Early Postoperative Low Expression of RAD50 in Rectal Cancer Patients Associates with Disease-Free Survival
Abstract
:1. Introduction
2. Results
2.1. Study Population
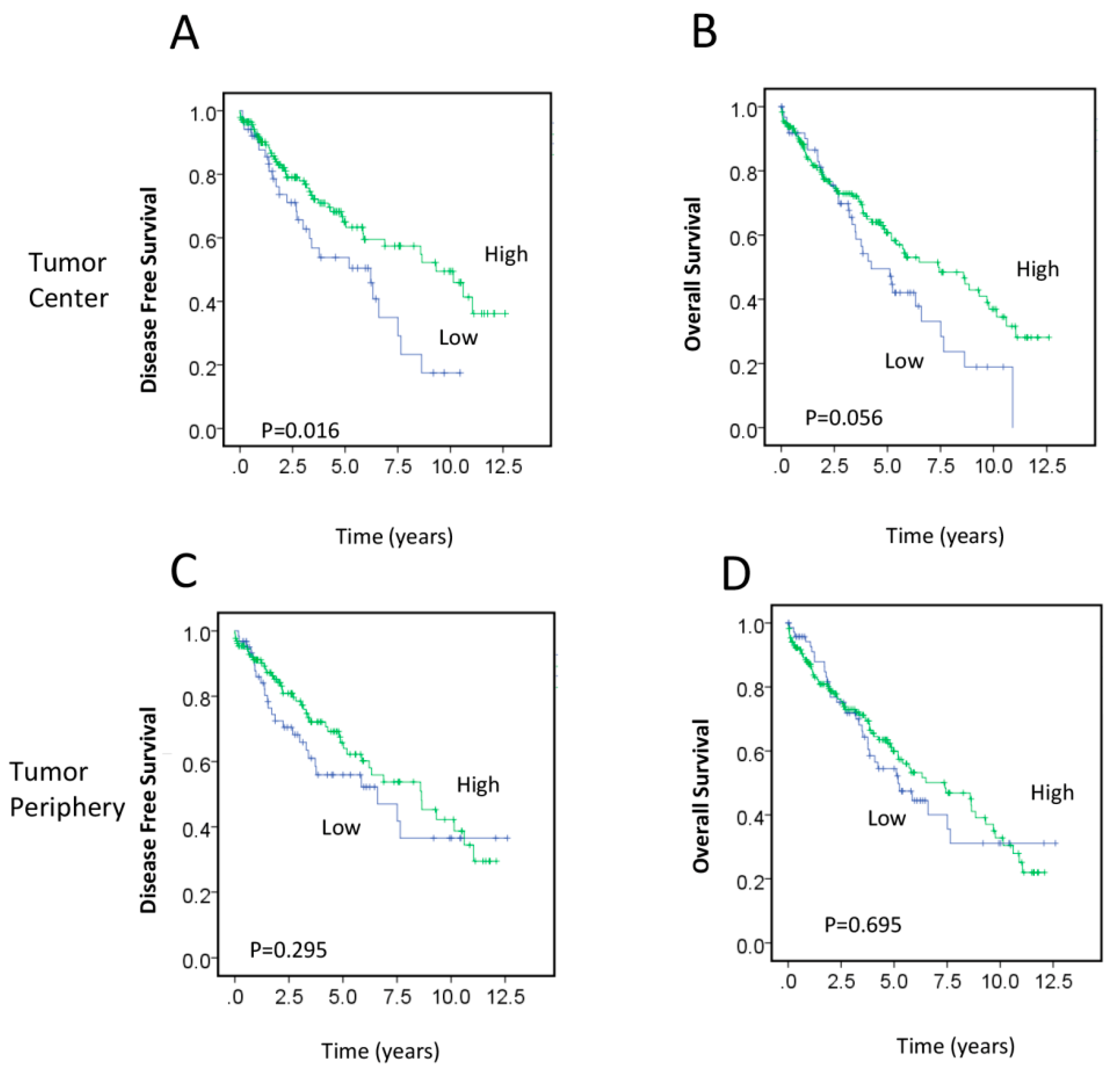
2.2. Association between RAD50 Expression and Clinicopathological Features and Prognosis
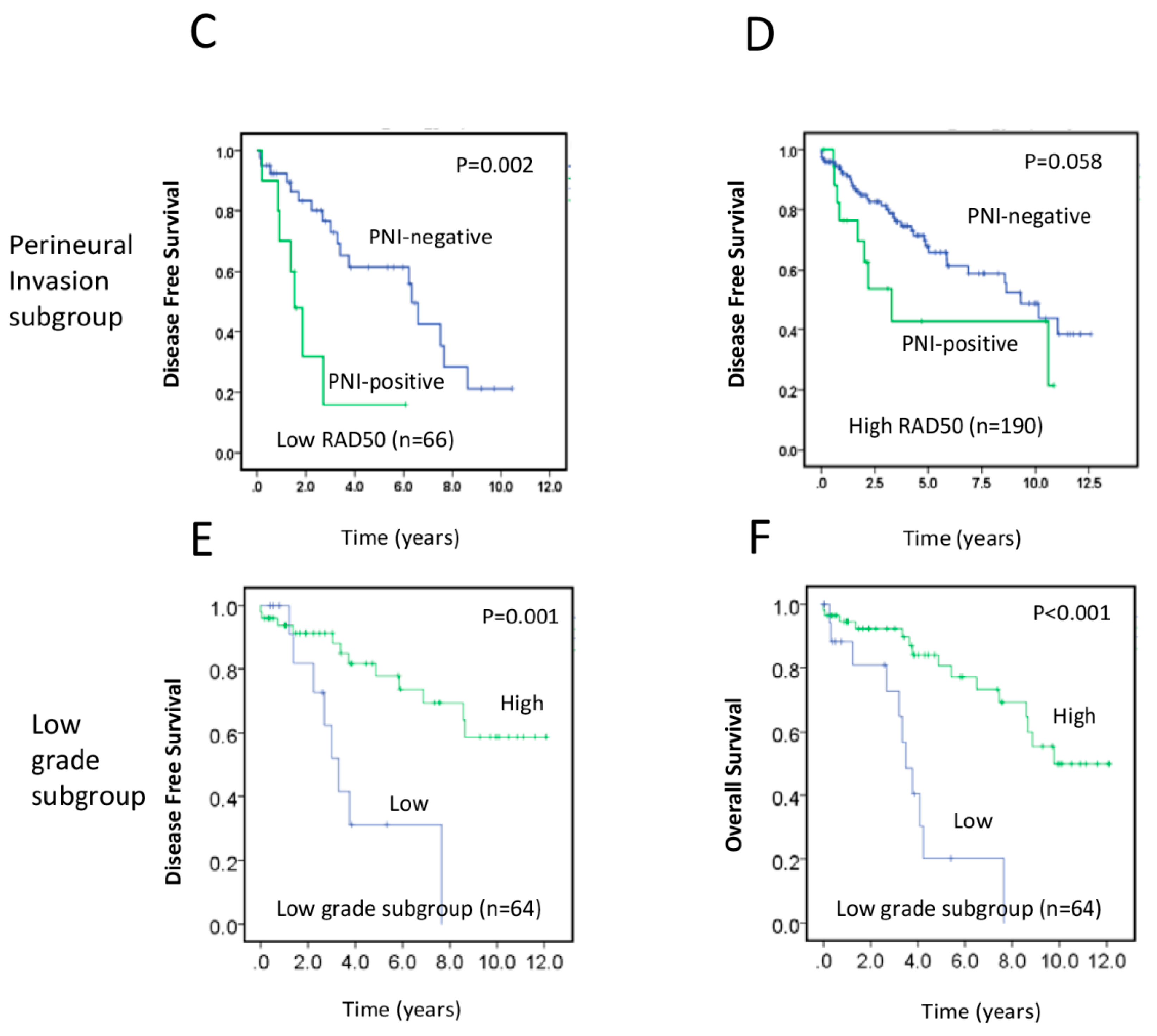
2.3. RAD50 Expression as a Putative Prognostic Factor for Early Stage Rectal Cancer
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Response and Outcomes of Interest
4.3. Sample Preparation and Tissue Microarrays
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Colon and Rectum Cancer. Available online: https://seer.Cancer.Gov/statfacts/html/colorect (accessed on 13 April 2017).
- Breugom, A.J.; van Gijn, W.; Muller, E.W.; Berglund, A.; van den Broek, C.B.; Fokstuen, T.; Gelderblom, H.; Kapiteijn, E.; Leer, J.W.; Marijnen, C.A.; et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann. Oncol. 2015, 26, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ma, Q.; Yu, Y.Y.; Wang, C.; Meng, W.J.; Adell, G.; Albertsson, M.; Arbman, G.; Jarlsfelt, I.; Peng, Z.H.; et al. Efficacy of surgery and adjuvant therapy in older patients with colorectal cancer: A strobe-compliant article. Medicine 2014, 93, e266. [Google Scholar] [CrossRef] [PubMed]
- Orsini, R.G.; Wiggers, T.; DeRuiter, M.C.; Quirke, P.; Beets-Tan, R.G.; van de Velde, C.J.; Rutten, H.J. The modern anatomical surgical approach to localised rectal cancer. EJC Suppl. 2013, 11, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, Y.L.; Chuang, J.P.; Lee, J.C. Differences in survival between colon and rectal cancer from SEER data. PLoS ONE 2013, 8, e78709. [Google Scholar] [CrossRef] [PubMed]
- Camma, C.; Giunta, M.; Fiorica, F.; Pagliaro, L.; Craxi, A.; Cottone, M. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA 2000, 284, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8, 507 patients from 22 randomised trials. Lancet 2001, 358, 1291–1304. [Google Scholar]
- Forker, L.J.; Choudhury, A.; Kiltie, A.E. Biomarkers of tumour radiosensitivity and predicting benefit from radiotherapy. Clin. Oncol. 2015, 27, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Colussi, D.; Brandi, G.; Bazzoli, F.; Ricciardiello, L. Molecular pathways involved in colorectal cancer: Implications for disease behavior and prevention. Int. J. Mol. Sci. 2013, 14, 16365–16385. [Google Scholar] [CrossRef] [PubMed]
- Vilar, E.; Gruber, S.B. Microsatellite instability in colorectal cancer-the stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Miquel, C.; Jacob, S.; Grandjouan, S.; Aime, A.; Viguier, J.; Sabourin, J.C.; Sarasin, A.; Duval, A.; Praz, F. Frequent alteration of DNA damage signalling and repair pathways in human colorectal cancers with microsatellite instability. Oncogene 2007, 26, 5919–5926. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Tommiska, J.; Oplustilova, L.; Aaltonen, K.; Tamminen, A.; Heikkinen, T.; Mistrik, M.; Aittomaki, K.; Blomqvist, C.; Heikkila, P.; et al. Aberrations of the MRE11-RAD50-NBS1 DNA damage sensor complex in human breast cancer: MRE11 as a candidate familial cancer-predisposing gene. Mol. Oncol. 2008, 2, 296–316. [Google Scholar] [CrossRef] [PubMed]
- Paull, T.T.; Deshpande, R.A. The Mre11/Rad50/Nbs1 complex: Recent insights into catalytic activities and ATP-driven conformational changes. Exp. Cell Res. 2014, 329, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Rein, K.; Stracker, T.H. The MRE11 complex: An important source of stress relief. Exp. Cell Res. 2014, 329, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Vilar, E.; Bartnik, C.M.; Stenzel, S.L.; Raskin, L.; Ahn, J.; Moreno, V.; Mukherjee, B.; Iniesta, M.D.; Morgan, M.A.; Rennert, G.; et al. MRE11 deficiency increases sensitivity to poly(ADP-ribose) polymerase inhibition in microsatellite unstable colorectal cancers. Cancer Res. 2011, 71, 2632–2642. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, H.; Arbman, G.; Sun, X.F. The different roles of hRAD50 in microsatellite stable and unstable colorectal cancers. Dis. Mark. 2008, 24, 127–134. [Google Scholar] [CrossRef]
- Ho, V.; Chung, L.; Revoltar, M.; Lim, S.H.; Tut, T.G.; Abubakar, A.; Henderson, C.J.; Chua, W.; Ng, W.; Lee, M.; et al. MRE11 and ATM expression levels predict rectal cancer survival and their association with radiotherapy response. PLoS ONE 2016, 11, e0167675. [Google Scholar] [CrossRef] [PubMed]
- Pavelitz, T.; Renfro, L.; Foster, N.R.; Caracol, A.; Welsch, P.; Lao, V.V.; Grady, W.B.; Niedzwiecki, D.; Saltz, L.B.; Bertagnolli, M.M.; et al. MRE11-deficiency associated with improved long-term disease free survival and overall survival in a subset of stage III colon cancer patients in randomized CALGB 89803 trial. PLoS ONE 2014, 9, e108483. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, J.; Birgisson, H.; Pahlman, L.; Cedermark, B.; Glimelius, B.; Gunnarsson, U. Swedish rectal cancer trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J. Clin. Oncol. 2005, 23, 5644–5650. [Google Scholar] [CrossRef] [PubMed]
- Birgisson, H.; Pahlman, L.; Gunnarsson, U.; Glimelius, B.; Swedish Rectal Cancer Trial Group. Adverse effects of preoperative radiation therapy for rectal cancer: Long-term follow-up of the swedish rectal cancer trial. J. Clin. Oncol. 2005, 23, 8697–8705. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Elbers, H.; Askoxylakis, V.; Motschall, E.; Bork, U.; Buchler, M.W.; Weitz, J.; Koch, M. Neoadjuvant radiotherapy for rectal cancer: Meta-analysis of randomized controlled trials. Ann. Surg. Oncol. 2013, 20, 4169–4182. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, K.; Stal, O.; Skoog, L.; Rutqvist, L.E.; Nordenskjold, B.; Askmalm, M.S. Intact Mre11/Rad50/Nbs1 complex predicts good response to radiotherapy in early breast cancer. Int. J. Radiat. Oncol Biol. Phys. 2007, 68, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Chinnaiyan, P.; Fulp, W.J.; Eschrich, S.; Torres-Roca, J.F.; Caudell, J.J. The radiosensitivity index predicts for overall survival in glioblastoma. Oncotarget 2015, 6, 34414–34422. [Google Scholar] [PubMed]
- Choudhury, A.; Nelson, L.D.; Teo, M.T.; Chilka, S.; Bhattarai, S.; Johnston, C.F.; Elliott, F.; Lowery, J.; Taylor, C.F.; Churchman, M.; et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 2010, 70, 7017–7026. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Hansen, L.T.; Phear, G.; Scorah, J.; Spang-Thomsen, M.; Cox, A.; Helleday, T.; Meuth, M. Thymidine selectively enhances growth suppressive effects of camptothecin/irinotecan in MSI+ cells and tumors containing a mutation of MRE11. Clin. Cancer Res. 2008, 14, 5476–5483. [Google Scholar] [CrossRef] [PubMed]
- McPherson, L.A.; Shen, Y.; Ford, J.M. Poly (ADP-ribose) polymerase inhibitor LT-626: Sensitivity correlates with MRE11 mutations and synergizes with platinums and irinotecan in colorectal cancer cells. Cancer Lett. 2014, 343, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.; Samartzis, E.P.; Zimmermann, A.K.; Fink, D.; Moch, H.; Noske, A.; Dedes, K.J. Lack of MRE11-RAD50-NBS1 (MRN) complex detection occurs frequently in low-grade epithelial ovarian cancer. BMC Cancer 2017, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Ping, J.; Li, Y.; Holmqvist, A.; Adell, G.; Arbman, G.; Zhang, H.; Zhou, Z.G.; Sun, X.F. Prognostic significance and molecular features of colorectal mucinous adenocarcinomas: A strobe-compliant study. Medicine 2015, 94, e2350. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]

| Variables | All Patients (%) |
|---|---|
| Total, n | 266 |
| Age median | 72 |
| Sex | |
| Male | 176/266 (66.2) |
| Female | 90/266 (33.8) |
| Tumor stage | |
| T1–2 | 88/266 (33.1) |
| T3–4 | 172/266 (66.9) |
| Node stage | |
| N0 | 140/259 (54.1) |
| N1–2 | 119/259 (55.9) |
| Metastasis stage | |
| M0 | 223/240 (92.9) |
| M1 | 17/240 (7.1) |
| Histological Grade | |
| 1–2 | 246/266 (92.5) |
| 3 | 20/266 (7.5) |
| Vascular invasion | |
| Absent | 201/263 (76.4) |
| Present | 62/263 (23.6) |
| Perineural invasion | |
| Absent | 220/263 (83.7) |
| Present | 43/263 (16.3) |
| Radiotherapy | |
| Total | 77/246 (31.3) |
| Neoadjuvant | 55/77 (71.4) |
| Adjuvant | 22/77 (28.6) |
| Variables | Subgroups | Tumor Center | Tumor Periphery | ||||
|---|---|---|---|---|---|---|---|
| Low (%) | High (%) | p Value | Low (%) | High (%) | p Value | ||
| Sex | Male | 26.3 | 73.7 | 0.98 | 29.6 | 70.4 | 0.42 |
| Female | 26.1 | 73.9 | 34.4 | 65.6 | |||
| Age | ≤72 | 28.8 | 71.2 | 0.39 | 30.6 | 69.4 | 0.82 |
| >72 | 24.1 | 75.9 | 31.9 | 68.1 | |||
| Tumor stage | T1–2 | 23.5 | 76.5 | 0.65 | 32.1 | 67.9 | 0.68 |
| T3–4 | 26.2 | 73.8 | 29.6 | 70.4 | |||
| Node stage | Negative | 26.5 | 73.5 | 0.67 | 31.1 | 68.9 | 0.95 |
| Positive | 24.1 | 75.9 | 30.8 | 69.2 | |||
| Metastasis stage | M0 | 25.5 | 74.5 | 0.72 | 31.5 | 68.5 | 0.09 |
| M1 | 29.4 | 70.6 | 11.8 | 88.2 | |||
| Histological Grade | 1–2 | 26.1 | 73.9 | 0.88 | 31.3 | 68.7 | 0.98 |
| 3 | 27.8 | 72.2 | 31.6 | 68.4 | |||
| Vascular invasion | Absent | 26.3 | 73.7 | 0.74 | 31.9 | 68.1 | 0.50 |
| Present | 24.2 | 75.8 | 27.4 | 72.6 | |||
| Perineural invasion | Absent | 24.7 | 75.3 | 0.40 | 32.2 | 67.8 | 0.28 |
| Present | 31.1 | 68.9 | 23.8 | 76.2 | |||
| Adjuvant therapy | No | 25.2 | 74.8 | 0.83 | 24.8 | 75.2 | 0.04 |
| Yes | 26.6 | 73.4 | 38.8 | 61.2 | |||
| Neoadjuvant therapy | No | 23.8 | 76.2 | 0.14 | 27.1 | 72.9 | 0.16 |
| Yes | 34.1 | 65.9 | 37.3 | 62.7 | |||
| Tumor regression grade | 0–1 | 50 | 50 | 0.14 | 50 | 50 | 0.22 |
| 2–3 | 28.3 | 71.7 | 32.8 | 67.2 | |||
| MSH6 | Negative | 50.0 | 50.00 | 0.43 | 0 | 100 | 0.35 |
| Positive | 25.5 | 74.5 | 30.8 | 69.2 | |||
| PMS2 | Negative | 55.6 | 44.4 | 0.04 | 44.4 | 55.6 | 0.36 |
| Positive | 25.5 | 74.5 | 30.1 | 69.9 | |||
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% | p-Value | |
| RAD50 | ||||||
| Low | 0.552 | 0.339–0.899 | 0.017 | 0.567 | 0.345–0.931 | 0.025 |
| High | ||||||
| Age | ||||||
| ≤72 | 1.279 | 0.785–2.086 | 0.323 | |||
| >72 | ||||||
| Sex | ||||||
| Male | 1.074 | 0.657–1.754 | 0.776 | |||
| Female | ||||||
| Tumor stage | ||||||
| T1–2 | 1.643 | 0.983–2.747 | 0.058 | |||
| T3–4 | ||||||
| Node stage | ||||||
| Negative | 1.198 | 0.709–1.827 | 0.593 | |||
| Positive | ||||||
| Histological Grade | ||||||
| 1–2 | 1.646 | 0.712–3.804 | 0.244 | |||
| 3 | ||||||
| Vascular invasion | ||||||
| Absent | 1.888 | 0.650–0.2.171 | 0.575 | |||
| Present | ||||||
| Perineural invasion | ||||||
| Absent | 2.534 | 0.373–1.134 | 0.001 | 2.364 | 1.343–4.162 | 0.003 |
| Present | ||||||
| Adjuvant therapy | ||||||
| No | 0.65 | 0.373–1.134 | 0.129 | |||
| Yes | ||||||
| Neoadjuvant therapy | ||||||
| No | 1.147 | 0.672–0.957 | 0.616 | |||
| Yes | ||||||
| T1–2, G1–2, † RAD50 | 0.218 | 0.084–0.570 | 0.002 | |||
| T3–4, G3, † RAD50 | 0.401 | 0.065–2.471 | 0.324 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, V.; Chung, L.; Singh, A.; Lea, V.; Revoltar, M.; Lim, S.H.; Tut, T.-G.; Ng, W.; Lee, M.; De Souza, P.; et al. Early Postoperative Low Expression of RAD50 in Rectal Cancer Patients Associates with Disease-Free Survival. Cancers 2017, 9, 163. https://doi.org/10.3390/cancers9120163
Ho V, Chung L, Singh A, Lea V, Revoltar M, Lim SH, Tut T-G, Ng W, Lee M, De Souza P, et al. Early Postoperative Low Expression of RAD50 in Rectal Cancer Patients Associates with Disease-Free Survival. Cancers. 2017; 9(12):163. https://doi.org/10.3390/cancers9120163
Chicago/Turabian StyleHo, Vincent, Liping Chung, Amandeep Singh, Vivienne Lea, Maxine Revoltar, Stephanie H. Lim, Thein-Ga Tut, Weng Ng, Mark Lee, Paul De Souza, and et al. 2017. "Early Postoperative Low Expression of RAD50 in Rectal Cancer Patients Associates with Disease-Free Survival" Cancers 9, no. 12: 163. https://doi.org/10.3390/cancers9120163




