Carbon Ion Radiotherapy: A Review of Clinical Experiences and Preclinical Research, with an Emphasis on DNA Damage/Repair
Abstract
:1. Introduction
2. Rationale for Charged Particle Therapy
2.1. Physics Rationale: The Spread-Out Bragg Peak, Enhanced Dose Distribution, Lateral Focusing, Dose Verification, and Superior Linear Energy Transfer
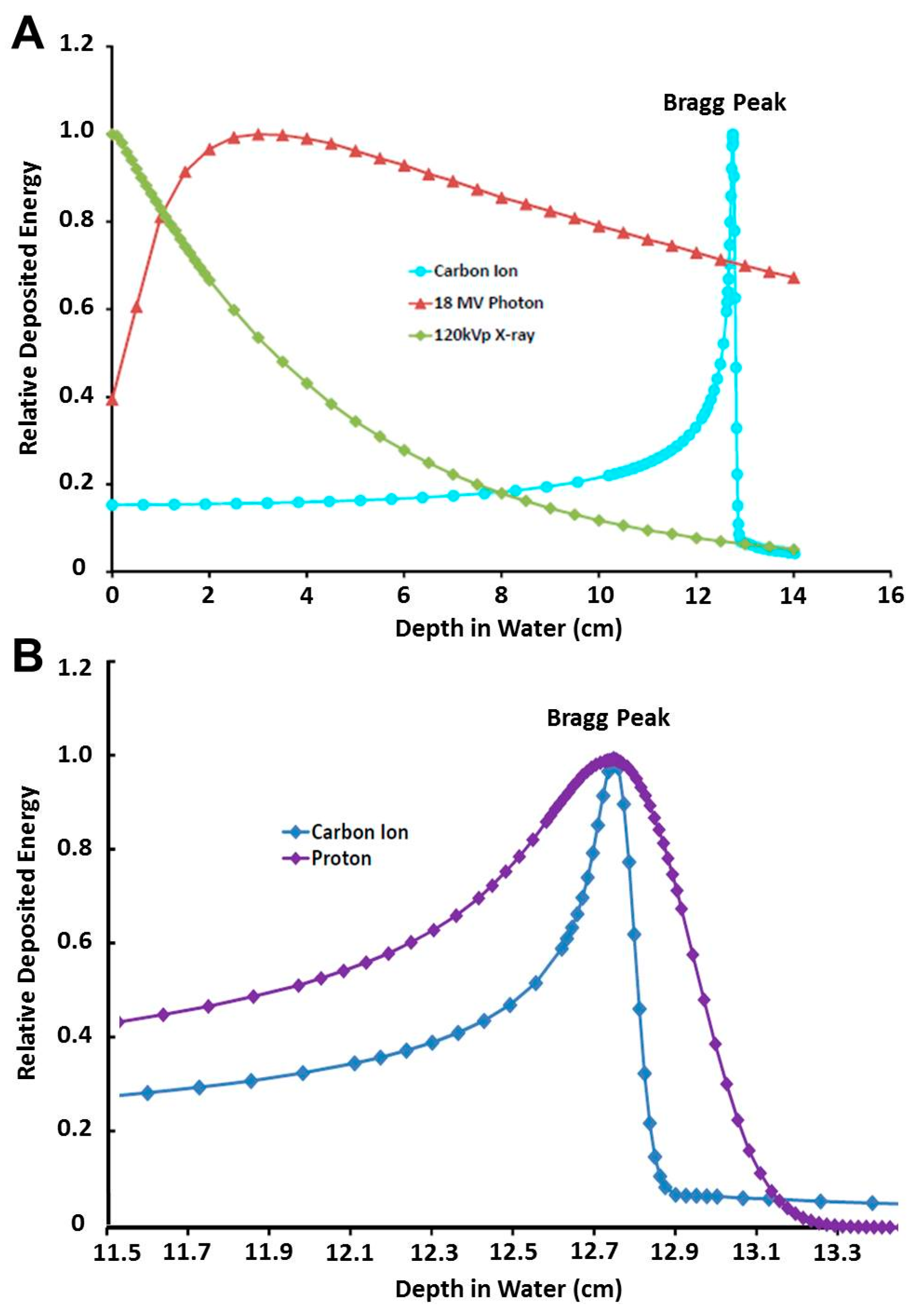
2.1.1. The Spread-Out Bragg Peak, Enhanced Dose Distribution, and Lateral Focusing
2.1.2. Dose Verification
2.1.3. Superior LET
2.2. Biological Rationale: Relative Biological Effectiveness, Complex DNA Damage, and Oxygen Enhancement Ratio
3. Complex DNA Damage and Repair
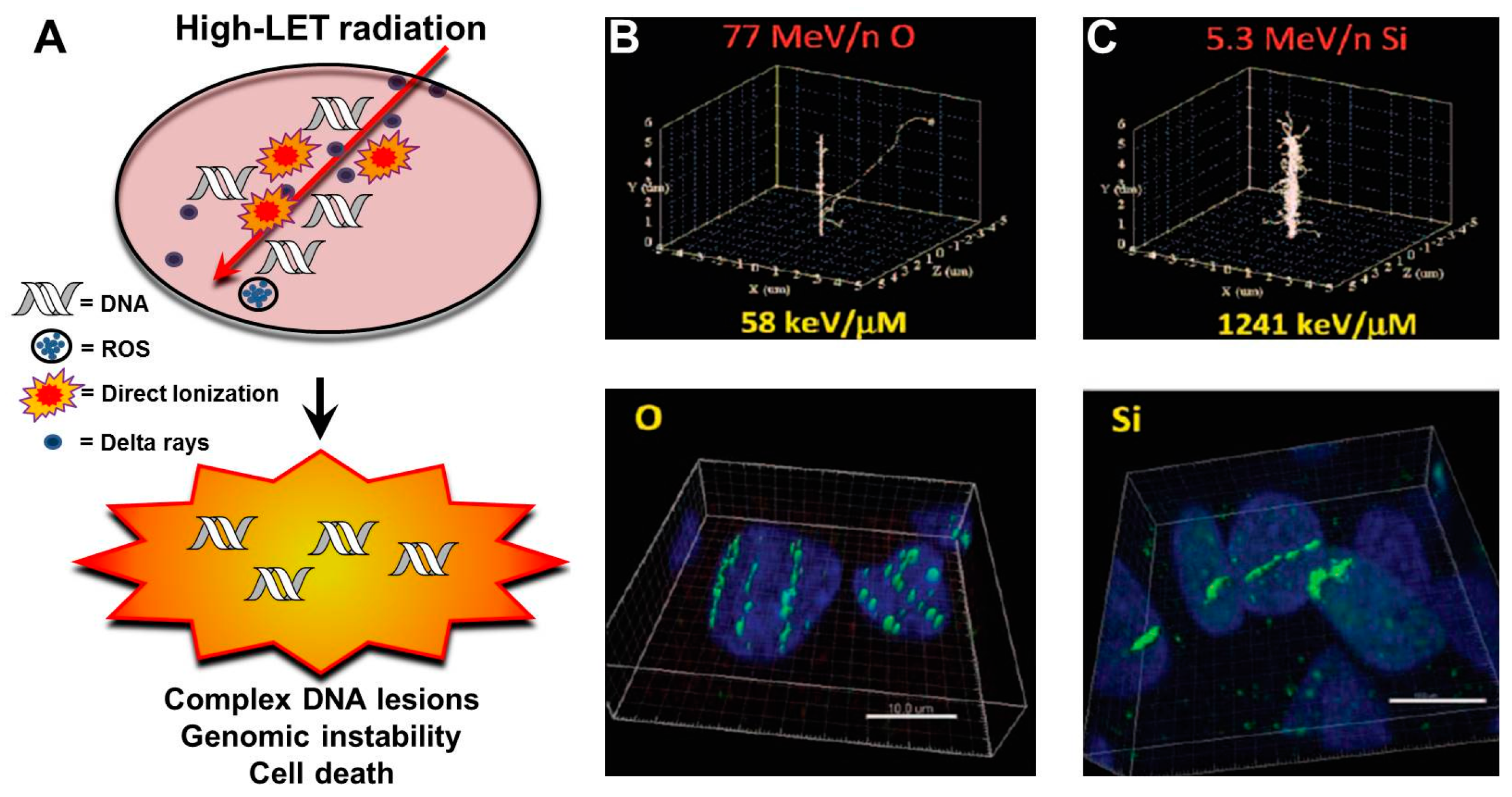
3.1. Charged Particles and Track Structures
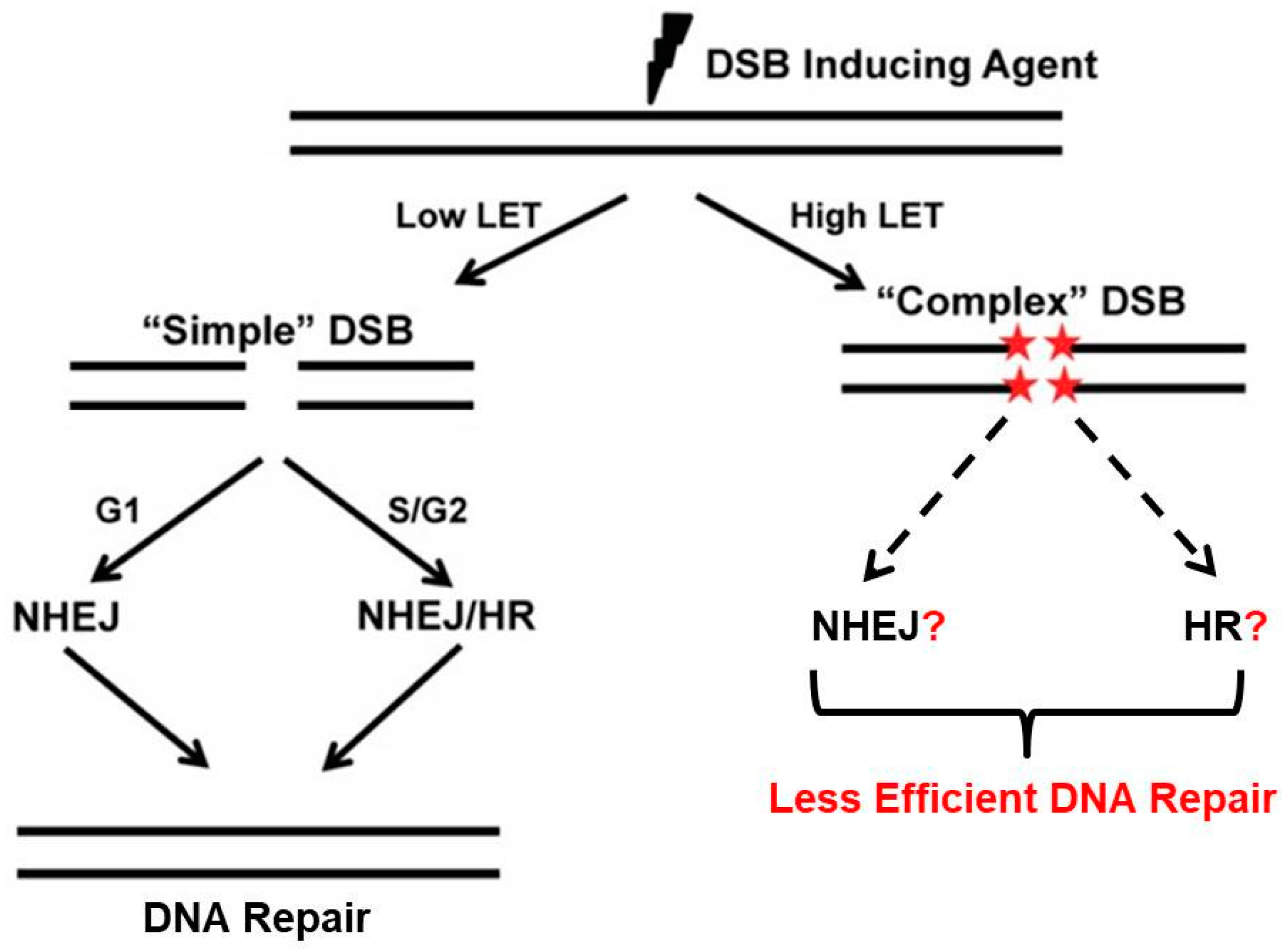
3.2. LET and Clustered DNA Damage
3.3. Clustered Damage, Chromosomes and DNA Repair
4. Preclinical Research in Carbon Ion Radiotherapy
4.1. DNA Damage and Repair after CIRT in Select Preclinical Models
4.2. LET-Dependent Differential Expression of DNA Repair Genes
4.3. CIRT and Chemotherapy
5. Clinical Experiences with CIRT
5.1. Brief History
5.2. Clinical Rationale
5.3. Clinical Experience by Disease Site
5.3.1. Osteosarcomas and Soft Tissue Sarcomas (STS)
5.3.2. Head and Neck (Including Skull Base) Cancers
5.3.3. Prostate Cancers
5.3.4. Cervical Cancers
5.3.5. Hepatocellular Carcinomas (HCC)
5.3.6. Pancreatic Cancers
5.3.7. Glioblastoma (GBM)
5.3.8. Pediatric Cancers
5.3.9. Recurrent and Previously Irradiated Cancers
5.4. Cost-Effectiveness of Carbon Ion Radiotherapy
5.5. Carcinogenesis after Carbon Ion Radiotherapy
5.6. Opportunities for Further Improving Efficacy of CIRT in the Clinic
6. Future Promises and Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Schulz-Ertner, D.; Jakel, O.; Schlegel, W. Radiation therapy with charged particles. Semin. Radiat. Oncol. 2006, 16, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Pompos, A.; Durante, M.; Choy, H. Heavy Ions in Cancer Therapy. JAMA Oncol. 2016, 2, 1539–1540. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.R. Radiological use of fast protons. Radiology 1946, 47, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Borak, T.B.; Tsujii, H.; Nickoloff, J.A. Heavy charged particle radiobiology: Using enhanced biological effectiveness and improved beam focusing to advance cancer therapy. Mutat. Res. 2011, 711, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Jereczek-Fossa, B.A.; Krengli, M.; Orecchia, R. Particle beam radiotherapy for head and neck tumors: Radiobiological basis and clinical experience. Head Neck 2006, 28, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Jermann, M. Particle Therapy Statistics in 2014. Int. J. Part. Ther. 2015, 2, 50–54. [Google Scholar] [CrossRef]
- Particle Therapy Patient Statistics (per end of 2015). Available online: https://www.ptcog.ch/archive/patient_statistics/Patientstatistics-updateDec2015.pdf (accessed on 27 March 2017).
- Mohan, R.; Grosshans, D. Proton therapy—Present and future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Bragg, W. On the ionization of various gases by the alpha particles of radium. Proc. Phys. Soc. Lond. 1907, 523–550. [Google Scholar]
- DeLaney, T. Proton and Charged Particle Radiotherapy; Lippincott, Williams and Wilkins: Philidephia, PA, USA, 2007. [Google Scholar]
- Lomax, A.J. Charged particle therapy: The physics of interaction. Cancer J. 2009, 15, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Weber, U.; Kraft, G. Comparison of carbon ions versus protons. Cancer J. 2009, 15, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Uzawa, A.; Ando, K.; Koike, S.; Furusawa, Y.; Matsumoto, Y.; Takai, N.; Hirayama, R.; Watanabe, M.; Scholz, M.; Elsasser, T.; et al. Comparison of biological effectiveness of carbon-ion beams in Japan and Germany. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Kraft, G.; An, H.; Engenhart-Cabillic, R. Ion beam radiobiology and cancer: Time to update ourselves. Biochim. Biophys. Acta 2009, 1796, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Significance and implementation of RBE variations in proton beam therapy. Technol. Cancer Res. Treat. 2003, 2, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H.; Niemierko, A.; Ancukiewicz, M.; Gerweck, L.E.; Goitein, M.; Loeffler, J.S.; Suit, H.D. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 407–421. [Google Scholar] [CrossRef]
- Weyrather, W.K.; Debus, J. Particle beams for cancer therapy. Clin. Oncol. 2003, 15, S23–S28. [Google Scholar] [CrossRef]
- Blaisdell, J.O.; Harrison, L.; Wallace, S.S. Base excision repair processing of radiation-induced clustered DNA lesions. Radiat. Prot. Dosim. 2001, 97, 25–31. [Google Scholar] [CrossRef]
- Harrison, L.; Hatahet, Z.; Purmal, A.A.; Wallace, S.S. Multiply damaged sites in DNA: Interactions with Escherichia coli endonucleases III and VIII. Nucl. Acids Res. 1998, 26, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Prog. Nucl. Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar]
- Asaithamby, A.; Hu, B.; Chen, D.J. Unrepaired clustered DNA lesions induce chromosome breakage in human cells. Proc. Natl. Acad. Sci. USA 2011, 108, 8293–8298. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P.; Burki, H.J. Survival of synchronized Chinese hamster cells exposed to radiation of different linear-energy transfer. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1975, 27, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Zhang, P.; Zhang, S.; Naidu, M.; Wang, H.; Wang, Y. S-phase cells are more sensitive to high-linear energy transfer radiation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Tamulevicius, P.; Wang, M.; Iliakis, G. Homology-directed repair is required for the development of radioresistance during S phase: Interplay between double-strand break repair and checkpoint response. Radiat. Res. 2007, 167, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Giaccia, A. Radiobiology for the Radiologist; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Tinganelli, W.; Ma, N.Y.; Von Neubeck, C.; Maier, A.; Schicker, C.; Kraft-Weyrather, W.; Durante, M. Influence of acute hypoxia and radiation quality on cell survival. J. Radiat. Res. 2013, 54 (Suppl. 1), i23–i30. [Google Scholar] [CrossRef] [PubMed]
- Wozny, A.S.; Lauret, A.; Battiston-Montagne, P.; Guy, J.B.; Beuve, M.; Cunha, M.; Saintigny, Y.; Blond, E.; Magne, N.; Lalle, P.; et al. Differential pattern of HIF-1alpha expression in HNSCC cancer stem cells after carbon ion or photon irradiation: One molecular explanation of the oxygen effect. Br. J. Cancer 2017, 116, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Plante, I.; Ponomarev, A.; Cucinotta, F.A. 3D visualisation of the stochastic patterns of the radial dose in nano-volumes by a Monte Carlo simulation of HZE ion track structure. Radiat. Prot. Dosim. 2011, 143, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Sutherland, B.M. Spectrum of complex DNA damages depends on the incident radiation. Radiat. Res. 2006, 165, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Holley, W.R. Biochemical mechanisms and clusters of damage for high-LET radiation. Adv. Space Res. 1992, 12, 33–43. [Google Scholar] [CrossRef]
- Ward, J.F. Biochemistry of DNA lesions. Radiat. Res. Suppl. 1985, 8, S103–S111. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. The complexity of DNA damage: Relevance to biological consequences. Int. J. Radiat. Biol. 1994, 66, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Georgakilas, A.G. Formation of clustered DNA damage after high-LET irradiation: A review. J. Radiat. Res. 2008, 49, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.M.; Bennett, P.V.; Schenk, H.; Sidorkina, O.; Laval, J.; Trunk, J.; Monteleone, D.; Sutherland, J. Clustered DNA damages induced by high and low LET radiation, including heavy ions. Phys. Med. 2001, 17 (Suppl. 1), 202–204. [Google Scholar] [PubMed]
- Ibanez, I.L.; Bracalente, C.; Molinari, B.L.; Palmieri, M.A.; Policastro, L.; Kreiner, A.J.; Burlon, A.A.; Valda, A.; Navalesi, D.; Davidson, J.; et al. Induction and rejoining of DNA double strand breaks assessed by H2AX phosphorylation in melanoma cells irradiated with proton and lithium beams. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Wilson, P.; Thieberger, P.; Lowenstein, D.; Wang, M.; Cucinotta, F.A. Biological characterization of low-energy ions with high-energy deposition on human cells. Radiat. Res. 2014, 182, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.A.; Rogakou, E.P.; Panyutin, I.G.; Bonner, W.M. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat. Res. 2002, 158, 486–492. [Google Scholar] [CrossRef]
- Cashman, J.; Dykstra, B.; Clark-Lewis, I.; Eaves, A.; Eaves, C. Changes in the proliferative activity of human hematopoietic stem cells in NOD/SCID mice and enhancement of their transplantability after in vivo treatment with cell cycle inhibitors. J. Exp. Med. 2002, 196, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Jakob, B.; Splinter, J.; Conrad, S.; Voss, K.O.; Zink, D.; Durante, M.; Lobrich, M.; Taucher-Scholz, G. DNA double-strand breaks in heterochromatin elicit fast repair protein recruitment, histone H2AX phosphorylation and relocation to euchromatin. Nucl. Acids Res. 2011, 39, 6489–6499. [Google Scholar] [CrossRef] [PubMed]
- Lorat, Y.; Timm, S.; Jakob, B.; Taucher-Scholz, G.; Rube, C.E. Clustered double-strand breaks in heterochromatin perturb DNA repair after high linear energy transfer irradiation. Radiother. Oncol. 2016, 121, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Zafar, F.; Seidler, S.B.; Kronenberg, A.; Schild, D.; Wiese, C. Homologous recombination contributes to the repair of DNA double-strand breaks induced by high-energy iron ions. Radiat. Res. 2010, 173, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Zhang, P.; Wang, Y. The Ku-dependent non-homologous end-joining but not other repair pathway is inhibited by high linear energy transfer ionizing radiation. DNA Repair 2008, 7, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Wang, P.; Yu, X.; Essers, J.; Chen, D.; Kanaar, R.; Takeda, S.; Wang, Y. Characteristics of DNA-binding proteins determine the biological sensitivity to high-linear energy transfer radiation. Nucl. Acids Res. 2010, 38, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Rostek, C.; Turner, E.L.; Robbins, M.; Rightnar, S.; Xiao, W.; Obenaus, A.; Harkness, T.A. Involvement of homologous recombination repair after proton-induced DNA damage. Mutagenesis 2008, 23, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Grosse, N.; Fontana, A.O.; Hug, E.B.; Lomax, A.; Coray, A.; Augsburger, M.; Paganetti, H.; Sartori, A.A.; Pruschy, M. Deficiency in homologous recombination renders Mammalian cells more sensitive to proton versus photon irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Gerelchuluun, A.; Manabe, E.; Ishikawa, T.; Sun, L.; Itoh, K.; Sakae, T.; Suzuki, K.; Hirayama, R.; Asaithamby, A.; Chen, D.J.; et al. The major DNA repair pathway after both proton and carbon-ion radiation is NHEJ, but the HR pathway is more relevant in carbon ions. Radiat. Res. 2015, 183, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, X.; Xie, Y.; Tanaka, K.; Wang, B.; Zhang, H. DNA-PKcs inhibition sensitizes cancer cells to carbon-ion irradiation via telomere capping disruption. PLoS ONE 2013, 8, e72641. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, R.; Okada, M.; Okabe, A.; Noguchi, M.; Takakura, K.; Takahashi, S. Repair of DNA damage induced by accelerated heavy ions in mammalian cells proficient and deficient in the non-homologous end-joining pathway. Radiat. Res. 2006, 165, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Eguchi-Kasai, K.; Murakami, M.; Itsukaichi, H.; Fukutsu, K.; Yatagai, F.; Kanai, T.; Ohara, H.; Sato, K. Repair of DNA double-strand breaks and cell killing by charged particles. Adv. Space Res. 1998, 22, 543–549. [Google Scholar] [CrossRef]
- Weyrather, W.K.; Ritter, S.; Scholz, M.; Kraft, G. RBE for carbon track-segment irradiation in cell lines of differing repair capacity. Int. J. Radiat. Biol. 1999, 75, 1357–1364. [Google Scholar] [PubMed]
- Saha, J.; Wang, M.; Cucinotta, F.A. Investigation of switch from ATM to ATR signaling at the sites of DNA damage induced by low and high LET radiation. DNA Repair 2013, 12, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Whalen, M.K.; Gurai, S.K.; Zahed-Kargaran, H.; Pluth, J.M. Specific ATM-mediated phosphorylation dependent on radiation quality. Radiat. Res. 2008, 170, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Goodhead, D.T. Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA. Int. J. Radiat. Biol. 1994, 65, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Imaoka, T.; Masunaga, S.; Ogata, T.; Okayasu, R.; Takahashi, A.; Kato, T.A.; Kobayashi, Y.; Ohnishi, T.; Ono, K.; et al. Recent advances in the biology of heavy-ion cancer therapy. J. Radiat. Res. 2010, 51, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Oike, T.; Shibata, A.; Niimi, A.; Kubota, Y.; Sakai, M.; Amornwhichet, N.; Yoshimoto, Y.; Hagiwara, Y.; Kimura, Y.; et al. Mitotic catastrophe is a putative mechanism underlying the weak correlation between sensitivity to carbon ions and cisplatin. Sci. Rep. 2017, 7, 40588. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, M.; Alphonse, G.; Colliaux, A.; Beuve, M.; Trajkovic-Bodennec, S.; Battiston-Montagne, P.; Testard, I.; Chapet, O.; Bajard, M.; Taucher-Scholz, G.; et al. Different mechanisms of cell death in radiosensitive and radioresistant p53 mutated head and neck squamous cell carcinoma cell lines exposed to carbon ions and x-rays. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, G.; Maalouf, M.; Boivin, A.; Battiston-Montagne, P.; Beuve, M.; Levy, A.; Jalade, P.; Fournier, C.; Ardail, D.; Magne, N.; et al. Targeting head and neck cancer stem cells to overcome resistance to photon and carbon ion radiation. Stem Cell Rev. 2014, 10, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Sommer, S.; Hartel, C.; Nasonova, E.; Durante, M.; Ritter, S. Complex exchanges are responsible for the increased effectiveness of C-ions compared to X-rays at the first post-irradiation mitosis. Mutat. Res. 2010, 701, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Elsasser, T.; Tonn, T.; Seifried, E.; Durante, M.; Ritter, S.; Fournier, C. Response of human hematopoietic stem and progenitor cells to energetic carbon ions. Int. J. Radiat. Biol. 2009, 85, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Lorat, Y.; Brunner, C.U.; Schanz, S.; Jakob, B.; Taucher-Scholz, G.; Rube, C.E. Nanoscale analysis of clustered DNA damage after high-LET irradiation by quantitative electron microscopy—The heavy burden to repair. DNA Repair 2015, 28, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Carante, M.P.; Altieri, S.; Bortolussi, S.; Postuma, I.; Protti, N.; Ballarini, F. Modeling radiation-induced cell death: Role of different levels of DNA damage clustering. Radiat. Environ. Biophys. 2015, 54, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Furusawa, Y.; George, K.; Kawata, T.; Cucinotta, F.A. Analysis of unrejoined chromosomal breakage in human fibroblast cells exposed to low- and high-LET radiation. J. Radiat. Res. 2002, 43 (Suppl.), S181–S185. [Google Scholar] [CrossRef] [PubMed]
- Kawata, T.; Durante, M.; Furusawa, Y.; George, K.; Ito, H.; Wu, H.; Cucinotta, F.A. Rejoining of isochromatid breaks induced by heavy ions in G2-phase normal human fibroblasts. Radiat. Res. 2001, 156, 598–602. [Google Scholar] [CrossRef]
- Kawata, T.; Gotoh, E.; Durante, M.; Wu, H.; George, K.; Furusawa, Y.; Cucinotta, F.A. High-LET radiation-induced aberrations in prematurely condensed G2 chromosomes of human fibroblasts. Int. J. Radiat. Biol. 2000, 76, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Zhang, Y.; Feiveson, A.; Cucinotta, F.A.; Wu, H. Association of inter- and intrachromosomal exchanges with the distribution of low- and high-LET radiation-induced breaks in chromosomes. Radiat. Res. 2011, 176, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Ferrandon, S.; Saultier, P.; Carras, J.; Battiston-Montagne, P.; Alphonse, G.; Beuve, M.; Malleval, C.; Honnorat, J.; Slatter, T.; Hung, N.; et al. Telomere profiling: Toward glioblastoma personalized medicine. Mol. Neurobiol. 2013, 47, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Hanot, M.; Boivin, A.; Malesys, C.; Beuve, M.; Colliaux, A.; Foray, N.; Douki, T.; Ardail, D.; Rodriguez-Lafrasse, C. Glutathione depletion and carbon ion radiation potentiate clustered DNA lesions, cell death and prevent chromosomal changes in cancer cells progeny. PLoS ONE 2012, 7, e44367. [Google Scholar] [CrossRef] [PubMed]
- Glowa, C.; Karger, C.P.; Brons, S.; Zhao, D.; Mason, R.P.; Huber, P.E.; Debus, J.; Peschke, P. Carbon ion radiotherapy decreases the impact of tumor heterogeneity on radiation response in experimental prostate tumors. Cancer Lett. 2016, 378, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Teshima, T.; Kagawa, K.; Hishikawa, Y.; Takahashi, Y.; Kawaguchi, A.; Suzumoto, Y.; Nojima, K.; Furusawa, Y.; Matsuura, N. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res. 2005, 65, 113–120. [Google Scholar] [PubMed]
- Akino, Y.; Teshima, T.; Kihara, A.; Kodera-Suzumoto, Y.; Inaoka, M.; Higashiyama, S.; Furusawa, Y.; Matsuura, N. Carbon-ion beam irradiation effectively suppresses migration and invasion of human non-small-cell lung cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Teshima, T.; Inaoka, M.; Minami, K.; Tsuchiya, T.; Isono, M.; Furusawa, Y.; Matsuura, N. Carbon ion irradiation suppresses metastatic potential of human non-small cell lung cancer A549 cells through the phosphatidylinositol-3-kinase/Akt signaling pathway. J. Radiat. Res. 2011, 52, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Rieken, S.; Rieber, J.; Brons, S.; Habermehl, D.; Rief, H.; Orschiedt, L.; Lindel, K.; Weber, K.J.; Debus, J.; Combs, S.E. Radiation-induced motility alterations in medulloblastoma cells. J. Radiat. Res. 2015, 56, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Iwakawa, M.; Ohno, T.; Imadome, K.; Nakawatari, M.; Sakai, M.; Tsujii, H.; Nakano, T.; Imai, T. Application of carbon-ion beams or gamma-rays on primary tumors does not change the expression profiles of metastatic tumors in an in vivo murine model. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Imadome, K.; Shoji, Y.; Isozaki, T.; Endo, S.; Yamada, S.; Imai, T. Carbon-Ion Irradiation Suppresses Migration and Invasiveness of Human Pancreatic Carcinoma Cells MIAPaCa-2 via Rac1 and RhoA Degradation. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Goetze, K.; Scholz, M.; Taucher-Scholz, G.; Mueller-Klieser, W. The impact of conventional and heavy ion irradiation on tumor cell migration in vitro. Int. J. Radiat. Biol. 2007, 83, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Stahler, C.; Roth, J.; Cordes, N.; Taucher-Scholz, G.; Mueller-Klieser, W. Impact of carbon ion irradiation on epidermal growth factor receptor signaling and glioma cell migration in comparison to conventional photon irradiation. Int. J. Radiat. Biol. 2013, 89, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, A.; Ueda, Y.; Yamada, S.; Harada, Y.; Shimada, H.; Hasegawa, M.; Tsujii, H.; Ochiai, T.; Yonemitsu, Y. Carbon-ion beam treatment induces systemic antitumor immunity against murine squamous cell carcinoma. Cancer 2010, 116, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, Y.; Iwakawa, M.; Seino, K.; Nakawatari, M.; Wada, H.; Kamijuku, H.; Nakamura, E.; Nakano, T.; Imai, T. Combining carbon ion radiotherapy and local injection of alpha-galactosylceramide-pulsed dendritic cells inhibits lung metastases in an in vivo murine model. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Dokic, I.; Mairani, A.; Niklas, M.; Zimmermann, F.; Chaudhri, N.; Krunic, D.; Tessonnier, T.; Ferrari, A.; Parodi, K.; Jakel, O.; et al. Next generation multi-scale biophysical characterization of high precision cancer particle radiotherapy using clinical proton, helium-, carbon- and oxygen ion beams. Oncotarget 2016, 7, 56676–56689. [Google Scholar] [CrossRef] [PubMed]
- Oike, T.; Niimi, A.; Okonogi, N.; Murata, K.; Matsumura, A.; Noda, S.E.; Kobayashi, D.; Iwanaga, M.; Tsuchida, K.; Kanai, T.; et al. Visualization of complex DNA double-strand breaks in a tumor treated with carbon ion radiotherapy. Sci. Rep. 2016, 6, 22275. [Google Scholar] [CrossRef] [PubMed]
- Masunaga, S.; Ando, K.; Uzawa, A.; Hirayama, R.; Furusawa, Y.; Koike, S.; Ono, K. The radiosensitivity of total and quiescent cell populations in solid tumors to 290 MeV/u carbon ion beam irradiation in vivo. Acta Oncol. 2008, 47, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Chiblak, S.; Tang, Z.; Campos, B.; Gal, Z.; Unterberg, A.; Debus, J.; Herold-Mende, C.; Abdollahi, A. Radiosensitivity of Patient-Derived Glioma Stem Cell 3-Dimensional Cultures to Photon, Proton, and Carbon Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Hartel, C.; Pignalosa, D.; Kraft-Weyrather, W.; Jiang, G.L.; Diaz-Carballo, D.; Durante, M. The Effect of X-Ray and Heavy Ions Radiations on Chemotherapy Refractory Tumor Cells. Front. Oncol. 2016, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Dokic, I.; Mairani, A.; Brons, S.; Schoell, B.; Jauch, A.; Krunic, D.; Debus, J.; Regnier-Vigouroux, A.; Weber, K.J. High resistance to X-rays and therapeutic carbon ions in glioblastoma cells bearing dysfunctional ATM associates with intrinsic chromosomal instability. Int. J. Radiat. Biol. 2015, 91, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Imadome, K.; Iwakawa, M.; Nojiri, K.; Tamaki, T.; Sakai, M.; Nakawatari, M.; Moritake, T.; Yanagisawa, M.; Nakamura, E.; Tsujii, H.; et al. Upregulation of stress-response genes with cell cycle arrest induced by carbon ion irradiation in multiple murine tumors models. Cancer Biol. Ther. 2008, 7, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Iwakawa, M.; Furusawa, Y.; Ishikawa, K.; Aoki, M.; Imadome, K.; Matsumoto, I.; Tsujii, H.; Ando, K.; Imai, T. Gene expression analysis in human malignant melanoma cell lines exposed to carbon beams. Int. J. Radiat. Biol. 2008, 84, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; You, A.; Juricko, J.; Engenhart-Cabillic, R.; An, H.X. Genetic alterations after carbon ion irradiation in human lung adenocarcinoma cells. Int. J. Oncol. 2011, 38, 161–168. [Google Scholar] [PubMed]
- Suetens, A.; Moreels, M.; Quintens, R.; Chiriotti, S.; Tabury, K.; Michaux, A.; Gregoire, V.; Baatout, S. Carbon ion irradiation of the human prostate cancer cell line PC3: A whole genome microarray study. Int. J. Oncol. 2014, 44, 1056–1072. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.H.; Park, S.; Peyton, M.; Girard, L.; Xie, Y.; Minna, J.D.; Story, M.D. Distinct transcriptome profiles identified in normal human bronchial epithelial cells after exposure to gamma-rays and different elemental particles of high Z and energy. BMC Genomics 2013, 14, 372. [Google Scholar] [CrossRef] [PubMed]
- Shinoto, M.; Yamada, S.; Terashima, K.; Yasuda, S.; Shioyama, Y.; Honda, H.; Kamada, T.; Tsujii, H.; Saisho, H. Carbon Ion Radiation Therapy With Concurrent Gemcitabine for Patients With Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Shiba, S.; Wakatsuki, M.; Kato, S.; Ohno, T.; Okonogi, N.; Karasawa, K.; Kiyohara, H.; Tsujii, H.; Nakano, T.; Kamada, T.; et al. Carbon-ion radiotherapy for locally advanced cervical cancer with bladder invasion. J. Radiat. Res. 2016, 57, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gao, J.; Hu, J.; Hu, W.; Guan, X.; Lu, R.; Lu, J.J. Phase I/II trial evaluating concurrent carbon-ion radiotherapy plus chemotherapy for salvage treatment of locally recurrent nasopharyngeal carcinoma. Chin. J. Cancer 2016, 35, 101. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, F.; Brons, S.; Haberer, T.; Debus, J.; Combs, S.E.; Weber, K.J. Comparison of the effects of photon versus carbon ion irradiation when combined with chemotherapy in vitro. Radiat. Oncol. 2013, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- El Shafie, R.A.; Habermehl, D.; Rieken, S.; Mairani, A.; Orschiedt, L.; Brons, S.; Haberer, T.; Weber, K.J.; Debus, J.; Combs, S.E. In vitro evaluation of photon and raster-scanned carbon ion radiotherapy in combination with gemcitabine in pancreatic cancer cell lines. J. Radiat. Res. 2013, 54 (Suppl. 1), i113–i119. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Zipp, L.; Rieken, S.; Habermehl, D.; Brons, S.; Winter, M.; Haberer, T.; Debus, J.; Weber, K.J. In vitro evaluation of photon and carbon ion radiotherapy in combination with chemotherapy in glioblastoma cells. Radiat. Oncol. 2012, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Bohl, J.; Elsasser, T.; Weber, K.J.; Schulz-Ertner, D.; Debus, J.; Weyrather, W.K. Radiobiological evaluation and correlation with the local effect model (LEM) of carbon ion radiation therapy and temozolomide in glioblastoma cell lines. Int. J. Radiat. Biol. 2009, 85, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Adeberg, S.; Baris, D.; Habermehl, D.; Rieken, S.; Brons, S.; Weber, K.J.; Roth, W.; Debus, J.; Combs, S.E. Evaluation of chemoradiotherapy with carbon ions and the influence of p53 mutational status in the colorectal carcinoma cell line HCT 116. Tumori 2014, 100, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Harrabi, S.; Combs, S.E.; Brons, S.; Haberer, T.; Debus, J.; Weber, K.J. Temozolomide in combination with carbon ion or photon irradiation in glioblastoma multiforme cell lines—Does scheduling matter? Int. J. Radiat. Biol. 2013, 89, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, H.; Shimada, H.; Yamada, S.; Yasuda, S.; Kamata, T.; Ando, K.; Tsujii, H.; Ochiai, T. Synergistic growth suppression induced in esophageal squamous cell carcinoma cells by combined treatment with docetaxel and heavy carbon-ion beam irradiation. Oncol. Rep. 2006, 15, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Harrabi, S.B.; Adeberg, S.; Winter, M.; Haberer, T.; Debus, J.; Weber, K.J. S-phase-specific radiosensitization by gemcitabine for therapeutic carbon ion exposure in vitro. J. Radiat. Res. 2016, 57, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Fujimoto, T.; Matsuba, S.; Fujita, T.; Sato, S.; Furukawa, T.; Hara, Y.; Mizushima, K.; Saraya, Y.; Tansho, R.; et al. Beam commissioning of a superconducting rotating-gantry for carbon-ion radiotherapy. Nucl. Instrum. Methods Phys. Res. 2016, 834, 71–80. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Tsujii, H. Particle radiation therapy using proton and heavier ion beams. J. Clin. Oncol. 2007, 25, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.M.; Pompos, A.; Timmerman, R.; Jiang, S.; Story, M.D.; Pistenmaa, D.; Choy, H. The Role of Hypofractionated Radiation Therapy with Photons, Protons, and Heavy Ions for Treating Extracranial Lesions. Front. Oncol. 2015, 5, 302. [Google Scholar] [CrossRef] [PubMed]
- Story, M.; Pompos, A.; Timmerman, R. On the value of carbon-ion therapy. Phys. Today 2016, 69. [Google Scholar] [CrossRef]
- Tsujii, H.; Mizoe, J.E.; Kamada, T.; Baba, M.; Kato, S.; Kato, H.; Tsuji, H.; Yamada, S.; Yasuda, S.; Ohno, T.; et al. Overview of clinical experiences on carbon ion radiotherapy at NIRS. Radiother. Oncol. 2004, 73 (Suppl. 2), S41–S49. [Google Scholar] [CrossRef]
- Kamada, T.; Tsujii, H.; Blakely, E.A.; Debus, J.; De Neve, W.; Durante, M.; Jakel, O.; Mayer, R.; Orecchia, R.; Potter, R.; et al. Carbon ion radiotherapy in Japan: An assessment of 20 years of clinical experience. Lancet Oncol. 2015, 16, e93–e100. [Google Scholar] [CrossRef]
- Kamada, T.; Tsujii, H.; Tsuji, H.; Yanagi, T.; Mizoe, J.E.; Miyamoto, T.; Kato, H.; Yamada, S.; Morita, S.; Yoshikawa, K.; et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J. Clin. Oncol. 2002, 20, 4466–4471. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, I.; Kagei, K.; Kamada, T.; Imai, R.; Sugahara, S.; Okada, T.; Tsuji, H.; Ito, H.; Tsujii, H. Carbon ion radiotherapy for unresectable retroperitoneal sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Matsunobu, A.; Imai, R.; Kamada, T.; Imaizumi, T.; Tsuji, H.; Tsujii, H.; Shioyama, Y.; Honda, H.; Tatezaki, S. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer 2012, 118, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, S.; Kamada, T.; Imai, R.; Tsuji, H.; Kameda, N.; Okada, T.; Tsujii, H.; Tatezaki, S. Carbon ion radiotherapy for localized primary sarcoma of the extremities: Results of a phase I/II trial. Radiother. Oncol. 2012, 105, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Imai, R.; Kamada, T.; Maruyama, K.; Tsuji, H.; Tsujii, H.; Shioyama, Y.; Honda, H.; Isu, K. Impact of carbon ion radiotherapy for primary spinal sarcoma. Cancer 2013, 119, 3496–3503. [Google Scholar] [CrossRef] [PubMed]
- DeLaney, T.F.; Liebsch, N.J.; Pedlow, F.X.; Adams, J.; Dean, S.; Yeap, B.Y.; McManus, P.; Rosenberg, A.E.; Nielsen, G.P.; Harmon, D.C.; et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Imai, R.; Kamada, T.; Araki, N. Carbon Ion Radiation Therapy for Unresectable Sacral Chordoma: An Analysis of 188 Cases. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Imai, R.; Kamada, T.; Tsuji, H.; Tsujii, H. Carbon Ion Radiation Therapy for Chondrosarcoma. Int. J. Radiat. Oncol. 2012, 84 (Suppl.), S139. [Google Scholar] [CrossRef]
- Outani, H.; Hamada, K.; Imura, Y.; Oshima, K.; Sotobori, T.; Demizu, Y.; Kakunaga, S.; Joyama, S.; Imai, R.; Okimoto, T.; et al. Comparison of clinical and functional outcome between surgical treatment and carbon ion radiotherapy for pelvic chondrosarcoma. Int. J. Clin. Oncol. 2016, 21, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Aoka, Y.; Kamada, T.; Kawana, M.; Yamada, Y.; Nishikawa, T.; Kasanuki, H.; Tsujii, H. Primary cardiac angiosarcoma treated with carbon-ion radiotherapy. Lancet Oncol. 2004, 5, 636–638. [Google Scholar] [CrossRef]
- Iwata, S.; Yonemoto, T.; Ishii, T.; Kumagai, K.; Imai, R.; Hagiwara, Y.; Kamada, T.; Tatezaki, S. Efficacy of carbon-ion radiotherapy and high-dose chemotherapy for patients with unresectable Ewing’s sarcoma family of tumors. Int. J. Clin. Oncol. 2013, 18, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.D.; Uhl, M.; Chaudhri, N.; Herfarth, K.K.; Debus, J.; Roeder, F. Carbon Ion irradiation in the treatment of grossly incomplete or unresectable malignant peripheral nerve sheaths tumors: Acute toxicity and preliminary outcome. Radiat. Oncol. 2015, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Blattmann, C.; Oertel, S.; Schulz-Ertner, D.; Rieken, S.; Haufe, S.; Ewerbeck, V.; Unterberg, A.; Karapanagiotou-Schenkel, I.; Combs, S.E.; Nikoghosyan, A.; et al. Non-randomized therapy trial to determine the safety and efficacy of heavy ion radiotherapy in patients with non-resectable osteosarcoma. BMC Cancer 2010, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Mizoe, J.E.; Hasegawa, A.; Jingu, K.; Takagi, R.; Bessyo, H.; Morikawa, T.; Tonoki, M.; Tsuji, H.; Kamada, T.; Tsujii, H.; et al. Results of carbon ion radiotherapy for head and neck cancer. Radiother. Oncol. 2012, 103, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Chaudhri, N.; Hassel, J.C.; Federspil, P.A.; Vanoni, V.; Debus, J.; Jensen, A.D. Raster-scanned intensity-controlled carbon ion therapy for mucosal melanoma of the paranasal sinus. Head Neck 2016, 38 (Suppl. 1), E1445–E1451. [Google Scholar] [CrossRef] [PubMed]
- Demizu, Y.; Fujii, O.; Terashima, K.; Mima, M.; Hashimoto, N.; Niwa, Y.; Akagi, T.; Daimon, T.; Murakami, M.; Fuwa, N. Particle therapy for mucosal melanoma of the head and neck. A single-institution retrospective comparison of proton and carbon ion therapy. Strahlentherapie und Onkologie 2014, 190, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Toyama, S.; Tsuji, H.; Mizoguchi, N.; Nomiya, T.; Kamada, T.; Tokumaru, S.; Mizota, A.; Ohnishi, Y.; Tsujii, H. Long-term results of carbon ion radiation therapy for locally advanced or unfavorably located choroidal melanoma: Usefulness of CT-based 2-port orthogonal therapy for reducing the incidence of neovascular glaucoma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.D.; Nikoghosyan, A.V.; Lossner, K.; Haberer, T.; Jakel, O.; Munter, M.W.; Debus, J. COSMIC: A Regimen of Intensity Modulated Radiation Therapy Plus Dose-Escalated, Raster-Scanned Carbon Ion Boost for Malignant Salivary Gland Tumors: Results of the Prospective Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Koto, M.; Hasegawa, A.; Takagi, R.; Sasahara, G.; Ikawa, H.; Mizoe, J.E.; Jingu, K.; Tsujii, H.; Kamada, T.; Okamoto, Y. Carbon ion radiotherapy for locally advanced squamous cell carcinoma of the external auditory canal and middle ear. Head Neck 2016, 38, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, N.; Tsuji, H.; Toyama, S.; Kamada, T.; Tsujii, H.; Nakayama, Y.; Mizota, A.; Ohnishi, Y. Carbon-ion radiotherapy for locally advanced primary or postoperative recurrent epithelial carcinoma of the lacrimal gland. Radiother. Oncol. 2015, 114, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, T.; Mizoe, J.E.; Hasegawa, A.; Takagi, R.; Bessho, H.; Onda, T.; Kamada, T.; Okamoto, Y.; Tsujii, H. Mucosal malignant melanoma of the head and neck treated by carbon ion radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Koto, M.; Hasegawa, A.; Takagi, R.; Sasahara, G.; Ikawa, H.; Mizoe, J.E.; Jingu, K.; Tsujii, H.; Kamada, T.; Okamoto, Y. Feasibility of carbon ion radiotherapy for locally advanced sinonasal adenocarcinoma. Radiother. Oncol. 2014, 113, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Koto, M.; Hasegawa, A.; Takagi, R.; Ikawa, H.; Naganawa, K.; Mizoe, J.E.; Jingu, K.; Tsujii, H.; Tsuji, H.; Kamada, T.; et al. Evaluation of the safety and efficacy of carbon ion radiotherapy for locally advanced adenoid cystic carcinoma of the tongue base. Head Neck 2016, 38 (Suppl. 1), E2122–E2126. [Google Scholar] [CrossRef] [PubMed]
- Debus, J.; Haberer, T.; Schulz-Ertner, D.; Jakel, O.; Wenz, F.; Enghardt, W.; Schlegel, W.; Kraft, G.; Wannenmacher, M. Carbon ion irradiation of skull base tumors at GSI. First clinical results and future perspectives. Strahlentherapie und Onkologie 2000, 176, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Haberer, T.; Jakel, O.; Thilmann, C.; Kramer, M.; Enghardt, W.; Kraft, G.; Wannenmacher, M.; Debus, J. Radiotherapy for chordomas and low-grade chondrosarcomas of the skull base with carbon ions. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 36–42. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Haberer, T.; Scholz, M.; Thilmann, C.; Wenz, F.; Jakel, O.; Kraft, G.; Wannenmacher, M.; Debus, J. Acute radiation-induced toxicity of heavy ion radiotherapy delivered with intensity modulated pencil beam scanning in patients with base of skull tumors. Radiother. Oncol. 2002, 64, 189–195. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Nikoghosyan, A.; Thilmann, C.; Haberer, T.; Jakel, O.; Karger, C.; Scholz, M.; Kraft, G.; Wannenmacher, M.; Debus, J. Carbon ion radiotherapy for chordomas and low-grade chondrosarcomas of the skull base. Results in 67 patients. Strahlentherapie und Onkologie 2003, 179, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Karger, C.P.; Feuerhake, A.; Nikoghosyan, A.; Combs, S.E.; Jakel, O.; Edler, L.; Scholz, M.; Debus, J. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Nikoghosyan, A.; Hof, H.; Didinger, B.; Combs, S.E.; Jakel, O.; Karger, C.P.; Edler, L.; Debus, J. Carbon ion radiotherapy of skull base chondrosarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Mattke, M.; Welzel, T.; Roeder, F.; Oelmann, J.; Habl, G.; Jensen, A.; Ellerbrock, M.; Jakel, O.; Haberer, T.; et al. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: First long-term results. Cancer 2014, 120, 3410–3417. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Mattke, M.; Welzel, T.; Oelmann, J.; Habl, G.; Jensen, A.D.; Ellerbrock, M.; Haberer, T.; Herfarth, K.K.; Debus, J. High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: First report of long-term results. Cancer 2014, 120, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Ares, C.; Hug, E.B.; Lomax, A.J.; Bolsi, A.; Timmermann, B.; Rutz, H.P.; Schuller, J.C.; Pedroni, E.; Goitein, G. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: First long-term report. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Jingu, K.; Tsujii, H.; Mizoe, J.E.; Hasegawa, A.; Bessho, H.; Takagi, R.; Morikawa, T.; Tonogi, M.; Tsuji, H.; Kamada, T.; et al. Carbon ion radiation therapy improves the prognosis of unresectable adult bone and soft-tissue sarcoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Nikoghosyan, A.V.; Rauch, G.; Munter, M.W.; Jensen, A.D.; Combs, S.E.; Kieser, M.; Debus, J. Randomised trial of proton vs. carbon ion radiation therapy in patients with low and intermediate grade chondrosarcoma of the skull base, clinical phase III study. BMC Cancer 2010, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Nikoghosyan, A.V.; Karapanagiotou-Schenkel, I.; Munter, M.W.; Jensen, A.D.; Combs, S.E.; Debus, J. Randomised trial of proton vs. carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-Study. BMC Cancer 2010, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Hu, J.; Guan, X.; Gao, J.; Lu, R.; Lu, J.J. Phase I/II Trial Evaluating Carbon Ion Radiotherapy for Salvaging Treatment of Locally Recurrent Nasopharyngeal Carcinoma. J. Cancer 2016, 7, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Akakura, K.; Tsujii, H.; Morita, S.; Tsuji, H.; Yagishita, T.; Isaka, S.; Ito, H.; Akaza, H.; Hata, M.; Fujime, M.; et al. Phase I/II clinical trials of carbon ion therapy for prostate cancer. Prostate 2004, 58, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Tsuji, H.; Kamada, T.; Yanagi, T.; Mizoe, J.E.; Kanai, T.; Morita, S.; Wakatsuki, M.; Shimazaki, J.; Tsujii, H. Carbon ion radiation therapy for prostate cancer: Results of a prospective phase II study. Radiother. Oncol. 2006, 81, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Tsuji, H.; Kamada, T.; Hirasawa, N.; Yanagi, T.; Mizoe, J.E.; Akakura, K.; Suzuki, H.; Shimazaki, J.; Tsujii, H. Risk factors of late rectal bleeding after carbon ion therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Tsuji, H.; Kamada, T.; Hirasawa, N.; Yanagi, T.; Mizoe, J.E.; Akakura, K.; Suzuki, H.; Shimazaki, J.; Nakano, T.; et al. Adverse effects of androgen deprivation therapy on persistent genitourinary complications after carbon ion radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Tsuji, H.; Ishikawa, H.; Okada, T.; Akakura, K.; Suzuki, H.; Harada, M.; Tsujii, H. Carbon ion radiotherapy for treatment of prostate cancer and subsequent outcomes after biochemical failure. Anticancer Res. 2010, 30, 5105–5111. [Google Scholar] [PubMed]
- Shimazaki, J.; Tsuji, H.; Ishikawa, H.; Kamada, T.; Harada, M.; Akakura, K.; Suzuki, H.; Ichikawa, T.; Tsujii, H. Biochemical failure after carbon ion radiotherapy for prostate cancer. Anticancer Res. 2012, 32, 3267–3273. [Google Scholar] [PubMed]
- Maruyama, K.; Tsuji, H.; Nomiya, T.; Katoh, H.; Ishikawa, H.; Kamada, T.; Wakatsuki, M.; Akakura, K.; Shimazaki, J.; Aoyama, H.; et al. Five-year quality of life assessment after carbon ion radiotherapy for prostate cancer. J. Radiat. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, T.; Tsuji, H.; Maruyama, K.; Toyama, S.; Suzuki, H.; Akakura, K.; Shimazaki, J.; Nemoto, K.; Kamada, T.; Tsujii, H. Phase I/II trial of definitive carbon ion radiotherapy for prostate cancer: Evaluation of shortening of treatment period to 3 weeks. Br. J. Cancer 2014, 110, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Tsuji, H.; Kamada, T.; Akakura, K.; Suzuki, H.; Shimazaki, J.; Tsujii, H. Carbon ion radiotherapy in advanced hypofractionated regimens for prostate cancer: From 20 to 16 fractions. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, T.; Tsuji, H.; Kawamura, H.; Ohno, T.; Toyama, S.; Shioyama, Y.; Nakayama, Y.; Nemoto, K.; Tsujii, H.; Kamada, T. A multi-institutional analysis of prospective studies of carbon ion radiotherapy for prostate cancer: A report from the Japan Carbon ion Radiation Oncology Study Group (J-CROS). Radiother. Oncol. 2016, 121, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar] [CrossRef]
- Kato, S.; Ohno, T.; Tsujii, H.; Nakano, T.; Mizoe, J.E.; Kamada, T.; Miyamoto, T.; Tsuji, H.; Kato, H.; Yamada, S.; et al. Dose escalation study of carbon ion radiotherapy for locally advanced carcinoma of the uterine cervix. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, M.; Kato, S.; Ohno, T.; Karasawa, K.; Ando, K.; Kiyohara, H.; Tsujii, H.; Nakano, T.; Kamada, T.; Shozu, M. Dose-escalation study of carbon ion radiotherapy for locally advanced squamous cell carcinoma of the uterine cervix (9902). Gynecol. Oncol. 2014, 132, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, M.; Kato, S.; Ohno, T.; Karasawa, K.; Kiyohara, H.; Tamaki, T.; Ando, K.; Tsujii, H.; Nakano, T.; Kamada, T.; et al. Clinical outcomes of carbon ion radiotherapy for locally advanced adenocarcinoma of the uterine cervix in phase 1/2 clinical trial (protocol 9704). Cancer 2014, 120, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, M.; Kato, S.; Ohno, T.; Kiyohara, H.; Karasawa, K.; Tamaki, T.; Ando, K.; Irie, D.; Shiba, S.; Tsujii, H.; et al. Difference in distant failure site between locally advanced squamous cell carcinoma and adenocarcinoma of the uterine cervix after C-ion RT. J. Radiat. Res. 2015, 56, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, M.; Kato, S.; Kiyohara, H.; Ohno, T.; Karasawa, K.; Tamaki, T.; Ando, K.; Tsujii, H.; Nakano, T.; Kamada, T.; et al. Clinical trial of prophylactic extended-field carbon-ion radiotherapy for locally advanced uterine cervical cancer (protocol 0508). PLoS ONE 2015, 10, e0127587. [Google Scholar] [CrossRef] [PubMed]
- Bujold, A.; Massey, C.A.; Kim, J.J.; Brierley, J.; Cho, C.; Wong, R.K.; Dinniwell, R.E.; Kassam, Z.; Ringash, J.; Cummings, B.; et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J. Clin. Oncol. 2013, 31, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Tsujii, H.; Miyamoto, T.; Mizoe, J.E.; Kamada, T.; Tsuji, H.; Yamada, S.; Kandatsu, S.; Yoshikawa, K.; Obata, T.; et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Imada, H.; Kato, H.; Yasuda, S.; Yamada, S.; Yanagi, T.; Kishimoto, R.; Kandatsu, S.; Mizoe, J.E.; Kamada, T.; Yokosuka, O.; et al. Comparison of efficacy and toxicity of short-course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity to the porta hepatis. Radiother. Oncol. 2010, 96, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S. Hepatocellular Carcinoma. In Carbon-Ion Radiotherapy: Principles, Practices and Treatment Planning; Tsujii, H., Kamada, T., Shirai, T., Noda, K., Tsuji, H., Karasawa, K., Eds.; Springer Japan: Tokyo, Jappan, 2014; pp. 213–218. [Google Scholar]
- Habermehl, D.; Debus, J.; Ganten, T.; Ganten, M.K.; Bauer, J.; Brecht, I.C.; Brons, S.; Haberer, T.; Haertig, M.; Jakel, O.; et al. Hypofractionated carbon ion therapy delivered with scanned ion beams for patients with hepatocellular carcinoma—Feasibility and clinical response. Radiat. Oncol. 2013, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Habermehl, D.; Ganten, T.; Schmidt, J.; Edler, L.; Burkholder, I.; Jakel, O.; Haberer, T.; Debus, J. Phase I study evaluating the treatment of patients with hepatocellular carcinoma (HCC) with carbon ion radiotherapy: The PROMETHEUS-01 trial. BMC Cancer 2011, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Saitoh, J.; Kobayashi, D.; Shibuya, K.; Koyama, Y.; Shimada, H.; Shirai, K.; Ohno, T.; Nakano, T. Dosimetric comparison of carbon ion radiotherapy and stereotactic body radiotherapy with photon beams for the treatment of hepatocellular carcinoma. Radiat. Oncol. 2015, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.X.; Fu, S.; Zhang, Q.; Guo, X.M. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: A systematic review and meta-analysis. Radiother. Oncol. 2015, 114, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.F.; Smalley, S.R.; Jewell, W.; Paradelo, J.C.; Reymond, R.D.; Hassanein, R.E.; Evans, R.G. Patterns of failure after curative resection of pancreatic carcinoma. Cancer 1990, 66, 56–61. [Google Scholar] [CrossRef]
- Shinoto, M.; Shioyama, Y.; Matsunobu, A.; Okamoto, K.; Suefuji, H.; Toyama, S.; Honda, H.; Kudo, S. Dosimetric analysis of upper gastrointestinal ulcer after carbon-ion radiotherapy for pancreatic cancer. Radiother. Oncol. 2016, 120, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Shinoto, M.; Yamada, S.; Yasuda, S.; Imada, H.; Shioyama, Y.; Honda, H.; Kamada, T.; Tsujii, H.; Saisho, H. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer 2013, 119, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Habermehl, D.; Kieser, M.; Dreher, C.; Werner, J.; Haselmann, R.; Jakel, O.; Jager, D.; Buchler, M.W.; Debus, J. Phase I study evaluating the treatment of patients with locally advanced pancreatic cancer with carbon ion radiotherapy: The PHOENIX-01 trial. BMC Cancer 2013, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Mizoe, J.E.; Tsujii, H.; Hasegawa, A.; Yanagi, T.; Takagi, R.; Kamada, T.; Tsuji, H.; Takakura, K. Phase I/II clinical trial of carbon ion radiotherapy for malignant gliomas: Combined X-ray radiotherapy, chemotherapy, and carbon ion radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Bruckner, T.; Mizoe, J.E.; Kamada, T.; Tsujii, H.; Kieser, M.; Debus, J. Comparison of carbon ion radiotherapy to photon radiation alone or in combination with temozolomide in patients with high-grade gliomas: Explorative hypothesis-generating retrospective analysis. Radiother. Oncol. 2013, 108, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Kieser, M.; Rieken, S.; Habermehl, D.; Jakel, O.; Haberer, T.; Nikoghosyan, A.; Haselmann, R.; Unterberg, A.; Wick, W.; et al. Randomized phase II study evaluating a carbon ion boost applied after combined radiochemotherapy with temozolomide versus a proton boost after radiochemotherapy with temozolomide in patients with primary glioblastoma: The CLEOPATRA trial. BMC Cancer 2010, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Nikoghosyan, A.; Jaekel, O.; Karger, C.P.; Haberer, T.; Munter, M.W.; Huber, P.E.; Debus, J.; Schulz-Ertner, D. Carbon ion radiotherapy for pediatric patients and young adults treated for tumors of the skull base. Cancer 2009, 115, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Kessel, K.A.; Herfarth, K.; Jensen, A.; Oertel, S.; Blattmann, C.; Ecker, S.; Hoess, A.; Martin, E.; Witt, O.; et al. Treatment of pediatric patients and young adults with particle therapy at the Heidelberg Ion Therapy Center (HIT): Establishment of workflow and initial clinical data. Radiat. Oncol. 2012, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.D.; Poulakis, M.; Nikoghosyan, A.V.; Chaudhri, N.; Uhl, M.; Munter, M.W.; Herfarth, K.K.; Debus, J. Re-irradiation of adenoid cystic carcinoma: Analysis and evaluation of outcome in 52 consecutive patients treated with raster-scanned carbon ion therapy. Radiother. Oncol. 2015, 114, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Kamada, T.; Ebner, D.K.; Shinoto, M.; Terashima, K.; Isozaki, Y.; Yasuda, S.; Makishima, H.; Tsuji, H.; Tsujii, H.; et al. Carbon-Ion Radiation Therapy for Pelvic Recurrence of Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Habermehl, D.; Wagner, M.; Ellerbrock, M.; Buchler, M.W.; Jakel, O.; Debus, J.; Combs, S.E. Reirradiation Using Carbon Ions in Patients with Locally Recurrent Rectal Cancer at HIT: First Results. Ann. Surg. Oncol. 2015, 22, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Kieser, M.; Habermehl, D.; Weitz, J.; Jager, D.; Fossati, P.; Orrechia, R.; Engenhart-Cabillic, R.; Potter, R.; Dosanjh, M.; et al. Phase I/II trial evaluating carbon ion radiotherapy for the treatment of recurrent rectal cancer: The PANDORA-01 trial. BMC Cancer 2012, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Welzel, T.; Jensen, A.; Ellerbrock, M.; Haberer, T.; Jakel, O.; Herfarth, K.; Debus, J. Carbon ion beam treatment in patients with primary and recurrent sacrococcygeal chordoma. Strahlentherapie und Onkologie 2015, 191, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Welzel, T.; Oelmann, J.; Habl, G.; Hauswald, H.; Jensen, A.; Ellerbrock, M.; Debus, J.; Herfarth, K. Active raster scanning with carbon ions: Reirradiation in patients with recurrent skull base chordomas and chondrosarcomas. Strahlentherapie und Onkologie 2014, 190, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, W.; Nakajima, M.; Yamamoto, N.; Yamada, S.; Yamashita, H.; Nakagawa, K.; Tsuji, H.; Kamada, T. Carbon ion radiotherapy for oligo-recurrent lung metastases from colorectal cancer: A feasibility study. Radiat. Oncol. 2014, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Morikawa, S.; Kato, S.; Kajiyama, H.; Nawa, A.; Kikkawa, F. Carbon ion radiotherapy for recurrent malignant transformation from mature cystic teratoma of the ovary. J. Obstet. Gynaecol. Res. 2012, 38, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Kalbe, A.; Nikoghosyan, A.; Ackermann, B.; Jakel, O.; Haberer, T.; Debus, J. Carbon ion radiotherapy performed as re-irradiation using active beam delivery in patients with tumors of the brain, skull base and sacral region. Radiother. Oncol. 2011, 98, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Burkholder, I.; Edler, L.; Rieken, S.; Habermehl, D.; Jakel, O.; Haberer, T.; Haselmann, R.; Unterberg, A.; Wick, W.; et al. Randomised phase I/II study to evaluate carbon ion radiotherapy versus fractionated stereotactic radiotherapy in patients with recurrent or progressive gliomas: The CINDERELLA trial. BMC Cancer 2010, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Jakel, O.; Land, B.; Combs, S.E.; Schulz-Ertner, D.; Debus, J. On the cost-effectiveness of Carbon ion radiation therapy for skull base chordoma. Radiother. Oncol. 2007, 83, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, A.; Ohno, T.; Yamada, S.; Sakurai, H.; Nakano, T. Cost-effectiveness of carbon ion radiation therapy for locally recurrent rectal cancer. Cancer Sci. 2010, 101, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Grutters, J.P.; Pijls-Johannesma, M.; Ruysscher, D.D.; Peeters, A.; Reimoser, S.; Severens, J.L.; Lambin, P.; Joore, M.A. The cost-effectiveness of particle therapy in non-small cell lung cancer: Exploring decision uncertainty and areas for future research. Cancer Treat. Rev. 2010, 36, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Miyamoto, T.; Nakajima, M.; Karube, M.; Hayashi, K.; Tsuji, H.; Tsujii, H.; Kamada, T.; Fujisawa, T. A Dose Escalation Clinical Trial of Single-Fraction Carbon Ion Radiotherapy for Peripheral Stage I Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Pignalosa, D.; Lee, R.; Hartel, C.; Sommer, S.; Nikoghosyan, A.; Debus, J.; Ritter, S.; Durante, M. Chromosome inversions in lymphocytes of prostate cancer patients treated with X-rays and carbon ions. Radiother. Oncol. 2013, 109, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Blyth, B.J.; Kakinuma, S.; Sunaoshi, M.; Amasaki, Y.; Hirano-Sakairi, S.; Ogawa, K.; Shirakami, A.; Shang, Y.; Tsuruoka, C.; Nishimura, M.; et al. Genetic Analysis of T Cell Lymphomas in Carbon Ion-Irradiated Mice Reveals Frequent Interstitial Chromosome Deletions: Implications for Second Cancer Induction in Normal Tissues during Carbon Ion Radiotherapy. PLoS ONE 2015, 10, e0130666. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U. Modeling the risk of secondary malignancies after radiotherapy. Genes 2011, 2, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Eley, J.G.; Friedrich, T.; Homann, K.L.; Howell, R.M.; Scholz, M.; Durante, M.; Newhauser, W.D. Comparative Risk Predictions of Second Cancers After Carbon-Ion Therapy Versus Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ghosh, P.; Magpayo, N.; Testa, M.; Tang, S.; Gheorghiu, L.; Biggs, P.; Paganetti, H.; Efstathiou, J.A.; Lu, H.M.; et al. Lung cancer cell line screen links fanconi anemia/BRCA pathway defects to increased relative biological effectiveness of proton radiation. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Oonishi, K.; Cui, X.; Hirakawa, H.; Fujimori, A.; Kamijo, T.; Yamada, S.; Yokosuka, O.; Kamada, T. Different effects of carbon ion beams and X-rays on clonogenic survival and DNA repair in human pancreatic cancer stem-like cells. Radiother. Oncol. 2012, 105, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Oonishi, K.; Tsujii, H.; Yasuda, T.; Matsumoto, Y.; Furusawa, Y.; Akashi, M.; Kamada, T.; Okayasu, R. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res. 2011, 71, 3676–3687. [Google Scholar] [CrossRef] [PubMed]
- Sai, S.; Wakai, T.; Vares, G.; Yamada, S.; Kamijo, T.; Kamada, T.; Shirai, T. Combination of carbon ion beam and gemcitabine causes irreparable DNA damage and death of radioresistant pancreatic cancer stem-like cells in vitro and in vivo. Oncotarget 2015, 6, 5517–5535. [Google Scholar] [CrossRef] [PubMed]
- Yajima, H.; Fujisawa, H.; Nakajima, N.I.; Hirakawa, H.; Jeggo, P.A.; Okayasu, R.; Fujimori, A. The complexity of DNA double strand breaks is a critical factor enhancing end-resection. DNA Repair 2013, 12, 936–946. [Google Scholar] [CrossRef] [PubMed]
| Central Location | Trial Name (Group) | Cancer Histology/Site | Trial Design | Trial Arms | Primary End-Point |
|---|---|---|---|---|---|
| National Institute of Radiological Sciences, Chiba, Japan | JCROS-1502 (Multi-institutional) | Pancreatic cancer, T4M0 | Phase II | Single arm: carbon ion therapy (55.2 GyE/12 fractions) and gemcitabine | 2-year overall survival |
| JCROS-1509 (Multi-institutional) | High-risk prostate cancer | Phase II | Single arm: carbon ion therapy (51.6 GyE/12 fractions) and hormone therapy | 5-year biochemical relapse-free survival | |
| Locally advanced cervical adenocarcinoma | Phase I/II | Single arm: carbon ion therapy (20 fractions) and concurrent cisplatin | Acute toxicity and response rate | ||
| Esophageal squamous cell carcinoma, stage II/III | Phase I/II | Single arm: preoperative carbon ion therapy (8 fractions) and concurrent cisplatin and 5-FU, followed by surgery | Acute toxicity and response rate | ||
| Gunma University Heavy Ion Medical Center, Gunma, Japan | JCROS-1505 (Multi-institutional) | Hepatocellular carcinoma, inoperable | Phase II | Single arm: carbon ion therapy (60 GyE/4 fractions or 60 GyE/12 fractions if near digestive tract) | 3-year overall survival |
| GUNMA-1102 | Primary malignant bone and soft tissue tumor in the childhood | Phase I | Single arm: carbon ion therapy | Acute complication rate | |
| GUNMA-0801 | Rectal cancer, post-operative pelvic recurrence | Phase I/II | Single arm: carbon ion therapy in 16 fractions | 3-year local control | |
| GUNMA-0904 | Primary malignant bone and soft tissue tumor | Phase I/II | Single arm: carbon ion therapy in 16 fractions | 2-year local control | |
| GUNMA-0703 | Hepatocellular carcinoma | Single arm: carbon ion therapy in 4 fractions | 3-year local control | ||
| Heavy Ion Medical Accelerator (HIMAT), Saga, Japan | JCROS-1501 (Multi-institutional) | Lung cancer, inoperable, stage I | Phase II | Single arm: carbon ion therapy (60 GyE/4 fractions) | 3-year overall survival |
| HIMAT-1351 | Rectal cancer, local recurrence after surgery | Phase II | Single arm: carbon ion therapy | 3-year local control | |
| HIMAT-1341 | Bone and soft tissue sarcoma, inoperable | Phase II | Single arm: carbon ion therapy | 2-year local control | |
| HIMAT-1342 | Chordoma, inoperable | Phase II | Single arm: carbon ion therapy | 2-year local control | |
| HIMAT-1326 | Pancreatic cancer, locally advanced | Phase II | Single arm: carbon ion therapy with concurrent chemotherapy | 2-year overall survival | |
| Hepatocellular carcinoma (>3 cm) | Phase II | Single arm: carbon ion therapy in 4 fractions | 3-year overall survival and cause-specific survival | ||
| Hepatocellular carcinoma (≤3 cm) | Phase II | Single arm: carbon ion therapy in 2 fractions | 3-year local control | ||
| Non-small cell lung cancer, central, stage I | Phase II | Single arm: Carbon ion therapy (12 fractions) | 3-year local control | ||
| Non-small cell lung cancer, peripheral, stage I | Phase II | Single arm: carbon ion therapy (4 fractions) | 3-year local control | ||
| Ion Beam Radiation Oncology Center in Kanagawa (iROCK), Kanagawa, Japan | iROCK-1601LI and iROCK-1604LI | Hepatocellular carcinoma | Phase II | Single arm: carbon ion therapy in 2 or 4 fractions | 3-year local control |
| iROCK-1504LU | Non-small cell lung cancer, small, peripheral, stage IA | Non-randomized, phase II | Arm 1: carbon ion therapy Arm 2: surgical resection | 5-year overall survival | |
| iROCK-1605PA | Pancreatic cancer, locally advanced | Phase II | Single arm: carbon ion therapy (12 fractions) and gemcitabine | 3-year overall survival | |
| iROCK-1603HN | Mucosal malignant melanoma of the head and neck | Phase II | Single arm: carbon ion therapy (16 fractions) combined with anti-tumor agents | 3-year overall survival | |
| iROCK-1501PR | Prostate cancer, T1c-T3N0M0 | Phase II | Single arm: carbon ion therapy (12 fractions) | 5-year biochemical relapse-free survival | |
| Prostate cancer, T1b-T3N0M0 | Phase II | Single arm: carbon ion therapy (12 fractions) with hormone therapy | 5-year biochemical relapse-free survival | ||
| Non-squamous cell carcinoma of head and neck (no melanoma nor sarcoma) | Phase II | Single arm: carbon ion therapy (16 fractions) | 3-year local control | ||
| Heidelberg University, Germany | HIT-1 | Chordoma of the skull base | Randomized, phase III | Standard arm: proton therapy Experimental arm: carbon ion therapy | Local-progression-free survival |
| CSP12C | Low and intermediate grade chondrosarcoma of the skull base | Randomized, phase III | Standard arm: proton therapy Experimental arm: carbon ion therapy | Local-progression-free survival | |
| ISAC | Sacrococcygeal chordoma | Randomized | Standard arm: proton therapy Experimental arm: carbon ion therapy | Toxicity | |
| ACCEPT | Adenoid cystic carcinoma | Phase I/II | Single arm: cetuximab and IMRT plus carbon ion boost | Toxicity | |
| Shanghai Heavy Ion Center | Hepatocellular carcinoma | Non-randomized, phase II | Single arm: Carbon ion therapy with GM-CSF | Progression-free survival | |
| Hepatocellular carcinoma | Phase I | Carbon ion therapy or carbon plus proton therapy depending on proximity to bowel | Toxicity | ||
| Localized prostate cancer | Phase I/II | Single arm: carbon ion therapy | Toxicity | ||
| Oligo-metastatic prostate cancer | Phase II | Single arm: carbon ion therapy to prostate plus chemotherapy or hormonal therapy | Time to PSA relapse | ||
| Locally recurrent nasopharyngeal carcinoma | Non-randomized, phase I/II | Single arm: carbon ion therapy, 2.5 GyE or 3 GyE per fraction | Toxicity | ||
| National Center of Oncological Hadrontherapy (CNAO), Italy | High risk prostate cancer | Non-randomized, phase II | Single arm: carbon ion boost followed by conventional photon RT | Toxicity | |
| SACRO | Localized sacral chordoma | Randomized | Standard arm: surgery Experimental arm: radiotherapy including option for carbon ion therapy | Relapse-free survival |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad, O.; Sishc, B.J.; Saha, J.; Pompos, A.; Rahimi, A.; Story, M.D.; Davis, A.J.; Kim, D.W.N. Carbon Ion Radiotherapy: A Review of Clinical Experiences and Preclinical Research, with an Emphasis on DNA Damage/Repair. Cancers 2017, 9, 66. https://doi.org/10.3390/cancers9060066
Mohamad O, Sishc BJ, Saha J, Pompos A, Rahimi A, Story MD, Davis AJ, Kim DWN. Carbon Ion Radiotherapy: A Review of Clinical Experiences and Preclinical Research, with an Emphasis on DNA Damage/Repair. Cancers. 2017; 9(6):66. https://doi.org/10.3390/cancers9060066
Chicago/Turabian StyleMohamad, Osama, Brock J. Sishc, Janapriya Saha, Arnold Pompos, Asal Rahimi, Michael D. Story, Anthony J. Davis, and D.W. Nathan Kim. 2017. "Carbon Ion Radiotherapy: A Review of Clinical Experiences and Preclinical Research, with an Emphasis on DNA Damage/Repair" Cancers 9, no. 6: 66. https://doi.org/10.3390/cancers9060066





