Selection of Nucleic Acid Aptamers Targeting Tumor Cell-Surface Protein Biomarkers
Abstract
:1. Introduction
2. Generalities on Aptamers
3. Protein- and Cell-Based SELEX Processes
3.1. Aptamers Selected by Protein-Based SELEX
3.2. Aptamers Selected by Cell-Based SELEX
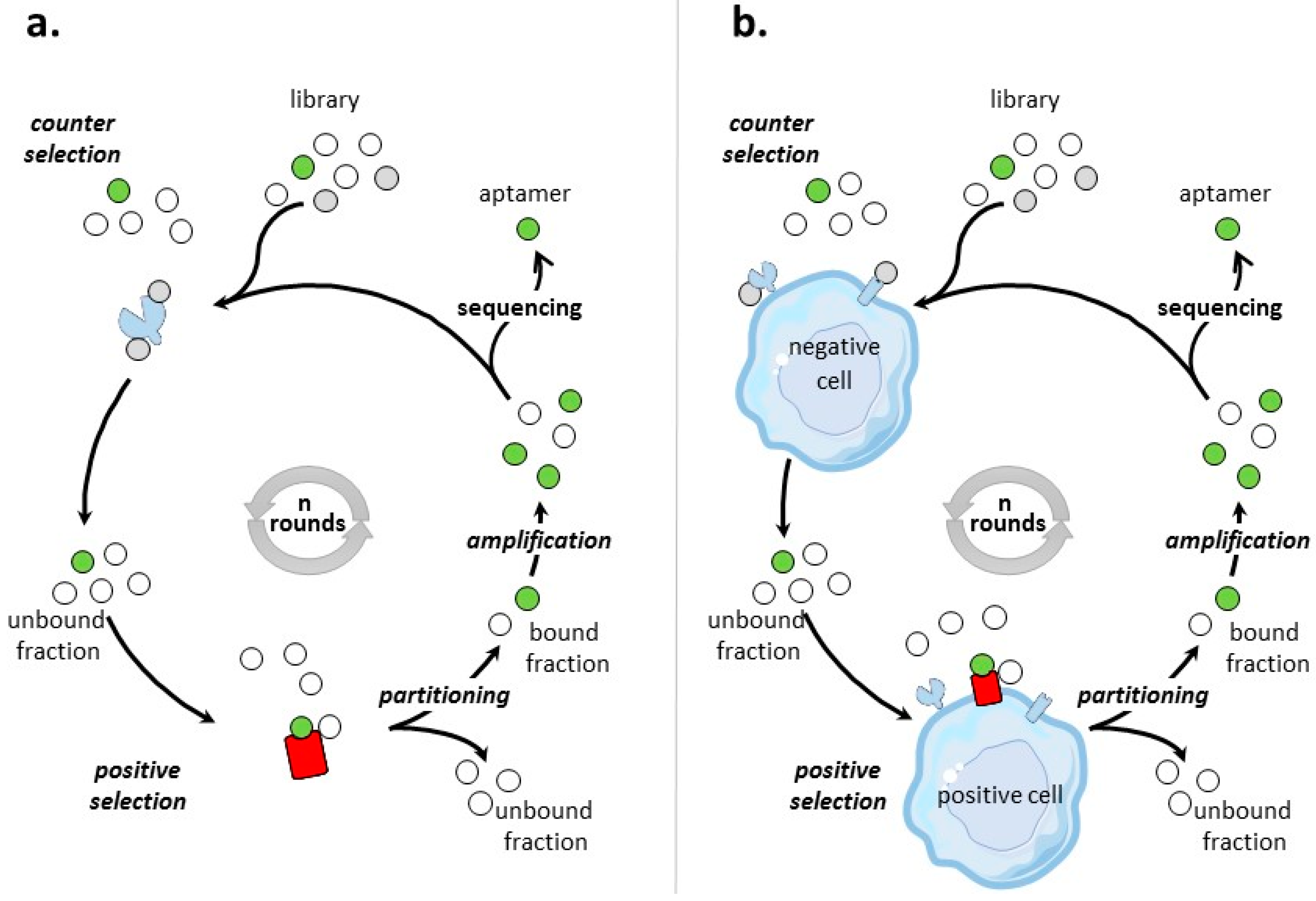
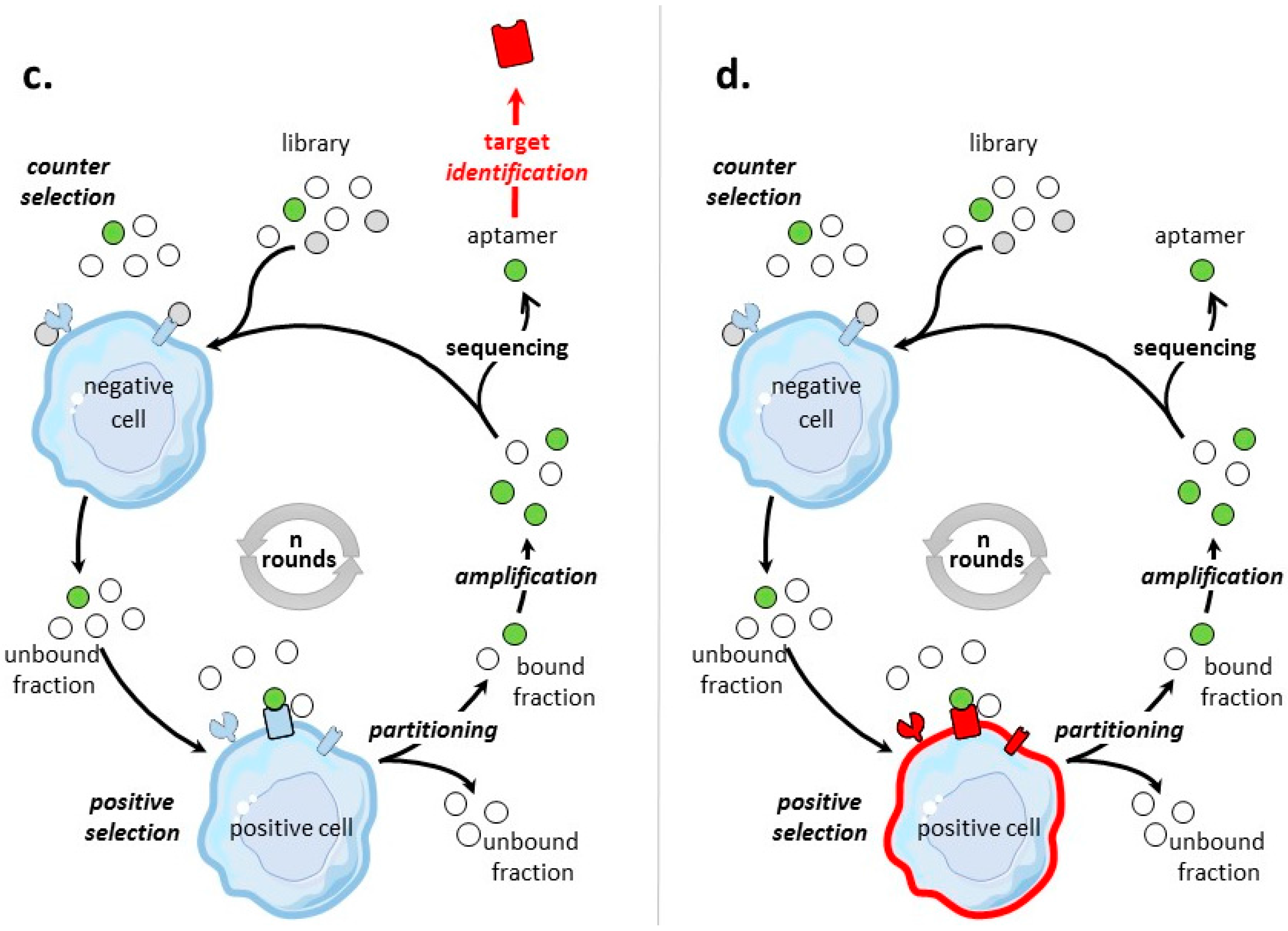
3.2.1. Cell-Based SELEX to Identify Aptamers to Tumor Cell-Surface Protein Biomarkers
Pre-Identified Tumor Cell-Surface Biomarkers
Post-Identified Tumor Cell-Surface Biomarkers—Discovery of Tumor Biomarkers
Undetermined Targets
3.2.2. Advantages and Limitations of Cell-Based SELEX
4. Applications of Aptamers in Oncology
4.1. Aptamers as Detection and Imaging Reagent
4.2. Aptamers in Therapy
5. Aptamers in Clinical Trials
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.L.; Joyce, G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 1990, 344, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, J.V.; Orlova, G.V.; Frolov, A.S.; Gelfand, M.S.; Ponomarenko, M.P. SELEX_DB: A database on in vitro selected oligomers adapted for recognizing natural sites and for analyzing both SNPs and site-directed mutagenesis data. Nucleic Acids Res. 2002, 30, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Aptamer Database Apta-Index™. Available online: http://www.aptagen.com/aptamer-index/aptamer-list.aspx (accessed on 1 June 2017).
- Cruz-Toledo, J.; McKeague, M.; Zhang, X.; Giamberardino, A.; McConnell, E.; Francis, T.; DeRosa, M.C.; Dumontier, M. Aptamer base: A collaborative knowledge base to describe aptamers and SELEX experiments. Database 2012, 2012, bas006. [Google Scholar] [CrossRef] [PubMed]
- Lao, Y.-H.; Phua, K.K.L.; Leong, K.W. Aptamer nanomedicine for cancer therapeutics: Barriers and potential for translation. ACS Nano 2015, 9, 2235–2254. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, N.; Seeboeck, R.; Hofmann, E.; Eger, A. ErbB family signalling: A paradigm for oncogene addiction and personalized oncology. Cancers 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Lemech, C.; Arkenau, H.-T. Biomarkers in advanced colorectal cancer: Challenges in translating clinical research into practice. Cancers 2011, 3, 1844–1860. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, G.; Miller, M.L.; Aksoy, B.A.; Senbabaoglu, Y.; Schultz, N.; Sander, C. Emerging landscape of oncogenic signatures across human cancers. Nat. Genet. 2013, 45, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Crescenzi, E.; Gramanzini, M.; Fedele, M.; Zannetti, A.; Cerchia, L. Aptamer-mediated impairment of EGFR-integrin αvβ3 complex inhibits vasculogenic mimicry and growth of triple-negative breast cancers. Sci. Rep. 2017, 7, 46659. [Google Scholar] [CrossRef] [PubMed]
- Szász, A.M.; Győrffy, B.; Marko-Varga, G. Cancer heterogeneity determined by functional proteomics. Semin. Cell Dev. Biol. 2017, 64, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Monaco, I.; Camorani, S.; Colecchia, D.; LOCATELLI, E.; Calandro, P.; Oudin, A.; Niclou, S.; Arra, C.; Chiariello, M.; Cerchia, L.; et al. Aptamer functionalization of nanosystems for glioblastoma targeting through the blood-brain-barrier. J. Med. Chem. 2017, 60, 4510–4516. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wilson, G.; Hebbard, L.; Duan, W.; Liddle, C.; George, J.; Qiao, L. Aptamers: A promising chemical antibody for cancer therapy. Oncotarget 2016, 7, 13446–13463. [Google Scholar] [PubMed]
- Xiang, D.; Zheng, C.; Zhou, S.-F.; Qiao, S.; Tran, P.H.-L.; Pu, C.; Li, Y.; Kong, L.; Kouzani, A.Z.; Lin, J.; et al. Superior performance of aptamer in tumor penetration over antibody: Implication of aptamer-based theranostics in solid tumors. Theranostics 2015, 5, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Avci-Adali, M.; Paul, A.; Wilhelm, N.; Ziemer, G.; Wendel, H.P. Upgrading SELEX technology by using lambda exonuclease digestion for Single-Stranded DNA generation. Molecules 2009, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Avci-Adali, M.; Ziemer, G.; Wendel, H.P. Streptavidin-coated magnetic beads for DNA strand separation implicate a multitude of problems during cell-SELEX. Oligonucleotides 2009, 19, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Eulberg, D.; Klussmann, S. Spiegelmers: Biostable aptamers. ChemBioChem 2003, 4, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Padilla, R.; Sousa, R. Efficient synthesis of nucleic acids heavily modified with non-canonical ribose 2′-groups using a mutant T7 RNA polymerase (RNAP). Nucleic Acids Res. 1999, 27, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, P.E.; Lewis, S.D.; Silva, R.F.; Preiss, J.R.; Horwitz, L.R.; Pendergrast, P.S.; McCauley, T.G.; Kurz, J.C.; Epstein, D.M.; Wilson, C.; et al. Direct in vitro selection of a 2′-O-methyl aptamer to VEGF. Chem. Biol. 2005, 12, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, V.B.; Taylor, A.I.; Cozens, C.; Abramov, M.; Renders, M.; Zhang, S.; Chaput, J.C.; Wengel, J.; Peak-Chew, S.-Y.; McLaughlin, S.H.; et al. Synthetic genetic polymers capable of heredity and evolution. Science 2012, 336, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Obika, S. In vitro selection of BNA (LNA) aptamers. Artif. DNA PNA XNA 2013, 4, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, Y.; Irisawa, Y.; Ozaki, H.; Obika, S.; Kuwahara, M. 2′,4′-BNA/LNA aptamers: CE-SELEX using a DNA-based library of full-length 2′-O,4′-C-methylene-bridged/linked bicyclic ribonucleotides. Bioorg. Med. Chem. Lett. 2013, 23, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Veedu, R.N.; Wengel, J. Locked nucleic acid nucleoside triphosphates and polymerases: On the way towards evolution of LNAaptamers. Mol. Biosyst. 2009, 5, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Shigdar, S.; Macdonald, J.; O’Connor, M.; Wang, T.; Xiang, D.; Al.Shamaileh, H.; Qiao, L.; Wei, M.; Zhou, S.-F.; Zhu, Y.; et al. Aptamers as theranostic agents: Modifications, serum stability and functionalisation. Sensors 2013, 13, 13624–13637. [Google Scholar] [CrossRef] [PubMed]
- Anosova, I.; Kowal, E.A.; Dunn, M.R.; Chaput, J.C.; Van Horn, W.D.; Egli, M. The structural diversity of artificial genetic polymers. Nucleic Acids Res. 2016, 44, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, V.B.; Holliger, P. Towards XNA nanotechnology: New materials from synthetic genetic polymers. Trends Biotechnol. 2014, 32, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Dalby, A.B.; Eaton, B.E.; Fitzwater, T.; et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 2010, 5, e15004. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.E. The joys of in vitro selection: Chemically dressing oligonucleotides to satiate protein targets. Curr. Opin. Chem. Biol. 1997, 1, 10–16. [Google Scholar] [CrossRef]
- Sun, H.; Zu, Y. A Highlight of recent advances in aptamer technology and its application. Molecules 2015, 20, 11959–11980. [Google Scholar] [CrossRef] [PubMed]
- Andreola, M.-L.; Calmels, C.; Michel, J.; Toulmé, J.-J.; Litvak, S. Towards the selection of phosphorothioate aptamers. Eur. J. Biochem. 2000, 267, 5032–5040. [Google Scholar] [CrossRef] [PubMed]
- Lato, S.M.; Ozerova, N.D.S.; He, K.; Sergueeva, Z.; Shaw, B.R.; Burke, D.H. Boron-containing aptamers to ATP. Nucleic Acids Res. 2002, 30, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Cload, S.T. SELEX with modified nucleotides. Curr. Opin. Chem. Biol. 2008, 12, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Vater, A.; Klussmann, S. Turning mirror-image oligonucleotides into drugs: The evolution of Spiegelmer® therapeutics. Drug Discov. Today 2015, 20, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Cheng, J.; Teply, B.A.; Sherifi, I.; Jon, S.; Kantoff, P.W.; Richie, J.P.; Langer, R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 6315–6320. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-H.; An, S.; Yu, M.K.; Kwon, H.-K.; Im, S.-H.; Jon, S. Targeted chemoimmunotherapy using drug-loaded aptamer–dendrimer bioconjugates. J. Control. Release 2011, 155, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.E.; Lee, K.H.; Park, Y.S.; Oh, D.-K.; Oh, S.; Kim, K.-S.; Kim, D.-E. RNA aptamer-conjugated liposome as an efficient anticancer drug delivery vehicle targeting cancer cells in vivo. J. Control. Release 2014, 196, 234–242. [Google Scholar]
- Willis, M.C.; Collins, B.; Zhang, T.; Green, L.S.; Sebesta, D.P.; Bell, C.; Kellogg, E.; Gill, S.C.; Magallanez, A.; Knauer, S.; et al. Liposome-anchored vascular endothelial growth factor aptamers. Bioconjug. Chem. 1998, 9, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Jon, S.; Khademhosseini, A.; Tran, T.-N.T.; LaVan, D.A.; Langer, R. Nanoparticle-aptamer bioconjugates. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.M.; Lewis, S.D.; Kurz, M.; Boomer, R.M.; Thompson, K.M.; Wilson, C.; McCauley, T.G. Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm. Res. 2004, 21, 2234–2246. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G. Aptamers against cell surface receptors: Selection, modification and application. Curr. Med. Chem. 2011, 18, 4107–4116. [Google Scholar] [CrossRef] [PubMed]
- Dua, P.; Kim, S.; Lee, D. Nucleic acid aptamers targeting cell-surface proteins. Methods 2011, 54, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Cibiel, A.; Dupont, D.M.; Ducongé, F. Methods to identify aptamers against cell surface biomarkers. Pharmaceuticals 2011, 4, 1216–1235. [Google Scholar] [CrossRef]
- Shigdar, S.; Lin, J.; Yu, Y.; Pastuovic, M.; Wei, M.; Duan, W. RNA aptamer against a cancer stem cell marker epithelial cell adhesion molecule. Cancer Sci. 2011, 102, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, Z.; An, Y.; Zhang, W.; Zhang, H.; Liu, D.; Yu, C.; Duan, W.; Yang, C.J. Selection of DNA aptamers against epithelial cell adhesion molecule for cancer cell imaging and circulating tumor cell capture. Anal. Chem. 2013, 85, 4141–4149. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Han, S.R.; Kim, N.Y.; Lee, S.; Jeong, J.; Lee, S. An RNA aptamer that binds carcinoembryonic antigen inhibits hepatic metastasis of colon cancer cells in mice. Gastroenterology 2012, 143, 155–165.e8. [Google Scholar] [CrossRef] [PubMed]
- Ruckman, J.; Gold, L.; Stephens, A.; Janjic, N. Nucleic acid ligands to integrins. U.S. Patent 6,331,394, 18 December 2001. [Google Scholar]
- Mi, J.; Zhang, X.; Giangrande, P.H.; McNamara II, J.O.; Nimjee, S.M.; Sarraf-Yazdi, S.; Sullenger, B.A.; Clary, B.M. Targeted inhibition of αvβ3 integrin with an RNA aptamer impairs endothelial cell growth and survival. Biochem. Biophys. Res. Commun. 2005, 338, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Wang, J.; Ahmad, K.M.; Csordas, A.; Zhou, J.; Nie, J.; Stewart, R.; Thomson, J.A.; Rossi, J.J.; Soh, H.T. Selection strategy to generate aptamer pairs that bind to distinct sites on protein targets. Anal. Chem. 2012, 84, 5365–5371. [Google Scholar] [CrossRef] [PubMed]
- Faryammanesh, R.; Lange, T.; Magbanua, E.; Haas, S.; Meyer, C.; Wicklein, D.; Schumacher, U.; Hahn, U. SDA, a DNA aptamer Inhibiting E- and P-Selectin mediated adhesion of cancer and leukemia cells, the first and pivotal step in transendothelial migration during metastasis formation. PLoS ONE 2014, 9, e93173. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, D.; Koenig, A.; Jennings, S.; Hicke, B.; Han, H.L.; Fitzwater, T.; Chang, Y.F.; Varki, N.; Parma, D.; Varki, A. Calcium-dependent oligonucleotide antagonists specific for L-selectin. Proc. Natl. Acad. Sci. USA 1996, 93, 5883–5887. [Google Scholar] [CrossRef] [PubMed]
- Hicke, B.J.; Watson, S.R.; Koenig, A.; Lynott, C.K.; Bargatze, R.F.; Chang, Y.F.; Ringquist, S.; Moon-McDermott, L.; Jennings, S.; Fitzwater, T.; et al. DNA aptamers block L-selectin function in vivo. Inhibition of human lymphocyte trafficking in SCID mice. J. Clin. Invest. 1996, 98, 2688–2692. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Larson, T.; Nguyen, H.H.; Sokolov, K.V.; Ellington, A.D. Directed evolution of gold nanoparticle delivery to cells. Chem. Commun. Camb. Engl. 2010, 46, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Nguyen, H.H.; Byrom, M.; Ellington, A.D. Inhibition of cell proliferation by an anti-EGFR aptamer. PLoS ONE 2011, 6, e20299. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Cheek, M.A.; Sharaf, M.L.; Li, N.; Ellington, A.D.; Sullenger, B.A.; Shaw, B.R.; White, R.R. Aptamer-mediated delivery of chemotherapy to pancreatic cancer cells. Nucleic Acid Ther. 2012, 22, 295–305. [Google Scholar] [PubMed]
- Wang, D.-L.; Song, Y.-L.; Zhu, Z.; Li, X.-L.; Zou, Y.; Yang, H.-T.; Wang, J.-J.; Yao, P.-S.; Pan, R.-J.; Yang, C.J.; et al. Selection of DNA aptamers against epidermal growth factor receptor with high affinity and specificity. Biochem. Biophys. Res. Commun. 2014, 453, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kuan, C.-T.; Mi, J.; Zhang, X.; Clary, B.M.; Bigner, D.D.; Sullenger, B.A. Aptamers selected against the unglycosylated EGFRvIII ectodomain and delivered intracellularly reduce membrane-bound EGFRvIII and induce apoptosis. Biol. Chem. 2009, 390, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Jeong, S. In Vitro Selection of RNA Aptamer and Specific Targeting of ErbB2 in Breast Cancer Cells. Nucleic Acid Ther. 2011, 21, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Duan, J.-H.; Song, Y.-M.; Ma, J.; Wang, F.-D.; Lu, X.; Yang, X.-D. Novel HER2 aptamer selectively delivers cytotoxic drug to HER2-positive breast cancer cells in vitro. J. Transl. Med. 2012, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Duan, J.; Cao, B.; Zhang, L.; Lu, X.; Wang, F.; Yao, F.; Zhu, Z.; Yuan, W.; Wang, C.; et al. Selection of a novel DNA thioaptamer against HER2 structure. Clin. Transl. Oncol. 2015, 17, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Sett, A.; Borthakur, B.B.; Bora, U. Selection of DNA aptamers for extra cellular domain of human epidermal growth factor receptor 2 to detect HER2 positive carcinomas. Clin. Transl. Oncol. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.B.; Chernis, G.A.; Hoang, V.Q.; Landgraf, R. Inhibition of heregulin signaling by an aptamer that preferentially binds to the oligomeric form of human epidermal growth factor receptor-3. Proc. Natl. Acad. Sci. USA 2003, 100, 9226–9231. [Google Scholar] [CrossRef] [PubMed]
- Boltz, A.; Piater, B.; Toleikis, L.; Guenther, R.; Kolmar, H.; Hock, B. Bi-specific aptamers mediating tumor cell lysis. J. Biol. Chem. 2011, 286, 21896–21905. [Google Scholar] [CrossRef] [PubMed]
- Piater, B.; Doerner, A.; Guenther, R.; Kolmar, H.; Hock, B. Aptamers binding to c-Met inhibiting tumor cell migration. PLoS ONE 2015, 10, e0142412. [Google Scholar] [CrossRef] [PubMed]
- Lupold, S.E.; Hicke, B.J.; Lin, Y.; Coffey, D.S. Identification and characterization of nuclease-stabilized rna molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002, 62, 4029–4033. [Google Scholar] [PubMed]
- McNamara, J.O.; Andrechek, E.R.; Wang, Y.; Viles, K.D.; Rempel, R.E.; Gilboa, E.; Sullenger, B.A.; Giangrande, P.H. Cell type–specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 2006, 24, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.M.; Matthews, C.S.; Missailidis, S. DNA aptamers that bind to MUC1 tumour marker: design and characterization of MUC1-binding single-stranded DNA aptamers. Tumour Biol. 2006, 27, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.M.; Papamichael, K.; Guilbault, G.; Schwarzacher, T.; Gariepy, J.; Missailidis, S. DNA aptamers against the MUC1 tumour marker: Design of aptamer–antibody sandwich ELISA for the early diagnosis of epithelial tumours. Anal. Bioanal. Chem. 2008, 390, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Pratico, E.D.; Sullenger, B.A.; Nair, S.K. Identification and characterization of an agonistic aptamer against the T Cell costimulatory receptor, OX40. Nucleic Acid Ther. 2013, 23, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dollins, C.M.; Nair, S.; Boczkowski, D.; Lee, J.; Layzer, J.M.; Gilboa, E.; Sullenger, B.A. Assembling OX40 Aptamers on a molecular scaffold to create a receptor-activating aptamer. Chem. Biol. 2008, 15, 675–682. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.O.; Kolonias, D.; Pastor, F.; Mittler, R.S.; Chen, L.; Giangrande, P.H.; Sullenger, B.; Gilboa, E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Investig. 2008, 118, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Oguro, A.; Ohtsu, T.; Nakamura, Y. RNA aptamers selected against the receptor activator of NF-κB acquire general affinity to proteins of the tumor necrosis factor receptor family. Nucleic Acids Res. 2004, 32, 6120–6128. [Google Scholar] [CrossRef] [PubMed]
- Pastor, F.; Soldevilla, M.M.; Villanueva, H.; Kolonias, D.; Inoges, S.; de Cerio, A.L.; Kandzia, R.; Klimyuk, V.; Gleba, Y.; Gilboa, E.; et al. CD28 aptamers as powerful immune response modulators. Mol. Ther. Nucleic Acids 2013, 2, e98. [Google Scholar] [CrossRef] [PubMed]
- Santulli-Marotto, S.; Nair, S.K.; Rusconi, C.; Sullenger, B.; Gilboa, E. Multivalent RNA aptamers that inhibit Ctla-4 and enhance tumor immunity. Cancer Res. 2003, 63, 7483–7489. [Google Scholar] [PubMed]
- Zhou, J.; Tiemann, K.; Chomchan, P.; Alluin, J.; Swiderski, P.; Burnett, J.; Zhang, X.; Forman, S.; Chen, R.; Rossi, J. Dual functional BAFF receptor aptamers inhibit ligand-induced proliferation and deliver siRNAs to NHL cells. Nucleic Acids Res. 2013, 41, 4266–4283. [Google Scholar] [CrossRef] [PubMed]
- Roth, F.; Fuente, A.C.D.L.; Vella, J.L.; Zoso, A.; Inverardi, L.; Serafini, P. Aptamer-mediated blockade of IL4Rα triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012, 72, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, F.; Aissouni, Y.; Hamm, J.; Boutin, H.; Libri, D.; Ducongé, F.; Tavitian, B. Binding of an aptamer to the N-terminal fragment of VCAM-1. Bioorg. Med. Chem. Lett. 2007, 17, 6119–6122. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Ito, K.; Matsumoto, M.; Seya, T.; Nishikawa, S.; Hasegawa, T.; Fukuda, K. Isolation of RNA aptamers against human Toll-like receptor 3 ectodomain. Nucleic Acids Symp. Ser. 2006, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Somasunderam, A.; Thiviyanathan, V.; Tanaka, T.; Li, X.; Neerathilingam, M.; Lokesh, G.L.R.; Mann, A.; Peng, Y.; Ferrari, M.; Klostergaard, J.; et al. Combinatorial selection of DNA thioaptamers targeted towards the HA binding domain of human CD44. Biochemistry 2010, 49, 9106–9112. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, N.; Alshaer, W.; Allozi, O.; Mahafzah, A.; El-Khateeb, M.; Hillaireau, H.; Noiray, M.; Fattal, E.; Ismail, S. In vitro selection of modified RNA aptamers against CD44 cancer stem cell marker. Nucleic Acid Ther. 2013, 23, 401–407. [Google Scholar] [CrossRef] [PubMed]
- White, R.R.; Roy, J.A.; Viles, K.D.; Sullenger, B.A.; Kontos, C.D. A nuclease-resistant RNA aptamer specifically inhibits angiopoietin-1-mediated Tie2 activation and function. Angiogenesis 2008, 11, 395–401. [Google Scholar] [CrossRef] [PubMed]
- White, R.R.; Shan, S.; Rusconi, C.P.; Shetty, G.; Dewhirst, M.W.; Kontos, C.D.; Sullenger, B.A. Inhibition of rat corneal angiogenesis by a nuclease-resistant RNA aptamer specific for angiopoietin-2. Proc. Natl. Acad. Sci. USA 2003, 100, 5028–5033. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.J.; Laber, D.A.; Miller, D.M.; Thomas, S.D.; Trent, J.O. Discovery and development of the g-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009, 86, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Tam, R.C.; Phan, U.T.; Milovanovic, T.; Pai, B.; Lim, C.; Bard, J.; He, L. Oligonucleotide-mediated inhibition of CD28 expression induces human T-cell hyporesponsiveness and manifests impaired contact hypersensitivity in mice. J. Immunol. 1997, 158, 200–208. [Google Scholar] [PubMed]
- Spencer, D.I.; Missailidis, S.; Denton, G.; Murray, A.; Brady, K.; Matteis, C.I.; Searle, M.S.; Tendler, S.J.; Price, M.R. Structure/activity studies of the anti-MUC1 monoclonal antibody C595 and synthetic MUC1 mucin-core-related peptides and glycopeptides. Biospectroscopy 1999, 5, 79–91. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, X.; Lu, P.Y.; Rosato, R.R.; Tan, W.; Zu, Y. Oligonucleotide aptamers: New tools for targeted cancer therapy. Mol. Ther. Nucleic Acids 2014, 3, e182. [Google Scholar] [CrossRef] [PubMed]
- Blind, M.; Blank, M. Aptamer selection technology and recent advances. Mol. Ther. Nucleic Acids 2015, 4, e223. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, D.; Bing, T.; Zhang, N. Cell-SELEX: Aptamer selection against whole cells. In Aptamers Selected by Cell-SELEX for Theranostics; Tan, W., Fang, X., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 13–33. [Google Scholar]
- Mallikaratchy, P. Evolution of complex target SELEX to identify aptamers against mammalian cell-surface antigens. Molecules 2017, 22, 215. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.; Ahmed, M.-S.L.; Dolf, A.; Endl, E.; Knolle, P.A.; Famulok, M. Fluorescence-activated cell sorting for aptamer SELEX with cell mixtures. Nat. Protoc. 2010, 5, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, M.-S.L.; Dolf, A.; Endl, E.; Knolle, P.; Famulok, M.; Mayer, G. Enrichment of cell-targeting and population-specific aptamers by fluorescence-activated cell sorting. Angew. Chem. Int. Ed. 2008, 47, 5190–5193. [Google Scholar] [CrossRef] [PubMed]
- Avci-Adali, M.; Metzger, M.; Perle, N.; Ziemer, G.; Wendel, H.P. Pitfalls of cell-systematic evolution of ligands by exponential enrichment (SELEX): Existing dead cells during in vitro selection anticipate the enrichment of specific aptamers. Oligonucleotides 2010, 20, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wilner, S.E.; Wengerter, B.; Maier, K.; de Lourdes Borba Magalhães, M.; Del Amo, D.S.; Pai, S.; Opazo, F.; Rizzoli, S.O.; Yan, A.; Levy, M. An RNA alternative to human transferrin: a new tool for targeting human cells. Mol. Ther. Nucleic Acids 2012, 1, e21. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Levy, M. Cell Internalization SELEX: in vitro selection for molecules that internalize into cells. In Therapeutic Applications of Ribozymes and Riboswitches; Lafontaine, D., Dubé, A., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2014; pp. 241–265. [Google Scholar]
- Gourronc, F.A.; Rockey, W.M.; Thiel, W.H.; Giangrande, P.H.; Klingelhutz, A.J. Identification of RNA aptamers that Internalize into HPV-16 E6/E7 transformed tonsillar epithelial cells. Virology 2013, 446, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Bobbin, M.L.; Burnett, J.C.; Rossi, J.J. Current progress of RNA aptamer-based therapeutics. Front. Genet. 2012, 3, 234. [Google Scholar] [CrossRef] [PubMed]
- Thiel, W.H.; Bair, T.; Peek, A.S.; Liu, X.; Dassie, J.; Stockdale, K.R.; Behlke, M.A.; Miller, F.J.; Giangrande, P.H. Rapid identification of cell-specific, internalizing RNA aptamers with bioinformatics analyses of a cell-based aptamer selection. PLoS ONE 2012, 7, e43836. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Esposito, C.L.; Rienzo, A.; Catuogno, S.; Iaboni, M.; Condorelli, G.; de Franciscis, V.; Cerchia, L. Inhibition of receptor signaling and of glioblastoma-derived tumor growth by a novel PDGFRβ aptamer. Mol. Ther. 2014, 22, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Thiel, K.W.; Hernandez, L.I.; Dassie, J.P.; Thiel, W.H.; Liu, X.; Stockdale, K.R.; Rothman, A.M.; Hernandez, F.J.; McNamara, J.O.; Giangrande, P.H. Delivery of chemo-sensitizing siRNAs to HER2+-breast cancer cells using RNA aptamers. Nucleic Acids Res. 2012, 40, 6319–6337. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.G.; Marangoni, K.; Fujimura, P.T.; Alves, P.T.; Silva, M.J.; Bastos, V.A.F.; Goulart, L.R.; Goulart, V.A. 3D cell-SELEX: Development of RNA aptamers as molecular probes for PC-3 tumor cell line. Exp. Cell Res. 2016, 341, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, S.P.; Ohtsu, T.; Nakamura, Y. Selection of RNA aptamers against recombinant transforming growth factor-β type III receptor displayed on cell surface. Biochimie 2006, 88, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Delač, M.; Motaln, H.; Ulrich, H.; Lah, T.T. Aptamer for imaging and therapeutic targeting of brain tumor glioblastoma. Cytometry A 2015, 87, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Wadajkar, A.S.; Dancy, J.G.; Hersh, D.S.; Anastasiadis, P.; Tran, N.L.; Woodworth, G.F.; Winkles, J.A.; Kim, A.J. Tumor-targeted nanotherapeutics: overcoming treatment barriers for glioblastoma. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zumrut, H.E.; Ara, M.N.; Maio, G.E.; Van, N.A.; Batool, S.; Mallikaratchy, P.R. Ligand-guided selection of aptamers against T-cell receptor-cluster of differentiation 3 (TCR-CD3) expressed on Jurkat.E6 cells. Anal. Biochem. 2016, 512, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zumrut, H.E.; Ara, M.N.; Fraile, M.; Maio, G.; Mallikaratchy, P. Ligand-guided selection of target-specific aptamers: A screening technology for identifying specific aptamers against cell-surface proteins. Nucleic Acid Ther. 2016, 26, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Kao, W.-C.; Wang, W.-Y.; Wang, W.-Y.; Yang, R.-B.; Peck, K. Identification and characterization of oligonucleotides that inhibit Toll-like receptor 2-associated immune responses. FASEB J. 2009, 23, 3078–3088. [Google Scholar] [CrossRef] [PubMed]
- Pestourie, C.; Cerchia, L.; Gombert, K.; Aissouni, Y.; Boulay, J.; Franciscis, V.D.; Libri, D.; Tavitian, B.; Ducongé, F. Comparison of different strategies to select aptamers against a transmembrane protein target. Oligonucleotides 2006, 16, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, H.; Jacobson, O.; Wang, Z.; Chen, H.; Yang, X.; Niu, G.; Chen, X. Combinatorial screening of DNA aptamers for molecular imaging of HER2 in cancer. Bioconjug. Chem. 2017, 28, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Lange, T.; Mittelberger, F.; Schumacher, U.; Hahn, U. Selection and characterization of an α6β4 Integrin blocking DNA aptamer. Mol. Ther. Nucleic Acids 2016, 5, e294. [Google Scholar] [CrossRef] [PubMed]
- Soldevilla, M.M.; Villanueva, H.; Casares, N.; Lasarte, J.J.; Bendandi, M.; Inoges, S.; de Cerio, A.L.-D.; Pastor, F. MRP1-CD28 bi-specific oligonucleotide aptamers: Target costimulation to drug-resistant melanoma cancer stem cells. Oncotarget 2016, 7, 23182–23196. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Sakota, E.; Nakamura, Y. The efficient cell-SELEX strategy, Icell-SELEX, using isogenic cell lines for selection and counter-selection to generate RNA aptamers to cell surface proteins. Biochimie 2016, 131, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Huh, Y.M.; Lee, D. Isolation of RNA aptamers targeting HER-2-overexpressing breast cancer cells using cell-SELEX. Bull. Korean Chem. Soc. 2009, 30, 1827–1831. [Google Scholar]
- Dastjerdi, K.; Tabar, G.H.; Dehghani, H.; Haghparast, A. Generation of an enriched pool of DNA aptamers for an HER2-overexpressing cell line selected by Cell SELEX. Biotechnol. Appl. Biochem. 2011, 58, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.; Kamble, S.; Zhao, N.; Zeng, Z.; Portier, B.P.; Zu, Y. Immunotherapy of CD30-expressing lymphoma using a highly stable ssDNA aptamer. Biomaterials 2013, 34, 8909–8917. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, G.; Maron, R.; Mancini, M.; Schechter, B.; Sela, M.; Yarden, Y. Aptamer to ErbB-2/HER2 enhances degradation of the target and inhibits tumorigenic growth. Proc. Natl. Acad. Sci. USA 2013, 110, 8170–8175. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Shi, Y.; Wu, X.; Liang, H.; Gao, Y.; Li, S.; Zhang, X.; Wang, F.; Gao, T. DNA aptamers that target human glioblastoma multiforme cells overexpressing epidermal growth factor receptor variant III in vitro. Acta Pharmacol. Sin. 2013, 34, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liang, H.; Tan, Y.; Yuan, C.; Li, S.; Li, X.; Li, G.; Shi, Y.; Zhang, X. Cell-SELEX aptamer for highly specific radionuclide molecular imaging of glioblastoma in vivo. PLoS ONE 2014, 9, e90752. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Hernandez, F.J.; Gu, B.; Stockdale, K.R.; Nanapaneni, K.; Scheetz, T.E.; Behlke, M.A.; Peek, A.S.; Bair, T.; Giangrande, P.H.; et al. RNA Aptamer-Based Functional Ligands of the Neurotrophin Receptor, TrkB. Mol. Pharmacol. 2012, 82, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, E.Y.; Kim, S.Y.; Byun, S.K.; Lee, D.; Oh, K.-J.; Kim, W.K.; Han, B.S.; Chi, S.-W.; Lee, S.C.; et al. Identification of DNA aptamers toward epithelial cell adhesion molecule via cell-SELEX. Mol. Cells 2014, 37, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Shigdar, S.; Qiao, L.; Zhou, S.-F.; Xiang, D.; Wang, T.; Li, Y.; Lim, L.Y.; Kong, L.; Li, L.; Duan, W. RNA aptamers targeting cancer stem cell marker CD133. Cancer Lett. 2013, 330, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, Y.; Jiang, F.; Zhou, J.; Li, Y.; Liang, C.; Dang, L.; Lu, A.; Zhang, G. Development of cell-SELEX technology and its application in cancer diagnosis and therapy. Int. J. Mol. Sci. 2016, 17, 2079. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.M.; Donovan, M.J.; Tan, W. Using aptamers for cancer biomarker discovery. J. Nucleic Acids 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Qiu, L.; Li, J.; Fu, T.; Zhang, X.; Tan, W. Cancer Biomarker Discovery Using DNA Aptamers. Analyst 2016, 141, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.A.; Chen, H.; Hicke, B.J.; Swiderek, K.M.; Gold, L. A tenascin-C aptamer identified by tumor cell-SELEX: Systematic evolution of ligands by exponential enrichment. Proc. Natl. Acad. Sci. USA 2003, 100, 15416–15421. [Google Scholar] [CrossRef] [PubMed]
- Ireson, C.R.; Kelland, L.R. Discovery and development of anticancer aptamers. Mol. Cancer Ther. 2006, 5, 2957–2962. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, S.; Chen, W.; Spicer, E.K.; Courtenay-Luck, N.; Fernandes, D.J. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008, 68, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, S.; Wang, L.; Sridharan, V.; Chen, W.; Courtenay-Luck, N.; Jones, D.; Spicer, E.K.; Fernandes, D.J. Plasma membrane nucleolin is a receptor for the anticancer aptamer AS1411 in MV4-11 leukemia cells. Mol. Pharmacol. 2009, 76, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.J.; Reyes-Reyes, E.M.; Malik, M.T.; Murphy, E.M.; O’Toole, M.G.; Trent, J.O. G-quadruplex oligonucleotide AS1411 as a cancer-targeting agent: Uses and mechanisms. Biochim. Biophys. Acta 2017, 1861, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.L.; Passaro, D.; Longobardo, I.; Condorelli, G.; Marotta, P.; Affuso, A.; de Franciscis, V.; Cerchia, L. A neutralizing rna aptamer against egfr causes selective apoptotic cell death. PLoS ONE 2011, 6, e24071. [Google Scholar] [CrossRef] [PubMed]
- Dua, P.; Kang, H.S.; Hong, S.-M.; Tsao, M.-S.; Kim, S.; Lee, D. Alkaline phosphatase ALPPL-2 Is a novel pancreatic carcinoma-associated protein. Cancer Res. 2013, 73, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Bing, T.; Shangguan, D.; Wang, Y. Facile discovery of cell-surface protein targets of cancer cell aptamers. Mol. Cell. Proteom. 2015, 14, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhou, L.; Zheng, X.; Quan, Y.; Wang, X.; Zhou, X.; Ren, J. A novel molecular marker of breast cancer stem cells identified by cell-SELEX method. Cancer Biomark. 2015, 15, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Sefah, K.; Bae, K.-M.; Phillips, J.A.; Siemann, D.W.; Su, Z.; McClellan, S.; Vieweg, J.; Tan, W. Cell-based selection provides novel molecular probes for cancer stem cells. Int. J. Cancer J. Int. Cancer 2013, 132, 2578–2588. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Shangguan, D.; Cao, Z.; Fang, X.; Tan, W. Cell-specific internalization study of an aptamer from whole cell selection. Chem. Weinh. Bergstr. Ger. 2008, 14, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Iaboni, M.; Fontanella, R.; Rienzo, A.; Capuozzo, M.; Nuzzo, S.; Santamaria, G.; Catuogno, S.; Condorelli, G.; de Franciscis, V.; Esposito, C.L. Targeting insulin receptor with a novel internalizing aptamer. Mol. Ther. Nucleic Acids 2016, 5, e365. [Google Scholar] [CrossRef] [PubMed]
- Cerchia, L.; Esposito, C.L.; Camorani, S.; Rienzo, A.; Stasio, L.; Insabato, L.; Affuso, A.; de Franciscis, V. Targeting axl with an high-affinity inhibitory aptamer. Mol. Ther. 2012, 20, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- Mallikaratchy, P.; Tang, Z.; Kwame, S.; Meng, L.; Shangguan, D.; Tan, W. Aptamer directly evolved from live cells recognizes membrane bound immunoglobin heavy mu chain in burkitt’s lymphoma cells. Mol. Cell. Proteom. 2007, 6, 2230–2238. [Google Scholar] [CrossRef] [PubMed]
- Berezovski, M.V.; Lechmann, M.; Musheev, M.U.; Mak, T.W.; Krylov, S.N. Aptamer-facilitated biomarker discovery (AptaBiD). J. Am. Chem. Soc. 2008, 130, 9137–9143. [Google Scholar] [CrossRef] [PubMed]
- Zamay, G.S.; Kolovskaya, O.S.; Zamay, T.N.; Glazyrin, Y.E.; Krat, A.V.; Zubkova, O.; Spivak, E.; Wehbe, M.; Gargaun, A.; Muharemagic, D.; et al. Aptamers selected to postoperative lung adenocarcinoma detect circulating tumor cells in human blood. Mol. Ther. 2015, 23, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tang, L.; Sefah, K.; Zhao, Z.; Zhu, G.; Sun, W.; Goodison, S.; Tan, W. Novel aptamer developed for breast cancer cell internalization. Chem. Med. Chem. 2012, 7, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Sefah, K.; Meng, L.; Lopez-Colon, D.; Jimenez, E.; Liu, C.; Tan, W. DNA aptamers as molecular probes for colorectal cancer study. PLoS ONE 2010, 5, e14269. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Medley, C.D.; Smith, J.E.; Sefah, K.; Shangguan, D.; Tang, Z.; Meng, L.; Tan, W. Molecular recognition of small cell lung cancer cells using aptamers. Chem. Med. Chem. 2008, 3, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Kunii, T.; Ogura, S.; Mie, M.; Kobatake, E. Selection of DNA aptamers recognizing small cell lung cancer using living cell-SELEX. Analyst 2011, 136, 1310–1312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, L.; Shi, X.; Tan, W.; Fang, X.; Shangguan, D. Recognition of subtype non-small cell lung cancer by DNA aptamers selected from living cells. Analyst 2009, 134, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, D.; Meng, L.; Cao, Z.C.; Xiao, Z.; Fang, X.; Li, Y.; Cardona, D.; Witek, R.P.; Liu, C.; Tan, W. Identification of liver cancer-specific aptamers using whole live cells. Anal. Chem. 2008, 80, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, D.; Li, Y.; Tang, Z.; Cao, Z.C.; Chen, H.W.; Mallikaratchy, P.; Sefah, K.; Yang, C.J.; Tan, W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl. Acad. Sci. USA 2006, 103, 11838–11843. [Google Scholar] [CrossRef] [PubMed]
- Sefah, K.; Tang, Z.; Shangguan, D.; Chen, H.; Lopez-Colon, D.; Li, Y.; Parekh, P.; Martin, J.; Meng, L.; Tan, W. Molecular recognition of acute myeloid leukemia using aptamers. Leukemia 2009, 23, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Ma, Y.; Pei, X.; Liu, Q.; Lu, B.; Jin, L.; Wang, J.; Liu, J. A cell-based single-stranded DNA aptamer specifically targets gastric cancer. Int. J. Biochem. Cell Biol. 2014, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, Z.; Bai, H.; Fu, T.; Yang, C.; Hu, X.; Liu, Q.; Champanhac, C.; Teng, I.-T.; Ye, M.; Tan, W. DNA aptamer selected against pancreatic ductal adenocarcinoma for in vivo imaging and clinical tissue recognition. Theranostics 2015, 5, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Chen, H.; Zhou, X.-F.; Yin, C.-Q.; Wang, B.-C.; Peng, C.-W.; Liu, S.-P.; Wang, F.-B. Identification of an aptamer through whole cell-SELEX for targeting high metastatic liver cancers. Oncotarget 2016, 7, 8282–8294. [Google Scholar] [PubMed]
- Zueva, E.; Rubio, L.I.; Ducongé, F.; Tavitian, B. Metastasis-focused cell-based SELEX generates aptamers inhibiting cell migration and invasion. Int. J. Cancer 2011, 128, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wu, Q.; Hamerlik, P.; Hitomi, M.; Sloan, A.E.; Barnett, G.H.; Weil, R.J.; Leahy, P.; Hjelmeland, A.B.; Rich, J.N. Aptamer Identification of Brain Tumor Initiating Cells. Cancer Res. 2013, 73, 4923–4936. [Google Scholar] [CrossRef] [PubMed]
- Quang, N.; Miodek, A.; Cibiel, A.; Ducongé, F. Selection of aptamers against whole living cells: From cell-SELEX to identification of biomarkers. In Synthetic Antibodies; Tiller, T., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; pp. 253–272. [Google Scholar]
- Griffin, N.M.; Schnitzer, J.E. Overcoming key technological challenges in using mass spectrometry for mapping cell surfaces in tissues. Mol. Cell. Proteom. 2011, 10, R110.000935. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Crescenzi, E.; Colecchia, D.; Carpentieri, A.; Amoresano, A.; Fedele, M.; Chiariello, M.; Cerchia, L. Aptamer targeting EGFRvIII mutant hampers its constitutive autophosphorylation and affects migration, invasion and proliferation of glioblastoma cells. Oncotarget 2015, 6, 37570–37587. [Google Scholar] [PubMed]
- Cibiel, A.; Nguyen Quang, N.; Gombert, K.; Thézé, B.; Garofalakis, A.; Ducongé, F. From Ugly Duckling to Swan: Unexpected identification from Cell-SELEX of an anti-annexin A2 aptamer targeting tumors. PLoS ONE 2014, 9, e87002. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Z.; Zhao, Z.; Liu, Q.; Tan, W.; Fang, X. Cellular internalization and cytotoxicity of aptamers selected from lung cancer cell. Am. J. Biomed. Sci. 2013, 5, 47–58. [Google Scholar]
- Nimse, S.B.; Sonawane, M.D.; Song, K.-S.; Kim, T. Biomarker detection technologies and future directions. Analyst 2016, 141, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, V.S.P.K.S.A.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ozalp, V.C.; Kavruk, M.; Dilek, O.; Tahir, A. Aptamers: Molecular Tools for Medical Diagnosis. Curr. Top. Med. Chem. 2015, 15, 1125–1137. [Google Scholar] [CrossRef]
- Prakash, J.S.; Rajamanickam, K. Aptamers and Their Significant Role in Cancer Therapy and Diagnosis. Biomedicines 2015, 3, 248–269. [Google Scholar] [CrossRef] [PubMed]
- Bukari, B.A.; Citartan, M.; Ch’ng, E.S.; Bilibana, M.P.; Rozhdestvensky, T.; Tang, T.-H. Aptahistochemistry in diagnostic pathology: technical scrutiny and feasibility. Histochem. Cell Biol. 2017, 147, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tan, W.; Zu, Y. Aptamers: versatile molecular recognition probes for cancer detection. Analyst 2016, 141, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Dickey, D.D.; Giangrande, P.H. Oligonucleotide Aptamers: a Next-Generation Technology for the Capture and Detection of Circulating Tumor Cells. Methods 2016, 97, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Zamay, A.S.; Zamay, G.S.; Kolovskaya, O.S.; Zamay, T.N.; Berezovski, M.V. Aptamer-Based Methods for Detection of Circulating Tumor Cells and Their Potential for Personalized Diagnostics. Adv. Exp. Med. Biol. 2017, 994, 67–81. [Google Scholar] [PubMed]
- Hong, P.; Li, W.; Li, J. Applications of Aptasensors in Clinical Diagnostics. Sensors 2012, 12, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Ravalli, A.; Voccia, D.; Palchetti, I.; Marrazza, G. Electrochemical, Electrochemiluminescence, and Photoelectrochemical Aptamer-Based Nanostructured Sensors for Biomarker Analysis. Biosensors 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Rahimian, A.; Son, K.; Shin, D.-S.; Patel, T.; Revzin, A. Development of an aptasensor for electrochemical detection of exosomes. Methods 2016, 97, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zheng, J.; Li, C.; Qiu, L.; Zhang, X.; Tan, W. Aptamers Selected by Cell-SELEX for Molecular Imaging. J. Mol. Evol. 2015, 81, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, C.A.; Cai, W.; Hong, H. Applications of Aptamers in Targeted Imaging: State of the Art. Curr. Top. Med. Chem. 2015, 15, 1138–1152. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.-H.; Zhang, T.; Luo, H.; Yen, T.M.; Chen, P.-W.; Han, Y.; Lo, Y.-H. Nucleic acid aptamers: An emerging tool for biotechnology and biomedical sensing. Sensors 2015, 15, 16281–16313. [Google Scholar] [CrossRef] [PubMed]
- Mankoff, D.A.; Link, J.M.; Linden, H.M.; Sundararajan, L.; Krohn, K.A. Tumor receptor imaging. J. Nucl. Med. 2008, 49, 149S–163S. [Google Scholar] [CrossRef] [PubMed]
- Wiraja, C.; Yeo, D.; Lio, D.; Labanieh, L.; Lu, M.; Zhao, W.; Xu, C. Aptamer technology for tracking cells’ status & function. Mol. Cell. Ther. 2014, 2. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Shigdar, S.; Qiao, G.; Wang, T.; Kouzani, A.Z.; Zhou, S.-F.; Kong, L.; Li, Y.; Pu, C.; Duan, W. Nucleic acid aptamer-guided cancer therapeutics and diagnostics: the next generation of cancer medicine. Theranostics 2015, 5, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Pastor, F. Aptamers: A New Technological Platform in Cancer Immunotherapy. Pharmaceuticals 2016, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Soldevilla, M.M.; Villanueva, H.; Pastor, F. Aptamers: A Feasible Technology in Cancer Immunotherapy. J. Immunol. Res. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Khedri, M.; Rafatpanah, H.; Abnous, K.; Ramezani, P.; Ramezani, M. Cancer immunotherapy via nucleic acid aptamers. Int. Immunopharmacol. 2015, 29, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, J.; Wu, M.; Zhao, J.X. Aptamers: Active targeting ligands for cancer diagnosis and therapy. Theranostics 2015, 5, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, H.J.; Heo, K. Therapeutic aptamers: Developmental potential as anticancer drugs. BMB Rep. 2015, 48, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, J.; Zhang, X.-B.; Ye, M.; Tan, W. Nucleic acid aptamer-mediated drug delivery for targeted cancer therapy. ChemMedChem 2015, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Catuogno, S.; Esposito, C.L.; de Franciscis, V. Aptamer-mediated targeted delivery of therapeutics: An update. Pharmaceuticals 2016, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.C.B.; Lakshmipriya, T.; Chen, Y.; Arshad, M.K.M.; Kerishnan, J.P.; Ruslinda, A.R.; Al-Douri, Y.; Voon, C.H.; Hashim, U. Cell-targeting aptamers act as intracellular delivery vehicles. Appl. Microbiol. Biotechnol. 2016, 100, 6955–6969. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Shigdar, S.; Qiao, G.; Zhou, S.-F.; Li, Y.; Wei, M.Q.; Qiao, L.; Al. Shamaileh, H.; Zhu, Y.; Zheng, C.; et al. Aptamer-mediated cancer gene therapy. Curr. Gene Ther. 2015, 15, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Parashar, A. Aptamers in Therapeutics. J. Clin. Diagn. Res. JCDR 2016, 10, BE01–BE06. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Niu, G.; Chen, X. Aptamer–Drug Conjugates. Bioconjug. Chem. 2015, 26, 2186–2197. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Liao, J.; Liu, J.; Chen, K.; Tong, G.; Yuan, P.; Liu, Z.; Pu, Y.; Liu, H. Aptamer-Functionalized Nanoparticles for Drug Delivery. J. Biomed. Nanotechnol. 2014, 10, 3189–3203. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.-M.; Liu, H.; Kuai, H.; Peng, R.; Mo, L.; Zhang, X.-B. Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem. Soc. Rev. 2016, 45, 2583–2602. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, G.; Vestal, C.G.; Richardson, C. Aptamer-Functionalized Nanoparticles as “Smart Bombs”: The Unrealized Potential for Personalized Medicine and Targeted Cancer Treatment. Target. Oncol. 2015, 10, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, H.; Dong, S.; Ge, L.; Zhang, Y. Progress in Aptamer-Mediated Drug Delivery Vehicles for Cancer Targeting and Its Implications in Addressing Chemotherapeutic Challenges. Theranostics 2014, 4, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Cansiz, S.; Zhang, L.; Wu, C.; Wu, Y.; Teng, I.-T.; Hou, W.; Wang, Y.; Wan, S.; Cai, R.; Jin, C.; et al. DNA Aptamer Based Nanodrugs: Molecular engineering for efficiency. Chem. Asian J. 2015, 10, 2084–2094. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Maekawa, T.; Sakthikumar, D. Aptamers in Targeted Nanotherapy. Curr. Top. Med. Chem. 2015, 15, 1102–1114. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wu, S.-M.; Li, H.-W.; Chang, H.-T. Biomedical Applications of DNA-Conjugated Gold Nanoparticles. ChemBioChem 2016, 17, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, C.P.; Scardino, E.; Layzer, J.; Pitoc, G.A.; Ortel, T.L.; Monroe, D.; Sullenger, B.A. RNA aptamers as reversible antagonists of coagulation factor IXa. Nature 2002, 419, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Vavalle, J.P.; Rusconi, C.P.; Zelenkofske, S.; Wargin, W.A.; Ortel, T.L.; Alexander, J.H.; Povsic, T.J.; Becker, R.C. The effect of the REG2 Anticoagulation System on thrombin generation kinetics: a pharmacodynamic and pharmacokinetic first-in-human study. J. Thromb. Thrombolysis 2014, 38, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.S.; Sullenger, B.A. Modulation of the coagulation cascade using aptamers. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Neoh, K.G.; Kang, E.-T.; Choe, W.S.; Su, X. PEGylated Anti-MUC1 Aptamer-Doxorubicin Complex for Targeted Drug Delivery to MCF7 Breast Cancer Cells. Macromol. Biosci. 2011, 11, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Emrani, A.S.; Tabrizian, K.; ZandKarimi, M.; Ramezani, M.; Abnous, K. Targeted delivery of Epirubicin to cancer cells by PEGylated A10 aptamer. J. Drug Target. 2013, 21, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Povsic, T.J.; Vavalle, J.P.; Aberle, L.H.; Kasprzak, J.D.; Cohen, M.G.; Mehran, R.; Bode, C.; Buller, C.E.; Montalescot, G.; Cornel, J.H.; et al. A Phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the REG1 system in patients with acute coronary syndromes: results of the RADAR trial. Eur. Heart J. 2013, 34, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Povsic, T.J.; Lawrence, M.G.; Lincoff, A.M.; Mehran, R.; Rusconi, C.P.; Zelenkofske, S.L.; Huang, Z.; Sailstad, J.; Armstrong, P.W.; et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J. Allergy Clin. Immunol. 2016, 138, 1712–1715. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Mehran, R.; Povsic, T.J.; Zelenkofske, S.L.; Huang, Z.; Armstrong, P.W.; Steg, P.G.; Bode, C.; Cohen, M.G.; Buller, C.; et al. Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention (REGULATE-PCI): A randomised clinical trial. Lancet 2016, 387, 349–356. [Google Scholar] [CrossRef]
- Verheugt, F.W.A. An anticoagulant too good to be true for revascularisation. Lancet 2016, 387, 314–315. [Google Scholar] [CrossRef]
- Ganson, N.J.; Povsic, T.J.; Sullenger, B.A.; Alexander, J.H.; Zelenkofske, S.L.; Sailstad, J.M.; Rusconi, C.P.; Hershfield, M.S. Pre-existing anti–polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J. Allergy Clin. Immunol. 2016, 137, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.W.; Akerblom, E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int. Arch. Allergy Appl. Immunol. 1983, 70, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ganson, N.J.; Kelly, S.J.; Scarlett, E.; Sundy, J.S.; Hershfield, M.S. Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res. Ther. 2006, 8, R12. [Google Scholar] [CrossRef] [PubMed]
- Hershfield, M.S.; Ganson, N.J.; Kelly, S.J.; Scarlett, E.L.; Jaggers, D.A.; Sundy, J.S. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res. Ther. 2014, 16, R63. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.K.; Hempel, G.; Koling, S.; Chan, L.S.; Fisher, T.; Meiselman, H.J.; Garratty, G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 2007, 110, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ruckman, J.; Green, L.S.; Beeson, J.; Waugh, S.; Gillette, W.L.; Henninger, D.D.; Claesson-Welsh, L.; Janjic, N. 2′-Fluoropyrimidine RNA-based Aptamers to the 165-Amino Acid Form of Vascular Endothelial Growth Factor (VEGF165) Inhibition of Receptor Binding and VEGF-induced Vascular Permeability through Interactions Requiring the Exon 7-encoded Domain. J. Biol. Chem. 1998, 273, 20556–20567. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sridharan, V.; Soundararajan, S.; Otake, Y.; Stuart, R.; Jones, D.; Fernandes, D. Activity and Mechanism of Action of AS1411 in Acute Myeloid Leukemia Cells. Blood 2007, 110, 1604. [Google Scholar]
- Xu, X.; Hamhouyia, F.; Thomas, S.D.; Burke, T.J.; Girvan, A.C.; McGregor, W.G.; Trent, J.O.; Miller, D.M.; Bates, P.J. Inhibition of DNA Replication and Induction of S Phase Cell Cycle Arrest by G-rich Oligonucleotides. J. Biol. Chem. 2001, 276, 43221–43230. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, K.; Gorospe, M. RNA-binding protein nucleolin in disease. RNA Biol. 2012, 9, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Joshi, N.; Agarwal, A.; Dahiya, S.; Bittner, P.; Smith, E.; Taylor, S.; Piwnica-Worms, D.; Weber, J.; Leonard, J.R. Knocking down nucleolin expression in gliomas inhibits tumor growth and induces cell cycle arrest. J. Neurooncol. 2012, 108, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Laber, D.A.; Sharma, V.R.; Bhupalam, L.; Taft, B.; Hendler, F.J.; Barnhart, K.M. Update on the first phase I study of AGRO100 in advanced cancer. J. Clin. Oncol. 2005, 23, 3064. [Google Scholar] [CrossRef]
- Laber, D.A.; Taft, B.S.; Kloecker, G.H.; Bates, P.J.; Trent, J.O.; Miller, D.M. Extended phase I study of AS1411 in renal and non-small cell lung cancers. J. Clin. Oncol. 2006, 24, 13098. [Google Scholar]
- Rosenberg, J.E.; Bambury, R.; Drabkin, H.A.; Lara, P.N.; Harzstark, A.L.; Figlin, R.A.; Smith, G.W.; Choueiri, T.; Erlandsson, F.; Laber, D.A. A phase II trial of the nucleolin-targeted DNA aptamer AS1411 in metastatic refractory renal cell carcinoma. Investig. New Drugs 2014, 32, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Stuart, R.K.; Stockerl-Goldstein, K.; Cooper, M.; Devetten, M.; Herzig, R.; Medeiros, B.; Schiller, G.; Wei, A.; Acton, G.; Rizzieri, D. Randomized phase II trial of the nucleolin targeting aptamer AS1411 combined with high-dose cytarabine in relapsed/refractory acute myeloid leukemia (AML). J. Clin. Oncol. 2009, 27, 15s. [Google Scholar]
- Vater, A.; Sahlmann, J.; Kröger, N.; Zöllner, S.; Lioznov, M.; Maasch, C.; Buchner, K.; Vossmeyer, D.; Schwoebel, F.; Purschke, W.G.; et al. Hematopoietic Stem and Progenitor Cell Mobilization in Mice and Humans by a First-in-Class Mirror-Image Oligonucleotide Inhibitor of CXCL12. Clin. Pharmacol. Ther. 2013, 94, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C.; Alomran, R.; Chernikova, S.B.; Lartey, F.; Stafford, J.; Jang, T.; Merchant, M.; Zboralski, D.; Zöllner, S.; Kruschinski, A.; et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro-Oncol. 2014, 16, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hoellenriegel, J.; Zboralski, D.; Maasch, C.; Rosin, N.Y.; Wierda, W.G.; Keating, M.J.; Kruschinski, A.; Burger, J.A. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood 2014, 123, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Steurer, M.; Montillo, M.; Scarfò, L.; Mauro, F.R.; Andel, J.; Wildner, S.; Trentin, L.; Janssens, A.; Burgstaller, S.; Kruschinski, A.; et al. Results from a Phase IIa Study of the Anti-CXCL12 Spiegelmer Olaptesed Pegol (NOX-A12) in Combination with Bendamustine/Rituximab in Patients with Chronic Lymphocytic Leukemia. Blood 2014, 124, 1996. [Google Scholar]
- Ludwig, H.; Weisel, K.; Petrucci, M.T.; Leleu, X.; Cafro, A.M.; Garderet, L.; Leitgeb, C.; Foa, R.; Greil, R.; Yakoub-Agha, I.; et al. Olaptesed pegol, an anti-CXCL12/SDF-1 Spiegelmer, alone and with bortezomib–dexamethasone in relapsed/refractory multiple myeloma: A Phase IIa Study. Leukemia 2017, 31, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, H.; Ding, H.; Huang, Y.; Cao, X.; Yang, G.; Li, J.; Xie, Z.; Meng, Y.; Li, X.; Zhao, Q.; Shen, B.; Shao, N. Identification of an aptamer targeting hnRNP A1 by tissue slide-based SELEX. J. Pathol. 2009, 218, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Liu, F.; Zhou, L.; Shao, N.; Zhao, X. SELEX aptamer used as a probe to detect circulating tumor cells in peripheral blood of pancreatic cancer patients. PLoS ONE 2015, 10, e0121920. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Volk, D.E.; Lokesh, G.L.-R.; Elizondo-Riojas, M.-A.; Li, L.; Nick, A.M.; Sood, A.K.; Rosenblatt, K.P.; Gorenstein, D.G. Morph-X-Select: Morphology-based tissue aptamer selection for ovarian cancer biomarker discovery. BioTechniques 2016, 61, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Liu, Y.; Rabbani, Z.N.; Yang, Z.; Urban, J.H.; Sullenger, B.A.; Clary, B.M. In vivo selection of tumor-targeting RNA motifs. Nat. Chem. Biol. 2010, 6, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Chen, Y.H.; Lennox, K.A.; Behlke, M.A.; Davidson, B.L. In vivo SELEX for Identification of Brain-penetrating Aptamers. Mol. Ther. Nucleic Acids 2013, 2, e67. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Major Functions of Biomarker in Cancer |
|---|---|
| Cell-adhesion molecules | |
| Epithelial cell adhesion molecule-EpCAM (CD326) | EpCam is a pleiotropic molecule able to promote and prevent epithelial cell-cell adhesion. Induces proliferation, up-regulates proto-oncogene (c-myc, cyclins A/E) and is involved in migration, proliferation, differentiation and metastasis. Circulating tumor cell marker Implicated in self-renewal of stem cells |
| Carcinoembryonic antigen-CEA (CD66e) | Implicated in cell adhesion, anoikis resistance, promotion of metastasis |
| Integrin αvβ3 | Essential for endothelial cell survival. Involved in cell growth and migration, tumor invasion, metastasis and angiogenesis |
| Integrin α6β4 | Implicated in tumor cell growth, invasion and metastasis |
| Integrin αv | Endothelial adhesion receptor. Implicated in angiogenesis |
| Integrin α4 | Prognostic marker in lymphocytic leukemia |
| E-and P-Selectin | Implicated in tumor cell adhesion to vascular endothelium, migration, proliferation of cancer cells and step of metastasis formation |
| L-Selectin | Roles in cell adhesion and in initiation of cell-cell interaction in vasculature system. Initiates events leading to leukocyte extravasations for the blood. Biomarker of naïve T cells |
| Tyrosine kinase receptors | |
| EGFR | EGFR is a receptor tyrosine kinase expressed at the surface of cell. Involved in proliferation, invasion, angiogenesis and metastasis. Associated with poor prognosis |
| EGFRVIII | The most common form of EGFR mutation. Associated with poor survival of patients. Promotes tumor cell motility |
| PDGFR β | Cell surface tyrosine kinase receptor. Implicated in proliferation, migration and angiogenesis |
| HER-2 | Belongs to ErbB family (EGFR, ErbB2). Implicated in formation, progression of human tumor, aggressiveness and resistance to therapy. Implicated in poor prognosis |
| HER-3 | Member of the receptor tyrosine kinase. Contributes to increased drug resistance in HER2 cells |
| c-MET | Receptor tyrosine kinase involved in development and progression of cancer. Induces cell proliferation, cell survival, cell motility and invasion |
| Receptor tyrosine kinase-RETC634Y | Mutated form of RET (proto-oncogene) |
| Neurotrophin receptor TrkB | Cancer cell resistance to chemotherapy. Promotes growth of cancer cells. Implicated in cell proliferation, differentiation, survival, and invasion |
| TGFβ III receptor | Role in signal transduction. Role in disease is poorly understood but correlates with higher tumor grade |
| Protein Tyrosine Kinase 7-PTK7 | Biomarker for leukemia |
| Insulin Receptor | Receptor tyrosine kinase. Implicated in activation of MAPK/ERK and PI3K/AKT pathways Implicated in cancer development and progression |
| Axl | Belongs to TAM family of tyrosine kinase receptors. Implicated in invasiveness and metastasis |
| Cell membrane-associated enzyme | |
| Prostate specific membrane antigen-PSMA | Prostate tumor cell marker |
| Mucines | |
| MUC-1 | Cell surface mucin glycoprotein |
| Tumor necrosis factor receptor (TNF-R) and co-stimulatory receptors | |
| T-cell receptor OX40 | Member of TNF family receptors. Co-stimulatory receptor on CD4+ T cells. Increases immune response to cancer and foreign entities. Implicated in exacerbating effects of inflammation through the increased cytokine production (IL2) and in cell survival of CD4+ OX40+ T cells |
| T-cell receptor 4-1BB | Co-stimulatory receptor implicated in survival and expansion of activated T cells |
| Receptor activator of NF-κB-RANK | NFkB is a member of the tumor necrosis factor (TNF) receptor family. Implicated in osteoclast differentiation and in focal bone erosion and bone malignancies |
| CD28 | Co-stimulatory receptor implicated in activation of T lymphocytes |
| Surface transmembrane glycoproteins | |
| CD133 | Cancer stem cells marker |
| CD30 TNFRSF8 | Hematological malignancies biomarker |
| Others | |
| Cytotoxic T cell antigen-4-CTLA-4 | Receptor expressed on activated T cells. Reduction in T-cell responses and activation |
| B-cell–activating factor (BAFF)-receptor (BAFF-R) | BAFF is a member of the tumor necrosis factor (TNF) family cytokines. BAFF receptor is expressed on B-cells. Implicated in proliferation, maturation and cell survival of cancer B cells |
| CD124 (IL-4Rα) | Implicated in survival and in suppressor activity of myeloid derived suppressor cells |
| VCAM-1 | Member of the immunoglobulin-like super family. Implicated in recruitment of immune cells to inflammation sites |
| Toll-like receptor 3 ectodomain | Implicated in the production of INF-β and inflammatory cytokine |
| hyaluronic acid (HA) binding domain of CD44 | Belongs to the proteoglycan family of transmembrane glycoproteins. Receptor for hyaluronic acid. Role in tumor growth and metastasis. Marker for subpopulations of tumor initiating cells and implicated in chemo resistance |
| Angiopoietin-1 | Promotes endothelial cell survival and vascular impermeability. Prevents increase in endothelial cell adhesion molecule expression |
| Angiopoietin-2 | Potentialisation of proangiogenic growth factors |
| CD71 | Responsible for cellular iron transport. Rapidly proliferative cells require more iron. |
| Cofactor for enzyme involved in DNA synthesis of proliferative cells | |
| Multidrug resistant-associated protein 1-MRP1 | MRP1 correlates with chemotherapy drug resistance. Expressed in cancer stem cells |
| CD16α | Implicated in antibody dependent cellular cytotoxicity and recruitment of NK cells. NK cells are important for suppressing tumor metastasis and outgrowth |
| Alkaline phosphatase placental-like 2-ALPPL-2 | Implicated in cell growth and invasion. Ectopically expressed on cell surface and in cell secretion |
| Nucleolin | Multifunctional protein interacting with DNA and RNA. Involved in rRNA maturation, ribosome assembly and exportation of ribosome components to the cytoplasm |
| Role in mRNA stabilization (example: role in stabilization of bcl-2 mRNA in human leukemia). Overexpressed on the surface of certain cancer cells | |
| Immunoglobulin heavy mu chain | Major component of the B-cell antigen receptor in Burkitt-s lymphoma cells |
| Implicated in transforming healthy cells and in cancer progression | |
| Biomarker | Target Used for Protein-SELEX | Aptamer Library | Number of Variable Nucleotides | Number of Rounds of Selection | Separation Method | Name of Selected Aptamers | KD | Applications | Year | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Cell-adhesion molecules | ||||||||||
| Epithelial cell adhesion molecule-EpCAM (CD326) | His-tagged C-terminal domain of EpCAM | 2′F-RNA | 40 | 12 | affinity chromatography | Truncated EpCAM RNA aptamer | 55 nM to human cells | Target stem cell marker. Potential applications: development of targeted cancer nanomedecine and molecular imaging agents | 2011 | [45] |
| EpCAM | His-tagged C-terminal domain of EpCAM | DNA | 40 | 12 | affinity chromatography | Truncated aptamer SYL3C | 38 and 67 nM to cells | Imaging (confocal) Cancer cell capture | 2013 | [46] |
| Carcinoembryonic antigen- CEA (CD66e) | His-tagged recombinant protein of full-length CEA | 2′F-RNA | 40 | 17 | affinity chromatography | Group I, II and III | low nM range | Inhibition of cell migration/invasion in vivo. Promotion of cell anoikis resistance in vitro Inhibition of liver metastasis in vivo | 2012 | [47] |
| Integrin αvβ3 | purified αvβ3 integrin | 2′F-RNA | 50 | 15–17 | affinity chromatography | Apt-avb3 | nM range | Inhibition of endothelial cell adhesion and proliferation Reduction of endothelial cell tube formation Inhibition of cancer cell proliferation and adhesion (HUVEC) Increases endothelial cell apoptosis | 2005 | [48,49] |
| Integrin αvβ3 | purified αvβ3 integrin | 2′F-RNA | 50 | 6 | MAI-SELEX 1 | αV-1 and β3-1 | 8.9–10.5 nM | Recognizes distinct binding sites on a single target ( αV or β3) with minimal cross-reactivity Potential applications: molecular diagnosis and targeted therapies | 2012 | [50] |
| E-and P-Selectin | recombinant human E-selectin/IgG-Fc-chimeras | DNA | 50 | 17 | affinity chromatography | SDA | 100 nM | Inhibition of cancer cell adhesion Potential applications in therapies during metastasis formation | 2014 | [51] |
| L-Selectin | L-selectin-Ig chimera | 2′-NH2 RNA | 40 | 14 | affinity chromatography | 14.12 | 0.2–3 nM range | Preferential blokade of a specific selectin | 1996 | [52] |
| L-Selectin | L-selectin–IgG fusion protein | DNA | 40 | 17 | affinity chromatography | LD201, LD174 and LD196 | 1.8, 5.5 and 3.1 nM | Inhibition of lymphocyte rolling on endothelial cells | 1996 | [53] |
| Tyrosine kinase receptors | ||||||||||
| EGFR | purified extracellular domain of human EGFR | RNA | 62 | 12 | not documented | J18 | 7 nM | Drug delivery (internalization of gold nanoparticules) Potential application: delivery of siRNA and cancer detection | 2010 | [54] |
| EGFR | human EGFR-Fc protein | 2′F-RNA | 62 | 9 + 7–9 rounds with a 30% doped sequence (from aptamer E01) | affinity chromatography | E07 Internalized | 2.4 nM | Prevention of proliferation of tumor cells (blocks receptor autophosphorylation) Drug delivery (Gemcitabine) and induces cell death | 2011 | [55,56] |
| EGFR | purified extracellular domain of EGFR | DNA | 40 | 11 | affinity chromatography | Tutu-22 | 56 nM | Regognizes EGFR-positive cancer cells with strong affinity and selectivity Potential applications: development of novel targeted cancer detection, imaging and therapy | 2014 | [57] |
| EGFRVIII | histidine-tagged EGFRvIII ectodomain (E. coli. system) | 2′F-RNA | 40 | 12 | affinity chromatography | E21 | 33 nM | Disruption of post-translational modifications of immature EGFRvIII Induction of apoptosis | 2009 | [58] |
| HER-2 | recombinant glutathione S-transferase (GST)-tagged ErbB2 protein (22–122 amino acids) | 2′F-RNA | 50 | 15 | affinity chromatography | SE15-8 | low nM range | High specificity to ErbB2 and not to other members of the ErbB family Potential applications: drug delivery and imaging for in vivo diagnosis | 2011 | [59] |
| HER-2 | peptide from the juxtamembrane region of HER2 extracellular domain | DNA | 40 | multiple | affinity chromatography | HB5 | 18.9 nM | Drug delivery (Doxorubicin) | 2012 | [60] |
| HER-2 | 20-amino acid HER2 peptide | Thio-DNA | 21 | 12 | affinity chromatography | HY6 | 172 nM | Potential application: targeted therapy | 2015 | [61] |
| HER-2 | His-tagged Her2–extra cellular domain (E. coli system) | DNA | 40 | 15 | membrane filtration | ECD_Apt1 | 6.33 nM | Potential applications: theranostic (non invasive cancer diagnosis), therapeutics and monitoring patient compliance | 2017 | [62] |
| HER-3 | extracellular domains of HER3 produced in S2 insect cells | RNA | 49 | 15 | membrane filtration and gel shift assay | A30 | 0.1 nM range | Inhibition of HER3 activation and growth of tumor cells Potential application: anticancer drug | 2003 | [63] |
| c-MET | c-Met-Fc | DNA | 40 | 12 | membrane filtration | CLN3 and CLN4 | 91 pm and 11 nM | Mediates tumor cell lysis Recruits NK cells to tumor and induces ADCC | 2011 | [64] |
| c-MET | c-Met-Fc | 2′F-RNA | 40 | 16 | membrane filtration | CLN64 | 1 nM | Inhibition of tumor cell migration Potential application: therapeutics and diagnosis | 2015 | [65] |
| Cell membrane-associated enzyme | ||||||||||
| Prostate specific membrane antigen-PSMA | 706 extracellular amino acids of PMSA | 2′F-RNA | 40 | 6 | affinity chromatography | A9, A10, A10-3 after minimization & optimization | low nM range | Promotion of tumor regression Delivery of siRNA Potential application: diagnosis and therapies | 2002 | [66,67] |
| Mucins | ||||||||||
| MUC-1 | MUC-1 peptides of 2 lenghts: 9 and 60 amino acids | DNA | 25 | 10 | affinity chromatography | S1.3/S2.2 | low nM range to 0.1 nM | Potential application: detection by fluorescent microscopy | 2006 | [68] |
| MUC-1 | His-tagged unglycosylated form of the MUC1 protein containing five tandem repeats of the VTR (E. coli system) | DNA | 25 | 10 | affinity chromatography | MUC1-5TR-1, 2, 3, 4 | 47–85 nM | Potential application: diagnosis assays for early or metastatic diseases | 2008 | [69] |
| Tumor necrosis factor receptor (TNF-R) and co-stimulatory receptors | ||||||||||
| T-cell receptor OX40 | extracellular domain of OX40-Fc fusion protein | 2′F-RNA | 40 | 9–11 | affinity chromatography | 9C7, 11F11 | 2-10 nM for purified OX40 protein and # 50 nM for OX40 on activated T cells | Increasing proliferation of T lymphocytes and production of IFN-γ. Potential application: therapeutics in association with dendritic cell-based vaccines (adoptive cellular therapy) | 2013 | [70] |
| T-cell receptor OX40 | murine extracellular domain of OX40-Fc fusion protein | 2′F-RNA | 40 | 11 | affinity chromatography | 9.8 | 8 nM | Induces nuclear localization of NFκB, cytokine production and cell proliferation. Increases dendritic cell based tumor vaccine effects | 2008 | [71] |
| T-cell receptor 4-1BB | murine extracellular domain of 4-1BB-Fc fusion protein | 2′F-RNA | 40 | 12 | affinity chromatography | M12-23 (multimeric aptamer) | 40 nM | Inhibition of tumor growth in vivo. Potential application: therapeutic manipulation of the immune system | 2008 | [72] |
| Receptor activator of NF-κB-RANK | recombinant human soluble RANK/IgG1Fc chimera | RNA | 40 | 7 | affinity chromatography | apt1, apt2 and apt3 | 0.33, 1.8 and 5.8 μM. 100 nM for the 2′-F version of aptamers | Potential application: therapeutics against osteoclastogenesis | 2004 | [73] |
| CD28 2 | murine recombinant CD28-Fc fusion protein | 2′F-RNA | 25 | 9 | affinity chromatography | CD28Apt2 and CD28Apt7 | 60 nM for CD28Apt7-dimer | Potentialisation of antitumor vaccine efficacy Reduction of tumor progression and increased overall survival (in vivo) Potential application: enhancing vaccine-induced immune responses | 2013 | [74] |
| Others | ||||||||||
| Cytotoxic T cell Antigen-4-CTLA-4 | murine CTLA-4/Fc fusion protein | 2′F-RNA | 40 | 9 | membrane filtration | M9-9 | 30–60 nM | Increases tumor immunity (in vivo) Potential application: immunotherapy | 2003 | [75] |
| B-cell–activating factor (BAFF)-receptor (BAFF-R) | Human recombinant BAFF-R protein | 2′F-RNA | 50 | 12 | membrane filtration | R-1, R-2 and R-14 | 47, 95 and 96 nM | Delivery of siRNA. Potential application: combinatorial therapeutics | 2013 | [76] |
| CD124 (IL-4Rα) | recombinant ILR4α protein enzymatically cleaved | 2′F-RNA | 40 | 5 | affinity chromatography | cL42 | 14 nM for recombinant protein and 788 nM for MCS2 cells | Induction of MDSCS apoptosis Promotes CD8+ T cell infiltration and reduces the number of MDSCs infiltration. Reduction of tumor progression in vivo | 2012 | [77] |
| VCAM-1 | N-terminal fragment of VCAM-1 | 2′F-RNA | 40 | 12 | affinity chromatography | 12.11 | 10 nM | Potential application: imaging | 2007 | [78] |
| Toll-like receptor 3 ectodomain | Toll-like receptor 3 ectodomain with N-terminal FLAG and C-terminal His | RNA | 40 | 7 | membrane filtration | Family-I and Family II | # 3 nM | Aptamer without agonist and antagonist effects | 2006 | [79] |
| hyaluronic acid (HA) binding domain of CD44 | HA-binding domain of human CD44 (cell-free expression system) | Thio-DNA | 30 | 10 | affinity chromatography | TA1-TA6 | 180–295 nM | Potential applications targeted therapy and imaging | 2010 | [80] |
| CD44 | GST-tagged human recombinant full length CD44 protein | 2′F-RNA | 45 | 11 | affinity chromatography | Apt1 | 81.3 nM | Potential applications therapeutic (targeted delivery againt stem cells) and diagnosis | 2013 | [81] |
| Angiopoietin-1 | recombinant human Ang1 | 2′F-RNA | 40 | 9 | membrane filtration | ANG9-4 | 2.8 nM | Inhibition of cell endothelial cell survival | 2008 | [82] |
| Angiopoietin-2 | recombinant human Ang2 | 2′F-RNA | 40 | 11 | membrane filtration | 11-1 and truncated 11-1.41 | 3.1 and 2.2 nM | Inhibition of angiogeneis (in vivo) | 2003 | [83] |
| Biomarker | Aptamer Library | Number of Variable Positions | KD Range | Number of Positive Selection Rounds (selection method) 1 | Cells Used for Positive Selection | Cells Used for Counter-SELEX | Applications | References |
|---|---|---|---|---|---|---|---|---|
| Tyrosine kinase receptors | ||||||||
| EGFRvIII | DNA | 30 | 0.62–37.57 nM | 14 | U87-EGFRvIII, U87-MG, GBM cells overexpressing EGFRvIII | U87-MG | Potential applications: delivery of chemical drug and diagnosis | [117] |
| EGFRvIII | DNA | 40 | 3–16 nM | 11 | U87-MG, GBM cells overexpressing EGFRvIII | U87-MG | Imaging (radiolabeled 188Re, in vivo) Potential applications: diagnosis in stratifying patient and monitoring treatment | [118] |
| HER-2 | RNA | 40 | 94.6 nM | 20 | HER-2-overexpressing SK-BR-3 cell line | MDA-MB-231, a HER-2-underexpressing breast cancer cell line | Potential application: therapy | [113] |
| HER-2 | 2′F-RNA | 20 | 46–82 nM | 9 (cell-internalization SELEX) | N202.1A mammary carcinoma clonal cell lines expressing high levels of surface HER-2/neu. | N202.1 E clonal cell line has no detectable surface expression of the HER2/neu oncoprotein. | Drug delivery (Bcl-2 siRNA) Induces chemosensibilisation and reduces drug resistance | [100] |
| HER-2 | DNA | 50 | Not documented | 4 rounds | Cleared extract of ErbB-2-overexpressing N87 cells | Not documented | Acceleration of ErbB-2 degradation in lysosomes Inhibition of growth of tumor cell in vitro and tumor mass in vivo | [116] |
| HER-2 | DNA | 30 | 5–23 nM | 8 rounds of protein SELEX followed by 7 rounds of cell -SELEX (hybrid-SELEX) | HER-2 overexpressed in SKOV3 ovarian cancer cells | No cells | PET imaging (radio labeled, in vivo) | [109] |
| HER-2 | DNA | 40 | Not documented | 16 | HER2- overexpressing breast cancer cell line, SK-BR3 | HER2 negative breast cancer cell line, MDA-MB468 | Development of a new method to monitor the enrichment of aptamers in a given round of cell-SELEX | [114] |
| Receptor tyrosine kinase-RETC634Y | 2′F RNA | 50 | Tens of nM | 15 rounds of selection on cells, followed by 7 rounds on purified protein (hybrid-SELEX) | RETC634Y mutant receptor expressed in PC12 cells (PC12/MEN2A) | PC12 | Potential applications: diagnosis, imaging and therapy | [108] |
| Neurotrophin receptor TrkB | 2′F RNA | 20 | 2 nM | 4 (cell-internalization SELEX) | TrkB-expressing HEK cells | HEK | Neuroprotective effects Potential in therapy for neuro degenerative disease | [119] |
| Other kinase receptors | ||||||||
| TGFβ III receptor | 2′F RNA | 60 | 1 nM | 11 | TGFβ III receptor ectopically expressed on CHO-K1 cells | CHO-K1 | Potential application: therapy through inhibition of TGFβIII receptor | [102] |
| Transferrin receptors (TfR) | ||||||||
| CD71 | 2′F-RNA | 50 | nM range | 4 rounds of protein-SELEX on the His-tagged recombinant protein and 1 round on cells (hybrid SELEX combined with cell-internalization SELEX) | HeLa cells, a human cervical cancer cell line known to express TfR | NO cells | Delivery of siRNA Targets cell in liposomes | [94] |
| ATP-binding cassette (ABC) transporters | ||||||||
| Multidrug resistant-associated protein 1-MRP1 | 2′F-RNA | 25 | 50 nM | 10 rounds of peptide-SELEX followed by 1 round of cell-SELEX (hybrid-SELEX) | chemotherapy-resistant tumor cell line that has high MRP1 expression (H69AR) | Parental cell line H69 | Reduction of cell growth in vitro and improved survival in vivo | [111] |
| Cell adhesion molecules | ||||||||
| Epithelial cell adhesion molecule-EpCAM (CD326) | DNA | 40 | μM range | 7 (FACS-SELEX) | EpCAM over-expressed in HepG2 cells | HepG2 cells | Potential application: stem cell marker | [120] |
| Integrin α6β4 | DNA | 39 | 139 nM | 5 rounds of cell-SELEX followed by 7 rounds of protein-SELEX (hybrid-SELEX) | PC-3 cells | PC-3 β4 integrin (ITGB4) knockdown cells | Imaging (confocal) Potential application: drug delivery | [110] |
| Integrin αv | RNA | 35 & 40 | 359–408 nM | 13 (Isogenic cell-SELEX) | Human HEK293 cells manipulated to generate positive αv selection cells by overexpressing ITGAV | Human HEK293 cells depleted in ITGAV with microRNA-mediated silencing. | Potential application: targeting channels, transporters… | [112] |
| Fc receptors | ||||||||
| CD16α | DNA | 40 | 6–429 nM | 9 rounds of protein-SELEX and 6 rounds of cell-SELEX (hybrid-SELEX) | CD16_-6His and CD16_ Val-158 or Phe-158 alloforms expressed on recombinant Jurkat cells | Jurkat E6.1 cell line | ADCC (tumor cell lysis) | [64] |
| Surface transmembrane glycoproteins | ||||||||
| CD133 | 2′F RNA | 40 | Not determined | 6 | HEK293T expressing CD133 | His-tagged irrelevant protein expressed in HEK293T | Potential application: target cancer stem cells and molecular imaging | [121] |
| CD30 TNFRSF8 | DNA | 30 | nM range | 20 rounds of cell-SELEX followed by 5 rounds on purified His-tagged CD30 protein (hybrid-SELEX) | CD30-positive K299 T-cells lymphoma | Jurkat cells | Potential application: therapy | [115] |
| Biomarker | Aptamer Library | Applications | References |
|---|---|---|---|
| EGFR | 2′F-RNA | Induces EGFR-mediated signal pathways causing selective cell death Inhibits tumor growth in vivo Combined cetuximab-aptamer treatment shows clear synergy in inducing apoptosis in vitro and in vivo Potential application: translational therapy | [130] |
| PDGFR β | 2′F-RNA | Inhibition of receptor signaling and of glioblastoma-derived tumor growth Inhibition of cell migration and proliferation Induction of differentiation | [99] |
| Alkaline phosphatase placental-like 2-ALPPL-2 | 2′F-RNA | Targets ALPPL-2 in both membrane bound and secretary forms Potential applications: diagnosis, imaging and therapy | [131] |
| selectin L and integrin α4 | DNA | Potential application: therapeutic intervention | [132] |
| CD44/CD24 | DNA | Potential applications: disruption of therapeutic resistance, invasion and angiogenesis | [133] |
| CD44 | DNA | Potential applications: cancer detection, imaging and drug delivery | [134] |
| Protein Tyrosine Kinase 7-PTK7 | DNA | Potential application: drug delivery | [135] |
| Insulin Receptor | 2′-fluoro RNA | Inhibition of IR signaling Reduction of cell viability Potential applications: targeted therapies | [136] |
| Axl | 2′F-RNA | Interferes with cell migration and invasion Inhibition of spheroid formation and cell transformation Inhibition of tumor growth | [137] |
| Nucleolin | DNA | Interferes with multiple biological activities in tumor cells Induction of apoptosis via down-regulation of bcl-2 proteins | [84,127,128,129] |
| Immunoglobulin heavy mu chain | DNA | Role in identification of cell membrane receptor with increased expression levels Potential applications: early diagnosis, targeted therapy and mechanistic studies | [138] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercier, M.-C.; Dontenwill, M.; Choulier, L. Selection of Nucleic Acid Aptamers Targeting Tumor Cell-Surface Protein Biomarkers. Cancers 2017, 9, 69. https://doi.org/10.3390/cancers9060069
Mercier M-C, Dontenwill M, Choulier L. Selection of Nucleic Acid Aptamers Targeting Tumor Cell-Surface Protein Biomarkers. Cancers. 2017; 9(6):69. https://doi.org/10.3390/cancers9060069
Chicago/Turabian StyleMercier, Marie-Cécile, Monique Dontenwill, and Laurence Choulier. 2017. "Selection of Nucleic Acid Aptamers Targeting Tumor Cell-Surface Protein Biomarkers" Cancers 9, no. 6: 69. https://doi.org/10.3390/cancers9060069






