Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked
Abstract
:1. Introduction
2. Lipid Mediators Produced by Lipoxygenases

| Lipoxygenase | Substrate | Product | Physiologial function | Ref. |
|---|---|---|---|---|
| 5-lipoxygenase (5-LOX) | arachidonic acid | 5(S)-HPETE, Leukotriene A4 | Pro-inflammatory mediator | [8] |
| γ-linoleic acid | Dihomo-γ-linoleic acid (DGLA) | Inhibition of arachidonic acid conversion | [9] | |
| Eicosapentaenoic acid (EPA) | Leukotriene A5 | Anti-inflammatory mediator/inhibitor LTA4 hydrolase | [10] | |
| Platelet 12-lipoxygenase (p12-LOX) | arachidonic acid | 12(S)-HPETE | Modulation of platelet aggregation | [11,12,13] |
| Dihomo-γ-linoleic acid (DGLA) | 12(S)-HPETrE | |||
| Eicosapentaenoic acid (EPA) | 12(S)-HPEPE | |||
| α-linoleic acid | 12(S)-HPOTrE | |||
| 12R-lipoxygenase (12R-LOX) | arachidonic acid | 12(R)-HPETE | Epidermal barrier acquisition | [14] |
| Linoleyl-ω-hydroxy ceramide | 9(R)-hydroperoxyllinoleoyl-ω-hydroxy ceramide | |||
| epidermis LOX3 (eLOX3) | 9(R)-hydroperoxyllinoleoyl-ω-hydroxy ceramide | 9(R)-10(R)-trans-epoxy-11E-13(R)-hydroxylinoleoyl-ω-hydroxy ceramide | ||
| 15-lipoxygenase-1 (15-LOX1) | linoleic acid | 13(S)-HPODE | modulation of MAP kinase signaling pathways | [15,16,17] |
| arachidonic acid | 15(S)-HPETE | modulation of leukotriene B4, pro-inflammatory mediators | ||
| 15-lipoxygenase-2 (15-LOX2) | arachidonic acid | 15(S)-HPETE | negative cell cycle regulator and tumor supressor | [18,19] |
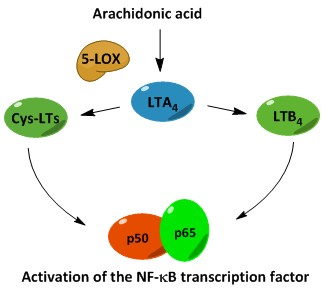
3. Biosynthesis of Leukotrienes: Initiation of Inflammatory Responses

4. Nuclear Factor κB (NF-κB) in Inflammation
4.1. The Canonical NF-κB Activation Pathway
4.2. The Non-Canonical NF-κB Activation Pathway
5. Role of Leukotrienes in Inflammatory Diseases
| Disease | Observations | Ref. |
|---|---|---|
| Asthma | Ectopic expression of 15-LOX induces NF-κB mediated reporter gene expression in epithelial cells. | [45] |
| Cardiovascular diseases | Increased levels of 5-LOX metabolites in patients with atherosclerosis. | [46] |
| The 15-LOX metabolite 15-HETE activates the NF-κB pathway and stimulates 15-LOX expression in a positive feedback loop. | [47] | |
| Rheumatoid Arthritis | The 15-LOX metabolite 15-HETE increases IκBα degradation and activation of the NF-κB pathway. | [48] |
| Cancer | The 5-LOX metabolite LTB4 is capable of activating the transcription factor NF-κB in cancer cells | [49] |
5.1. Asthma
5.2. Cardiovascular Diseases
5.3. Rheumatoid Arthritis
5.4. Inflammatory Bowel Disease
5.5. Lipoxygenase in Cancer
6. Biosynthesis of Lipoxins: Termination of Inflammatory Responses

7. Lipoxygenase Inhibitors

7.1. FLAP Inhibitors
7.2. Redox Inhibitors
7.3. Iron-Chelator Inhibitors
7.4. Non-Redox Inhibitors
7.5. Leukotriene Antagonist
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nowsheen, K.; Aziz, T.B.; Kryston, N.F.; Ferguson, N.F.; Georgakilas, A. The interplay between inflammation and oxidative stress and carcinogenesis. Curr. Mol. Med. 2012, 12, 672–680. [Google Scholar]
- Haining, J.L.; Axelrod, B. Induction period in the lipoxidase-catalyzed oxidation of linoleic acid and its abolition by substrate peroxide. J. Biol. Chem. 1958, 232, 193–202. [Google Scholar]
- Solomon, E.I.; Zhou, J.; Neese, F.; Pavel, E.G. New insights from spectroscopy into the structure/function relationships of lipoxygenases. Chem. Biol. 1997, 4, 795–808. [Google Scholar] [CrossRef]
- Brash, A.R. Lipoxygenases: Occurrence, Functions, Catalysis, and Acquisition of Substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sigal, E. The molecular biology of mammalian arachidonic acid metabolism. Am. J. Physiol. 1991, 260, L13–L28. [Google Scholar]
- Chen, X.; Funk, C.D. The N-terminal “β-Barrel” Domain of 5-Lipoxygenase is Essential for Nuclear Membrane Translocation. J. Biol. Chem. 2001, 276, 811–818. [Google Scholar] [CrossRef]
- Samuelsson, B. Leukotrienes: Mediators of immediate hypersensitivity reactions and inflammation. Science 1983, 220, 568–575. [Google Scholar]
- Iversen, L.; Fogh, K.; Bojesen, G.; Kragballe, K. Linoleic acid and dihomogammalinolenic acid inhibit leukotriene B4 formation and stimulate the formation of their 15-lipoxygenase products by human neutrophils in vitro. Evidence of formation of antiinflammatory compounds. Agents Actions 1991, 33, 286–291. [Google Scholar] [CrossRef]
- Nathaniel, D.J.; Evans, J.F.; Leblanc, Y.; Léveillé, C.; Fitzsimmons, B.J.; Ford-Hutchinson, A.W. Leukotriene A5 is a substrate and an inhibitor of rat and human neutrophil LTA4 hydrolase. Biochem. Biophys. Res. Commun. 1985, 131, 827–835. [Google Scholar] [CrossRef]
- Brüne, B.; Ullrich, V. 12-hydroperoxyeicosatetraenoic acid inhibits main platelet functions by activation of soluble guanylate cyclase. Mol. Pharmacol. 1991, 39, 671–678. [Google Scholar]
- Yeung, J.; Holinstat, M. 12-Lipoxygenase: A Potential Target for Novel Anti-Platelet Therapeutics. Cardiovasc. Hematol. Agents Med. Chem. 2011, 9, 154–164. [Google Scholar] [CrossRef]
- Ikei, K.N.; Yeung, J.; Apopa, P.L.; Ceja, J.; Vesci, J.; Holman, T.R.; Holinstat, M. Investigations of human platelet-type 12-lipoxygenase: Role of lipoxygenase products in platelet activation. J. Lipid Res. 2012, 53, 2546–2559. [Google Scholar] [CrossRef]
- Epp, N.; Fürstenberger, G.; Müller, K.; de Juanes, S.; Leitges, M.; Hausser, I.; Thieme, F.; Liebisch, G.; Schmitz, G.; Krieg, P. 12R-lipoxygenase deficiency disrupts epidermal barrier function. J. Cell Biol. 2007, 177, 173–182. [Google Scholar] [CrossRef]
- Profita, M.; Sala, A.; Riccobono, L.; Pace, E.; Paternò, A.; Zarini, S.; Siena, L.; Mirabella, A.; Bonsignore, G.; Vignola, A.M. 15(S)-HETE modulates LTB4 production and neutrophil chemotaxis in chronic bronchitis. Am. J. Physiol. Cell Physiol. 2000, 279, C1249–C1258. [Google Scholar]
- Sordillo, L.M.; Weaver, J.A.; Cao, Y.; Corl, C.; Sylte, M.J.; Mullarky, I.K. Enhanced 15-HPETE production during oxidant stress induces apoptosis of endothelial cells. Prostaglandins Other Lipid Mediat. 2005, 76, 19–34. [Google Scholar] [CrossRef]
- Hsi, L.C.; Wilson, L.C.; Eling, T.E. Opposing Effects of 15-Lipoxygenase-1 and -2 Metabolites on MAPK Signaling in Prostate: Alteration in peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2002, 277, 40549–40556. [Google Scholar] [CrossRef]
- Bhatia, B.; Maldonado, C.J.; Tang, S.; Chandra, D.; Klein, R.D.; Chopra, D.; Shappell, S.B.; Yang, P.; Newman, R.A.; Tang, D.G. Subcellular localization and tumor-suppressive functions of 15-lipoxygenase 2 (15-LOX2) and its splice variants. J. Biol. Chem. 2003, 278, 25091–25100. [Google Scholar] [CrossRef]
- Tang, S.; Bhatia, B.; Maldonado, C.J.; Yang, P.; Newman, R.A.; Liu, J.; Chandra, D.; Traag, J.; Klein, R.D.; Fischer, S.M.; et al. Evidence That Arachidonate 15-Lipoxygenase 2 Is a Negative Cell Cycle Regulator in Normal Prostate Epithelial Cells. J. Biol. Chem. 2002, 277, 16189–16201. [Google Scholar] [CrossRef]
- Rådmark, O. The Molecular Biology and Regulation of 5-Lipoxygenase. Am. J. Respir. Crit. Care Med. 2000, 161, S11–S15. [Google Scholar] [CrossRef]
- Noguchi, M.; Miyano, M.; Matsumoto, T. Physicochemical characterization of ATP binding to human 5-lipoxygenase. Lipids 1996, 31, 367–371. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Brock, T.G. 5-Lipoxygenase and FLAP. Prostaglandins Leukot. Essent. Fat. Acids 2003, 69, 99–109. [Google Scholar] [CrossRef]
- Hunter, J.A.; Finkbeiner, W.E.; Nadel, J.A.; Goetzl, E.J.; Holtzman, M.J. Predominant generation of 15-lipoxygenase metabolites of arachidonic acid by epithelial cells from human trachea. Proc. Natl. Acad. Sci. USA 1985, 82, 4633–4637. [Google Scholar]
- Nadel, J.A.; Conrad, D.J.; Ueki, I.F.; Schuster, A.; Sigal, E. Immunocytochemical localization of arachidonate 15-lipoxygenase in erythrocytes, leukocytes, and airway cells. J. Clin. Investig. 1991, 87, 1139–1145. [Google Scholar] [CrossRef]
- Brash, A.R.; Boeglin, W.E.; Chang, M.S. Discovery of a second 15S-lipoxygenase in humans. Proc. Natl. Acad. Sci. USA 1997, 94, 6148–6152. [Google Scholar] [CrossRef]
- Brown, C.D.; Kilty, I.; Yeadon, M.; Jenkinson, S. Regulation of 15-lipoxygenase isozymes and mucin secretion by cytokines in cultured normal human bronchial epithelial cells. Inflamm. Res. 2001, 50, 321–326. [Google Scholar] [CrossRef]
- Chanez, P.; Bonnans, C.; Chavis, C.; Vachier, I. 15-Lipoxygenase. Am. J. Respir. Cell Mol. Biol. 2002, 27, 655–658. [Google Scholar] [CrossRef]
- Haeggstrom, J.Z.; Wetterholm, A. Enzymes and receptors in the leukotriene cascade. Cell Mol. Life Sci. 2002, 59, 742–753. [Google Scholar] [CrossRef]
- Okamoto, F.; Saeki, K.; Sumimoto, H.; Yamasaki, S.; Yokomizo, T. Leukotriene B4 Augments and Restores FcγRs-dependent Phagocytosis in Macrophages. J. Biol. Chem. 2010, 285, 41113–41121. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, S.R.; Park, H.S.; Park, S.J.; Min, K.H.; Lee, K.Y.; Jin, S.M.; Lee, Y.C. Cysteinyl leukotriene upregulates IL-11 expression in allergic airway disease of mice. J. Allergy Clin. Immunol. 2007, 119, 141–149. [Google Scholar]
- Thompson, C.; Cloutier, A.; Bossé, Y.; Poisson, C.; Larivée, P.; McDonald, P.P.; Stankova, J.; Rola-Pleszczynski, M. Signaling by the Cysteinyl-Leukotriene Receptor 2: Involvement in chemokine gene transcription. J. Biol. Chem. 2008, 283, 1974–1984. [Google Scholar] [CrossRef]
- Kawano, T.; Matsuse, H.; Kondo, Y.; Machida, I.; Saeki, S.; Tomari, S.; Mitsuta, K.; Obase, Y.; Fukushima, C.; Shimoda, T.; et al. Cysteinyl leukotrienes induce nuclear factor κb activation and rantes production in a murine model of asthma. J. Allergy Clin. Immunol. 2003, 112, 369–374. [Google Scholar] [CrossRef]
- Hoffmann, A.; Natoli, G.; Ghosh, G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene 2006, 25, 6706–6716. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-κB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009. [Google Scholar] [CrossRef]
- Zheng, C.; Yin, Q.; Wu, H. Structural studies of NF-kappaB signaling. Cell Res. 2011, 21, 183–195. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation Meets Ubiquitination: The Control of NF-κB Activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar]
- Holgate, S.T. Cytokine and anti-cytokine therapy for the treatment of asthma and allergic disease. Cytokine 2004, 28, 152–157. [Google Scholar]
- Williams, R.O.; Paleolog, E.; Feldmann, M. Cytokine inhibitors in rheumatoid arthritis and other autoimmune diseases. Curr. Opin. Pharmacol. 2007, 7, 412–417. [Google Scholar]
- Chung, K.F. Cytokines as targets in chronic obstructive pulmonary disease. Curr. Drug Targets 2006, 7, 675–681. [Google Scholar] [CrossRef]
- Perkins, N.D.; Gilmore, T.D. Good cop, bad cop: The different faces of NF-kappaB. Cell Death Differ. 2006, 13, 759–772. [Google Scholar]
- Haller, D.; Russo, M.P.; Sartor, R.B.; Jobin, C. IKKβ and Phosphatidylinositol 3-Kinase/Akt Participate in Non-pathogenic Gram-negative Enteric Bacteria-induced RelA Phosphorylation and NF-κB Activation in Both Primary and Intestinal Epithelial Cell Lines. J. Biol. Chem. 2002, 277, 38168–38178. [Google Scholar]
- Madrid, L.V.; Mayo, M.W.; Reuther, J.Y.; Baldwin, A.S. Akt Stimulates the Transactivation Potential of the RelA/p65 Subunit of NF-κB through Utilization of the IκB Kinase and Activation of the Mitogen-activated Protein Kinase p38. J. Biol. Chem. 2001, 276, 18934–18940. [Google Scholar]
- Perkins, N.D. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene 2006, 25, 6717–6730. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Liu, L.; Schain, F.; Brunnström, A.; Björkholm, M.; Claesson, H.E.; Sjöberg, J. 15-Lipoxygenase-1 induces expression and release of chemokines in cultured. human lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L196–L203. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S.; Rosenfeld, M.E.; Parthasarathy, S.; Sigal, E.; Särkioja, T.; Witztum, J.L.; Steinberg, D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J. Clin. Investig. 1991, 87, 1146–1152. [Google Scholar] [CrossRef]
- Li, J.; Rao, J.; Liu, Y.; Cao, Y.; Zhang, Y.; Zhang, Q.; Zhu, D. 15-Lipoxygenase promotes chronic hypoxia-induced pulmonary artery inflammation via positive interaction with nuclear factor-κB. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 971–979. [Google Scholar] [CrossRef]
- Wu, M.; Lin, T.; Chiu, Y.; Liou, H.; Yang, R.; Fu, W. Involvement of 15-lipoxygenase in the inflammatory arthritis. J. Cell. Biochem. 2012, 113, 2279–2289. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Wang, Q.; Zhang, X.; Ye, L. Lipid metabolism enzyme 5-LOX and its metabolite LTB4 are capable of activating transcription factor NF-κB in hepatoma cells. Biochem. Biophys. Res. Commun. 2012, 418, 647–651. [Google Scholar] [CrossRef]
- Hui, A.Y.; McCarty, W.J.; Masuda, K.; Firestein, G.S.; Sah, R.L. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 15–37. [Google Scholar] [CrossRef]
- Serhan, C.N. Novel Lipid Mediators and Resolution Mechanisms in Acute Inflammation: To Resolve or Not? Am. J. Pathol. 2010, 177, 1576–1591. [Google Scholar] [CrossRef]
- Dahlen, S.E.; Bjork, J.; Hedqvist, P.; Arfors, K.E.; Hammarstrom, S.; Lindgren, J.A.; Samuelsson, B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: In vivo effects with relevance to the acute inflammatory response. Proc. Natl. Acad. Sci. USA 1981, 78, 3887–3891. [Google Scholar] [CrossRef]
- Chen, M.; Lam, B.K.; Kanaoka, Y.; Nigrovic, P.A.; Audoly, L.P.; Austen, K.F.; Lee, D.M. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 2006, 203, 837–842. [Google Scholar] [CrossRef]
- Gheorghe, K.R.; Korotkova, M.; Catrina, A.I.; Backman, L.; Klint, E.; Claesson, H.; Radmark, O.; Jakobsson, P. Expression of 5-lipoxygenase and 15-lipoxygenase in rheumatoid arthritis synovium and effects of intraarticular glucocorticoids. Arthritis Res. Ther. 2009, 11, R83. [Google Scholar] [CrossRef]
- Asahara, H.; Asanuma, M.; Ogawa, N.; Nishibayashi, S.; Inoue, H. High DNA-binding activity of transcription factor NF-kappa B in synovial membranes of patients with rheumatoid arthritis. Biochem. Mol. Biol. Int. 1995, 37, 827–832. [Google Scholar]
- Jupp, J.; Hillier, K.; Elliott, D.H.; Fine, D.R.; Bateman, A.C.; Johnson, P.A.; Cazaly, A.M.; Penrose, J.F.; Sampson, A.P. Colonic expression of leukotriene-pathway enzymes in inflammatory bowel diseases. Inflamm. Bowel Dis. 2007, 13, 537–546. [Google Scholar] [CrossRef]
- Stanke-Labesque, F.; Pofelski, J.; Moreau-Gaudry, A.; Bessard, G.; Bonaz, B. Urinary leukotriene E4 excretion: A biomarker of inflammatory bowel disease activity. Inflamm. Bowel Dis. 2008, 14, 769–774. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef]
- Pidgeon, G.P.; Tang, K.; Cai, Y.L.; Piasentin, E.; Honn, K.V. Overexpression of Platelet-type 12-Lipoxygenase Promotes Tumor Cell Survival by Enhancing αvβ3 and αvβ5 Integrin Expression. Cancer Res. 2003, 63, 4258–4267. [Google Scholar]
- Hennig, R.; Grippo, P.; Ding, X.; Rao, S.M.; Buchler, M.W.; Friess, H.; Talamonti, M.S.; Bell, R.H.; Adrian, T.E. 5-Lipoxygenase, a Marker for Early Pancreatic Intraepithelial Neoplastic Lesions. Cancer Res. 2005, 65, 6011–6016. [Google Scholar]
- Steiner, D.R.S.; Gonzalez, N.C.; Wood, J.G. Leukotriene B4 promotes reactive oxidant generation and leukocyte adherence during acute hypoxia. J. Appl. Physiol. 2001, 91, 1160–1167. [Google Scholar]
- Kerjaschki, D.; Bago-Horvath, Z.; Rudas, M.; Sexl, V.; Schneckenleithner, C.; Wolbank, S.; Bartel, G.; Krieger, S.; Kalt, R.; Hantusch, B.; et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Investig. 2011, 121, 2000–2012. [Google Scholar] [CrossRef]
- Wu, Y.; Mao, F.; Zuo, X.; Moussalli, M.J.; Elias, E.; Xu, W.; Shureiqi, I. 15-LOX-1 suppression of hypoxia-induced metastatic phenotype and HIF-1α expression in human colon cancer cells. Cancer Med. 2014, 3, 472–484. [Google Scholar] [CrossRef]
- Suraneni, M.V.; Schneider-Broussard, R.; Moore, J.R.; Davis, T.C.; Maldonado, C.J.; Li, H.; Newman, R.A.; Kusewitt, D.; Hu, J.; Yang, P.; et al. Transgenic expression of 15-lipoxygenase 2 (15-LOX2) in mouse prostate leads to hyperplasia and cell senescence. Oncogene 2010, 29, 4261–4275. [Google Scholar] [CrossRef]
- Serhan, C.N. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim. Biophys. Acta 1994, 1212, 1–25. [Google Scholar] [CrossRef]
- Chiang, N.; Arita, M.; Serhan, C.N. Anti-inflammatory circuitry: Lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 163–177. [Google Scholar] [CrossRef]
- Serhan, C.N. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Fierro, I.M.; Colgan, S.P.; Bernasconi, G.; Petasis, N.A.; Clish, C.B.; Arita, M.; Serhan, C.N. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin a4 inhibit human neutrophil migration: Comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. J. Immunol. 2003, 170, 2688–2694. [Google Scholar] [CrossRef]
- Clària, J.; Titos, E.; Jiménez, W.; Ros, J.; Ginès, P.; Arroyo, V.; Rivera, F.; Rodés, J. Altered biosynthesis of leukotrienes and lipoxins and host defense disorders in patients with cirrhosis and ascites. Gastroenterology 1998, 115, 147–156. [Google Scholar] [CrossRef]
- Karp, C.L. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat. Immunol. 2004, 5, 388–392. [Google Scholar] [CrossRef]
- Benson, M.; Carlsson, L.; Adner, M.; Jernås, M.; Rudemo, M.; Sjögren, A.; Svensson, P.A.; Uddman, R.; Cardell, L.O. Gene profiling reveals increased expression of uteroglobin and other anti-inflammatory genes in glucocorticoid-treated nasal polyps. J. Allergy Clin. Immunol. 2004, 113, 1137–1143. [Google Scholar] [CrossRef]
- Chavis, C.; Vachier, I.; Chanez, P.; Bousquet, J.; Godard, P. 5(S),15(S)-dihydroxyeicosatetraenoicacid and lipoxin generation in human polymorphonuclear cells: Dual specificity of 5-lipoxygenase towards endogenous and exogenous precursors. J. Exp. Med. 1996, 183, 1633–1643. [Google Scholar] [CrossRef]
- Basselin, M. Anti-inflammatory effects of chronic aspirin on brain arachidonic acid metabolites. Neurochem. Res. 2011, 36, 139–145. [Google Scholar] [CrossRef]
- Takeda, S.; Jiang, R.; Aramaki, H.; Imoto, M.; Toda, A.; Eyanagi, R.; Amamoto, T.; Yamamoto, I.; Watanabe, K. 9-tetrahydrocannabinol and its major metabolite 9-tetrahydrocannabinol-11-oic acid as 15-lipoxygenase inhibitors. J. Pharm. Sci. 2011, 100, 1206–1211. [Google Scholar] [CrossRef]
- Whitman, S. Structure-activity relationship studies of nordihydroguaiaretic acid inhibitors toward soybean, 12-human, and 15-human lipoxygenase. J. Med. Chem. 2002, 45, 2659–2661. [Google Scholar] [CrossRef]
- Moody, J.S.; Marnett, L.J. Kinetics of Inhibition of Leukocyte 12-Lipoxygenase by the Isoform-Specific Inhibitor 4-(2-Oxapentadeca-4-yne)phenylpropanoic Acid. Biochemistry 2002, 41, 10297–10303. [Google Scholar] [CrossRef]
- Hong, J.; Bose, M.; Ju, J.; Ryu, J.; Chen, X.; Sang, S.; Lee, M.; Yang, C.S. Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: Effects on cytosolic phospholipase A2, cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef]
- Gillard, J.; Ford-Hutchinson, A.W.; Chan, C.; Charleson, S.; Denis, D.; Foster, A.; Fortin, R.; Leger, S.; McFarlane, C.S.; Morton, H. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2—dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can. J. Physiol. Pharmacol. 1989, 67, 456–464. [Google Scholar] [CrossRef]
- Evans, J.F.; Ferguson, A.D.; Mosley, R.T.; Hutchinson, J.H. What’s all the FLAP about? 5-lipoxygenase-activating protein inhibitors for inflammatory diseases. Trends Pharmacol. Sci. 2008, 29, 72–78. [Google Scholar] [CrossRef]
- Friedman, B.S.; Bel, E.H.; Buntinx, A.; Tanaka, W.; Han, Y.R.; Shingo, S.; Spector, R.; Sterk, P. Oral Leukotriene Inhibitor (MK-886) Blocks Allergen-induced Airway Responses. Am. Rev. Respir. Dis. 1993, 147, 839–844. [Google Scholar] [CrossRef]
- Fruchtmann, R.; Mohrs, K.H.; Hatzelmann, A.; Raddatz, S.; Fugmann, B.; Junge, B.; Horstmann, H.; Muller-Peddinghaus, R. In vitro pharmacology of BAY X1005, a new inhibitor of leukotriene synthesis. Agents Actions 1993, 38, 188–195. [Google Scholar] [CrossRef]
- Mancini, J.A.; Prasit, P.; Coppolino, M.G.; Charleson, P.; Leger, S.; Evans, J.F.; Gillard, J.W.; Vickers, P.J. 5-Lipoxygenase-activating protein is the target of a novel hybrid of two classes of leukotriene biosynthesis inhibitors. Mol. Pharmacol. 1992, 41, 267–272. [Google Scholar]
- Charleson, S.; Evans, J.F.; Leger, S.; Perrier, H.; Prasit, P.; Wang, Z.; Vickers, P.J. Structural requirements for the binding of fatty acids to 5-lipoxygenase-activating protein. Eur. J. Pharmacol. 1994, 267, 275–280. [Google Scholar] [CrossRef]
- Werz, O. 5-Lipoxygenase: Cellular Biology and Molecular Pharmacology. Curr. Drug Targets Inflamm. Allergy 2002, 1, 23–44. [Google Scholar] [CrossRef]
- McMillan, R.M.; Walker, E.R.H. Designing therapeutically effective 5-lipoxygenase inhibitors. Trends Pharmacol. Sci. 1992, 13, 323–330. [Google Scholar] [CrossRef]
- Batt, D.G.; Maynard, G.D.; Petraitis, J.J.; Shaw, J.E.; Galbraith, W.; Harris, R.R. 2-Substituted-1-Naphthols as Potent 5-Lipoxygenase Inhibitors with Topical Antiinflammatory Activity. J. Med. Chem. 1990, 33, 360–370. [Google Scholar] [CrossRef]
- Corey, E.J.; Wright, S.W.; Matsuda, S.P.T. Stereochemistry and mechanism of the biosynthesis of leukotriene A4 from 5(S)-hydroperoxy-6(E),8,11,14(Z)-eicosatetraenoic acid. Evidence for an organoiron intermediate. J. Am. Chem. Soc. 1989, 111, 1452–1455. [Google Scholar]
- Doiron, J.; Boudreau, L.H.; Picot, N.; Villebonet, B.; Surette, M.E.; Touaibia, M. Synthesis and 5-lipoxygenase inhibitory activity of new cinnamoyl and caffeoylclusters. Bioorg. Med. Chem. Lett. 2009, 19, 1118–1121. [Google Scholar] [CrossRef]
- Ford-Hutchinson, A.; Gresser, M.; Young, R.N. 5-Lipoxygenase. Annu. Rev. Biochem. 1994, 63, 383–417. [Google Scholar] [CrossRef]
- Gillmor, S.A.; Villasenor, A.; Fletterick, R.; Sigal, E.; Browner, M.F. The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nat. Struct. Biol. 1997, 4, 1003–1009. [Google Scholar] [CrossRef]
- Xu, S.; Mueser, T.; Marnett, L.; Funk, M., Jr. Crystal structure of 12-lipoxygenase catalytic-domain-inhibitor complex identifies a substrate-binding channel for catalysis. Structure 2012, 20, 1490–1497. [Google Scholar] [CrossRef]
- Connolly, P.J.; Wetter, S.K.; Beers, K.N.; Hamel, S.C.; Chen, R.H.K.; Wachter, M.P.; Ansell, J.; Singer, M.M.; Steber, M.; Ritchie, D.M.; et al. N-Hydroxyurea and hydroxamic acid inhibitors of cyclooxygenase and 5-lipoxygenase. Bioorg. Med. Chem. Lett. 1999, 9, 979–984. [Google Scholar] [CrossRef]
- Nelson, H.; Kemp, J.; Berger, W.; Corren, J.; Casale, T.; Dube, L.; Walton-Bowen, K.; LaVallee, N.; Stepanians, M. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: A 3-month randomized controlled study. Ann. Allergy Asthma Immunol. 2007, 99, 178–184. [Google Scholar] [CrossRef]
- Brooks, C.D.; Stewart, A.O.; Basha, A.; Bhatia, P.; Ratajczyk, J.D.; Martin, J.G.; Craig, R.A.; Kolasa, T.; Bouska, J.B.; Lanni, C. (R)-(+)-N-[3-[5-[(4-fluorophenyl)methyl]-2-thienyl]-1-methyl- 2-propynyl]-N-hydroxyurea (ABT-761), a second-generation 5-lipoxygenase inhibitor. J. Med. Chem. 1995, 38, 4768–4775. [Google Scholar] [CrossRef]
- Back, M. Inhibitors of the 5-lipoxygenase pathway in atherosclerosis. Curr. Pharm. Des. 2009, 15, 3116–3132. [Google Scholar] [CrossRef]
- Gurjar, M.K.; Murugaiah, A.M.S.; Radhakrishna, P.; Ramana, C.V.; Chorghade, M.S. A novel and simple asymmetric synthesis of CMI-977 (LDP-977): A potent anti-asthmatic drug lead. Tetrahedron Asymmetry 2003, 14, 1363–1370. [Google Scholar] [CrossRef]
- Pergola, C.; Werz, O. 5-Lipoxygenase inhibitors: A review of recent developments and patents. Expert Opin. Ther. Patents 2010, 20, 355–375. [Google Scholar] [CrossRef]
- Werz, O. Pharmacological intervention with 5-lipoxygenase: New insights and novel compounds. Expert Opin. Ther. Patents 2005, 15, 505–519. [Google Scholar] [CrossRef]
- Bird, T.G.C.; Bruneau, P.; Crawley, G.C.; Edwards, M.P.; Foster, S.J.; Girodeau, J.M.; Kingston, J.F.; McMillan, R.M. (Methoxyalkyl)thiazoles: A new series of potent, selective, and orally active 5-lipoxygenase inhibitors displaying high enantioselectivity. J. Med. Chem. 1991, 34, 2176–2186. [Google Scholar] [CrossRef]
- Falgueyret, J.; Hutchinson, J.H.; Riendeau, D. Criteria for the identification of non-redox inhibitors of 5-lipoxygenase. Biochem. Pharmacol. 1993, 45, 978–981. [Google Scholar] [CrossRef]
- Crawley, G.C.; Dowell, R.I.; Edwards, P.N.; Foster, S.J.; McMillan, R.M.; Walker, E.R.H.; Waterson, D.; Bird, T.G.; Bruneau, P.; Girodeau, J.M. Methoxytetrahydropyrans. A new series of selective and orally potent 5-lipoxygenase inhibitors. J. Med. Chem. 1992, 35, 2600–2609. [Google Scholar] [CrossRef]
- Kusner, E.J.; Buckner, C.K.; Dea, D.M.; DeHaas, C.J.; Marks, R.L.; Krell, R.D. The 5-lipoxygenase inhibitors ZD2138 and ZM230487 are potent and selective inhibitors of several antigen-induced guinea-pig pulmonary responses. Eur. J. Pharmacol. 1994, 257, 285–292. [Google Scholar] [CrossRef]
- Young, R.N. Inhibitors of 5-lipoxygenase: A therapeutic potential yet to be fully realized? Eur. J. Med. Chem. 1999, 34, 671–685. [Google Scholar] [CrossRef]
- Wisastra, R.; Ghizzoni, M.; Boltjes, A.; Haisma, H.J.; Dekker, F.J. Anacardic acid derived salicylates are inhibitors or activators of lipoxygenases. Bioorg. Med. Chem. 2012, 20, 5027–5032. [Google Scholar] [CrossRef]
- Baggelaar, M.P.; Huang, Y.; Feringa, B.L.; Dekker, F.J.; Minnaard, A.J. Catalytic asymmetric total synthesis of (S)-(−)-zearalenone, a novel lipoxygenase inhibitor. Bioorg. Med. Chem. 2013, 21, 5271–5274. [Google Scholar] [CrossRef]
- Wisastra, R.; Kok, P.A.; Eleftheriadis, N.; Baumgartner, M.P.; Camacho, C.J.; Haisma, H.J.; Dekker, F.J. Discovery of a novel activator of 5-lipoxygenase from an anacardic acid derived compound collection. Bioorg. Med. Chem. 2013, 21, 7763–7778. [Google Scholar] [CrossRef]
- Sorkness, C.A. The use of 5-lipoxygenase inhibitors and leukotriene receptor antagonists in the treatment of chronic asthma. Pharmacotherapy 1997, 17, 50S–54S. [Google Scholar]
- Renzi, P.M. Antileukotriene agents in asthma: The dart that kills the elephant? CMAJ 1999, 160, 217–223. [Google Scholar]
- Tintinger, G.R.; Feldman, C.; Theron, A.J; Anderson, R. Montelukast: More than a cysteinyl leukotriene receptor antagonist? ScientificWorldJournal 2010, 10, 2403–2413. [Google Scholar] [CrossRef]
- Ramires, R.; Caiaffa, M.F.; Tursi, A.; Haeggström, J.Z.; Macchia, L. Novel inhibitory effect on 5-lipoxygenase activity by the anti-asthma drug montelukast. Biochem. Biophys. Res. Commun. 2004, 324, 815–821. [Google Scholar] [CrossRef]
- Tahan, F. Montelukast inhibits tumour necrosis factor-a-mediated interleukin-8 expression through inhibition of nuclear factor-kappaB p65-associated histone acetyltransferase activity. Clin. Exp. Allergy 2008, 38, 805–811. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wisastra, R.; Dekker, F.J. Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked. Cancers 2014, 6, 1500-1521. https://doi.org/10.3390/cancers6031500
Wisastra R, Dekker FJ. Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked. Cancers. 2014; 6(3):1500-1521. https://doi.org/10.3390/cancers6031500
Chicago/Turabian StyleWisastra, Rosalina, and Frank J. Dekker. 2014. "Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked" Cancers 6, no. 3: 1500-1521. https://doi.org/10.3390/cancers6031500