; Ohlfest, J.R.; Terai, K.; Zamora, E.A.; Vogel, R.I.; Gupta, K.; Hebbel, R.P.; Dudek, A.Z. Blood outgrowth endothelial cell-based systemic delivery of antiangiogenic gene therapy for solid tumors. Cancer Gene Ther. 2010, 17, 855–863. [Google Scholar] [CrossRef]
- Kang, K.; Allen, P.; Bischoff, J. Bioengineered human vascular networks transplanted into secondary mice reconnect with host vasculature and reestablish perfusion. Blood 2011, 118, 6718–6721. [Google Scholar] [CrossRef]
- Lin, Y.; Weisdorf, D.J.; Solovey, A.; Hebbel, R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 2000, 105, 71–77. [Google Scholar] [CrossRef]
- Peichev, M.; Naiyer, A.J.; Pereira, D.; Zhu, Z.; Lane, W.; Williams, M.; Oz, M.; Hicklin, D.; Witte, L.; Moore, M.; et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 2000, 95, 952–958. [Google Scholar]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1996, 275, 964–966. [Google Scholar]
- Shi, Q.; Rafii, S.; Wu, M.H.; Wijelath, E.S.; Yu, C.; Ishida, A.; Fujita, Y.; Kothari, S.; Mohle, R.; Sauvage, L.; et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998, 92, 362–367. [Google Scholar]
- Urbich, C.; Aicher, A.; Heeschen, C.; Dernbach, E.; Hofmann, W.; Zeiher, A.; Dimmeler, S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J. Mol. Cell. Cardiol. 2005, 39, 733–742. [Google Scholar] [CrossRef]
- Wu, X.; Rabkin-Aikawa, E.; Guleserian, K.J.; Perry, T.E.; Masuda, Y.; Sutherland, F.W.H.; Schoen, F.; Mayer, J.; Bischoff, J. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, 480–487. [Google Scholar] [CrossRef]
- Asahara, T.; Takahashi, T.; Masuda, H.; Kalka, C.; Chen, D.; Iwaguro, H.; Inai, Y.; Silver, M.; Isner, J. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999, 18, 3964–3972. [Google Scholar] [CrossRef]
- Zheng, H.; Fu, G.; Dai, T.; Huang, H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J. Cardiovasc. Pharmacol. 2007, 50, 274–280. [Google Scholar] [CrossRef]
- Nagasawa, T.; Kikutani, H.; Kishimoto, T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc. Natl. Acad. Sci. USA 1994, 91, 2305–2309. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, H.; Jin, C.; Zhou, K.; Li, K.; Su, H.; Chen, W.; Bai, J.; Wang, Z. High-intensity focused ultrasound (HIFU): Effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur. Radiol. 2009, 19, 437–445. [Google Scholar] [CrossRef]
- Lynn, J.G.; Zwemer, R.L.; Chick, A.J. A new method for the generation and use of focused ultrasound in experiment biology. J. Gen. Physiol. 1942, 26, 179–193. [Google Scholar] [CrossRef]
- Fry, W.J.; Mosberg, W.H., Jr.; Barnard, J.W.; Fry, F.J. Production of focal destructive lesions in the central nervous system with ultrasound. J. Neurosurg. 1954, 11, 471–478. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.B.; Chen, W.Z.; Zhu, H.; Bai, J.; Zou, J.; Li, K.; Jin, C.; Xie, F.; Su, H. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann. Surg. Oncol. 2004, 11, 1061–1069. [Google Scholar] [CrossRef]
- Lin, Y.; Weisdorf, D.J.; Solovey, A.; Hebbel, R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 2000, 105, 71. [Google Scholar] [CrossRef]
- Melero-Martin, J.M.; Khan, Z.A.; Picard, A.; Wu, X.; Paruchuri, S.; Bischoff, J. In vivo vasculogenic potential of human blood- derived endothelial progenitor cells. Blood 2007, 109, 4761–4768. [Google Scholar]
- Dudek, A.Z.; Bodempudi, V.; Welsh, B.W.; Jasinski, P.; Griffin, R.J.; Milbauer, L.; Hebbel, R.P. Systemic inhibition of tumour angiogenesis by endothelial cell-based gene therapy. Br. J. Cancer 2007, 97, 513–522. [Google Scholar] [CrossRef]
- Griffin, R.J.; Williams, B.W.; Wild, R.; Cherrington, J.M.; Park, H.; Song, C.W. Simultaneous inhibition of the receptor kinase activity of vascular endothelial, fibroblast, and platelet-derived growth factors suppresses tumor growth and enhances tumor radiation response. Cancer Res. 2002, 62, 1702–1706. [Google Scholar]
- Dings, R.M.; Williams, B.W.; Song, C.W.; Griffioen, A.W.; Mayo, K.H.; Griffin, R.J. Anginex synergizes with radiation therapy to inhibit tumor growth by radiosensitizing endothelial cells. Int. J. Cancer 2005, 115, 312–319. [Google Scholar] [CrossRef]
- Griffioen, A.W.; van der Schaft, D.W.; Barendsz-Janson, A.F.; Cox, A.; Struijker Boudier, H.A.; Hillen, H.F.; Mayo, K.H. Anginex, a designed peptide that inhibits angiogenesis. Biochem. J. 2001, 354, 233–242. [Google Scholar] [CrossRef]
- Dings, R.P.; van der Schaft, D.W.; Hargittai, B.; Haseman, J.; Griffioen, A.W.; Mayo, K.H. Anti-tumor activity of the novel angiogenesis inhibitor anginex. Cancer Lett. 2003, 194, 55–66. [Google Scholar] [CrossRef]
- Van der Schaft, D.W.; Dings, R.P.; de Lussanet, Q.G.; van Eijk, L.I.; Nap, A.W.; Beets-Tan, R.; Bouma-ter Steege, J.; Wagstaff, J.; Mayo, K.H.; Griffioen, A.W. The designer anti-angiogenic peptide anginex targets tumor endothelial cells and inhibits tumor growth in animal models. FASEB J. 2002, 16, 991–993. [Google Scholar]
- Dings, R.P.M.; Williams, B.W.; Song, C.W.; Griffioen, A.W.; Mayo, K.H.; Griffin, R.J. Anginex synergizes with radiation therapy to inhibit tumor growth by radiosensitizing endothelial cells. Int. J. Cancer 2005, 115, 312–319. [Google Scholar] [CrossRef]
- Rich, J.N. Cancer stem cells in radiation resistance. Cancer Res. 2007, 67, 8980–8984. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 274–284. [Google Scholar]
- Taylor, M.; Billiot, F.; Marty, V.; Rouffiac, V.; Cohen, P.; Tournay, E.; Opolon, P.; Louache, F.; Vassal, G.; Laplace-Buihe, C.; et al. Reversing resistance to vascular-disrupting agents by blocking late mobilization of circulating endothelial progenitor cells. Cancer Discov. 2012, 2, 434–449. [Google Scholar] [CrossRef]
- Nagesha, D.K.; Tada, D.B.; Stambaugh, C.K.; Gultepe, E.; Jost, E.; Levy, C.O.; Cormack, R.; Makrigiorgos, G.M.; Sridhar, S. Radiosensitizer-eluting nanocoatings on gold fiducials for biological in-situ image-guided radio therapy (BIS-IGRT). Phys. Med. Biol. 2010, 55, 6039–6052. [Google Scholar] [CrossRef]
- Byfield, J.E. 5-Fluorouracil radiation sensitization: A brief review. Invest. New Drugs 1989, 7, 111–116. [Google Scholar] [CrossRef]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, C.A. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Jiang, H.; de Ridder, M.; Verovski, V.N.; Zonveaux, P.; Jordan, B.F.; Law, K.; Monsaert, C.; van den Berge, D.; Verellen, D.; Feron, O.; et al. Activated macrophages as a novel determinant of tumor cell radioresponse: The role of nitric oxide-mediated inhibition of cellular respiration and oxygen sparing. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1520–1527. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.; Cao, Y.; Zhu, X.; Zhu, H.; Chen, W.; Zou, J. “Wide local ablation” of localized breast cancer using high intensity focused ultrasound. J. Surg. Oncol. 2007, 96, 130–136. [Google Scholar] [CrossRef]
- Jolesz, F.A.; McDannold, N. Current status and future potential of MRI-guided focused ultrasound surgery. J. Magn. Reson. Imaging 2008, 27, 391–399. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pagan, J.; Przybyla, B.; Jamshidi-Parsian, A.; Gupta, K.; Griffin, R.J. Blood Outgrowth Endothelial Cells Increase Tumor Growth Rates and Modify Tumor Physiology: Relevance for Therapeutic Targeting. Cancers 2013, 5, 205-217. https://doi.org/10.3390/cancers5010205
Pagan J, Przybyla B, Jamshidi-Parsian A, Gupta K, Griffin RJ. Blood Outgrowth Endothelial Cells Increase Tumor Growth Rates and Modify Tumor Physiology: Relevance for Therapeutic Targeting. Cancers. 2013; 5(1):205-217. https://doi.org/10.3390/cancers5010205
Chicago/Turabian StylePagan, Jonathan, Beata Przybyla, Azemat Jamshidi-Parsian, Kalpna Gupta, and Robert J. Griffin. 2013. "Blood Outgrowth Endothelial Cells Increase Tumor Growth Rates and Modify Tumor Physiology: Relevance for Therapeutic Targeting" Cancers 5, no. 1: 205-217. https://doi.org/10.3390/cancers5010205




