Retroareolar Carcinomas in Breast Ultrasound: Pearls and Pitfalls
Abstract
:1. Introduction
2. Anatomy
3. US Imaging Modalities
3.1. US Technical Challenges
- Acoustic shadowing is the main culprit and is related to two simultaneous factors:
- ○
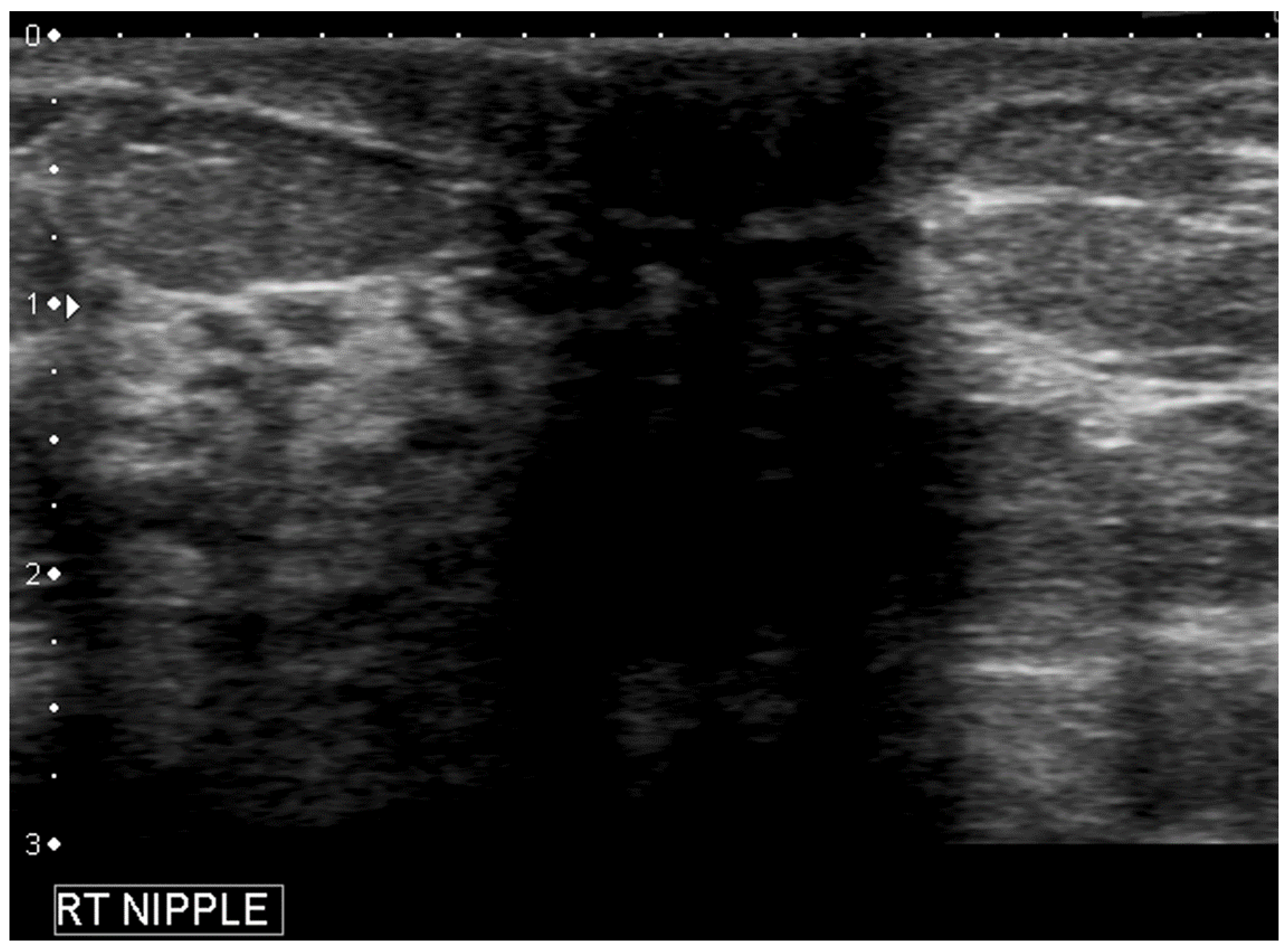
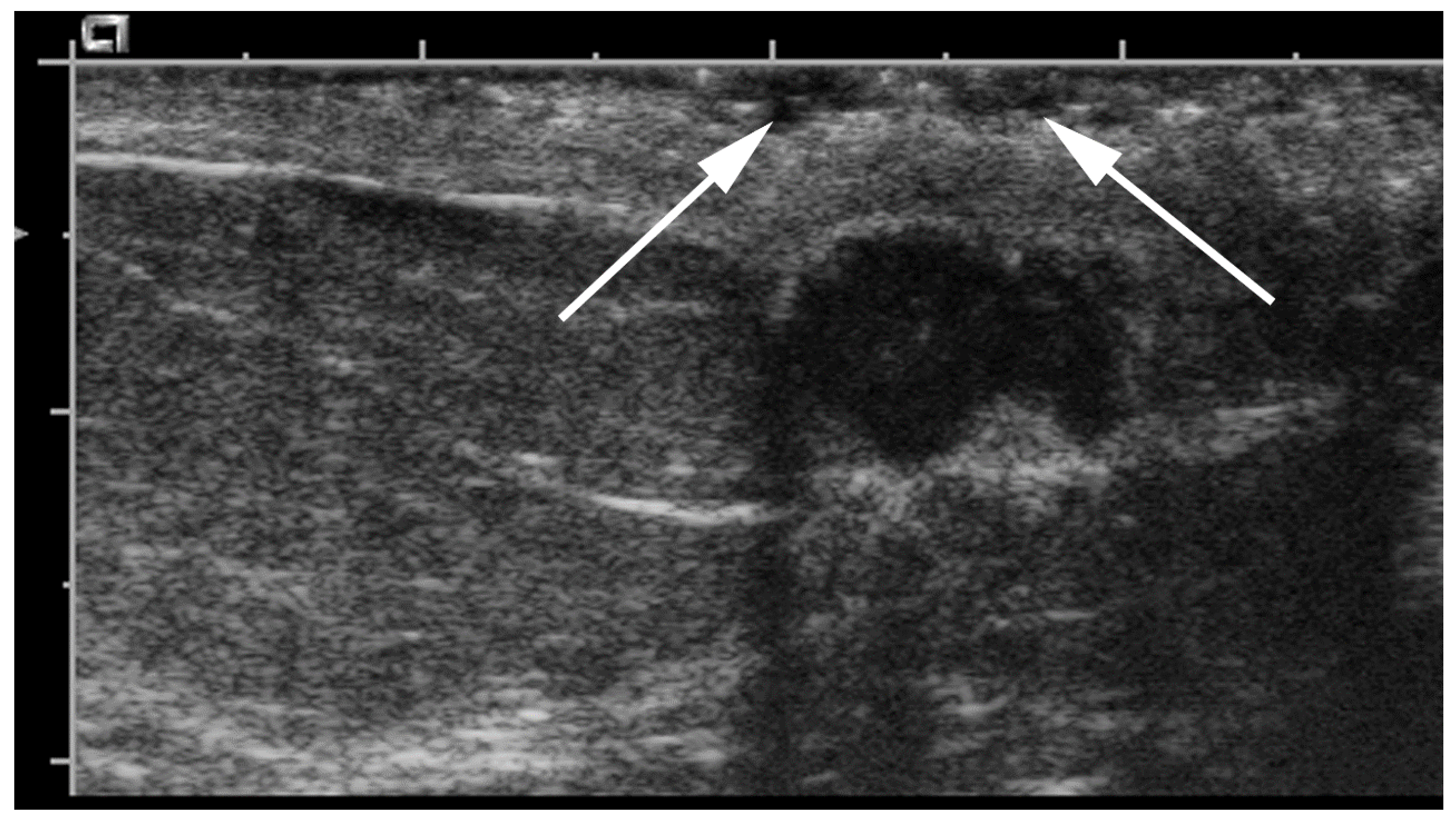
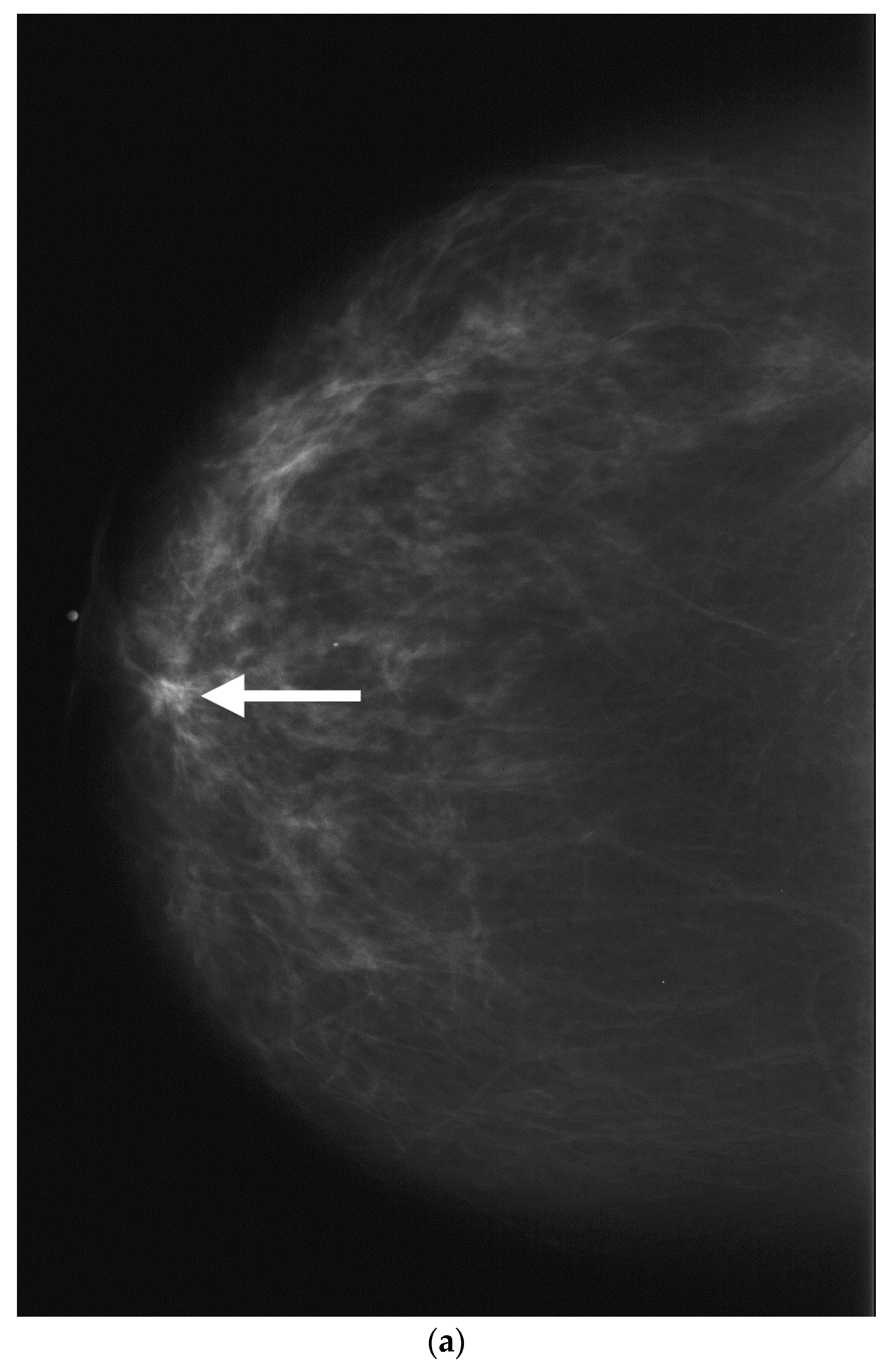
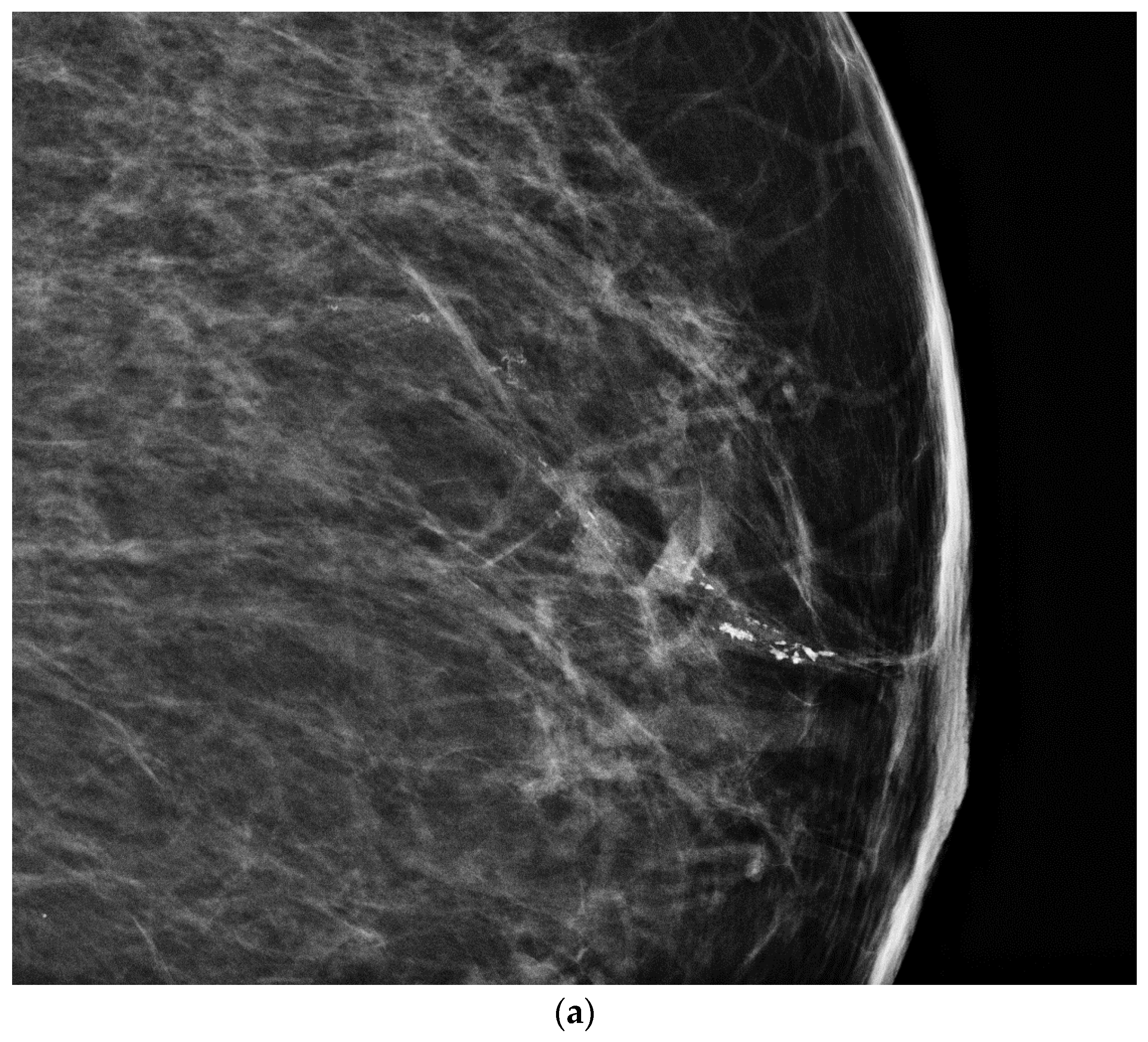
- Geometric shape of the nipple: the crevices and irregular surfaces may generate a mass like appearance with posterior acoustic shadowing (Figure 2).
- ○
- Ducts have a radial orientation that limits US evaluation given the beam direction.
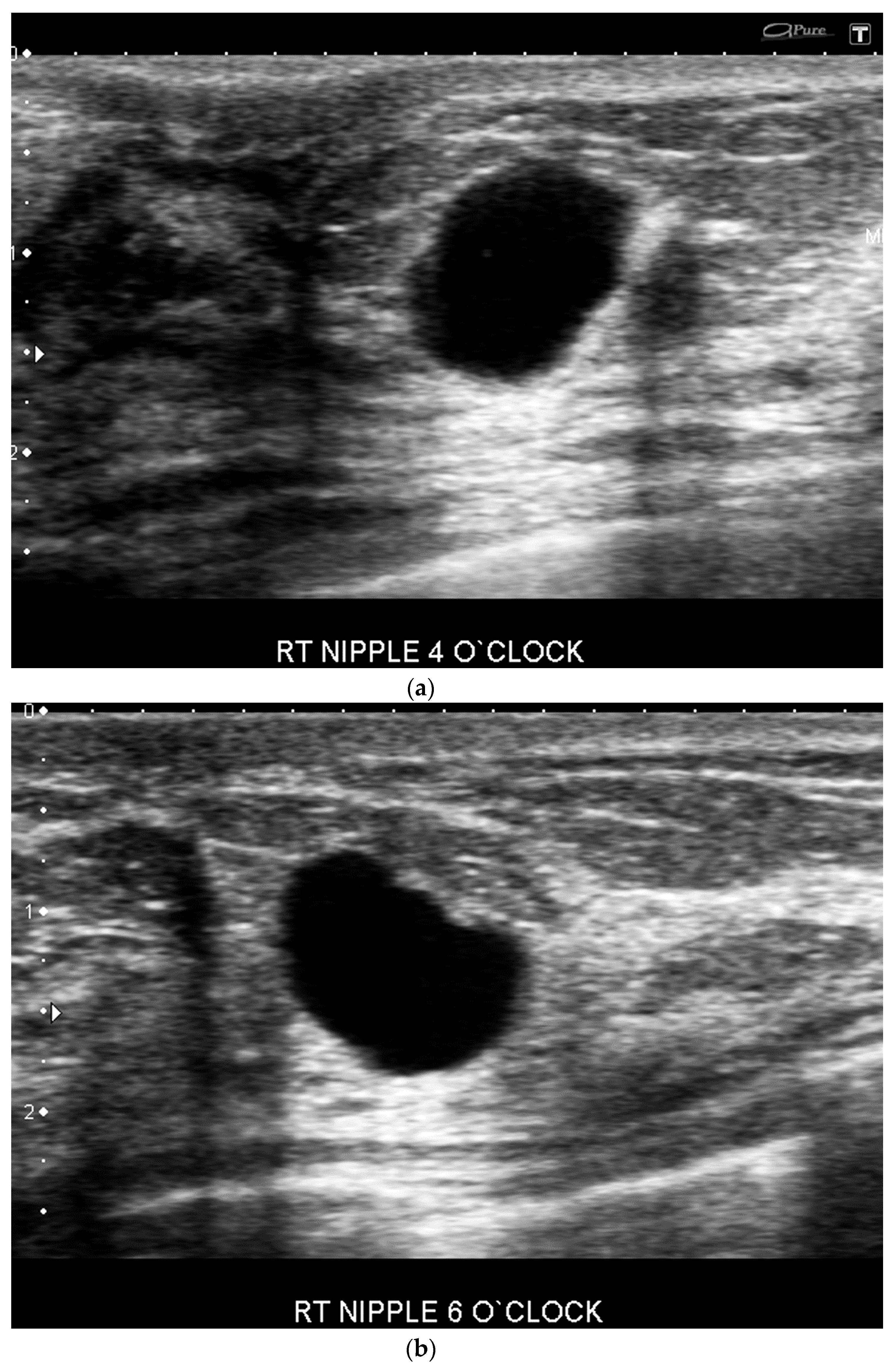
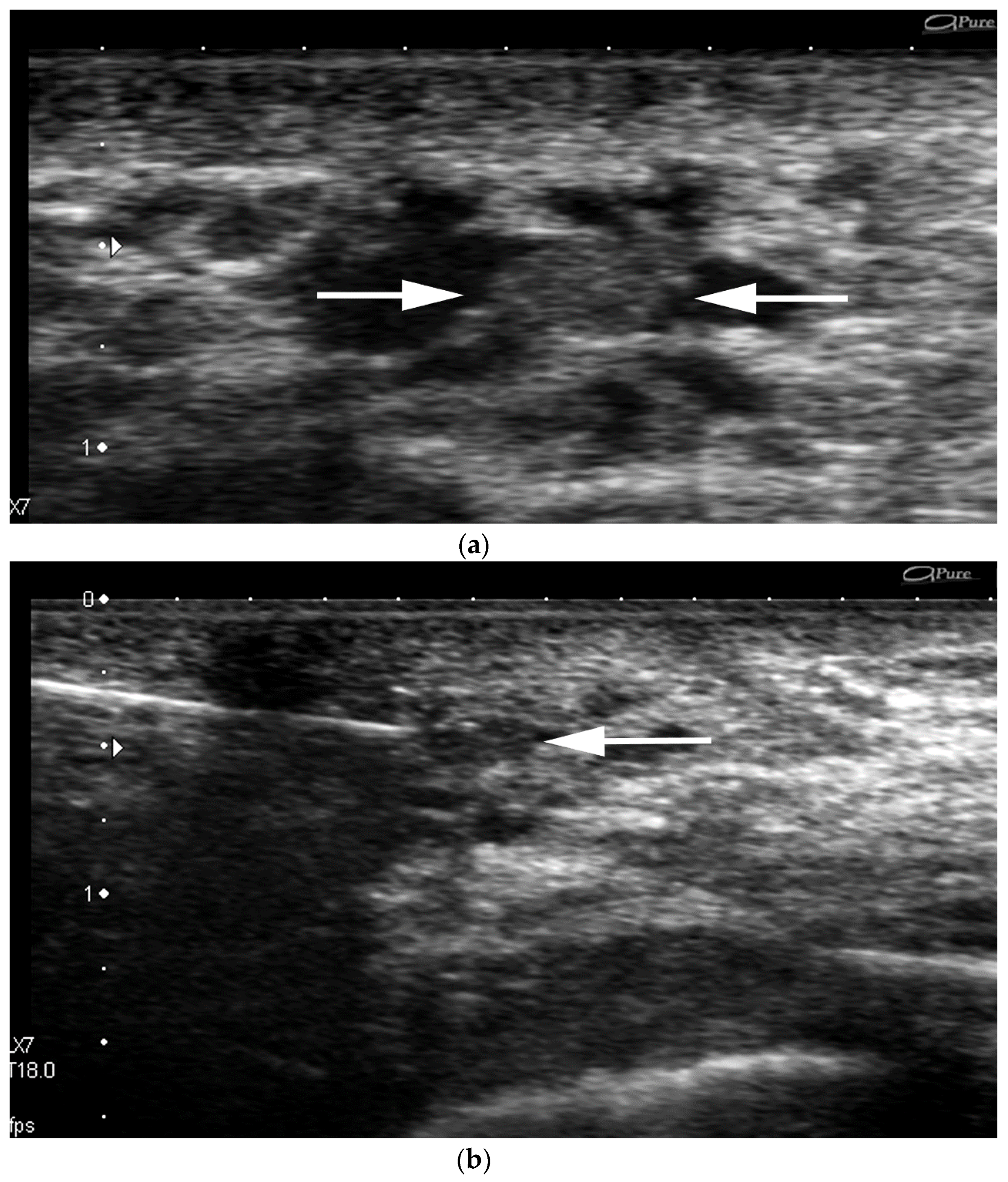
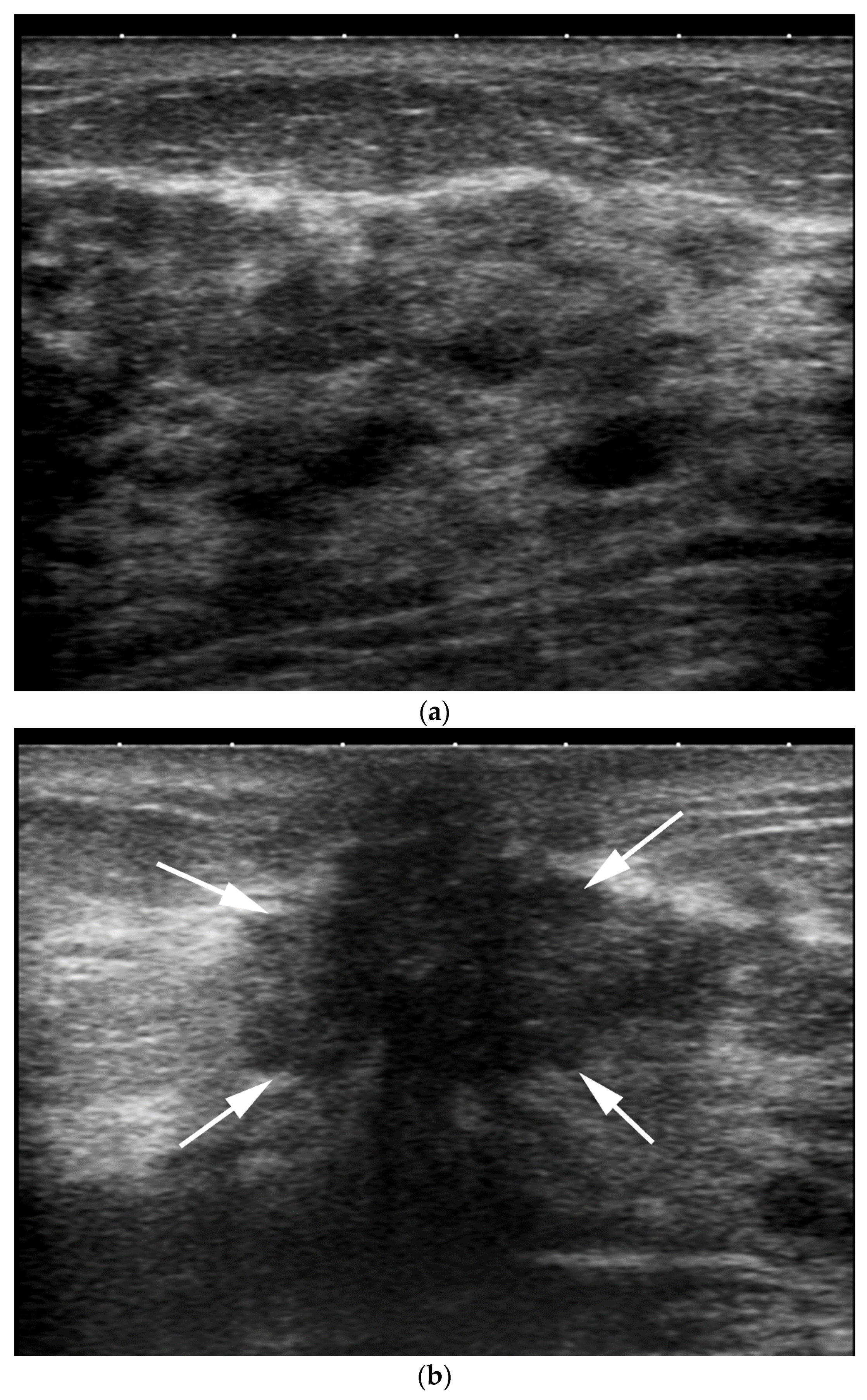
- Increased inter-observer variation in labelling the location. In our practice, we noted that a lesion adjacent to the nipple can be differently labeled with respect to location by different operators Depending on the probe position related to the nipple, the clock-face location can vary (e.g., the same retroareolar lesion can be labelled at 12 o’clock by one imager and 6 o’clock by a second one) (Figure 3).
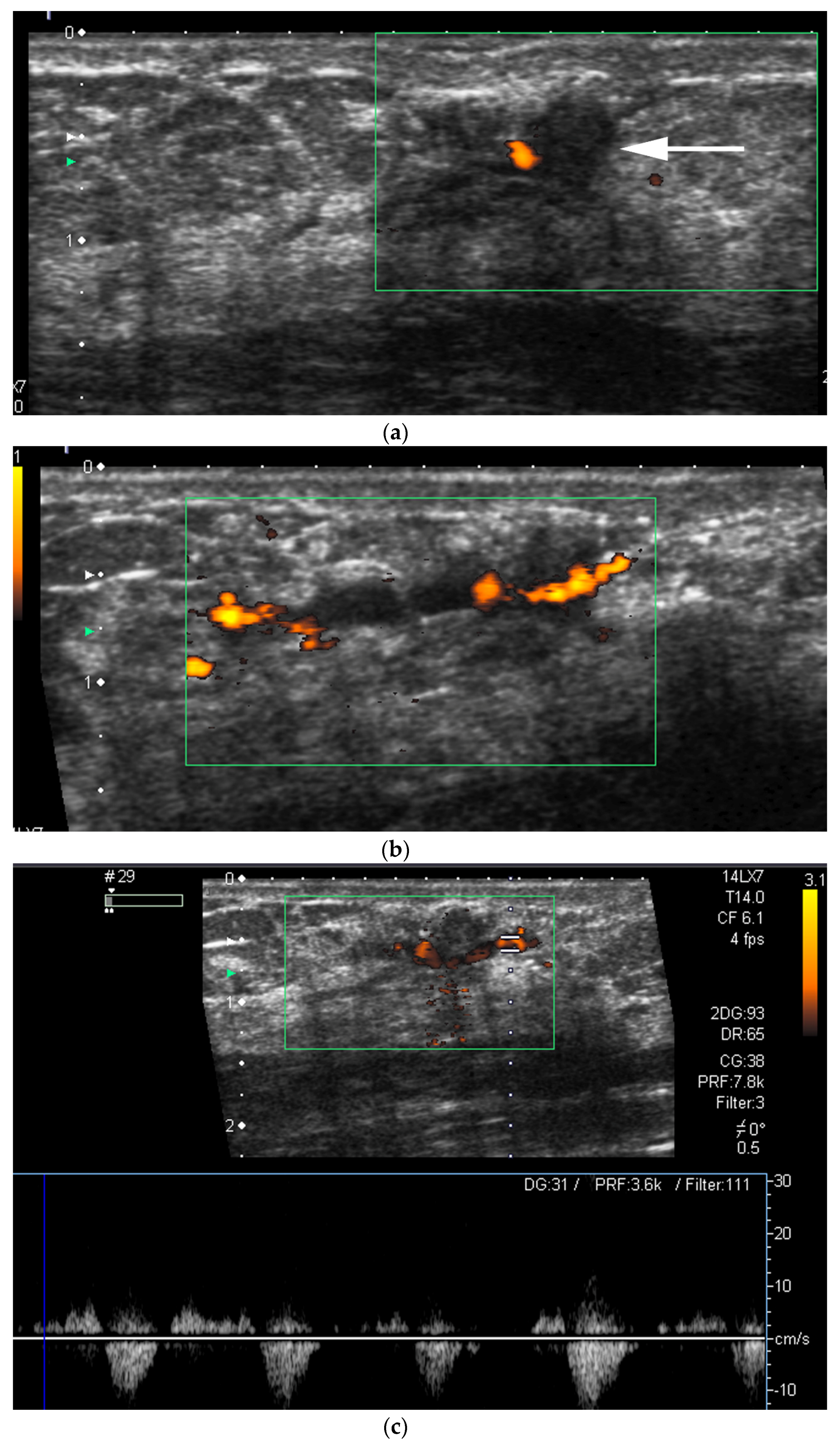
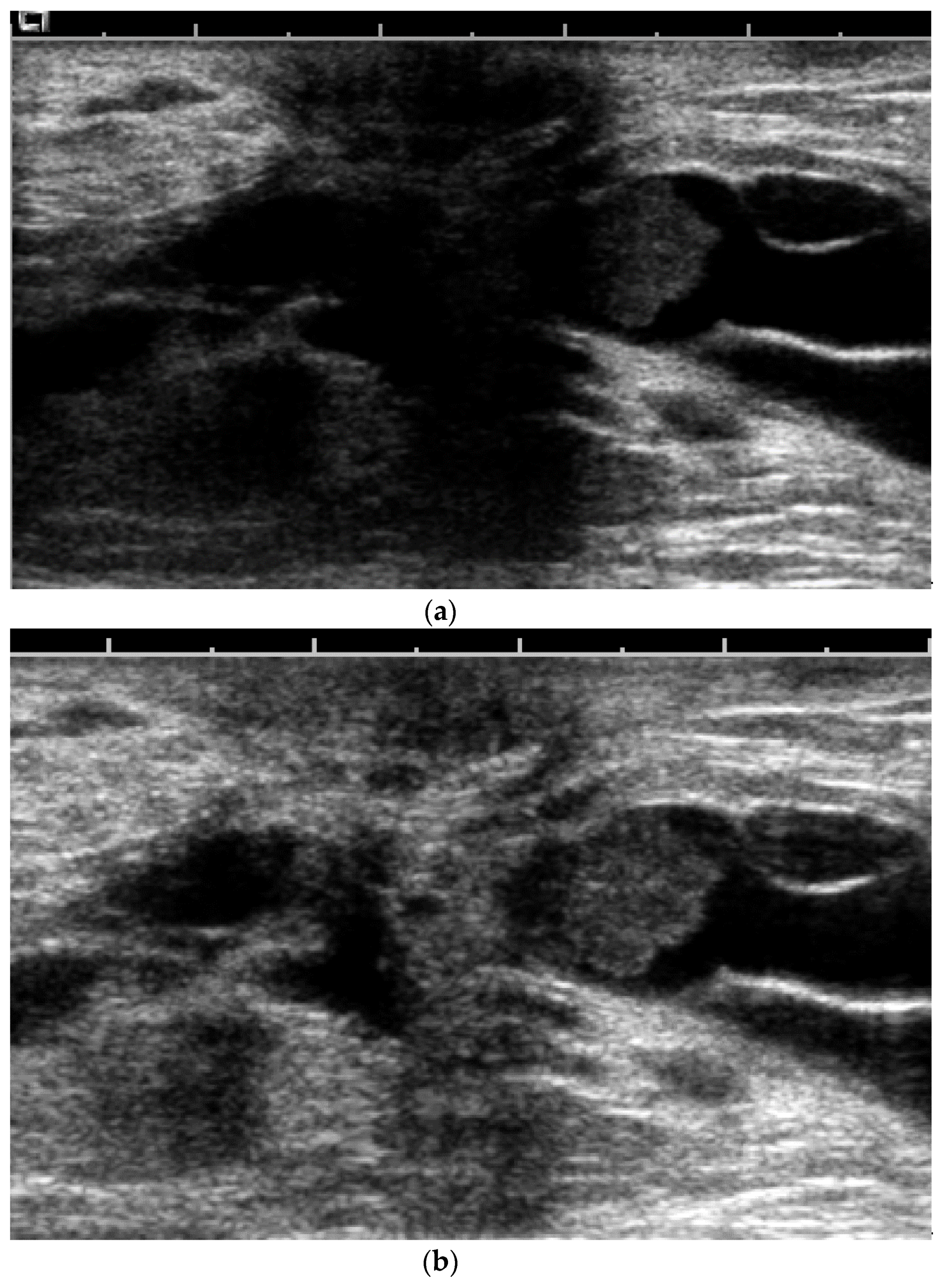
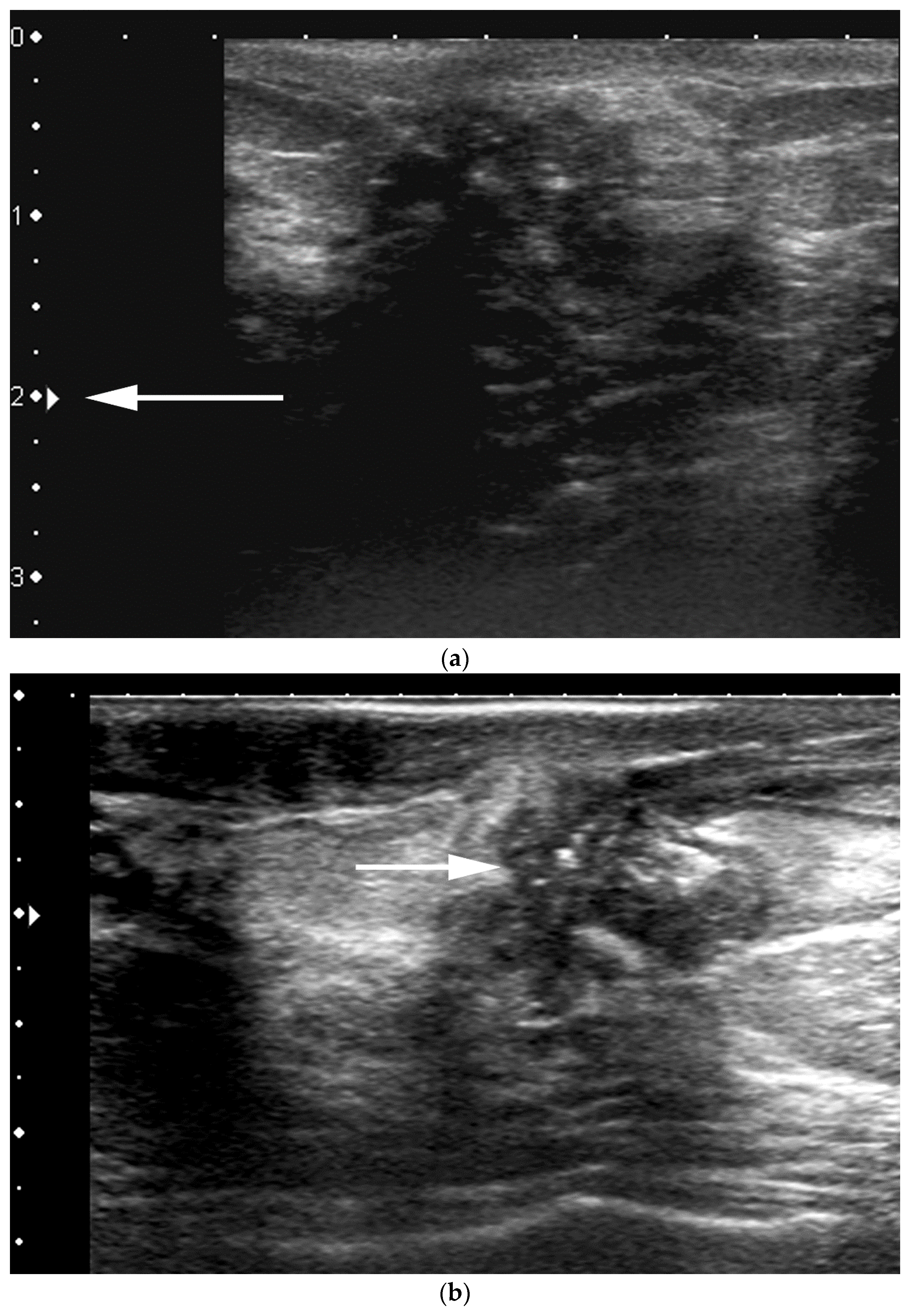
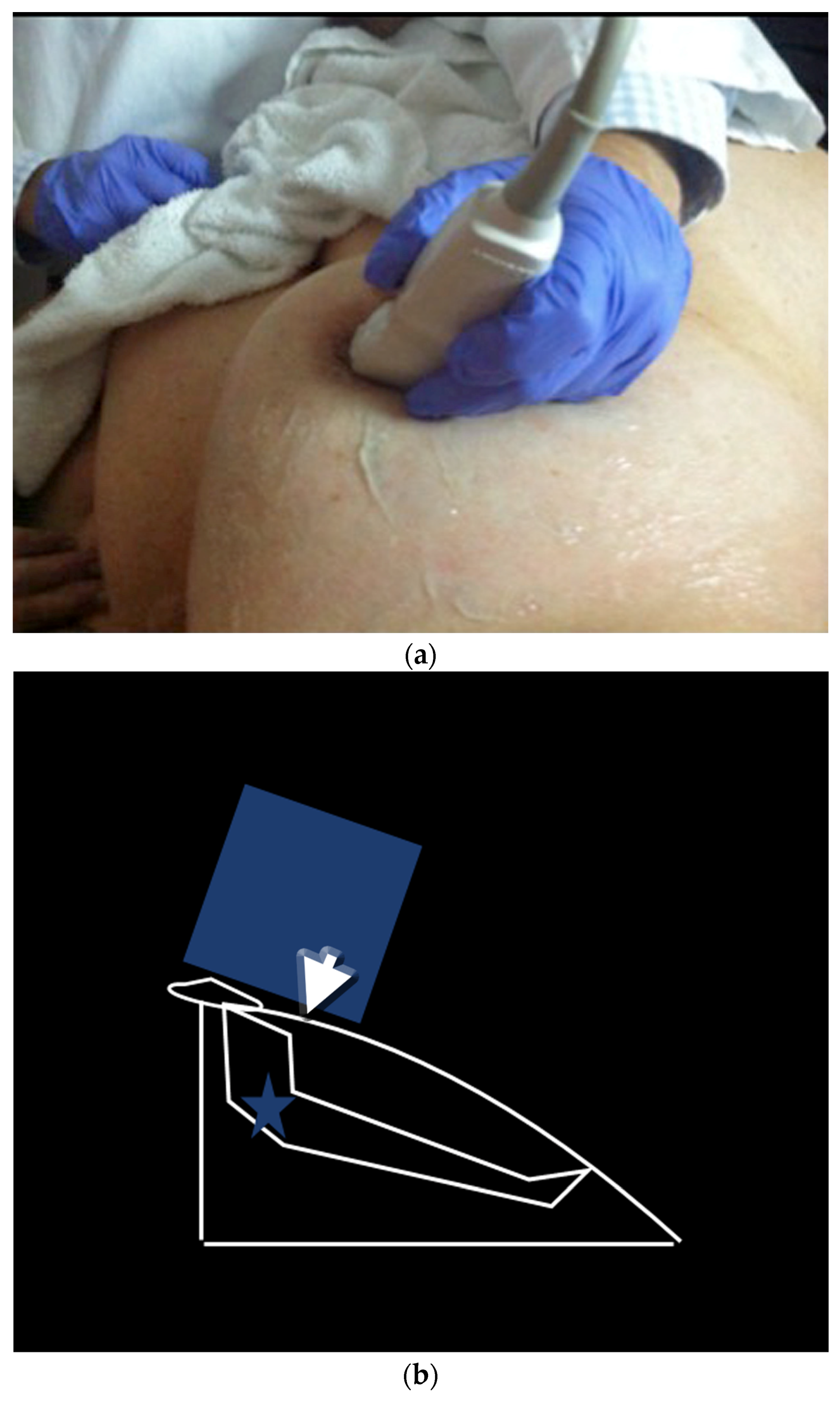
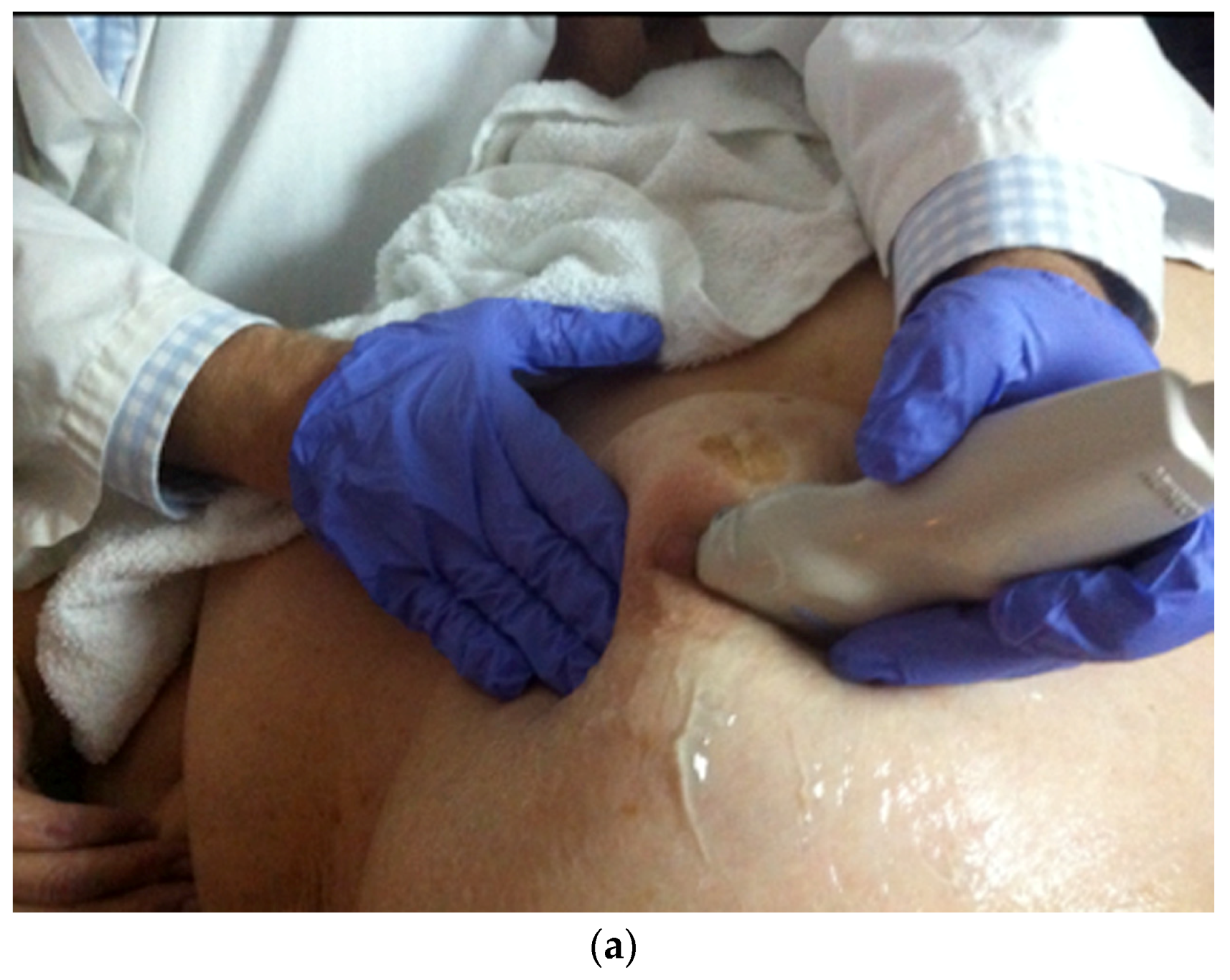
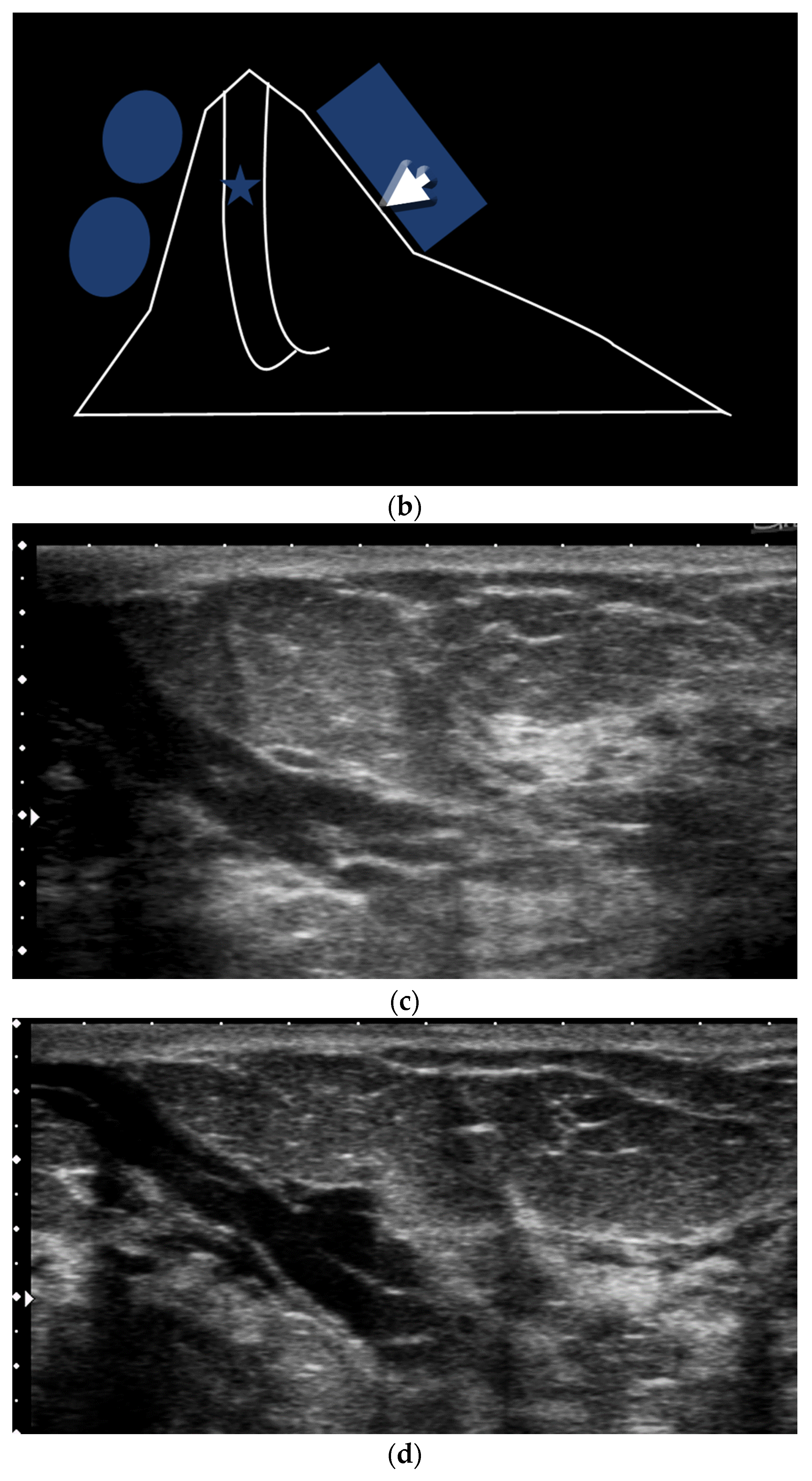
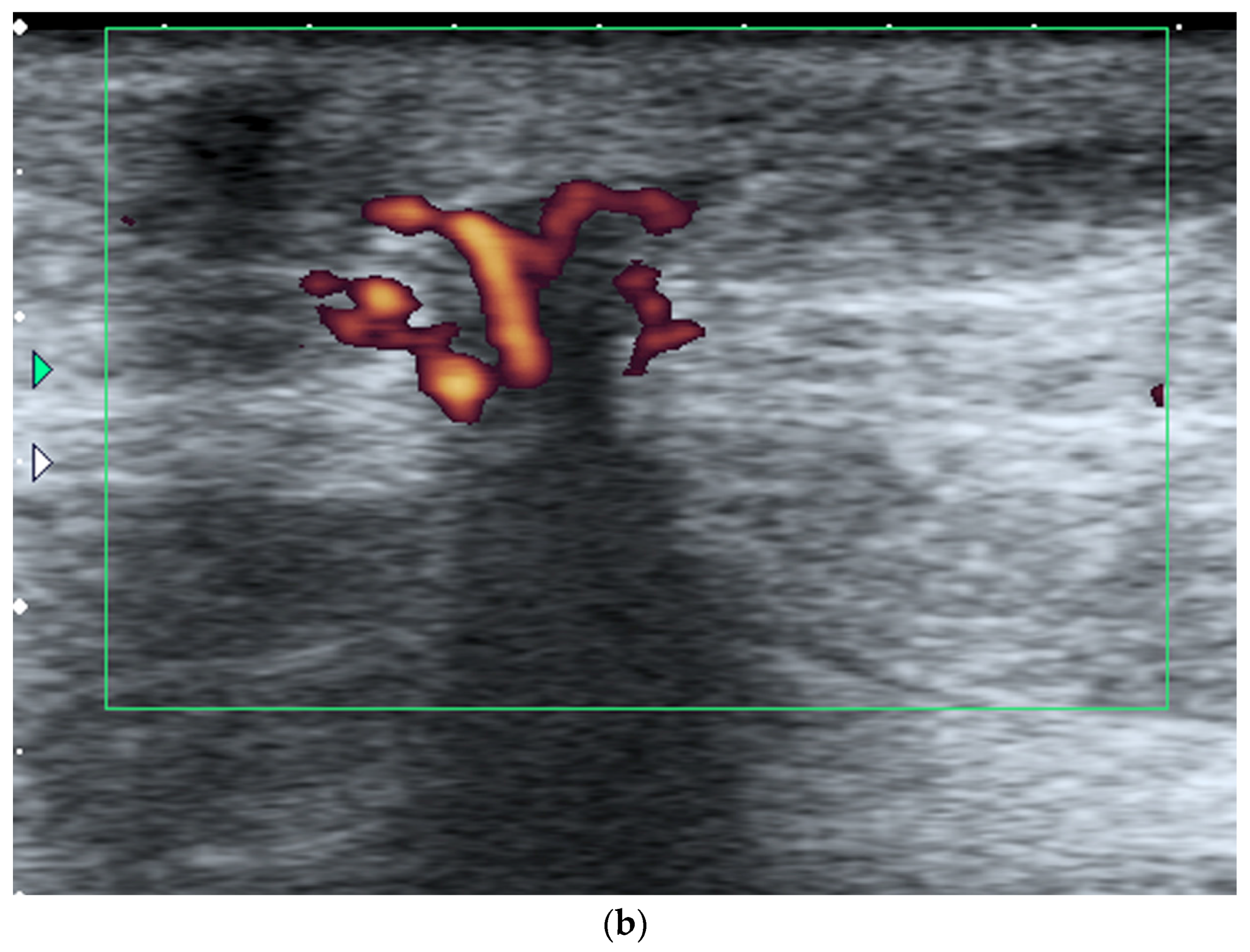
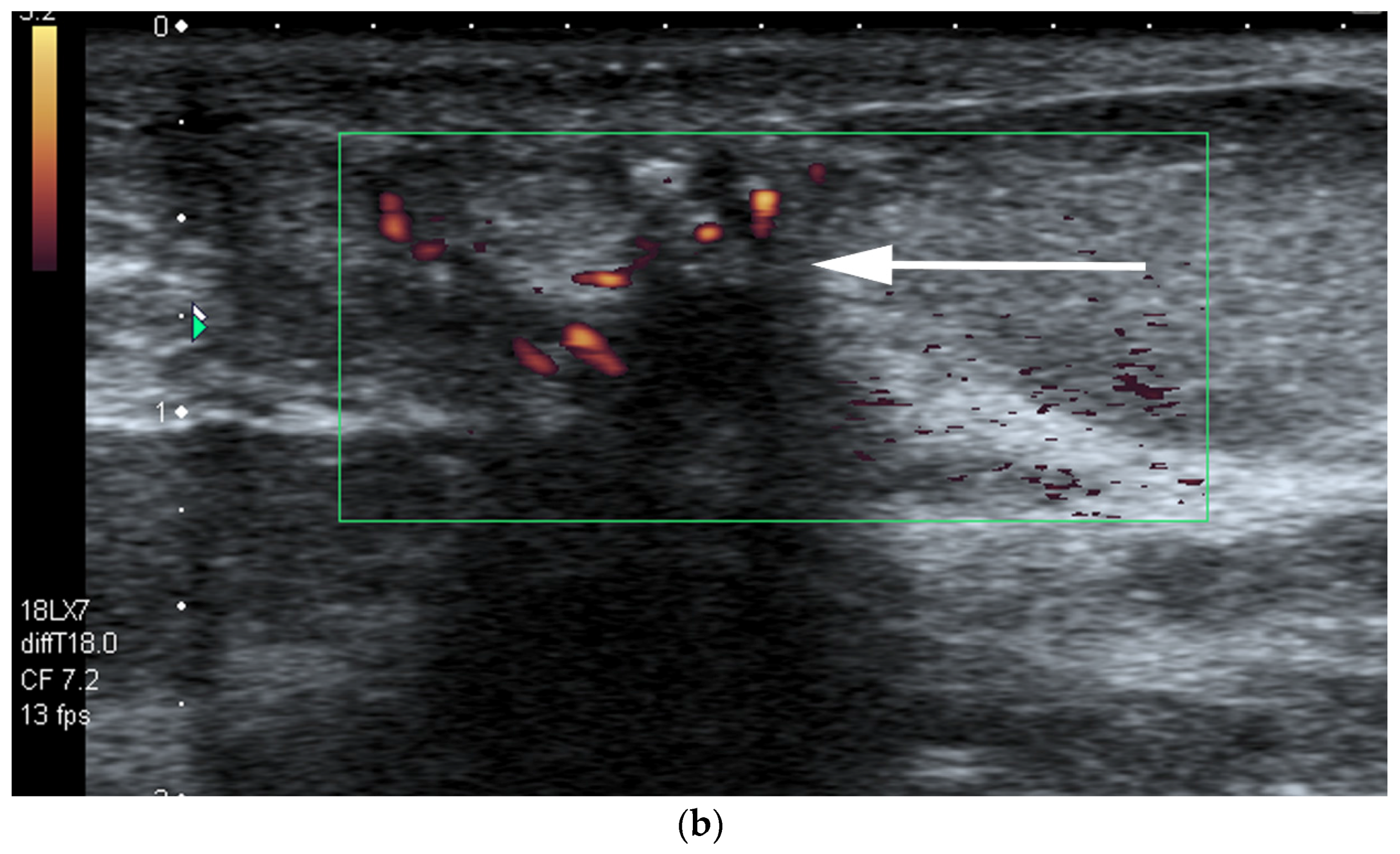
- US-guided procedures in the nipple-areolar region can be more complex, due to the increased sensitivity and vascularity of the area (Figure 4). The presence of shadowing, and the abundant amount of anesthetic necessary for the procedure can mask the targeted lesion. In addition, an intraductal lesion can become less visible after injection of local anesthetics (especially when injected into the ducts) and after multiple biopsy passes, when the cystic component collapses. Therefore, the sampling risks being less accurate (Figure 5).
3.2. Tips Proposed to Improve US Scanning of Retroareolar Structures
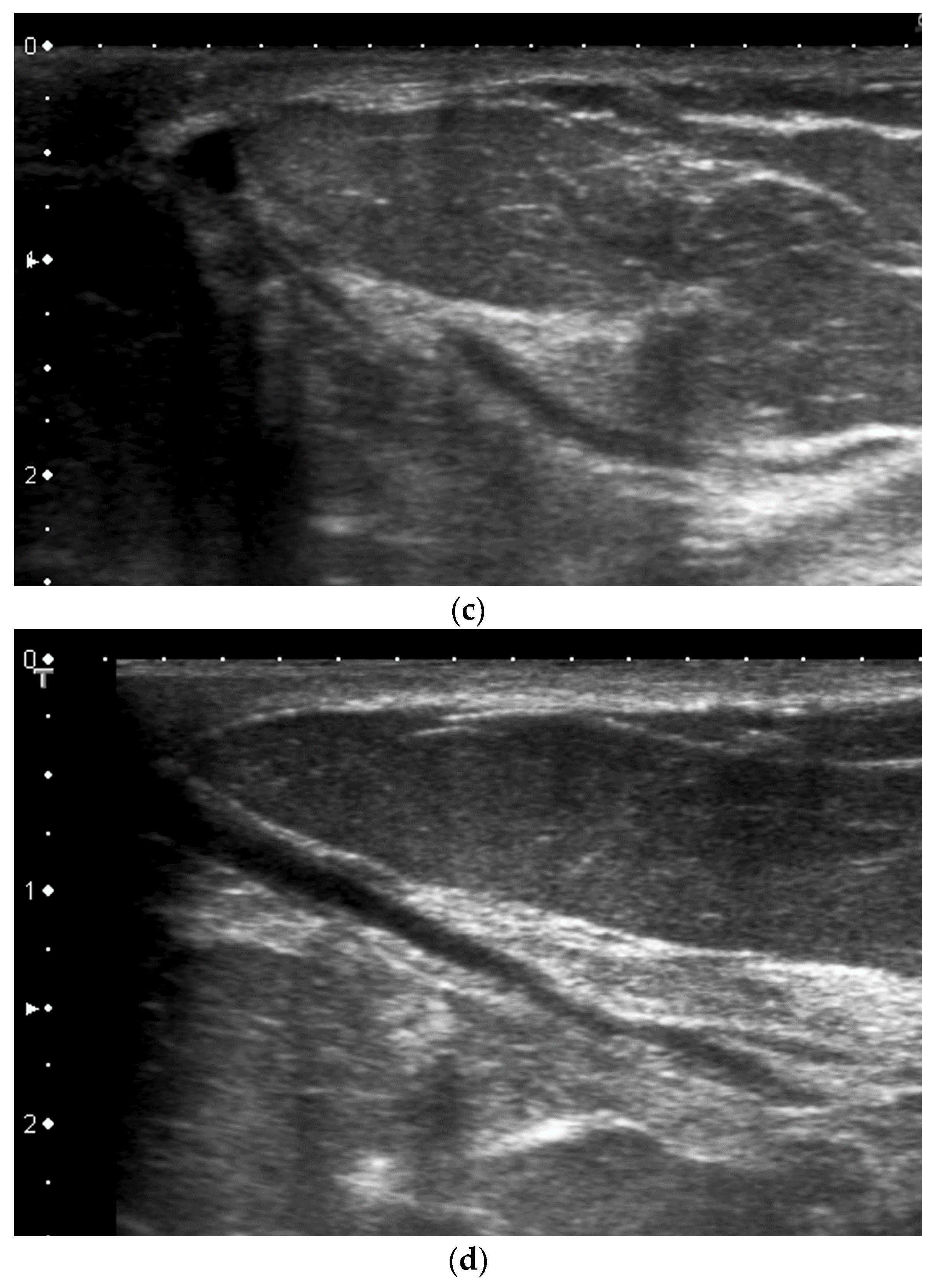
- A thick pad of gel can aid the operator to compensate for the geometric shape of the nipple. The abundant gel replaces the air trapped in the crevices and decreases the artifacts. The gel also allows the visualization of the more superficial structures (Figure 6).
- Comparison with the contralateral side is helpful, especially in case of subtle retroareolar findings (Figure 9).
3.3. Proper Scanning Techniques
- The peripheral compression technique (Figure 10)
- ○
- For visualization of the peripheral retroareolar duct segments
- ○
- Performed with a nipple compression on the lateral end of the probe. The tranducer is held with an angle. The beam is then perpendicular to the duct and simultaneously the probe maintains contact and pressure.
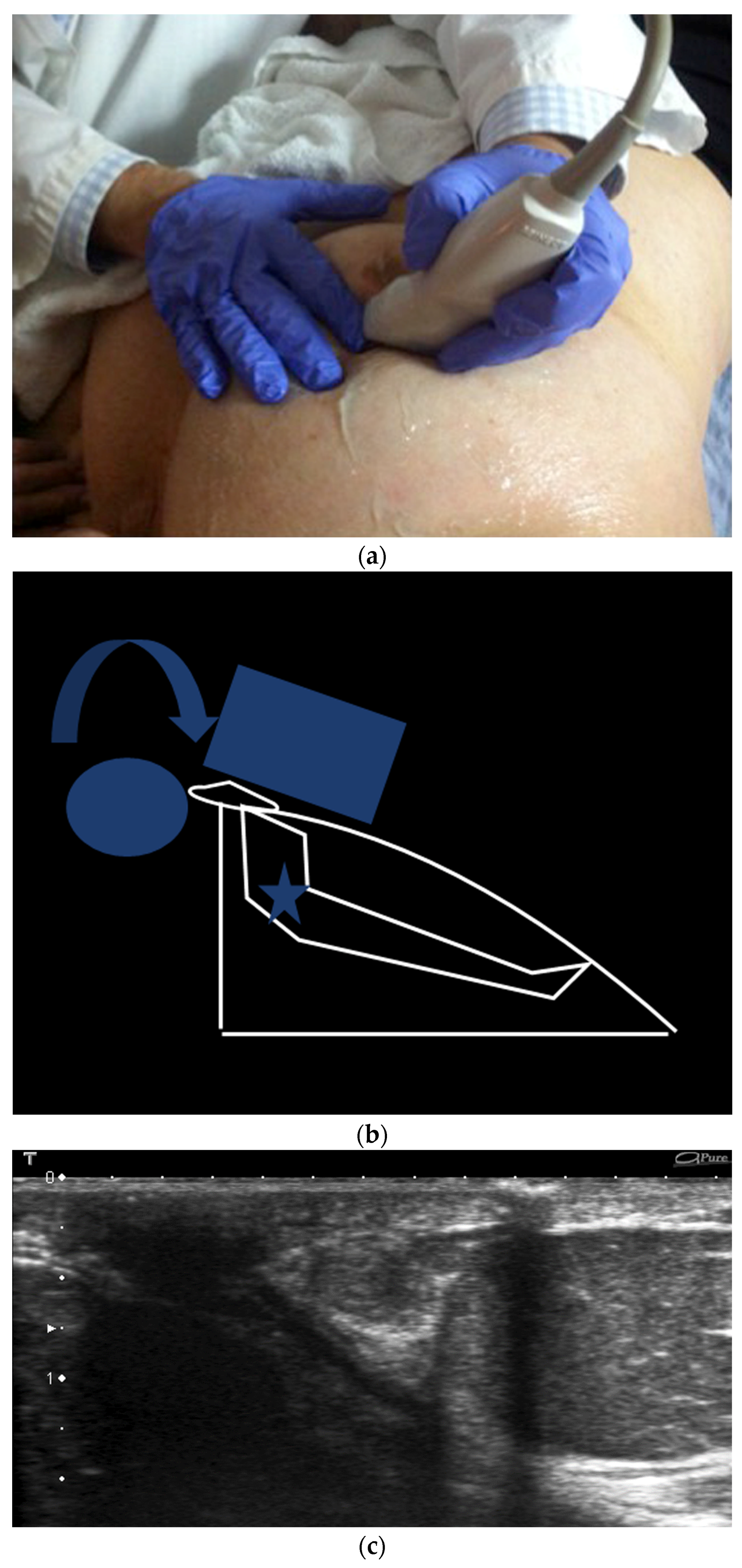
- The two-handed compression technique (Figure 11)
- ○
- For visualization of the central retroareolar duct segments
- ○
- The two-handed compression technique compresses the duct of interest between the non-scanning hand and the probe that is slid distally to include the nipple.
- The rolled nipple technique (Figure 12)
- ○
- Depicts the portion of the mammary duct within the nipple
- ○
- The probe rolls the nipple towards the finger of the contralateral hand.
- Ballottement maneuver:
- ○
- Helps to visualize whether the material contained in the ducts can be mobilized and is more suggestive of layering debris than of solid content [18]. With Doppler, the echogenic secretions show more color with compression due to the movement.
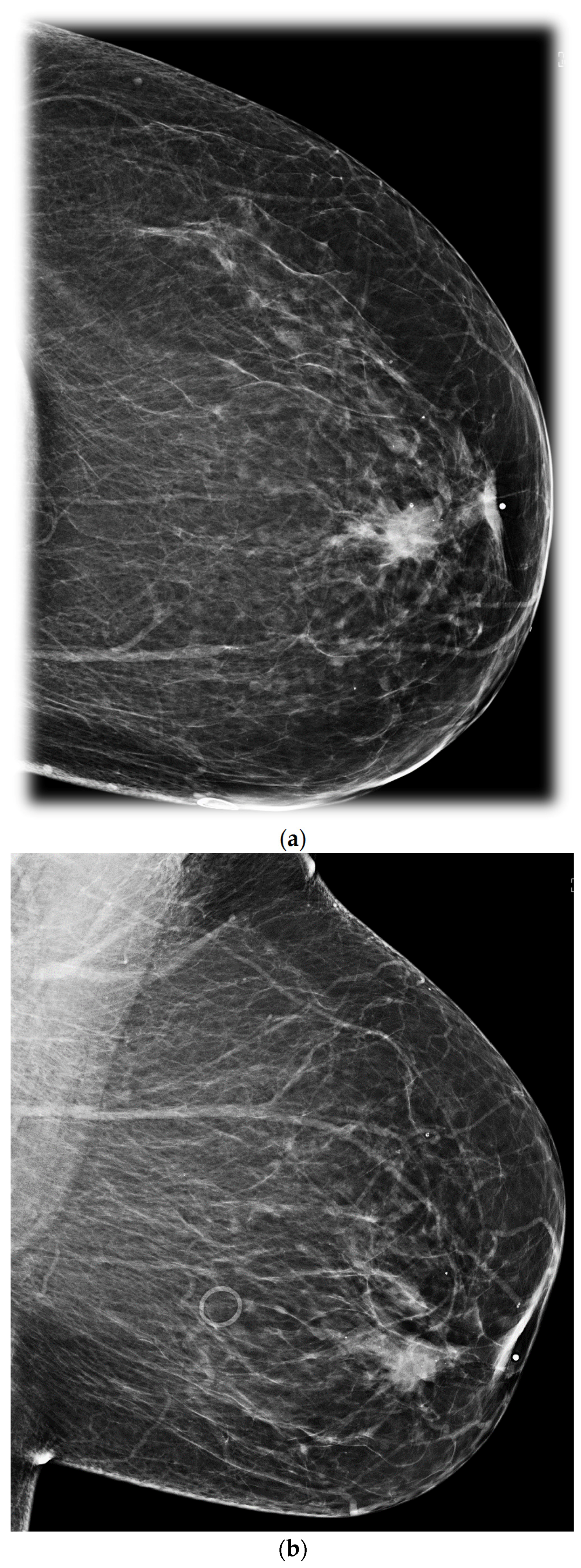
4. US and Mammogram/MRI Correlation of Retroareolar Masses
5. Role of the Retroareolar US in Clinical Practice
6. Conclusions
Conflicts of Interest
References
- Da Costa, D.; Taddese, A.; Cure, M.L.; Gerson, D.; Poppiti, R., Jr.; Esserman, L.E. Common and unusual diseases of the nipple-areolar complex. RadioGraphics 2007, 27, S65–S77. [Google Scholar] [CrossRef] [PubMed]
- An, H.Y.; Kim, K.S.; Yu, I.K.; Kim, K.W.; Kim, H.H. Image presentation. The nipple-areolar complex: A pictorial review of common and uncommon conditions. J. Ultrasound Med. 2010, 29, 949–962. [Google Scholar] [PubMed]
- Feig, S.A.; Shaber, G.S.; Patchefsky, A. Analysis of clinically occult and mammographically occult breast tumors. AJR Am. J. Roentgenol. 1977, 128, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.M.; Storm, E.S.; Atkinson, L.; Kenny, E.; Mitchell, L.S. Current breast imaging modalities, advances, and impact on breast care. Obstet. Gynecol. Clin. N. Am. 2013, 40, 429–457. [Google Scholar] [CrossRef] [PubMed]
- Giess, C.S.; Keating, D.M.; Osborne, M.P.; Ng, Y.Y.; Rosenblatt, R. Retroareolar breast carcinoma: Clinical, imaging, and histopathologic features. Radiology 1998, 207, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Rusby, J.E.; Smith, B.L.; Gui, G.P. Nipple-sparing mastectomy. Br. J. Surg. 2010, 97, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Fowble, B.; Solin, L.J.; Schultz, D.J.; Weiss, M.C. Breast recurrence and survival related to primary tumor location in patients undergoing conservative surgery and radiation for early-stage breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 933–939. [Google Scholar] [CrossRef]
- Mitnick, J.S.; Vazquez, M.F.; Plesser, K.P.; Roses, D.F. Breast cancer malpractice litigation in New York State. Radiology 1993, 189, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.T. Nipple-areolar complex: Normal anatomy and benign and malignant processes. RadioGraphics 2009, 29, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Mesurolle, B.; Bining, H.J.; El Khoury, M.; Barhdadi, A.; Kao, E. Contribution of tissue harmonic imaging and frequency compound imaging in interventional breast sonography. J. Ultrasound Med. 2006, 25, 845–855. [Google Scholar] [PubMed]
- Mesurolle, B.; Helou, T.; El Khoury, M.; Edwardes, M.; Sutton, E.J.; Kao, E. Tissue harmonic imaging, frequency compound imaging, and conventional imaging: Use and benefit in breast sonography. J. Ultrasound Med. 2007, 26, 1041–1051. [Google Scholar] [PubMed]
- Merry, G.M.; Mendelson, E.B. Update on screening breast ultrasonography. Radiol. Clin. N. Am. 2014, 52, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Mendelson, E.B. Technologist-performed handheld screening breast US imaging: How is it performed and what are the outcomes to date? Radiology 2014, 272, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Stavros, A.T. Breast anatomy: The basis for understanding sonography. In Breast Ultrasound; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; pp. 56–108. [Google Scholar]
- Sarica, O.; Zeybek, E.; Ozturk, E. Evaluation of nipple-areola complex with ultrasonography and magnetic resonance imaging. J. Comput. Assist. Tomogr. 2010, 34, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Stavros, A.T.; Thickman, D.; Rapp, C.L.; Dennis, M.A.; Parker, S.H.; Sisney, G.A. Solid breast nodules: Use of sonography to distinguish between benign and malignant lesions. Radiology 1995, 196, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Zhang, Z.; Lehrer, D.; Jong, R.A.; Pisano, E.D.; Barr, R.G.; Böhm-Vélez, M.; Mahoney, M.C.; Evans, W.P., 3rd; Larsen, L.H.; et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012, 307, 1394–1404. [Google Scholar] [PubMed]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A.; Bassett, L.W.; Mendelson, E.B.; Böhm-Vélez, M.; Berg, W.A.; Comstock, C.E.; Lee, C.H.; et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Park, V.Y.; Kim, M.J.; Kim, E.K.; Moon, H.J. Second Second-look US: How to find breast lesions with a suspicious MR imaging appearance. RadioGraphics 2013, 33, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Trop, I.; Labelle, M.; David, J.; Mayrand, M.H.; Lalonde, L. Second-look targeted studies after breast magnetic resonance imaging: Practical tips to improve lesion identification. Curr. Probl. Diagn. Radiol. 2010, 39, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, L.A.; Tannaphai, P.; Trimboli, R.M.; Verardi, N.; Fedeli, M.P.; Sardanelli, F. Contrast enhanced breast MRI: Spatial displacement from prone to supine patient’s position—Preliminary results. Eur. J. Radiol. 2012, 81, e771–e774. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Gutierrez, L.; Ness Aiver, M.S.; Carter, W.B.; Bhargavan, M.; Lewis, R.S.; Ioffe, O.B. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 2004, 233, 830–849. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferré, R.; Paré, M.; Smith, L.; Thériault, M.; Aldis, A.; Kao, E.; Mesurolle, B. Retroareolar Carcinomas in Breast Ultrasound: Pearls and Pitfalls. Cancers 2017, 9, 1. https://doi.org/10.3390/cancers9010001
Ferré R, Paré M, Smith L, Thériault M, Aldis A, Kao E, Mesurolle B. Retroareolar Carcinomas in Breast Ultrasound: Pearls and Pitfalls. Cancers. 2017; 9(1):1. https://doi.org/10.3390/cancers9010001
Chicago/Turabian StyleFerré, Romuald, Martine Paré, Lisa Smith, Mélanie Thériault, Ann Aldis, Ellen Kao, and Benoit Mesurolle. 2017. "Retroareolar Carcinomas in Breast Ultrasound: Pearls and Pitfalls" Cancers 9, no. 1: 1. https://doi.org/10.3390/cancers9010001




