EMT/MET at the Crossroad of Stemness, Regeneration and Oncogenesis: The Ying-Yang Equilibrium Recapitulated in Cell Spheroids
Abstract
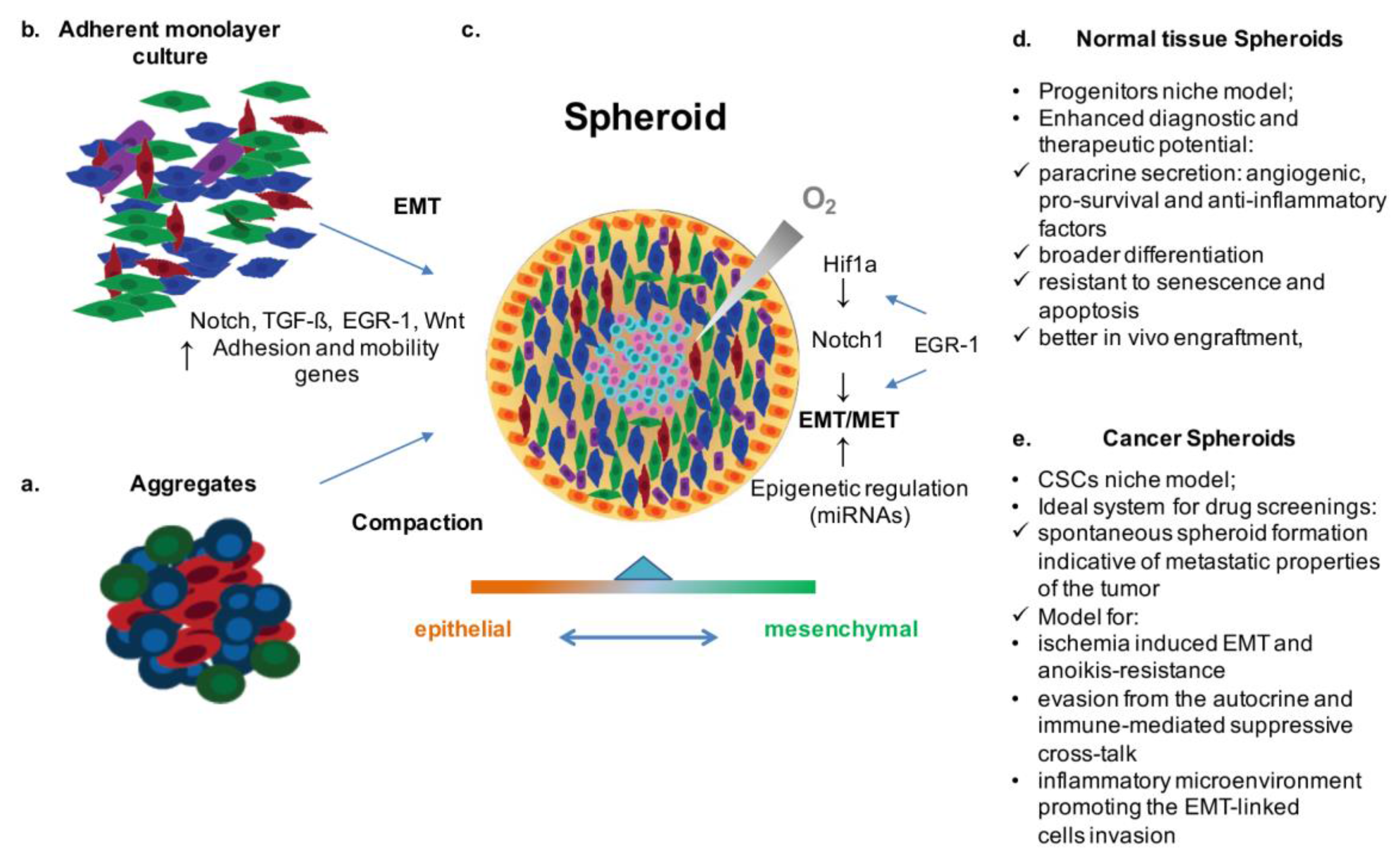
:Highlights:
- ●
- EMT/MET play a pivotal role in cell fate decision making for both normal and transformed cells.
- ●
- Thus, these mechanisms represent a strategic target for preclinical (basic studies, pharmacologic screening, and biotechnology advances), as well as clinical applications (personalized diagnosis and therapy).
- ●
- EMT/MET are finely reproduced within cell spheroid systems, which, as in vitro models of normal and transformed stem cell (SC) niches, represent an adequate cost/benefit biotechnological tool to investigate disease mechanisms, therapeutic targets, and related applications.
1. Introduction
2. EMT and Stemness in Physiological and Transformed Tissues
3. EMT-Induced Spheroids as an in Vitro Model of Stem Cell Niches and Tumors
4. Discovering Pharmacological Targets in Spheroid Model: The Case of EGR-1/TGF-β Network
5. Conclusions and Future Perspectives
Conflicts of Interest
References
- Stankevicius, V.; Kunigenas, L.; Stankunas, E.; Kuodyte, K.; Strainiene, E.; Cicenas, J.; Samalavicius, N.E.; Suziedelis, K. The expression of cancer stem cell markers in human colorectal carcinoma cells in a microenvironment dependent manner. Biochem Biophys Res Commun 2017, 484, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.; Everett, W.; Lim, S.; Natasha, G.; Loizidou, M.; Jell, G.; Tan, A.; Seifalian, A.M. Personalized in vitro cancer modeling––Fantasy or reality? Transl. Oncol. 2014, 7, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.H.; Yu, S.; Hayward, S.W.; Chan, F.L. Development of a three-dimensional culture model of prostatic epithelial cells and its use for the study of epithelial-mesenchymal transition and inhibition of PI3K pathway in prostate cancer. Prostate 2009, 69, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, T.G.; Mujoomdar, M.L.; Nachtigal, M.W. Constitutive activation of bmp signalling abrogates experimental metastasis of ovca429 cells via reduced cell adhesion. J. Ovarian Res. 2010. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, A.; Santini, D.; Bonafe, M.; Taffurelli, M.; Avenia, N. Interleukin-6 and pro inflammatory status in the breast tumor microenvironment. World J. Surg. Oncol. 2015, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.; Posern, G. Tightly controlled mrtf-a activity regulates epithelial differentiation during formation of mammary acini. Breast Cancer Res. 2017, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The role of snail in EMT and tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Malfettone, A.; Soukupova, J. New insights into the crossroads between EMT and stemness in the context of cancer. J. Clin. Med. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brabletz, T. Emt and met in metastasis: Where are the cancer stem cells? Cancer Cell 2012, 22, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, E.; Silva-Vargas, V.; Doetsch, F. Eyes wide open: A critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 2011, 8, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.R.; McClay, D.R. Sub-circuits of a gene regulatory network control a developmental epithelial-mesenchymal transition. Development 2014, 141, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Wellner, U.; Schubert, J.; Burk, U.C.; Schmalhofer, O.; Zhu, F.; Sonntag, A.; Waldvogel, B.; Vannier, C.; Darling, D.; zur Hausen, A.; et al. The emt-activator zeb1 promotes tumorigenicity by repressing stemness-inhibiting micrornas. Nat. Cell Biol. 2009, 11, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Shimono, Y.; Zabala, M.; Cho, R.W.; Lobo, N.; Dalerba, P.; Qian, D.; Diehn, M.; Liu, H.; Panula, S.P.; Chiao, E.; et al. Downregulation of mirna-200c links breast cancer stem cells with normal stem cells. Cell 2009, 138, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The mir-200 family and mir-205 regulate epithelial to mesenchymal transition by targeting zeb1 and sip1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Chao, C.H.; Xia, W.; Yang, J.Y.; Xiong, Y.; Li, C.W.; Yu, W.H.; Rehman, S.K.; Hsu, J.L.; Lee, H.H.; et al. P53 regulates epithelial-mesenchymal transition and stem cell properties through modulating mirnas. Nat. Cell Biol. 2011, 13, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Nishino, J.; Kim, I.; Chada, K.; Morrison, S.J. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16ink4a and p19arf expression. Cell 2008, 135, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Saha, K.; Pando, B.; van Zon, J.; Lengner, C.J.; Creyghton, M.P.; van Oudenaarden, A.; Jaenisch, R. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 2009, 462, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, X.; Zhong, X.; Kaur, S.; Li, N.; Liang, S.; Lassus, H.; Wang, L.; Katsaros, D.; Montone, K.; et al. Double-negative feedback loop between reprogramming factor lin28 and microrna let-7 regulates aldehyde dehydrogenase 1-positive cancer stem cells. Cancer Res 2010, 70, 9463–9472. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Feng, J.; Cui, X.; Huang, W.; Li, Y.; Su, F.; Liu, Q.; Zhu, J.; Lv, X.; et al. Lin28 induces epithelial-to-mesenchymal transition and stemness via downregulation of let-7a in breast cancer cells. PLoS ONE 2013, 8, e83083. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Martin, P. Wound repair: A showcase for cell plasticity and migration. Curr. Opin. Cell Biol. 2016, 42, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Arnoux, V.; Nassour, M.; L'Helgoualc'h, A.; Hipskind, R.A.; Savagner, P. Erk5 controls slug expression and keratinocyte activation during wound healing. Mol. Biol. Cell 2008, 19, 4738–4749. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.S.; Zhang, Y.; Li, T.; Smith, R.R.; Chimenti, I.; Terrovitis, I.; Davis, D.R.; Kizana, E.; Ho, A.S.; O'Rourke, B.; et al. Functional impairment of human resident cardiac stem cells by the cardiotoxic antineoplastic agent trastuzumab. Stem Cells Transl. Med. 2012, 1, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Pagano, F.; Angelini, F.; Siciliano, C.; Tasciotti, J.; Mangino, G.; De Falco, E.; Carnevale, R.; Sciarretta, S.; Frati, G.; Chimenti, I. Beta2-adrenergic signaling affects the phenotype of human cardiac progenitor cells through emt modulation. Pharmacol Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.E.; Chapman, H.A. Regenerative activity of the lung after epithelial injury. Biochim. Biophys. Acta 2013, 1832, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, I.; Pagano, F.; Angelini, F.; Siciliano, C.; Mangino, G.; Picchio, V.; De Falco, E.; Peruzzi, M.; Carnevale, R.; Ibrahim, M.; et al. Human lung spheroids as in vitro niches of lung progenitor cells with distinctive paracrine and plasticity properties. Stem Cells Transl. Med. 2017, 6, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- McCoy, E.L.; Iwanaga, R.; Jedlicka, P.; Abbey, N.S.; Chodosh, L.A.; Heichman, K.A.; Welm, A.L.; Ford, H.L. Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 2663–2677. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Jiang, J.K.; Yang, S.H.; Huang, T.S.; Lan, H.Y.; Teng, H.W.; Yang, C.Y.; Tsai, Y.P.; Lin, C.H.; Wang, H.W.; et al. Microrna-146a directs the symmetric division of snail-dominant colorectal cancer stem cells. Nat. Cell Biol. 2014, 16, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Tam, W.L.; Shibue, T.; Kaygusuz, Y.; Reinhardt, F.; Ng Eaton, E.; Weinberg, R.A. Distinct emt programs control normal mammary stem cells and tumour-initiating cells. Nature 2015, 525, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, J.; Ni, S.; Zhou, T.; Qing, X.; Li, H.; He, W.; Chen, J.; Li, F.; Zhuang, Q.; et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 2010, 7, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Sasaki, A.; Yamamoto, M.; Nakamura, M.; Sutou, K.; Osafune, K.; Yamanaka, S. Induction of pluripotency in human somatic cells via a transient state resembling primitive streak-like mesendoderm. Nat Commun 2014, 5, 3678. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Abell, A.N.; Jordan, N.V.; Huang, W.; Prat, A.; Midland, A.A.; Johnson, N.L.; Granger, D.A.; Mieczkowski, P.A.; Perou, C.M.; Gomez, S.M.; et al. Map3k4/cbp-regulated h2b acetylation controls epithelial-mesenchymal transition in trophoblast stem cells. Cell Stem Cell 2011, 8, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.V.; Johnson, G.L.; Abell, A.N. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle 2011, 10, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Jia, D.; Boareto, M.; Mani, S.A.; Pienta, K.J.; Ben-Jacob, E.; Levine, H. Coupling the modules of emt and stemness: A tunable "stemness window" model. Oncotarget 2015, 6, 25161–25174. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C. Filling the gaps between the in vivo and in vitro microenvironment: Engineering of spheroids for stem cell technology. Curr. Stem Cell Res. Ther. 2016, 11, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.; Bernier, D.; Ouellet, C.; Goyette, R.; Marceau, N. Spheroidal aggregate culture of rat liver cells: Histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J. Cell Biol. 1985, 101, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.A.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef] [PubMed]
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.; Coletta, M.; et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, G.; Pagano, S.; Bez, A.; Benetti, A.; Pozzi, S.; Iannolo, G.; Baronio, M.; Invernici, G.; Caruso, A.; Muneretto, C.; et al. Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages. Lancet 2004, 364, 1872–1883. [Google Scholar] [CrossRef]
- Fierabracci, A.; Caione, P.; Di Giovine, M.; Zavaglia, D.; Bottazzo, G.F. Identification and characterization of adult stem/progenitor cells in the human bladder (bladder spheroids): Perspectives of application in pediatric surgery. Pediatr. Surg. Int. 2007, 23, 837–839. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, M.A.; Giuliani, L.; Fierabracci, A. Identification and characterization of a novel expandable adult stem/progenitor cell population in the human exocrine pancreas. J. Endocrinol. Investig. 2008, 31, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Fierabracci, A.; Puglisi, M.A.; Giuliani, L.; Mattarocci, S.; Gallinella-Muzi, M. Identification of an adult stem/progenitor cell-like population in the human thyroid. J. Endocrinol. 2008, 198, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Johnston, P.; Zhang, B.; Zakari, A.; Chowdhry, T.; Smith, R.R.; Marban, E.; Rabb, H.; Womer, K.L. Kidney-derived stromal cells modulate dendritic and t cell responses. J. Am. Soc. Nephrol. 2009, 20, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Farnie, G.; Clarke, R.B. Mammary stem cells and breast cancer––Role of notch signalling. Stem Cell Rev. 2007, 3, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.; Cores, J.; Hensley, M.T.; Anthony, S.; Vandergriff, A.; de Andrade, J.B.; Allen, T.; Caranasos, T.G.; Lobo, L.J.; Cheng, K. Adult lung spheroid cells contain progenitor cells and mediate regeneration in rodents with bleomycin-induced pulmonary fibrosis. Stem Cells Transl. Med. 2015, 4, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Cesarz, Z.; Tamama, K. Spheroid culture of mesenchymal stem cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.; Cheng, K.; Lee, S.T.; Matsushita, S.; Davis, D.; Malliaras, K.; Zhang, Y.; Matsushita, N.; Smith, R.R.; Marban, E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells 2010, 28, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, I.; Massai, D.; Morbiducci, U.; Beltrami, A.P.; Pesce, M.; Messina, E. Stem cell spheroids and ex vivo niche modeling: Rationalization and scaling-up. J. Cardiovasc. Transl. Res. 2017, 10, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Mohyeldin, A.; Garzon-Muvdi, T.; Quinones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Cipolleschi, M.G.; Dello Sbarba, P.; Olivotto, M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 1993, 82, 2031–2037. [Google Scholar] [PubMed]
- Kluppel, M.; Wrana, J.L. Turning it up a notch: Cross-talk between TGF beta and notch signaling. Bioessays 2005, 27, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.M.; Inch, W.R.; McCredie, J.A.; Kruuv, J. A multi-component radiation survival curve using an in vitro tumour model. Int J Radiat Biol Relat Stud Phys Chem Med 1970, 18, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, A.; Bussink, J.; Sweep, F.C.; Span, P.N. Generation of multicellular tumor spheroids of breast cancer cells: How to go three-dimensional. Anal Biochem 2013, 437, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Siu, A.; Ramos, D.M. Multicellular spheroids as a model for hypoxia-induced EMT. Anticancer Res. 2016, 36, 6259–6263. [Google Scholar] [CrossRef] [PubMed]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Hsu, S.H. Acquisition of epithelial-mesenchymal transition and cancer stem-like phenotypes within chitosan-hyaluronan membrane-derived 3d tumor spheroids. Biomaterials 2014, 35, 10070–10079. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, C.; Kapadia, A.; Zhou, Q.; Harper, M.K.; Schaack, J.; LaBarbera, D.V. 3d models of epithelial-mesenchymal transition in breast cancer metastasis: High-throughput screening assay development, validation, and pilot screen. J. Biomol. Screen. 2011, 16, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3d complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, I.; Gaetani, R.; Barile, L.; Forte, E.; Ionta, V.; Angelini, F.; Frati, G.; Messina, E.; Giacomello, A. Isolation and expansion of adult cardiac stem/progenitor cells in the form of cardiospheres from human cardiac biopsies and murine hearts. Methods Mol. Biol. 2012, 879, 327–338. [Google Scholar] [PubMed]
- Huang, G.S.; Dai, L.G.; Yen, B.L.; Hsu, S.H. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.Y.; Liu, B.H.; Sieber, M.; Hsu, S.H. Substrate-dependent gene regulation of self-assembled human msc spheroids on chitosan membranes. BMC Genom. 2014, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Miraldi, F.; Chimenti, I.; Angelini, F.; Zeuner, A.; Giacomello, A.; Mercola, M.; Messina, E. Tgfbeta-dependent epithelial-to-mesenchymal transition is required to generate cardiospheres from human adult heart biopsies. Stem Cells Dev. 2012, 21, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Pease, J.C.; Brewer, M.; Tirnauer, J.S. Spontaneous spheroid budding from monolayers: A potential contribution to ovarian cancer dissemination. Biol. Open 2012, 1, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, S.; Ramos Valdes, Y.; Bertrand, M.; McGee, J.; Prefontaine, M.; Sugimoto, A.; DiMattia, G.E.; Shepherd, T.G. TGFbeta signaling regulates epithelial-mesenchymal plasticity in ovarian cancer ascites-derived spheroids. Endocr. Relat. Cancer 2016, 23, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.A.; Rezende, C.O., Jr.; Chantarasriwong, O.; Corben, A.D.; Theodorakis, E.A.; Alpaugh, M.L. Spontaneously-forming spheroids as an in vitro cancer cell model for anticancer drug screening. Oncotarget 2015, 6, 21255–21267. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Beerling, E.; Seinstra, D.; de Wit, E.; Kester, L.; van der Velden, D.; Maynard, C.; Schafer, R.; van Diest, P.; Voest, E.; van Oudenaarden, A.; et al. Plasticity between epithelial and mesenchymal states unlinks emt from metastasis-enhancing stem cell capacity. Cell Rep. 2016, 14, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Preobrazhenska, O.; auf dem Keller, U.; Sutcliffe, M.; Barclay, L.; McDonald, P.C.; Roskelley, C.; Overall, C.M.; Dedhar, S. Epithelial-mesenchymal transition (emt) is not sufficient for spontaneous murine breast cancer metastasis. Dev. Dyn. 2008, 237, 2755–2768. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Wong, M.K.; Tan, T.Z.; Kuay, K.T.; Ng, A.H.; Chung, V.Y.; Chu, Y.S.; Matsumura, N.; Lai, H.C.; Lee, Y.F.; et al. An emt spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (azd0530). Cell Death Dis. 2013, 4, e915. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Kondratyev, M.; Kreso, A.; Hallett, R.M.; Girgis-Gabardo, A.; Barcelon, M.E.; Ilieva, D.; Ware, C.; Majumder, P.K.; Hassell, J.A. Gamma-secretase inhibitors target tumor-initiating cells in a mouse model of erbb2 breast cancer. Oncogene 2012, 31, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Yang, F.; Chen, B.F.; Lu, Z.; Huo, X.S.; Zhou, W.P.; Wang, F.; Sun, S.H. The histone deacetylase 4/sp1/microrna-200a regulatory network contributes to aberrant histone acetylation in hepatocellular carcinoma. Hepatology 2011, 54, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Celia-Terrassa, T.; Liu, D.D.; Choudhury, A.; Hang, X.; Wei, Y.; Zamalloa, J.; Alfaro-Aco, R.; Chakrabarti, R.; Jiang, Y.Z.; Koh, B.I.; et al. Normal and cancerous mammary stem cells evade interferon-induced constraint through the mir-199a-lcor axis. Nat. Cell Biol. 2017, 19, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.S.; Su, X.F.; Fu, X.L.; Wu, G.Z.; Luo, K.L.; Fang, Z.; Yu, F.; Liu, H.; Hu, H.J.; Chen, L.S.; et al. Mesenchymal stem cells promote pancreatic adenocarcinoma cells invasion by transforming growth factor-beta1 induced epithelial-mesenchymal transition. Oncotarget 2016, 7, 41294–41305. [Google Scholar] [CrossRef] [PubMed]
- Trivanovic, D.; Jaukovic, A.; Krstic, J.; Nikolic, S.; Okic Djordjevic, I.; Kukolj, T.; Obradovic, H.; Mojsilovic, S.; Ilic, V.; Santibanez, J.F.; et al. Inflammatory cytokines prime adipose tissue mesenchymal stem cells to enhance malignancy of mcf-7 breast cancer cells via transforming growth factor-beta1. IUBMB Life 2016, 68, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Dancea, H.C.; Shareef, M.M.; Ahmed, M.M. Role of radiation-induced tgf-beta signaling in cancer therapy. Mol. Cell Pharmacol. 2009, 1, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, X.; Jin, T.; Wang, Y.; Hong, C.S.; Tan, L.; Dai, T.; Wu, L.; Zhuang, Z.; Shi, C. Increase in the radioresistance of normal skin fibroblasts but not tumor cells by mechanical injury. Cell Death Dis. 2017, 8, e2573. [Google Scholar] [CrossRef] [PubMed]
- Bhola, N.E.; Balko, J.M.; Dugger, T.C.; Kuba, M.G.; Sanchez, V.; Sanders, M.; Stanford, J.; Cook, R.S.; Arteaga, C.L. Tgf-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J. Clin. Investig. 2013, 123, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Ning, H.; Ishida, W.; Sodin-Semrl, S.; Takagawa, S.; Mori, Y.; Varga, J. The early-immediate gene egr-1 is induced by transforming growth factor-beta and mediates stimulation of collagen gene expression. J. Biol. Chem. 2006, 281, 21183–21197. [Google Scholar] [CrossRef] [PubMed]
- Bastianelli, D.; Siciliano, C.; Puca, R.; Coccia, A.; Murdoch, C.; Bordin, A.; Mangino, G.; Pompilio, G.; Calogero, A.; De Falco, E. Influence of egr-1 in cardiac tissue-derived mesenchymal stem cells in response to glucose variations. Biomed. Res. Int. 2014, 2014, 254793. [Google Scholar] [CrossRef] [PubMed]
- Baron, V.; Adamson, E.D.; Calogero, A.; Ragona, G.; Mercola, D. The transcription factor egr1 is a direct regulator of multiple tumor suppressors including tgfbeta1, pten, p53, and fibronectin. Cancer Gene Ther. 2006, 13, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.; Arcella, A.; De Gregorio, G.; Porcellini, A.; Mercola, D.; Liu, C.; Lombari, V.; Zani, M.; Giannini, G.; Gagliardi, F.M.; et al. The early growth response gene egr-1 behaves as a suppressor gene that is down-regulated independent of arf/mdm2 but not p53 alterations in fresh human gliomas. Clin. Cancer Res. 2001, 7, 2788–2796. [Google Scholar] [PubMed]
- Calogero, A.; Lombari, V.; De Gregorio, G.; Porcellini, A.; Ucci, S.; Arcella, A.; Caruso, R.; Gagliardi, F.M.; Gulino, A.; Lanzetta, G.; et al. Inhibition of cell growth by egr-1 in human primary cultures from malignant glioma. Cancer Cell Int. 2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Zhang, S.S.; Saito, K.; Williams, S.; Arimura, Y.; Ma, Y.; Ke, Y.; Baron, V.; Mercola, D.; Feng, G.S.; et al. Pten regulation by akt-egr1-arf-pten axis. EMBO J. 2009, 28, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Deng, H.; Fang, Y. Pten deletion potentiates invasion of colorectal cancer spheroidal cells through 3d matrigel. Integr. Biol. (Camb.) 2015, 7, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, S.; Fortin, J.; Sasik, R.; Robitaille, L.; Corbeil, J.; de Belle, I. The transcription factor egr1 regulates the hif-1alpha gene during hypoxia. Mol. Carcinog. 2009, 48, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Shimoyamada, H.; Yazawa, T.; Sato, H.; Okudela, K.; Ishii, J.; Sakaeda, M.; Kashiwagi, K.; Suzuki, T.; Mitsui, H.; Woo, T.; et al. Early growth response-1 induces and enhances vascular endothelial growth factor-a expression in lung cancer cells. Am. J. Pathol. 2010, 177, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.M.; Lee, S.Y.; Ju, M.K.; Kim, C.H.; Park, H.G.; Kang, H.S. Early growth response 1 regulates glucose deprivation-induced necrosis. Oncol. Rep. 2013, 29, 669–675. [Google Scholar] [PubMed]
- Kim, J.; Kang, S.M.; Lee, H.J.; Choi, S.Y.; Hong, S.H. Oxytocin inhibits head and neck squamous cell carcinoma cell migration by early growth response-1 upregulation. Anticancer Drugs 2017, 28, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Sarver, A.L.; Li, L.; Subramanian, S. Microrna mir-183 functions as an oncogene by targeting the transcription factor egr1 and promoting tumor cell migration. Cancer Res. 2010, 70, 9570–9580. [Google Scholar] [CrossRef] [PubMed]
- Verduci, L.; Azzalin, G.; Gioiosa, S.; Carissimi, C.; Laudadio, I.; Fulci, V.; Macino, G. Microrna-181a enhances cell proliferation in acute lymphoblastic leukemia by targeting egr1. Leuk Res. 2015, 39, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Santillan, K.; Melendez-Zajgla, J.; Jimenez-Hernandez, L.E.; Gaytan-Cervantes, J.; Munoz-Galindo, L.; Pina-Sanchez, P.; Martinez-Ruiz, G.; Torres, J.; Garcia-Lopez, P.; Gonzalez-Torres, C.; et al. Nf-kappabeta-inducing kinase regulates stem cell phenotype in breast cancer. Sci. Rep. 2016, 6, 37340. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Dass, C.R.; Sun, L.Q.; Khachigian, L.M. Inhibition of human breast carcinoma proliferation, migration, chemoinvasion and solid tumour growth by dnazymes targeting the zinc finger transcription factor egr-1. Nucleic Acids Res. 2004, 32, 3065–3069. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.G.; Huang, R.; Yao, C.; Li, S.; Yang, W.; Yang, D.; Huang, R.P. Concurrent down-regulation of egr-1 and gelsolin in the majority of human breast cancer cells. Cancer Genom. Proteom. 2007, 4, 377–385. [Google Scholar] [PubMed]
- Irwin, M.E.; Johnson, B.P.; Manshouri, R.; Amin, H.M.; Chandra, J. A nox2/egr-1/fyn pathway delineates new targets for tki-resistant malignancies. Oncotarget 2015, 6, 23631–23646. [Google Scholar] [CrossRef] [PubMed]
- Grotegut, S.; von Schweinitz, D.; Christofori, G.; Lehembre, F. Hepatocyte growth factor induces cell scattering through mapk/egr-1-mediated upregulation of snail. EMBO J. 2006, 25, 3534–3545. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers (Basel) 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ayinde, O.; Wang, Z.; Griffin, M. Tissue transglutaminase induces epithelial-mesenchymal-transition and the acquisition of stem cell like characteristics in colorectal cancer cells. Oncotarget 2017, 8, 20025–20041. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Cho, S.J.; Chang, K.K.; Park, D.J.; Ryeom, S.W.; Yoon, S.S. Role of rac1 pathway in epithelial-to-mesenchymal transition and cancer stem-like cell phenotypes in gastric adenocarcinoma. Mol. Cancer Res. 2017. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forte, E.; Chimenti, I.; Rosa, P.; Angelini, F.; Pagano, F.; Calogero, A.; Giacomello, A.; Messina, E. EMT/MET at the Crossroad of Stemness, Regeneration and Oncogenesis: The Ying-Yang Equilibrium Recapitulated in Cell Spheroids. Cancers 2017, 9, 98. https://doi.org/10.3390/cancers9080098
Forte E, Chimenti I, Rosa P, Angelini F, Pagano F, Calogero A, Giacomello A, Messina E. EMT/MET at the Crossroad of Stemness, Regeneration and Oncogenesis: The Ying-Yang Equilibrium Recapitulated in Cell Spheroids. Cancers. 2017; 9(8):98. https://doi.org/10.3390/cancers9080098
Chicago/Turabian StyleForte, Elvira, Isotta Chimenti, Paolo Rosa, Francesco Angelini, Francesca Pagano, Antonella Calogero, Alessandro Giacomello, and Elisa Messina. 2017. "EMT/MET at the Crossroad of Stemness, Regeneration and Oncogenesis: The Ying-Yang Equilibrium Recapitulated in Cell Spheroids" Cancers 9, no. 8: 98. https://doi.org/10.3390/cancers9080098




