The PI3Kδ Inhibitor Idelalisib Inhibits Homing in an in Vitro and in Vivo Model of B ALL
Abstract
:1. Introduction
2. Results
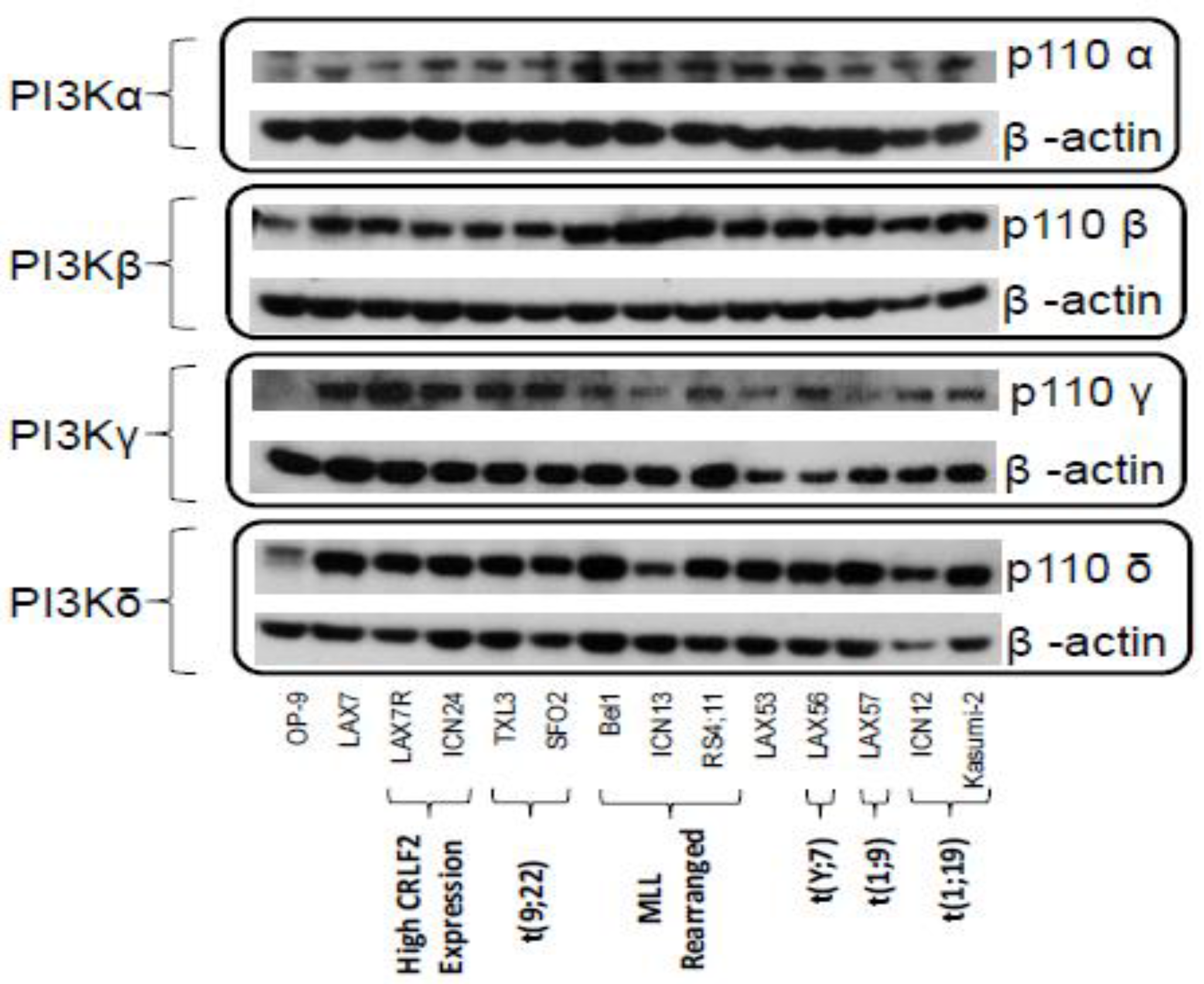
2.1. PI3K Isoforms are Expressed in B ALL Cells
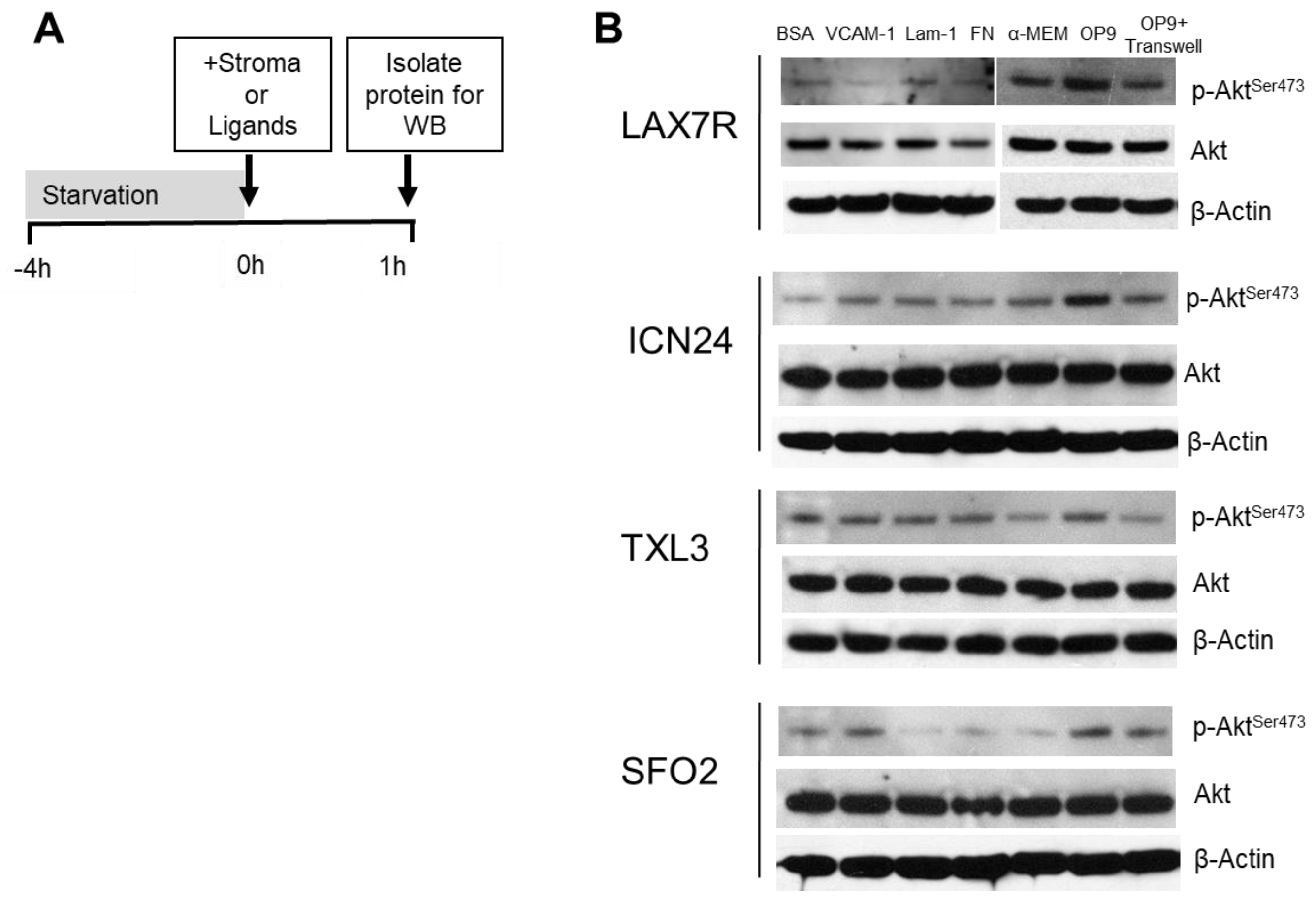
2.2. p-Aktser473 Activation by Ligands and Stroma
2.3. Idelalisib Decreases p-Aktser473
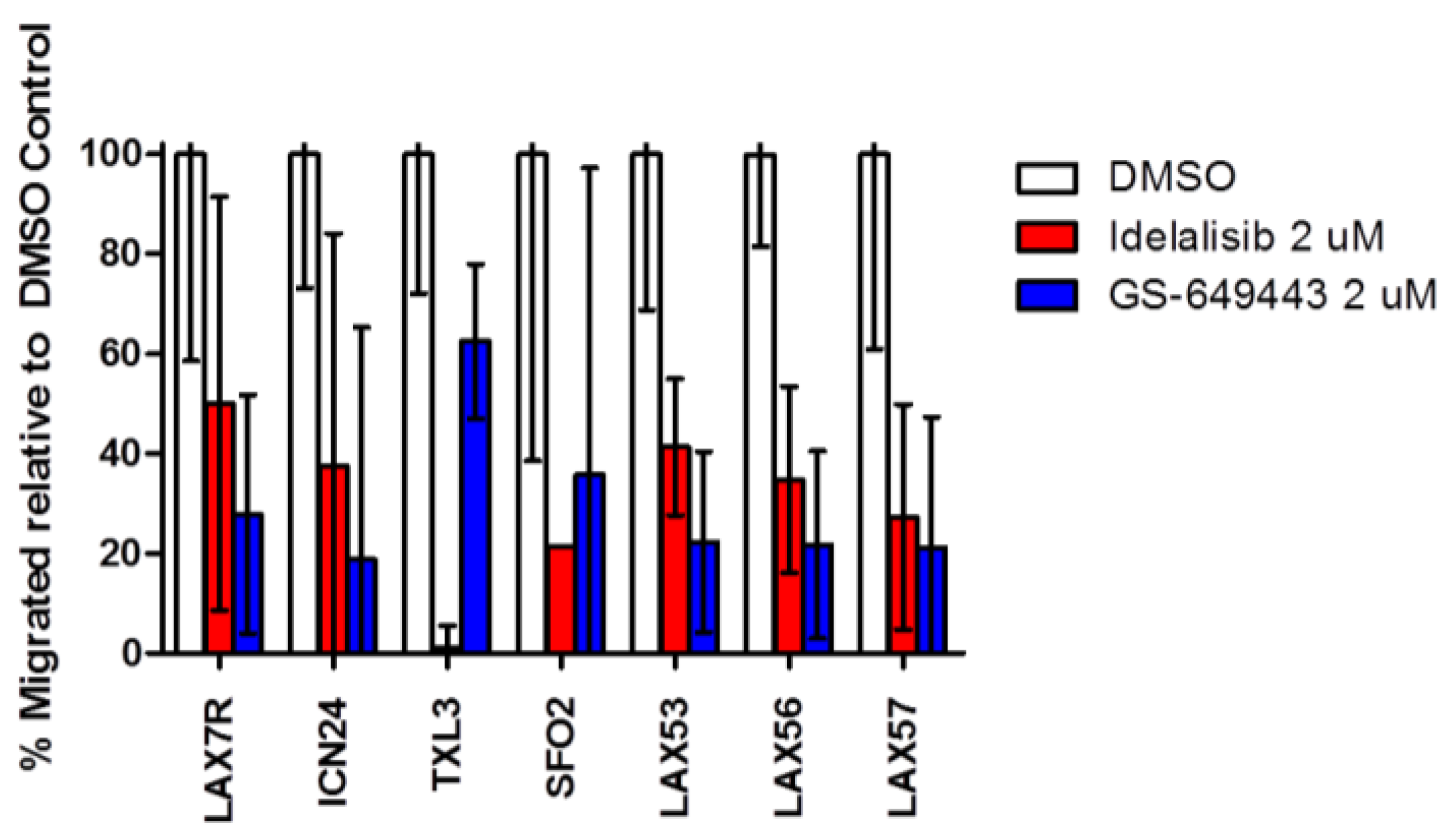
2.4. Idelalisib Inhibits Migration of ALL Cells to SDF-1α
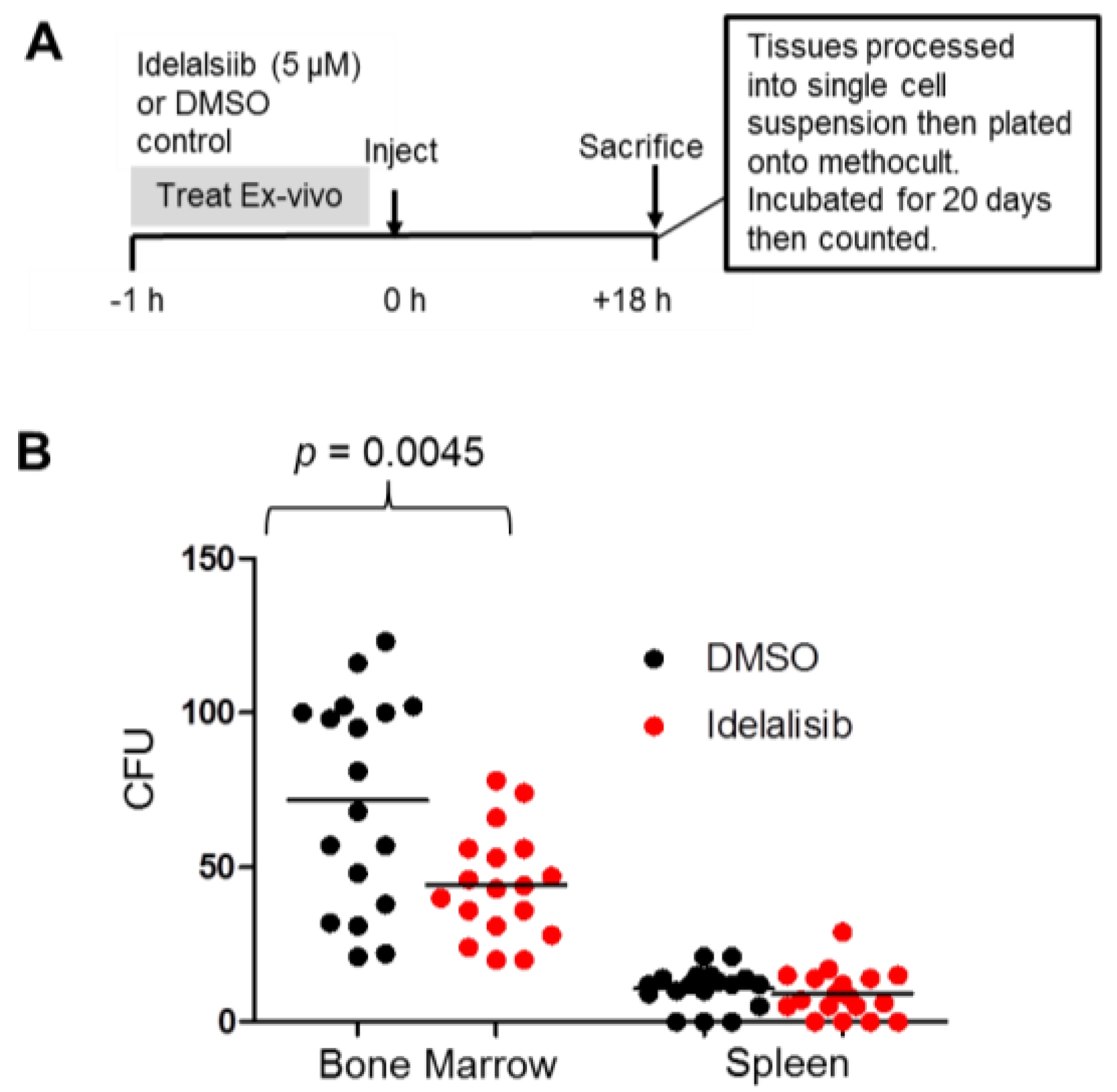
2.5. Idelalisib Inhibits Homing of ALL Cells to the Bone Marrow
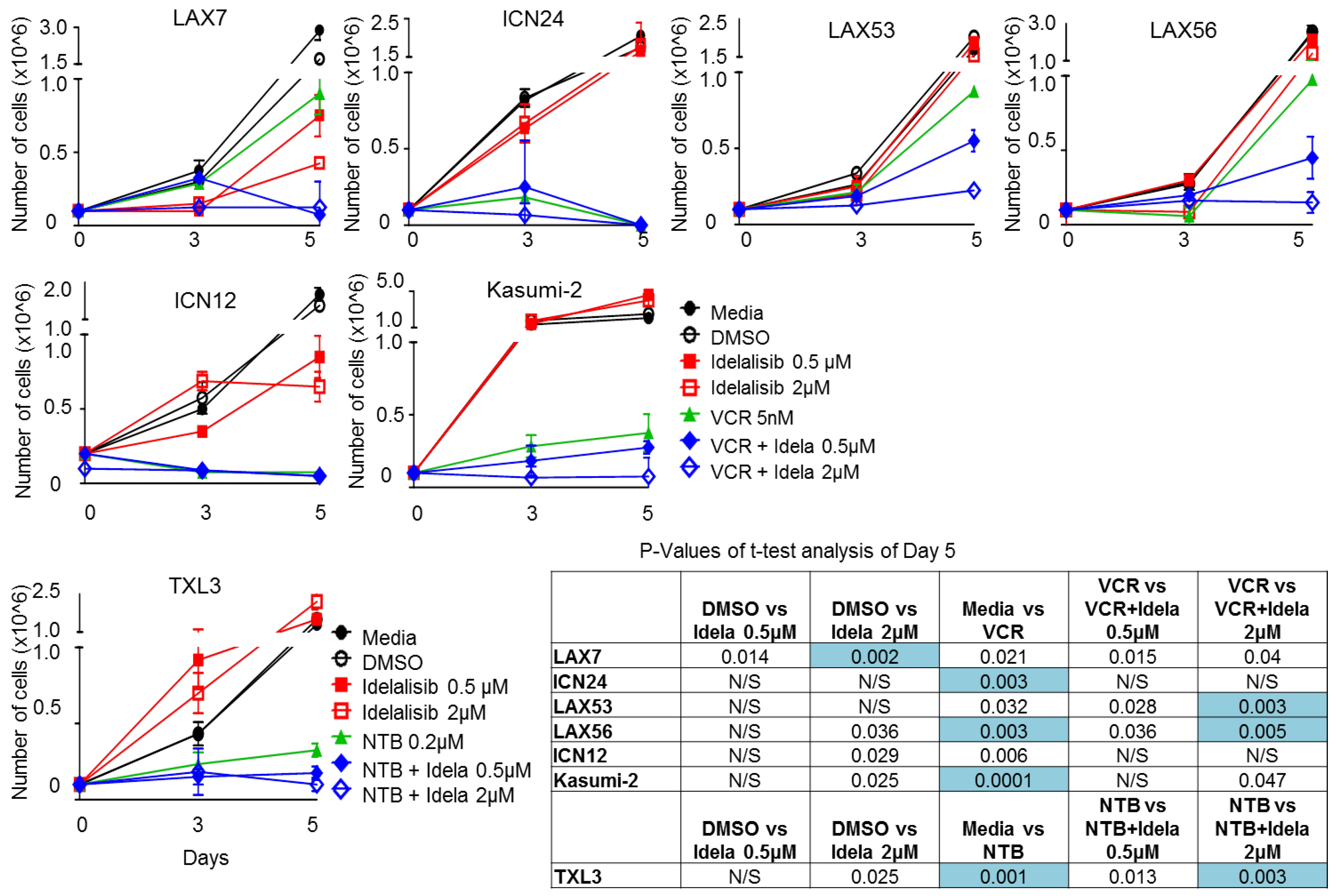
2.6. Effect of Idelalisib on Proliferation of ALL Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. PI3K Isoforms
4.3. p-Aktser473 Activation/Inhibition
4.4. Migration Assay
4.5. Homing and Colony Forming Assay
4.6. Proliferation Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Bhojwani, D.; Pui, C.H. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013, 14, e205–e217. [Google Scholar] [CrossRef]
- Shishido, S.; Bonig, H.; Kim, Y.M. Role of integrin alpha4 in drug resistance of leukemia. Front. Oncol. 2014, 4, 99. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-kinase akt pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Chantry, D.; Vojtek, A.; Kashishian, A.; Holtzman, D.A.; Wood, C.; Gray, P.W.; Cooper, J.A.; Hoekstra, M.F. P110delta, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J. Biol. Chem. 1997, 272, 19236–19241. [Google Scholar] [CrossRef] [PubMed]
- Jou, S.T.; Carpino, N.; Takahashi, Y.; Piekorz, R.; Chao, J.R.; Carpino, N.; Wang, D.; Ihle, J.N. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the b-cell receptor complex. Mol. Cell. Biol. 2002, 22, 8580–8591. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Khwaja, A. PI3Kδ inhibition hits a sensitive spot in b cell malignancies. Cancer Cell. 2014, 25, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Dienstmann, R.; Serra, V.; Tabernero, J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 2013, 10, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Janne, P.A.; Gray, N.; Settleman, J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat. Rev. Drug Discov. 2009, 8, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Okkenhaug, K. Haematological cancer: Idelalisib-targeting PI3Kδ in patients with b-cell malignancies. Nat. Rev. Clin. Oncol. 2014, 11, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Tasian, S.K.; Teachey, D.T.; Li, Y.; Shen, F.; Harvey, R.C.; Chen, I.M.; Ryan, T.; Vincent, T.L.; Willman, C.L.; Perl, A.E.; et al. Potent efficacy of combined PI3K/mTOR and JAK or ABL inhibition in murine xenograft models of ph-like acute lymphoblastic leukemia. Blood 2017, 129, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Rosin, N.Y.; Koehrer, S.; Kim, E.; O’Brien, S.; Wierda, W.G.; Thomas, D.A.; Estrov, Z.; Kantarjian, H.M.; Lannutti, B.J.; Burger, J.A. In vitro effects of pi3k delta inhibitor gs-1101 (Cal-101) in acute lymphoblastic leukemia (ALL). Blood 2012, 120, Abstract 3534. [Google Scholar]
- Eldfors, S.; Kuusanmaki, H.; Kontro, M.; Majumder, M.M.; Parsons, A.; Edgren, H.; Pemovska, T.; Kallioniemi, O.; Wennerberg, K.; Gokbuget, N.; et al. Idelalisib sensitivity and mechanisms of disease progression in relapsed TCF3-PBX1 acute lymphoblastic leukemia. Leukemia 2017, 31, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.; Chandrasekhar, J.; Evarts, J.; Forseth, K.; Haran, A.C.; Ip, C.; Kashishian, A.; Kim, M.; Koditek, D.; Koppenol, S.; et al. Discovery of orally efficacious phosphoinositide 3-kinase delta inhibitors with improved metabolic stability. J. Med. Chem. 2016, 59, 9228–9242. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, A.; Meadows, S.A.; Sorensen, R.A.; Cui, Z.H.; Keegan, K.S.; Brockett, R.; Chen, G.; Quéva, C.; Li, L.; Tannheimer, S.L. PI3Kδ inhibitor idelalisib in combination with BTK inhibitor ono/gs-4059 in diffuse large b cell lymphoma with acquired resistance to PI3Kδ and btk inhibitors. PLoS ONE 2017, 12, e0171221. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Price, T.; Olivere, L.; Warner, M.; Tannheimer, S.; Sipkins, D.A. PI3K delta inhibition suppresses central nervous system involvement of acute lymphoblastic leukemia. Blood 2016, 128, Abstract 282. [Google Scholar]
- Thomas, X.; Anglaret, B.; Bailly, M.; Maritaz, O.; Magaud, J.P.; Archimbaud, E. Differential adhesiveness between blood and marrow leukemic cells having similar pattern of vla adhesion molecule expression. Leuk. Res. 1998, 22, 953–960. [Google Scholar] [CrossRef]
- Hoellenriegel, J.; Meadows, S.A.; Sivina, M.; Wierda, W.G.; Kantarjian, H.; Keating, M.J.; Giese, N.; O’Brien, S.; Yu, A.; Miller, L.L.; et al. The phosphoinositide 3’-kinase delta inhibitor, cal-101, inhibits b-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 2011, 118, 3603–3612. [Google Scholar] [CrossRef] [PubMed]
- Fiorcari, S.; Brown, W.S.; McIntyre, B.W.; Estrov, Z.; Maffei, R.; O’Brien, S.; Sivina, M.; Hoellenriegel, J.; Wierda, W.G.; Keating, M.J.; et al. The PI3-kinase delta inhibitor idelalisib (GS-1101) targets integrin-mediated adhesion of chronic lymphocytic leukemia (CLL) cell to endothelial and marrow stromal cells. PLoS ONE 2013, 8, e83830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lannutti, B.J.; Meadows, S.A.; Herman, S.E.; Kashishian, A.; Steiner, B.; Johnson, A.J.; Byrd, J.C.; Tyner, J.W.; Loriaux, M.M.; Deininger, M.; et al. Cal-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of b-cell malignancies, inhibits pi3k signaling and cellular viability. Blood 2011, 117, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.; Kim, H.N.; Gang, E.J.; Schnair, C.; Lee, S.; Lee, S.; Khazal, S.; Kosoyan, O.; Konopleva, M.; Parekh, C.; et al. Department of Pediatrics, Division of Hematology, Oncology and Blood and Marrow Transplantation, Children’s Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, CA, USA. Unpublished work. 2017. [Google Scholar]
- Xue, G.; Hemmings, B.A. PKB/Akt-dependent regulation of cell motility. J. Natl. Cancer Inst. 2013, 105, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Reif, K.; Okkenhaug, K.; Sasaki, T.; Penninger, J.M.; Vanhaesebroeck, B.; Cyster, J.G. Cutting edge: Differential roles for phosphoinositide 3-kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J. Immunol. 2004, 173, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Byrd, J.C.; Coutre, S.E.; Benson, D.M.; Flinn, I.W.; Wagner-Johnston, N.D.; Spurgeon, S.E.; Kahl, B.S.; Bello, C.; Webb, H.K.; et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood 2014, 123, 3390–3397. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, E.; Kim, H.N.; Gang, E.J.; Schnair, C.; Lee, S.; Lee, S.; Khazal, S.; Kosoyan, O.; Konopleva, M.; Parekh, C.; et al. The PI3Kδ Inhibitor Idelalisib Inhibits Homing in an in Vitro and in Vivo Model of B ALL. Cancers 2017, 9, 121. https://doi.org/10.3390/cancers9090121
Adam E, Kim HN, Gang EJ, Schnair C, Lee S, Lee S, Khazal S, Kosoyan O, Konopleva M, Parekh C, et al. The PI3Kδ Inhibitor Idelalisib Inhibits Homing in an in Vitro and in Vivo Model of B ALL. Cancers. 2017; 9(9):121. https://doi.org/10.3390/cancers9090121
Chicago/Turabian StyleAdam, Etai, Hye Na Kim, Eun Ji Gang, Caitlin Schnair, Solomon Lee, Solah Lee, Sajad Khazal, Osanna Kosoyan, Marina Konopleva, Chintan Parekh, and et al. 2017. "The PI3Kδ Inhibitor Idelalisib Inhibits Homing in an in Vitro and in Vivo Model of B ALL" Cancers 9, no. 9: 121. https://doi.org/10.3390/cancers9090121




