Apc-Mutant Kyoto Apc Delta (KAD) Rats Are Susceptible to 4-NQO-Induced Tongue Carcinogenesis
Abstract
:1. Introduction
2. Results
2.1. Preneoplastic and Neoplastic Lesions in the Tongue in the KAD and F344/NS1c Rats that Received 4-NQO
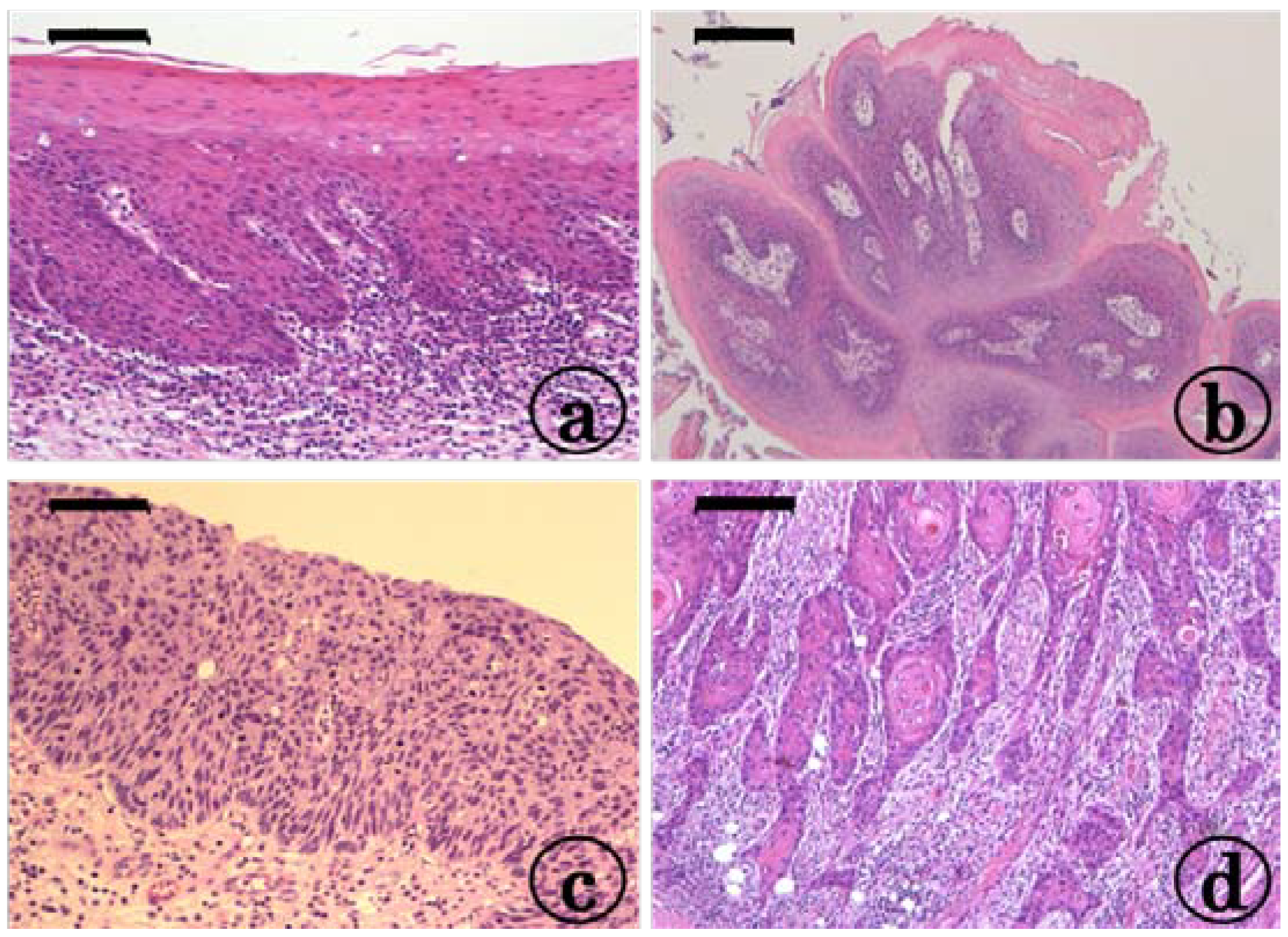

2.2. Inflammation in the Tongue in the KAD and F344/NS1c Rats that Received 4-NQO

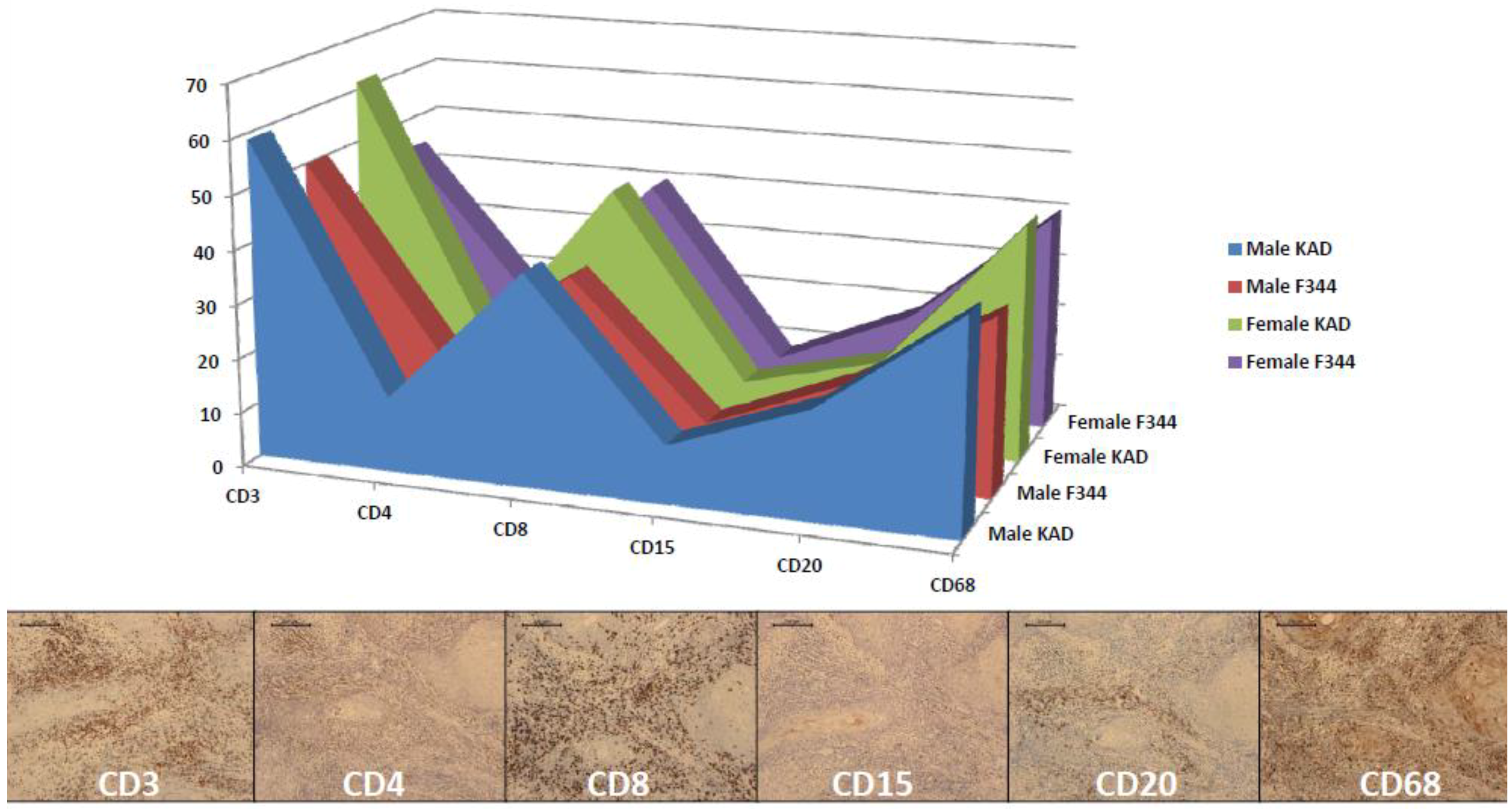
2.3. Classification of Inflammatory Lymphocytes in the Tongue on Immunohistochemistry of CDs
2.4. Mast Cell Density in the Tongue

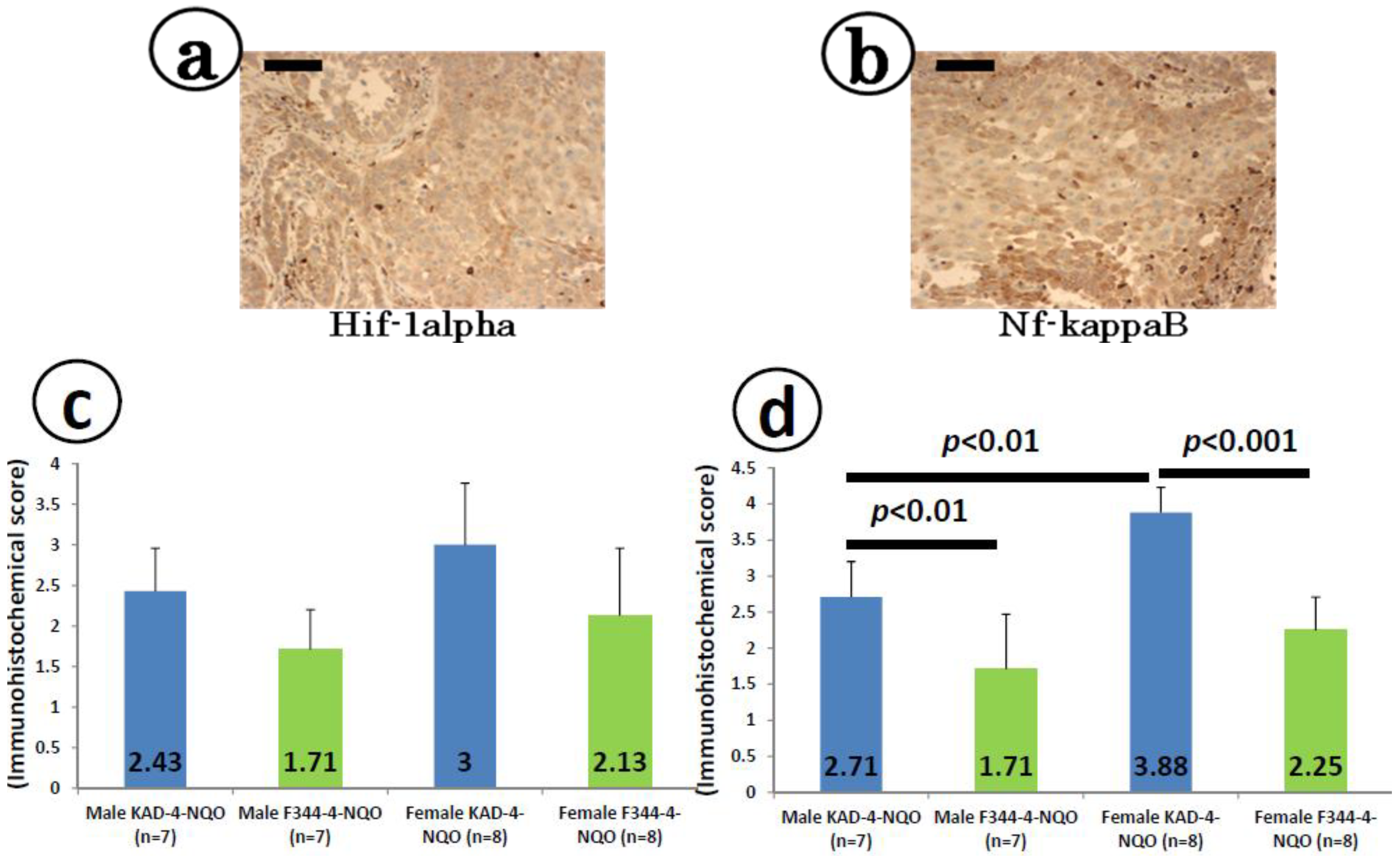
2.5. Immunohistochemical Expression of Hif-1α and Nf-κB in the Tongue SCCs
2.6. mRNA Expression Levels of Inducible Inflammatory Enzymes and Pro-Inflammatory Cytokines in the Tongue at Week 8

3. Discussion
4. Experimental
4.1. Animals, Chemicals and Diet
4.2. Experimental Protocol
4.3. Histopathological Diagnosis of the Tongue Lesions
4.4. Inflammation Scoring of the Tongue with or without Preneoplastic and Neoplastic Lesions
4.5. Immunohistochemistry and Histochemistry
4.6. Total RNA Extraction and Quantitative Real-Time PCR
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- Choi, S.; Myers, J.N. Molecular pathogenesis of oral squamous cell carcinoma: Implications for therapy. J. Dent. Res. 2008, 87, 14–32. [Google Scholar] [CrossRef]
- Hooper, S.J.; Wilson, M.J.; Crean, S.J. Exploring the link between microorganisms and oral cancer: A systematic review of the literature. Head Neck 2009, 31, 1228–1239. [Google Scholar]
- Meurman, J.H.; Uittamo, J. Oral micro-organisms in the etiology of cancer. Acta Odontol. Scand. 2008, 66, 321–326. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar]
- Sethi, G.; Sung, B.; Aggarwal, B.B. TNF: A master switch for inflammation to cancer. Front. Biosci. 2008, 13, 5094–5107. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Heidland, A.; Klassen, A.; Rutkowski, P.; Bahner, U. The contribution of Rudolf Virchow to the concept of inflammation: What is still of importance? J. Nephrol. 2006, 19, S102–S109. [Google Scholar]
- Schmidt, A.; Weber, O.F. In memoriam of Rudolf virchow: A historical retrospective including aspects of inflammation, infection and neoplasia. Contrib. Microbiol. 2006, 13, 1–15. [Google Scholar] [CrossRef]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar] [CrossRef]
- Tanaka, T. Inflammation and cancer. In Disease Progression and Chemoprevention; Tanaka, T., Ed.; Research Signpost: Kerala, India, 2007; pp. 27–44. [Google Scholar]
- Tanaka, T. Introduction for inflammation and cancer. Semin. Immunopathol. 2013, 35, 121–122. [Google Scholar] [CrossRef]
- Tanaka, T.; Shimizu, M.; Kochi, T.; Moriwaki, H. Chemical-induced Carcinogenesis. J. Exp. Clin. Med. 2013, 5, 203–209. [Google Scholar] [CrossRef]
- Feller, L.; Altini, M.; Lemmer, J. Inflammation in the context of oral cancer. Oral Oncol. 2013, 49, 887–892. [Google Scholar] [CrossRef]
- Forrest, J.L.; Horowitz, A.M.; Shmuely, Y. Dental hygienists’ knowledge, opinions, and practices related to oral and pharyngeal cancer risk assessment. J. Dent. Hyg. 2001, 75, 271–281. [Google Scholar]
- Meurman, J.H. Infectious and dietary risk factors of oral cancer. Oral Oncol. 2010, 46, 411–413. [Google Scholar] [CrossRef]
- Beroud, C.; Soussi, T. APC gene: Database of germline and somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1996, 24, 121–124. [Google Scholar] [CrossRef]
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M.; et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66, 589–600. [Google Scholar]
- Evers, C.; Gaspar, H.; Kloor, M.; Bozukova, G.; Kadmon, M.; Keller, M.; Sutter, C.; Moog, U. Hepatoblastoma in two siblings and familial adenomatous polyposis: Causal nexus or coincidence? Fam. Cancer 2012, 11, 529–533. [Google Scholar] [CrossRef]
- Tamura, G. Molecular pathogenesis of adenoma and differentiated adenocarcinoma of the stomach. Pathol. Int. 1996, 46, 834–841. [Google Scholar] [CrossRef]
- Imai, F.L.; Uzawa, K.; Shiiba, M.; Watanabe, T.; Miya, T.; Kubosawa, H.; Kondo, Y.; Tanzawa, H. Evidence of multi-step oncogenesis of oral squamous cell carcinoma. Oncol. Rep. 1998, 5, 1489–1491. [Google Scholar]
- Kannan, S.; Yokozaki, H.; Jayasree, K.; Sebastian, P.; Mathews, A.; Abraham, E.K.; Nair, M.K.; Tahara, E. Infrequent loss of heterozygosity of the major tumour suppressor genes in Indian oral cancers. Int. J. Oral Maxillofac. Surg. 2002, 31, 414–418. [Google Scholar] [CrossRef]
- Liao, P.H.; Lee, T.L.; Yang, L.C.; Yang, S.H.; Chen, S.L.; Chou, M.Y. Adenomatous polyposis coli gene mutation and decreased wild-type p53 protein expression in oral submucous fibrosis: A preliminary investigation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 202–207. [Google Scholar] [CrossRef]
- Pannone, G.; Bufo, P.; Santoro, A.; Franco, R.; Aquino, G.; Longo, F.; Botti, G.; Serpico, R.; Cafarelli, B.; Abbruzzese, A.; et al. WNT pathway in oral cancer: Epigenetic inactivation of WNT-inhibitors. Oncol. Rep. 2010, 24, 1035–1041. [Google Scholar]
- Yeh, K.T.; Chang, J.G.; Lin, T.H.; Wang, Y.F.; Chang, J.Y.; Shih, M.C.; Lin, C.C. Correlation between protein expression and epigenetic and mutation changes of Wnt pathway-related genes in oral cancer. Int. J. Oncol. 2003, 23, 1001–1007. [Google Scholar]
- Yoshimi, K.; Tanaka, T.; Takizawa, A.; Kato, M.; Hirabayashi, M.; Mashimo, T.; Serikawa, T.; Kuramoto, T. Enhanced colitis-associated colon carcinogenesis in a novel Apc mutant rat. Cancer Sci. 2009, 100, 2022–2027. [Google Scholar] [CrossRef]
- Yoshimi, K.; Hashimoto, T.; Niwa, Y.; Hata, K.; Serikawa, T.; Tanaka, T.; Kuramoto, T. Use of a chemically induced-colon carcinogenesis-prone Apc-mutant rat in a chemotherapeutic bioassay. BMC Cancer 2012, 12, 448. [Google Scholar]
- Yoshimi, K.; Tanaka, T.; Serikawa, T.; Kuramoto, T. Tumor suppressor APC protein is essential in mucosal repair from colonic inflammation through angiogenesis. Am. J. Pathol. 2013, 182, 1263–1274. [Google Scholar] [CrossRef]
- Tanaka, T.; Ishigamori, R. Understanding carcinogenesis for fighting oral cancer. J. Oncol. 2011, 2011, 603740. [Google Scholar]
- Tanaka, T.; Tanaka, M. Oral carcinogenesis and oral cancer chemoprevention: A review. Patholog. Res. Int. 2011, 2011, 431246. [Google Scholar]
- Van der Meij, E.H.; Mast, H.; van der Waal, I. The possible premalignant character of oral lichen planus and oral lichenoid lesions: A prospective five-year follow-up study of 192 patients. Oral Oncol. 2007, 43, 742–748. [Google Scholar] [CrossRef]
- Liu, Y.; Messadi, D.V.; Wu, H.; Hu, S. Oral lichen planus is a unique disease model for studying chronic inflammation and oral cancer. Med. Hypotheses 2010, 75, 492–494. [Google Scholar] [CrossRef]
- Khatri, M.J.; Desai, R.S.; Mamatha, G.S.; Kukarni, M.; Khatri, J. Immunohistochemical expression of mast cells using c-Kit in various grades of oral submucous fibrosis. ISRN Pathology 2013, 2013. Article ID 543976. [Google Scholar]
- Iamaroon, A.; Pongsiriwet, S.; Jittidecharaks, S.; Pattanaporn, K.; Prapayasatok, S.; Wanachantararak, S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J. Oral Pathol. Med. 2003, 32, 195–199. [Google Scholar] [CrossRef]
- Ranieri, G.; Labriola, A.; Achille, G.; Florio, G.; Zito, A.F.; Grammatica, L.; Paradiso, A. Microvessel density, mast cell density and thymidine phosphorylase expression in oral squamous carcinoma. Int. J. Oncol. 2002, 21, 1317–1323. [Google Scholar]
- Aromando, R.F.; Perez, M.A.; Heber, E.M.; Trivillin, V.A.; Tomasi, V.H.; Schwint, A.E.; Itoiz, M.E. Potential role of mast cells in hamster cheek pouch carcinogenesis. Oral Oncol. 2008, 44, 1080–1087. [Google Scholar] [CrossRef]
- Tanaka, T.; Ishikawa, H. Mast cells and inflammation-associated colorectal carcinogenesis. Semin. Immunopathol. 2013, 35, 245–254. [Google Scholar] [CrossRef]
- Eckert, A.W.; Schutze, A.; Lautner, M.H.; Taubert, H.; Schubert, J.; Bilkenroth, U. HIF-1α is a prognostic marker in oral squamous cell carcinomas. Int. J. Biol. Markers 2010, 25, 87–92. [Google Scholar]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef]
- Nagini, S.; Letchoumy, P.V.; A, T.; Cr, R. Of humans and hamsters: A comparative evaluation of carcinogen activation, DNA damage, cell proliferation, apoptosis, invasion, and angiogenesis in oral cancer patients and hamster buccal pouch carcinomas. Oral Oncol. 2009, 45, e31–37. [Google Scholar] [CrossRef]
- Gerhauser, C. Cancer chemoprevention and nutriepigenetics: State of the art and future challenges. Top. Curr. Chem. 2013, 329, 73–132. [Google Scholar] [CrossRef]
- Kimbro, K.S.; Simons, J.W. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr. Relat. Cancer 2006, 13, 739–749. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M.; Moriwaki, H. Cancer chemoprevention with green tea catechins: From bench to bed. Curr. Drug Targets 2012, 13, 1842–1857. [Google Scholar] [CrossRef]
- Jablonska, E.; Piotrowski, L.; Grabowska, Z. Serum levels of IL-1β, IL-6, TNF-α, sTNF-RI and CRP in patients with oral cavity cancer. Pathol. Oncol. Res. 1997, 3, 126–129. [Google Scholar] [CrossRef]
- Elashoff, D.; Zhou, H.; Reiss, J.; Wang, J.; Xiao, H.; Henson, B.; Hu, S.; Arellano, M.; Sinha, U.; Le, A.; et al. Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol. Biomarkers Prev. 2012, 21, 664–672. [Google Scholar] [CrossRef]
- Prasad, G.; McCullough, M. Chemokines and cytokines as salivary biomarkers for the early diagnosis of oral cancer. Int. J. Dent. 2013, 2013, 813756. [Google Scholar]
- Aivaliotis, I.L.; Pateras, I.S.; Papaioannou, M.; Glytsou, C.; Kontzoglou, K.; Johnson, E.O.; Zoumpourlis, V. How do cytokines trigger genomic instability? J. Biomed. Biotechnol. 2012, 2012, 536761. [Google Scholar]
- Suzuki, R.; Kohno, H.; Suzui, M.; Yoshimi, N.; Tsuda, H.; Wakabayashi, K.; Tanaka, T. An animal model for the rapid induction of tongue neoplasms in human c-Ha-ras proto-oncogene transgenic rats by 4-nitroquinoline 1-oxide: Its potential use for preclinical chemoprevention studies. Carcinogenesis 2006, 27, 619–630. [Google Scholar] [CrossRef]
- Tanaka, T.; Kohno, H.; Nomura, E.; Taniguchi, H.; Tsuno, T.; Tsuda, H. A novel geranylated derivative, ethyl 3-(4'-geranyloxy-3'-methoxyphenyl)-2-propenoate, synthesized from ferulic acid suppresses carcinogenesis and inducible nitric oxide synthase in rat tongue. Oncology 2003, 64, 166–175. [Google Scholar] [CrossRef]
- Yoshida, K.; Tanaka, T.; Kohno, H.; Sakata, K.; Kawamori, T.; Mori, H.; Wakabayashi, K. A COX-2 inhibitor, nimesulide, inhibits chemically-induced rat tongue carcinogenesis through suppression of cell proliferation activity and COX-2 and iNOS expression. Histol. Histopathol. 2003, 18, 39–48. [Google Scholar]
Appendix
| Genes (species) | Forward primer (5' to 3') | Reverse primer (5' to 3') |
|---|---|---|
| Tnf-α (rat) | GGCAGGTCTACTTTGGAGTCATTGC | ACATTCGAGGCTCCAGTGAATTCGG |
| Il-1β (rat) | ACCTGCTAGTGTGTGATGTTCCCA | AGGTGGAGAGCTTTCAGCTCACAT |
| Il-6 (rat) | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| Il-17 (rat) | GTCAATGCGGAGGGAAAG | CACGAAGCAGTTTGGGAC |
| KC (Il-8) (human) | TTGGCAGCCTTCCTGATT | AACTTCTCCACAACCCTCTG |
| Inf-γ (rat) | AAAGACAACCAGGCCATCAGCAAC | TCTGTGGGTTGTTCACCTCGAACT |
| Cox-2 (rat) | GCATTCTTTGCCCAGCACTTCACT | TTTAAGTCCACTCCATGGCCCAGT |
| iNos (rat) | AGAGAGATCGGGTTCACA | CACAGAACTGAGGGTACA |
| β-actin (rat) | TGGAATCCTGTGGCATCCA | TAACAGTCCGCCTAGAAGCA |

© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tanaka, T.; Shimizu, M.; Kochi, T.; Shirakami, Y.; Mori, T.; Watanabe, N.; Naiki, T.; Moriwaki, H.; Yoshimi, K.; Serikawa, T.; et al. Apc-Mutant Kyoto Apc Delta (KAD) Rats Are Susceptible to 4-NQO-Induced Tongue Carcinogenesis. Cancers 2014, 6, 1522-1539. https://doi.org/10.3390/cancers6031522
Tanaka T, Shimizu M, Kochi T, Shirakami Y, Mori T, Watanabe N, Naiki T, Moriwaki H, Yoshimi K, Serikawa T, et al. Apc-Mutant Kyoto Apc Delta (KAD) Rats Are Susceptible to 4-NQO-Induced Tongue Carcinogenesis. Cancers. 2014; 6(3):1522-1539. https://doi.org/10.3390/cancers6031522
Chicago/Turabian StyleTanaka, Takuji, Masahito Shimizu, Takahiro Kochi, Yohei Shirakami, Takayuki Mori, Naoki Watanabe, Takafumi Naiki, Hisataka Moriwaki, Kazuto Yoshimi, Tadao Serikawa, and et al. 2014. "Apc-Mutant Kyoto Apc Delta (KAD) Rats Are Susceptible to 4-NQO-Induced Tongue Carcinogenesis" Cancers 6, no. 3: 1522-1539. https://doi.org/10.3390/cancers6031522





