Recent Advances in Cancer Therapy Based on Dual Mode Gold Nanoparticles
Abstract
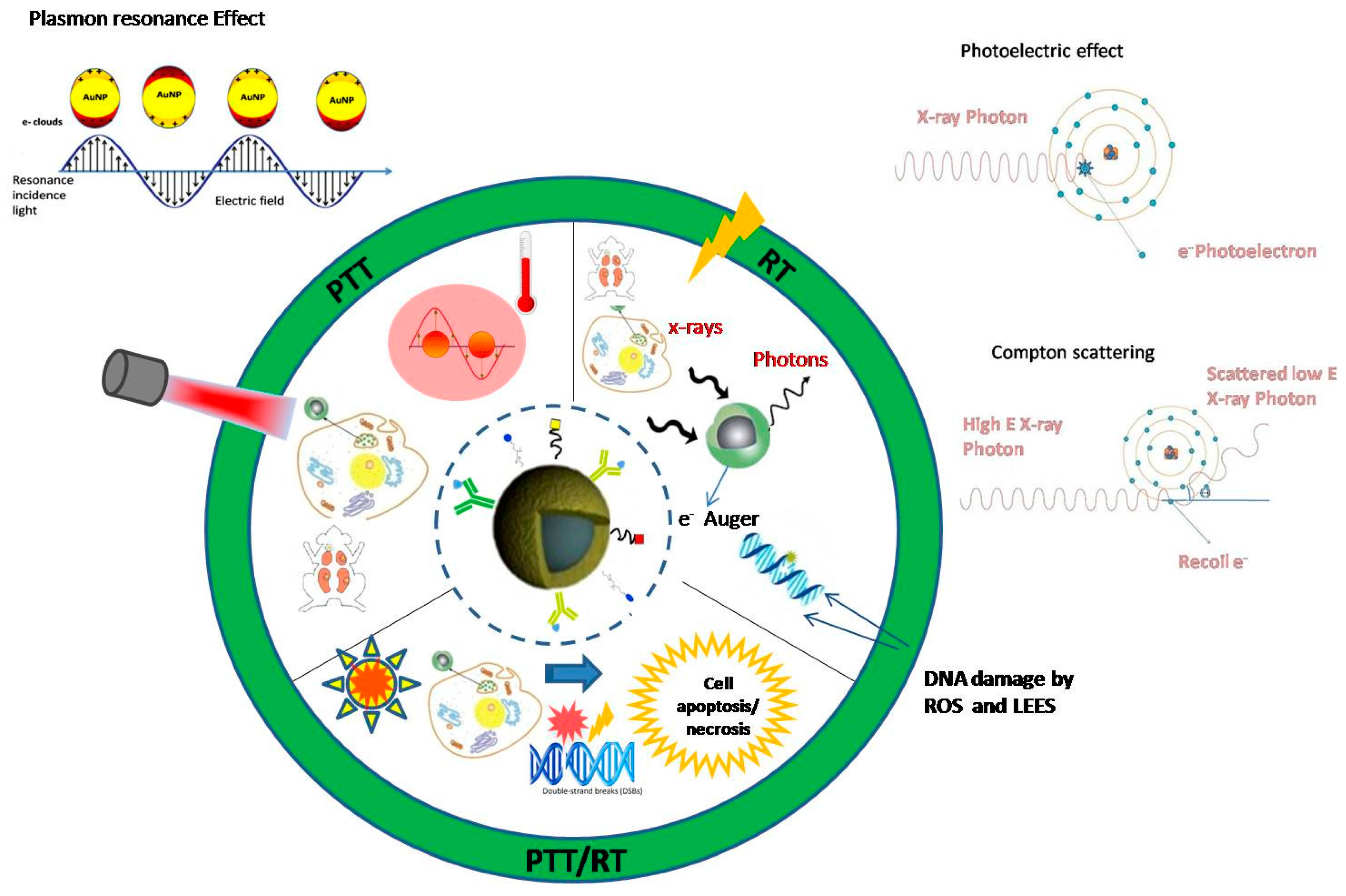
:1. Introduction
The Basic Physics of Interactions of AuNPs with Ionizing Radiation and Laser Radiation
2. Application of Interaction of AuNPs with Non-Ionizing Radiation (Non-IR) in Cancer Therapy
2.1. Introductory Remarks about Non-IR Cancer Treatment Modalities
2.2. Photothermal Therapy (PTT) and Gold NPs: Advantages and Limitations
2.2.1. Photothermal Therapy and Gold Nanoshells
2.2.2. Photothermal Therapy and Gold Nanorods
3. Application of Interaction of AuNPs in Combination with Ionizing Radiation (IR) in Cancer Therapy
3.1. The Combination of Metallic NPs with Radiotherapy
3.2. The Combination of Photothermal Therapy with Radiotherapy
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jaque, D.; Martinez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martin Rodriguez, E.; Garcia Sole, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solovyov, A.; Prise, K.M.; Golding, J.; Mason, N.M. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nano 2016, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for radiation therapy enhancement: The key parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.; Connolly, C.; Schettino, G.; Butterworth, K.T.; Prise, K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Su, M. Nanoparticle location and material dependent dose enhancement in x-ray radiation therapy. J. Phys. Chem. C Nanomater. Interfaces 2012, 116, 23047–23052. [Google Scholar] [CrossRef] [PubMed]
- Pustovalov, V.K. Theoretical study of heating of spherical nanoparticle in media by short laser pulses. Chem. Phys. 2005, 308, 103–108. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Dykman, L.A. Optical properties and biomedical applications of plasmonic nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1–35. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur optik trüber medien, speziell kolloidaler metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: Weinheim, Germany, 1998. [Google Scholar]
- Biswas, A.; Wang, T.; Biris, A.S. Single metal nanoparticle spectroscopy: Optical characterization of individual nanosystems for biomedical applications. Nanoscale 2010, 2, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.B. The surface plasmon resonance of supported noble metal nanoparticles: Characterization, laser tailoring, and SERS application. Ph.D. Thesis, University of Kassel, Kassel, Germany, 2007. [Google Scholar]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Pissuwan, D.; Valenzuela, S.M.; Cortie, M.B. Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol. 2006, 24, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito-Silva, A.M.; Sobral-Filho, R.G.; Barbosa-Silva, R.; de Araujo, C.B.; Galembeck, A.; Brolo, A.G. Improved synthesis of gold and silver nanoshells. Langmuir 2013, 29, 4366–4372. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Paganetti, H.; Prise, K.M. Optimising element choice for nanoparticle radiosensitisers. Nanoscale 2016, 8, 581–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makropoulou, M. Cancer and electromagnetic radiation therapy: Quo Vadis? 2016. Available online: https://pdfs.semanticscholar.org/b6e9/3da094b831761274ab304768306844fe2838.pdf (accessed on 18 December 2017).
- Spyratou, E.; Makropoulou, M.; Mourelatou, E.A.; Demetzos, C. Biophotonic techniques for manipulation and characterization of drug delivery nanosystems in cancer therapy. Cancer Lett. 2012, 327, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Fu, P.P.; Yu, H.; Ray, P.C. Theranostic nanomedicine for cancer detection and treatment. J. Food Drug Anal. 2014, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Serafetinides, A.A.; Makropoulou, M.; Kotsifaki, D.G.; Tsigaridas, G. Biophotonics for imaging and cell manipulation: Quo vadis? In Proceedings of the 19th International Conference and School on Quantum Electronics: Laser Physics and Applications, Sozopol, Bulgaria, 26–30 September 2016; Volume 1022613. [Google Scholar]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Ann. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed]
- Mouratidis, P.X.E.; Rivens, I.; Ter Haar, G. A study of thermal dose-induced autophagy, apoptosis and necroptosis in colon cancer cells. Int. J. Hyperth. 2015, 31, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 2012, 33, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Kodiha, M.; Wang, Y.M.; Hutter, E.; Maysinger, D.; Stochaj, U. Off to the organelles-killing cancer cells with targeted gold nanoparticles. Theranostics 2015, 5, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kang, B.; Qian, W.; Mackey, M.A.; Chen, P.C.; Oyelere, A.K.; El-Sayed, I.H.; El-Sayed, M.A. Comparative study of photothermolysis of cancer cells with nuclear-targeted or cytoplasm-targeted gold nanospheres: Continuous wave or pulsed lasers. J. Biomed. Opt. 2010, 15, 058002. [Google Scholar] [CrossRef] [PubMed]
- Rengan, A.K.; Kundu, G.; Banerjee, R.; Srivastava, R. Gold nanocages as effective photothermal transducers in killing highly tumorigenic cancer cells. Part. Part. Syst. Charact. 2014, 31, 398–405. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Pitsillides, C.M.; Joe, E.K.; Wei, X.; Anderson, R.R.; Lin, C.P. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys. J. 2003, 84, 4023–4032. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.K.; Gupta, B.; Ramasamy, T.; Thapa, R.K.; Pathak, S.; Oh, K.T.; Jeong, J.H.; Choi, H.G.; Yong, C.S.; Kim, J.O. PEGylated thermosensitive lipid-coated hollow gold nanoshells for effective combinational chemo-photothermal therapy of pancreatic cancer. Colloids Surf. B Biointerfaces 2017, 160, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Dai, L.; Li, J.; He, Q. Macrophage cell membrane camouflaged au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl. Mater Interfaces 2016, 8, 9610–9618. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Griffin, R.J.; Galanzha, E.I.; Kim, J.W.; Koonce, N.; Webber, J.; Mustafa, T.; Biris, A.S.; Nedosekin, D.A.; Zharov, V.P. Photothermal nanodrugs: Potential of TNF-gold nanospheres for cancer theranostics. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S.; Zhang, M.; Ma, Y.; Liu, Y.; Gao, W.; Zhang, J.; Gu, Y. Laser-triggered small interfering RNA releasing gold nanoshells against heat shock protein for sensitized photothermal therapy. Adv. Sci. 2017, 4, 1600327. [Google Scholar] [CrossRef] [PubMed]
- Von Maltzahn, G.; Park, J.H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhao, Y.; Huff, T.B.; Hansen, M.N.; Wei, A.; Cheng, J.X. Gold nanorods mediate tumor cell death by compromising membrane integrity. Adv. Mat. 2007, 19, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Ibrahim, I.M.; Ali, H.R.; Selim, S.A.; El-Sayed, M.A. Treatment of natural mammary gland tumors in canines and felines using gold nanorods-assisted plasmonic photothermal therapy to induce tumor apoptosis. Int. J. Nanomed. 2016, 11, 4849–4863. [Google Scholar]
- Yin, D.; Li, X.; Ma, Y.; Liu, Z. Targeted cancer imaging and photothermal therapy via monosaccharide-imprinted gold nanorods. Chem. Commun. 2017, 53, 6716–6719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kim, H.S.; Jin, T.; Woo, J.; Piao, Y.J.; Moon, W.K. Near-infrared photothermal therapy using anti-EGFR-gold nanorod conjugates for triple negative breast cancer. Oncotarget 2017, 8, 86566–86575. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, Y.H.; Wang, Z.; Kim, J.; Kim, J.S.; Kim, S.H.; Kim, K.; Kwon, I.C.; Kiesewetter, D.O.; Chen, X. Engineered Zn(II)-dipicolylamine-gold nanorod provides effective prostate cancer treatment by combining siRNA delivery and photothermal therapy. Theranostics 2017, 7, 4240–4254. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; La, W.G.; Hwang, S.; Ha, Y.S.; Park, N.; Won, N.; Jung, S.; Bhang, S.H.; Ma, Y.J.; Cho, Y.M.; et al. pH-responsive assembly of gold nanoparticles and “spatiotemporally concerted” drug release for synergistic cancer therapy. ACS Nano. 2013, 7, 3388–3402. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Wang, C.H.; Chen, S.T.; Chen, H.H.; Leng, W.H.; Chien, C.C.; Wang, C.L.; Kempson, I.M.; Hwu, Y.; Lai, T.C.; et al. Enhancement of cell radiation sensitivity by pegylated gold nanoparticles. Phys. Med. Biol. 2010, 55, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Au, L.; Zheng, D.; Zhou, F.; Li, Z.Y.; Li, X.; Xia, Y. A quantitative study on the photothermal effect of immuno gold nanocages targeted to breast cancer cells. ACS Nano 2008, 2, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Coulter, J.A.; Hounsell, A.R.; Butterworth, K.T.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.M.; Currell, F.J.; et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Khoshgard, K.; Hashemi, B.; Arbabi, A.; Rasaee, M.J.; Soleimani, M. Radiosensitization effect of folate-conjugated gold nanoparticles on HeLa cancer cells under orthovoltage superficial radiotherapy techniques. Phys. Med. Biol. 2014, 59, 2249–2263. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Toossi, M.T.; Sazgarnia, A.; Soleymanifard, S. The effect of glucose-coated gold nanoparticles on radiation bystander effect induced in MCF-7 and QUDB cell lines. Radiat. Environ. Biophys. 2016, 55, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, N.; Cai, Z.; Kwon, Y.L.; Lechtman, E.; Pignol, J.P.; Reilly, R.M. Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to x-radiation. Breast Cancer Res. Treat. 2013, 137, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Lin, L.; Slatkin, D.N.; Avraham Dilmanian, F.; Vadas, T.M.; Smilowitz, H.M. Gold nanoparticle hyperthermia reduces radiotherapy dose. Nanomedicine 2014, 10, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, T.; Chatterjee, D.; Lee, J.; Grant, J.D.; Bhatterai, S.; Tailor, R.; Goodrich, G.; Nicolucci, P.; Krishnan, S. Targeted gold nanoparticles enhance sensitization of prostate tumours to megavoltage radiation therapy in vivo. Nanomedicine 2015, 11, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Chen, J.; Luo, Z.; Wu, D.; Shen, X.; Song, S.S.; Sun, Y.M.; Liu, P.X.; Zhao, J.; Huo, S.; et al. Enhanced tumor accumulation of Sub-2 nm gold nanoclusters for cancer radiation therapy. Adv. Healthc. Mater 2014, 3, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Atun, R.; Jaffray, D.A.; Barton, M.B.; Bray, F.; Baumann, M.; Vikram, B.; Hanna, T.P.; Knaul, F.M.; Lievens, Y.; Lui, T.Y.; et al. Expanding global access to radiotherapy. Lancet Oncol. 2015, 16, 1153–1186. [Google Scholar] [CrossRef]
- Hawkins, R.B. A microdosimetric-kinetic theory of the dependence of the RBE for cell death on LET. Med. Phys. 1998, 25, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.B. A microdosimetric-kinetic model for the effect of non-poisson distribution of lethal lesions on the variation of RBE with LET. Radiat. Res. 2003, 160, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Inaniwa, T.; Furukawa, T.; Kase, Y.; Matsufuji, N.; Toshito, T.; Matsumoto, Y.; Furusawa, Y.; Noda, K. Treatment planning for a scanned carbon beam with a modified microdosimetric kinetic model. Phys. Med. Biol. 2010, 55, 6721–6737. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grun, R.; Friedrich, T.; Elsasser, T.; Kramer, M.; Zink, K.; Karger, C.P.; Durante, M.; Engenhart-Cabillic, R.; Scholz, M. Impact of enhancements in the local effect model (LEM) on the predicted RBE-weighted target dose distribution in carbon ion therapy. Phys. Med. Biol. 2012, 57, 7261–7274. [Google Scholar] [CrossRef] [PubMed]
- Elsasser, T.; Kramer, M.; Scholz, M. Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Shao, Z.; Vang, J.; Kaidar-Person, O.; Wang, A.Z. Application of nanotechnology to cancer radiotherapy. Cancer Nanotechnol. 2016, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Smilowitz, H.M.; O’Connor, M.J.; Dilmanian, F.A.; Slatkin, D.N. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine 2013, 8, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Sahu, P.K.; Beg, S.; Babu, S.M. Nanoparticles for cancer targeting: Current and future directions. Curr. Drug Deliv. 2016, 13, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Boateng, F.; Kumar, R.; Irvine, D.J.; Formenti, S.; Ngoma, T.; Herskind, C.; Veldwijk, M.R.; Hildenbrand, G.L.; Hausmann, M.; et al. Smart radiation therapy biomaterials. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Ganjalikhani, M. The potential effectiveness of nanoparticles as radio sensitizers for radiotherapy. BioImpacts 2014, 4, 15–20. [Google Scholar] [PubMed]
- Su, X.Y.; Liu, P.D.; Wu, H.; Gu, N. Enhancement of radiosensitization by metal-based nanoparticles in cancer radiation therapy. Cancer Biol. Med. 2014, 11, 86–91. [Google Scholar] [PubMed]
- Xu, W.; Luo, T.; Li, P.; Zhou, C.; Cui, D.; Pang, B.; Ren, Q.; Fu, S. RGD-conjugated gold nanorods induce radiosensitization in melanoma cancer cells by downregulating αvβ3 expression. Int. J. Nanomed. 2012, 7, 915–924. [Google Scholar]
- Koonce, N.A.; Quick, C.M.; Hardee, M.E.; Jamshidi-Parsian, A.; Dent, J.A.; Paciotti, G.F.; Nedosekin, D.; Dings, R.P.; Griffin, R.J. Combination of gold nanoparticle-conjugated tumor necrosis factor-α and radiation therapy results in a synergistic antitumor response in murine carcinoma models. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Wu, F.G.; Zhang, X.; Jiang, Y.W.; Jia, H.R.; Wang, H.Y.; Li, Y.H.; Liu, P.; Gu, N.; Chen, Z. Shape-dependent radiosensitization effect of gold nanostructures in cancer radiotherapy: Comparison of gold nanoparticles, nanospikes, and nanorods. ACS Appl. Mater. Interfaces 2017, 9, 13037–13048. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef] [PubMed]
- Berbeco, R.I.; Korideck, H.; Ngwa, W.; Kumar, R.; Patel, J.; Sridhar, S.; Johnson, S.; Price, B.D.; Kimmelman, A.; Makrigiorgos, G.M. DNA damage enhancement from gold nanoparticles for clinical MV photon beams. Radiat. Res. 2012, 178, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Marshall, T.I.; Currell, F.J.; Kacperek, A.; Schettino, G.; Prise, K.M. Variations in the processing of DNA double-strand breaks along 60-MeV therapeutic proton beams. Int. J. Radiat. Oncol. Biol. Phys 2016, 95, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, T.I.; Chaudhary, P.; Michaelidesova, A.; Vachelova, J.; Davidkova, M.; Vondracek, V.; Schettino, G.; Prise, K.M. Investigating the implications of a variable RBE on proton dose fractionation across a clinical pencil beam scanned spread-out Bragg peak. Int. J. Radiat. Oncol. Biol. Phys 2016, 95, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikitaki, Z.; Mavragani, I.V.; Laskaratou, D.A.; Gika, V.; Moskvin, V.P.; Theofilatos, K.; Vougas, K.; Stewart, R.D.; Georgakilas, A.G. Systemic mechanisms and effects of ionizing radiation: A new ‘old’ paradigm of how the bystanders and distant can become the players. Semin. Cancer Biol. 2016, 37, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, L. Gold nanoparticles enhancing protontherapy efficiency. Recent Pat. Nanotechnol. 2015, 9, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; McMahon, S.J.; Scarpelli, M.; Paganetti, H.; Schuemann, J. Comparing gold nano-particle enhanced radiotherapy with protons, megavoltage photons and kilovoltage photons: A monte carlo simulation. Phys. Med. Biol. 2014, 59, 7675–7689. [Google Scholar] [CrossRef] [PubMed]
- Schuemann, J.; Berbeco, R.; Chithrani, D.B.; Cho, S.H.; Kumar, R.; McMahon, S.J.; Sridhar, S.; Krishnan, S. Roadmap to clinical use of gold nanoparticles for radiation sensitization. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hunting, D.J.; Ayotte, P.; Sanche, L. Radiosensitization of DNA by gold nanoparticles irradiated with high-energy electrons. Radiat. Res. 2008, 169, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ionita, P.; Conte, M.; Gilbert, B.C.; Chechik, V. Gold nanoparticle-initiated free radical oxidations and halogen abstractions. Org. Biomol. Chem. 2007, 5, 3504–3509. [Google Scholar] [CrossRef] [PubMed]
- Chompoosor, A.; Saha, K.; Ghosh, P.S.; Macarthy, D.J.; Miranda, O.R.; Zhu, Z.J.; Arcaro, K.F.; Rotello, V.M. The role of surface functionality on acute cytotoxicity, ROS generation and DNA damage by cationic gold nanoparticles. Small 2010, 6, 2246–2249. [Google Scholar] [CrossRef] [PubMed]
- Mateo, D.; Morales, P.; Ávalos, A.; Haza, A.I. Oxidative stress contributes to gold nanoparticle-induced cytotoxicity in human tumor cells. Toxicol. Mech. Methods 2014, 24, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Huang, C.; Chen, X.; Yi, Z.; Sanche, L. Chemical radiosensitivity of DNA induced by gold nanoparticles. J. Biomed. Nanotechnol. 2015, 11, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; McMahon, S.J.; Paganetti, H.; Schuemann, J. Biological modeling of gold nanoparticle enhanced radiotherapy for proton therapy. Phys. Med. Biol. 2015, 60, 4149–4168. [Google Scholar] [CrossRef] [PubMed]
- Tsiamas, P.; Liu, B.; Cifter, F.; Ngwa, W.F.; Berbeco, R.I.; Kappas, C.; Theodorou, K.; Marcus, K.; Makrigiorgos, M.G.; Sajo, E.; et al. Impact of beam quality on megavoltage radiotherapy treatment techniques utilizing gold nanoparticles for dose enhancement. Phys. Med. Biol. 2013, 58, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, N.M.; Tsekenis, G.; Balanikas, E.C.; Pavlopoulou, A.; Mitsiogianni, M.; Mantso, T.; Pashos, G.; Boudouvis, A.G.; Lykakis, I.N.; Tsigaridas, G.; et al. Gold nanoparticles, radiations and the immune system: Current insights into the physical mechanisms and the biological interactions of this new alliance towards cancer therapy. Pharmacol. Ther. 2017, 178, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Stefancikova, L.; Lacombe, S.; Salado, D.; Porcel, E.; Pagacova, E.; Tillement, O.; Lux, F.; Depes, D.; Kozubek, S.; Falk, M. Effect of gadolinium-based nanoparticles on nuclear DNA damage and repair in glioblastoma tumor cells. J. Nanobiotechnol. 2016, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Mwakwari, S.C.; Sodji, Q.H.; Oyelere, A.K.; El-Sayed, M.A. Tamoxifen-poly(ethylene glycol)-thiol gold nanoparticle conjugates: Enhanced potency and selective delivery for breast cancer treatment. Bioconjug. Chem. 2009, 20, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Grall, R.; Girard, H.; Saad, L.; Petit, T.; Gesset, C.; Combis-Schlumberger, M.; Paget, V.; Delic, J.; Arnault, J.C.; Chevillard, S. Impairing the radioresistance of cancer cells by hydrogenated nanodiamonds. Biomaterials 2015, 61, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Heuskin, A.C.; Gallez, B.; Feron, O.; Martinive, P.; Michiels, C.; Lucas, S. Metallic nanoparticles irradiated by low-energy protons for radiation therapy: Are there significant physical effects to enhance the dose delivery? Med. Phys. 2017, 44, 4299–4312. [Google Scholar] [CrossRef] [PubMed]
- Ledingham, K.; Bolton, P.; Shikazono, N.; Ma, C.M. Towards laser driven hadron cancer radiotherapy: A review of progress. Appl. Sci. 2014, 4, 402–443. [Google Scholar] [CrossRef]
- Zhang, A.W.; Guo, W.H.; Qi, Y.F.; Wang, J.Z.; Ma, X.X.; Yu, D.X. Synergistic effects of gold nanocages in hyperthermia and radiotherapy treatment. Nanoscale Res. Lett. 2016, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.J.; Dings, R.P.M.; Jamshidi-Parsian, A.; Song, C.W. Mild temperature hyperthermia and radiation therapy: Role of tumour vascular thermotolerance and relevant physiological factors. Int. J. Hyperth. 2010, 26, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chatterjee, D.K.; Lee, M.H.; Krishnan, S. Gold nanoparticles in breast cancer treatment: Promise and potential pitfalls. Cancer Lett. 2014, 347, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.L.; Zhang, M.; Diagaradjane, P.; Peddibhotla, S.; Contreras, A.; Hilsenbeck, S.G.; Woodward, W.A.; Krishnan, S.; Chang, J.C.; Rosen, J.M. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci. Transl. Med. 2010, 2, 55ra79. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Toraya-Brown, S.; Fiering, S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int. J. Hyperth. 2014, 30, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, H.; Li, J.; Cheng, D.; Shuai, X. Co-delivery of doxorubicin and arsenite with reduction and pH dual-sensitive vesicle for synergistic cancer therapy. Nanoscale 2016, 8, 12608–12617. [Google Scholar] [CrossRef] [PubMed]
| Cellline | AuNP-Type | [CNP] | Up Take Time/Irradiation Time | Therapy Type | Shape | Size (nm) | Reference |
|---|---|---|---|---|---|---|---|
| Murine SCK tested in mice | TNF AuNPs | 0.1 mL/20 g bodyweight | 1 min | 690 nm Pulsed laser 0.1–1 J/cm2 | spheres | 33 nm | [37] |
| B16-F10 in vivo tested in nude mice | Doxorubicin-AuNPs | 1 μM | 24 h/5 min | 660 nm laser 8–20 W/cm2 | sphere | 20 nm | [45] |
| MIA PaCa-2 and PANC-1 cells | GNS-L/GB | 50 μg Auequiv | 48 h/3 min | 808 nm laser 4 W/cm2 | shells | 85 nm | [34] |
| MCF-7 tested female BALB/c mice | PEG-AuNSs-Transferrin | 1 mg/mL | 6 h/3 min | 808 nm laser 2 W/cm2 | shells | 30 nm | [46] |
| SK-BR-3 tested in mice | PEG-AuPs | 7.3 nM | 30 min/4–6 min | CW 820 nm 4 W/cm2 | shells | 110 (core) + 10 nm (shell) | [35] |
| 4T1 cancer cells tested in mice | MPCM-AuNSs | 1 mg/mL | 20 min/5 min | CW 808 nm 1 W/cm2 | shells | 80(core) + 7 nm (shell) | [36] |
| U87MG tumorstested in nude mice | HGN-siHsp70 | 1.5 × 10−9 M/Kg | 90 min/8 min | CW 808 nm 4 W/cm2 | shells | 70 nm | [38] |
| SK-BR3 | Anti-HER2-AuNPs | - | 5 min | 800 nm Pulsed laser 1.6 W/cm2 | nanocages | 65 nm | [47] |
| MDA-MB-435 tested in nude mice | PEG-AuNP | 14 μg/mL | 72 h/5 min | CW 810 nm laser 2 W/cm2 | nanorods | Aspect ratio 3.7 | [39] |
| Hs578T, HCC-38, MDA-MB-468 and MDAMB-231 | anti-EGFR-GN | 0.22 μg/mL | 48 h/3 min | 820 nm halogen lamp 1.5 W/cm2 | nanorods | Aspect ratio 4.0 | [43] |
| PC3 cells tested in nude mice | Zn(II)/DPA-AuNR | 250 μL | 24 h/10 min | 808 nm 0.5 W/cm2 | nanorods | Length 51.13 ± 5.2 nm | [44] |
| MDA-MB-231 | Thiol-AuNP | 12 μM | 24 h | X-radiation 160 kVp 4 Gy | sphere | 1.9 | [48] |
| ΕΜΤ-6 | PEG-AuNP | 500 μΜ | 48 h | X-radiation 10 Gy | sphere | 6.1 | [46] |
| HeLa | folic Acid-AuNP | 255 μΜ | 6, 12, 24, 48 h | X-radiation 180 kVp | _ | 50 | [49] |
| MCF-7 | Glu-AuNP | 100 μM | 2 h | X-radiation 100 kVp 10 Gy | sphere | 16 | [50] |
| MDA-MB-361 | anti-HER2-PEG-AuNP | 4.8 mg/(g tumor) | 48 h | X-radiation 100 kVp 11 Gy | sphere | 30 | [51] |
| SCCVII tested in mice | PEG-AuNPs | 1 μg/mL | 24 h/5 min | 820 nm halogen lamp 1.5 W/cm2 15 Gy | spheres | 15 nm | [52] |
| PC-3 cells tested in Foxn1 mice | Goserelin-PEG-AuNRs | 0.1–10 μg/g body weight * | 72 h/5 min | X-radiation 6 MV 5 Gy | nanorods | _ | [53] |
| 4T1 cells | CD44-Antibody-PEG-AuNPs | 3 nM | 24 h | CW 808 nm 2.5 W/cm2 X-radiation With 2, 4, 6, or 8 Gy of 6-MV | nanocages | 58.4 nm | [54] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spyratou, E.; Makropoulou, M.; Efstathopoulos, E.P.; Georgakilas, A.G.; Sihver, L. Recent Advances in Cancer Therapy Based on Dual Mode Gold Nanoparticles. Cancers 2017, 9, 173. https://doi.org/10.3390/cancers9120173
Spyratou E, Makropoulou M, Efstathopoulos EP, Georgakilas AG, Sihver L. Recent Advances in Cancer Therapy Based on Dual Mode Gold Nanoparticles. Cancers. 2017; 9(12):173. https://doi.org/10.3390/cancers9120173
Chicago/Turabian StyleSpyratou, Ellas, Mersini Makropoulou, Efstathios P. Efstathopoulos, Alexandros G. Georgakilas, and Lembit Sihver. 2017. "Recent Advances in Cancer Therapy Based on Dual Mode Gold Nanoparticles" Cancers 9, no. 12: 173. https://doi.org/10.3390/cancers9120173







