Mesoporous Silica Based Gold Catalysts: Novel Synthesis and Application in Catalytic Oxidation of CO and Volatile Organic Compounds (VOCs)
Abstract
:1. Introduction
2. Synthesis of Mesoporous Silica Based Gold Catalysts and Applications in CO Oxidation
2.1. Synthesis of Au/SiO2 by Colloidal Deposition

2.2. Synthesis of Au/Mesoporous Silica by Doping Oxides


2.3. Synthesis of Au/Mesoporous Silicas by Pre-Modification
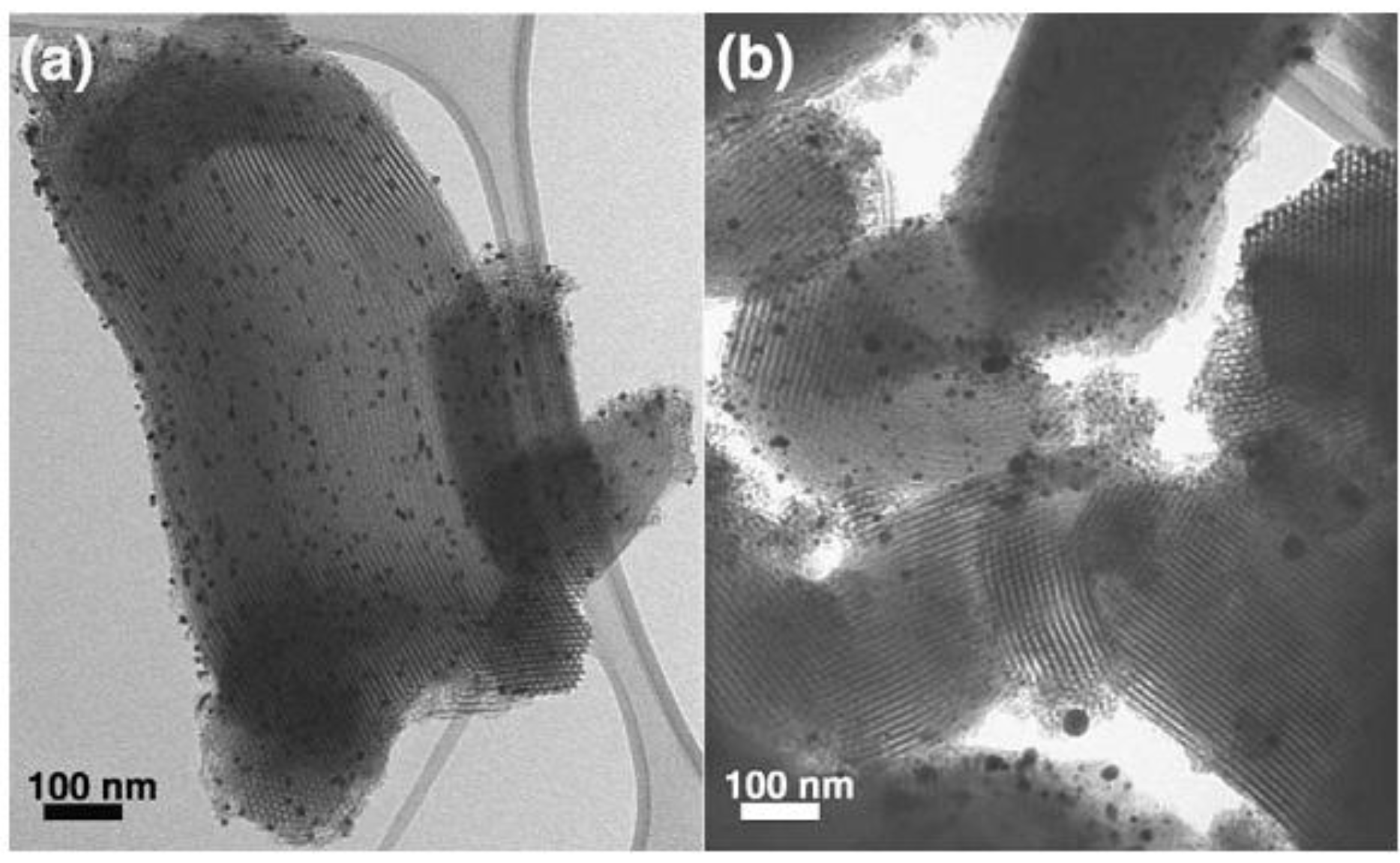





2.4. One-Pot Synthesis of Au/Mesoporous Silica

2.5. Colloidal Synthesis of Au-NPs Incorporated into Mesoporous Silica

2.6. Synthesis of Au/Mesoporous Silica by Gold Cationic Complex Precursor

2.7. Other Novel Synthesis of Au/Mesoporous Silica


| Catalysts | Preparation methods | Average Au particle size (nm) | Au loadings (wt.%) | T50 (°C) | Refs. |
|---|---|---|---|---|---|
| Au/SiO2 | Colloidal deposition | ~30–100 | 1.5 | - | [25,26] |
| Au/Ce20-SBA-15 | Doping oxide | ~3–5 | 2.0 | 81 | [27] |
| Au/mesoporous silica | Pre-modification | ~4–7 | 4–5 | ~80 | [34,35,36,37] |
| Au/mesoporous silica | One-pot synthesis | ~2–5 | 0.7–7.0 | - | [38,39,40] |
| Au/mesoporous silica | Colloidal synthesis | ~2–10 | 2.0 | - | [41,42] |
| Au/SBA-15 | Cationic complex precursor | ~3–4 | ~2.0–9.0 | 10 | [43,44,45] |
| Au/SBA-15 | Silver-catalyzed electroless deposition | ~5–9 | large | - | [46] |
3. Catalytic Oxidation of Volatile Organic Compounds (VOCs)
 ), C-Fe (●), C-FeAu (□), C-Fe Dp-Au (△), and Dp-Au (■). Reaction conditions: reactant mixture 0.3 vol% methanol, 10 vol.% O2, N2 balance. Space velocity (GHSV): 7.6 × 10−3 mol h−1 g−1. Reproduced with permission from [29]. Copyright 2011 Elsevier B.V.
), C-Fe (●), C-FeAu (□), C-Fe Dp-Au (△), and Dp-Au (■). Reaction conditions: reactant mixture 0.3 vol% methanol, 10 vol.% O2, N2 balance. Space velocity (GHSV): 7.6 × 10−3 mol h−1 g−1. Reproduced with permission from [29]. Copyright 2011 Elsevier B.V.
 ), C-Fe (●), C-FeAu (□), C-Fe Dp-Au (△), and Dp-Au (■). Reaction conditions: reactant mixture 0.3 vol% methanol, 10 vol.% O2, N2 balance. Space velocity (GHSV): 7.6 × 10−3 mol h−1 g−1. Reproduced with permission from [29]. Copyright 2011 Elsevier B.V.
), C-Fe (●), C-FeAu (□), C-Fe Dp-Au (△), and Dp-Au (■). Reaction conditions: reactant mixture 0.3 vol% methanol, 10 vol.% O2, N2 balance. Space velocity (GHSV): 7.6 × 10−3 mol h−1 g−1. Reproduced with permission from [29]. Copyright 2011 Elsevier B.V.


4. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 2, 405–408. [Google Scholar]
- Haruta, M.; Kageyama, H.; Kamijo, N.; Kobayashi, T.; Delannay, F. Fine Structure of novel gold catalysts prepared by coprecipitation. Stud. Surf. Sci. Catal. 1989, 44, 33–42. [Google Scholar] [CrossRef]
- Hutchings, G.J. Vapor phase hydrochlorination of acetylene: Correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Bond, G.C.; Thompson, D.T. Catalysis by gold. Catal. Rev. Sci. Eng. 1999, 41, 319–388. [Google Scholar] [CrossRef]
- Haruta, M.; Daté, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A 2001, 222, 427–437. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Goodman, D.W. Oxidation catalysis by supported gold nano-clusters. Top. Catal. 2002, 21, 25–34. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef]
- Kung, M.C.; Davis, R.J.; Kung, H.H. Understanding Au-catalyzed low-temperature CO oxidation. J. Phys. Chem. C 2007, 111, 11767–11775. [Google Scholar] [CrossRef]
- Della Pina, C.; Falletta, E.; Prati, L.; Rossi, M. Selective oxidation using gold. Chem. Soc. Rev. 2008, 37, 2077–2095. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported gold nanoparticles for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Li, D.; Nakagawa, Y.; Tomishige, K. Methane reforming to synthesis gas over Ni catalysts modified with noble metals. Appl. Catal. A 2011, 408, 1–24. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Yuan, Y.; Kozlova, A.P.; Asakura, K.; Wan, H.; Tsai, K.; Iwasawa, Y. Supported Au catalysts prepared from Au phosphine complexes and as-precipitated metal hydroxides: Characterization and low-temperature CO oxidation. J. Catal. 1997, 170, 191–199. [Google Scholar] [CrossRef]
- Lin, S.D.; Bollinger, M.; Vannice, M.A. Low temperature CO oxidation over Au/TiO2 and Au/SiO2 catalysts. Catal. Lett. 1993, 17, 245–262. [Google Scholar]
- Dekkers, M.A.P.; Lippits, M.J.; Nieuwenhuys, B.E. Supported gold/MOx catalysts for NO/H2 and CO/O2 reactions. Catal. Today 1999, 54, 381–390. [Google Scholar] [CrossRef]
- Oh, H.S.; Yang, J.H.; Costello, C.K.; Wang, Y.M.; Bare, S.R.; Kung, H.H.; Kung, M.C. Selective catalytic oxidation of CO: Effect of chloride on supported Au catalysts. J. Catal. 2002, 210, 375–386. [Google Scholar] [CrossRef]
- Song, S.W.; Hidajat, K.; Kawi, S. Functionalized SBA-15 materials as carriers for controlled drug delivery: Influence of surface properties on matrix-drug interactions. Langmuir 2005, 21, 9568–9575. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, H.; Yang, W.; Li, Y.; Sun, C. Gold nanoparticles mesoporous silica composite used as an enzyme immobilization matrix for amperometric glucose biosensor construction. Sens. Actuators B 2007, 124, 179–186. [Google Scholar] [CrossRef]
- Yiu, H.H.P.; Wright, P.A.; Botting, N.P. Enzyme immobilisation using SBA-15 mesoporous molecular sieves with functionalized surfaces. J. Mol. Catal. B 2001, 15, 81–92. [Google Scholar] [CrossRef]
- Okumura, M.; Nakamura, S.; Tsubota, S.; Nakamura, T.; Azuma, M.; Haruta, M. Chemical vapor deposition of gold on Al2O3, SiO2, and TiO2 for the oxidation of CO and of H2. Catal. Lett. 1998, 51, 53–58. [Google Scholar]
- Zhang, L.X.; Shi, J.L.; Yu, J.; Hua, Z.L.; Zhao, X.G.; Ruan, M.L. A new in-situ reduction route for the synthesis of Pt nanoclusters in the channels of mesoporous silica SBA-15. Adv. Mater. 2002, 14, 1510–1513. [Google Scholar] [CrossRef]
- Sun, J.; Ma, D.; Zhang, H.; Liu, X.; Han, X.; Bao, X.; Weinberg, G.; Pfander, N.; Su, D. Toward monodispersed silver nanoparticles with unusual thermal stability. J. Am. Chem. Soc. 2006, 128, 15756–15764. [Google Scholar]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Yan, W.F.; Chen, B.; Mahurin, S.M.; Hagaman, E.W.; Dai, S.; Overbury, S.H. Surface sol–gel modification of mesoporous silica materials with TiO2 for the assembly of ultrasmall gold nanoparticles. J. Phys. Chem. B 2004, 108, 2793–2796. [Google Scholar] [CrossRef]
- Wu, H.J.; Wang, L.D.; Zhang, J.Q.; Shen, Z.Y.; Zhao, J.H. Catalytic oxidation of benzene, toluene and p-xylene over colloidal gold supported on zinc oxide catalyst. Catal. Commun. 2011, 12, 859–865. [Google Scholar] [CrossRef]
- Comotti, M.; Li, W.C.; Spliethoff, B.; Schüth, F. Support effect in high activity gold catalyst for CO oxidation. J. Am. Chem. Soc. 2006, 128, 917–924. [Google Scholar] [CrossRef]
- Liotta, L.F.; Pantaleo, G.; Puleo, F.; Venezia, A.M. Au/CeO2-SBA-15 catalysts for CO oxidation: Effect of ceria loading on physic-chemical properties and catalytic performances. Catal. Today 2012, 187, 10–19. [Google Scholar] [CrossRef]
- Beck, A.; Horvath, A.; Stefler, G.; Katona, R.; Geszti, O.; Tolnai, G.; Liotta, L.F.; Guczi, L. Formation and structure of Au/TiO2 and Au/CeO2 nanostructures in mesoporous SBA-15. Catal. Today 2008, 139, 180–187. [Google Scholar] [CrossRef]
- Bonelli, R.; Lucarelli, C.; Pasini, T.; Liotta, L.F.; Zacchini, S.; Albonetti, S. Total oxidation of volatile organic compounds on Au/FeOx catalysts supported on mesoporous SBA-15 silica. Appl. Catal. A 2011, 400, 54–60. [Google Scholar] [CrossRef]
- Xu, X.X.; Hao, Z.P.; Zhao, W.; Hu, C. Characterization and catalytic performance of Co/SBA-15 supported gold catalysts for CO oxidation. Mater. Res. Bull. 2006, 41, 406–413. [Google Scholar] [CrossRef]
- Escamilla-Perea, L.; Peza-Ledesma, C.L.; Nava, R.; Rivera-Munoz, E.M.; Pawelec, B.; Fierro, J.L.G. CO oxidation at 20 °C over Au/SBA-15 catalysts decorated by Fe2O3 nanoparticles. Catal. Commun. 2011, 15, 108–112. [Google Scholar]
- Ren, L.H.; Zhang, H.L.; Lu, A.H.; Hao, Y.; Li, W.C. Porous silica as supports for controlled fabrication of Au/CeO2/SiO2 catalysts for CO oxidation: Influence of the silica nanostructures. Micropor. Mesopor. Mater. 2012, 158, 7–12. [Google Scholar] [CrossRef]
- Ma, G.; Binder, A.; Chi, M.; Liu, C.; Jin, R.; Jiang, D.; Fan, J.; Dai, S. Stabilizing gold clusters by heterostructured transition-metal oxide–mesoporous silica supports for enhanced catalytic activities for CO oxidation. Chem. Commun. 2012, 48, 11413–11415. [Google Scholar]
- Yang, C.M.; Sheu, H.S.; Chao, K.J. Templated synthesis and structural study of densely packed metal nanostructures in MCM-41 and MCM-48. Adv. Funct. Mater. 2002, 12, 143–148. [Google Scholar] [CrossRef]
- Yang, C.M.; Kalwei, M.; Schüth, F.; Chao, K.J. Gold nanoparticles in SBA-15 showing catalytic activity in CO oxidation. Appl. Catal. A 2003, 254, 289–296. [Google Scholar] [CrossRef]
- Chi, Y.S.; Lin, H.P.; Mou, C.Y. CO oxidation over gold nanocatalyst confined in mesoporous silica. Appl. Catal. A 2005, 284, 199–206. [Google Scholar]
- Lee, B.; Ma, Z.; Zhang, Z.T.; Park, C.; Dai, S. Influences of synthesis conditions and mesoporous structures on the gold nanoparticles supported on mesoporous silica hosts. Micropor. Mesopor. Mater. 2009, 122, 160–167. [Google Scholar] [CrossRef]
- Zhu, H.G.; Lee, B.; Dai, S.; Overbury, S.H. Coassembly synthesis of ordered mesoporous silica materials containing Au nanoparticles. Langmuir 2003, 19, 3974–3980. [Google Scholar] [CrossRef]
- Song, H.Y.; Li, G.; Wang, X.S. In situ synthesis of Au/Ti-HMS and its catalytic performance in oxidation of bulky sulfur compounds using in situ generated H2O2 in the presence of H2/O2. Micropor. Mesopor. Mater. 2009, 120, 346–350. [Google Scholar] [CrossRef]
- Song, H.Y.; Li, G.; Wang, X.S.; Xu, Y.J. Characterization and catalytic performance of Au/Ti-HMS catalysts on the oxidative desulphurization using in situ H2O2: Effect of method catalysts preparation. Catal. Today 2010, 149, 127–131. [Google Scholar] [CrossRef]
- Zhu, J.; Kónya, Z.; Puntes, V.F.; Kiricsi, I.; Miao, C.X.; Ager, J.W.; Alivisatos, A.P.; Somorjai, G.A. Encapsulation of metal (Au, Ag, Pt) nanoparticles into the mesoporous SBA-15 structure. Langmuir 2003, 19, 4396–4401. [Google Scholar] [CrossRef]
- Song, H.Y.; Li, G.; Wang, X.S.; Chen, Y.Y. Characterization and catalytic performance of Au/Ti-HMS for direct generation of H2O2 and in situ-H2O2-ODS from H2 and O2: An in situ-reduction synthesis and a recycle study of catalyst. Micropor. Mesopor. Mater. 2011, 139, 104–109. [Google Scholar] [CrossRef]
- Zhu, H.G.; Liang, C.D.; Yan, W.F.; Overbury, S.H.; Dai, S. Preparation of highly active silica-supported Au catalysts for CO oxidation by a solution-based technique. J. Phys. Chem. B 2006, 110, 10842–10848. [Google Scholar]
- Zhu, H.G.; Ma, Z.; Clark, J.C.; Pan, Z.W.; Overbury, S.H.; Dai, S. Low-temperature CO oxidation on Au/fumed SiO2-based catalysts prepared from Au(en)2Cl3 precursor. Appl. Catal. A 2007, 326, 89–99. [Google Scholar] [CrossRef]
- Yin, H.F.; Ma, Z.; Zhu, H.G.; Chi, M.F.; Dai, S. Evidence for and mitigation of the encapsulation of gold nanoparticles within silica supports upon high-temperature treatment of Au/SiO2 catalysts: Implication to catalyst deactivation. Appl. Catal. A 2010, 386, 147–156. [Google Scholar] [CrossRef]
- Asefa, T.; Lennox, R.B. Synthesis of gold nanoparticles via electroless deposition in SBA-15. Chem. Mater. 2005, 17, 2481–2483. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xie, Y.B.; Liu, C.J. Synthesis and characterization of noble metal (Pd, Pt, Au, Ag) nanostructured materials confined in the channels of mesoporous SBA-15. J. Phys. Chem. C 2008, 112, 19818–19824. [Google Scholar] [CrossRef]
- Shironita, S.; Takasaki, T.; Kamegawa, T.; Mori, K.; Yamashita, H. Application of microwave-assisted deposition for the synthesis of noble metal particles on Ti-containing mesoporous silica. Catal. Lett. 2009, 129, 404–407. [Google Scholar] [CrossRef]
- Haruta, M. Size-and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Sobczak, I.; Kieronczyk, N.; Trejda, M.; Ziolek, M. Gold, vanadium and niobium containing MCM-41 materials-Catalytic properties in methanol oxidation. Catal. Today 2008, 139, 188–195. [Google Scholar] [CrossRef]
- Solsona, B.; Perez-Cabero, M.; Vazquez, I.; Dejoz, A.; Garcia, T.; Alvarez-Rodriguez, J.; El-Haskouri, J.; Beltran, D.; Amoros, P. Total oxidation of VOCs on Au nanoparticles anchored on Co doped mesoporous UVM-7 silica. Chem. Eng. J. 2012, 187, 391–400. [Google Scholar] [CrossRef]
- Kucherov, A.V.; Sinev, I.M.; Ojala, S.; Keiski, R.; Kustov, L.M. Adsorptive-catalytic removal of CH3OH, CH3SH, and CH3SSCH3 from air over the bifunctional system noble metals/HZSM-5. Studies Surf. Sci. Catal. 2007, 170, 1129–1136. [Google Scholar] [CrossRef]
- Kucherov, A.V.; Tkachenko, O.P.; Kirichenko, O.A.; Kapustin, G.I.; Mishin, I.V.; Klementiev, K.V.; Ojala, S.; Kustov, L.M.; Keiski, R. Nanogold-containing catalysts for low-temperature removal of S-VOC from air. Top Catal. 2009, 52, 351–358. [Google Scholar] [CrossRef]
- Wu, S.H.; Mou, C.Y.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Ma, Z.; Dai, S. Development of novel supported gold catalysts: A materials perspective. Nano Res. 2011, 4, 3–32. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, H.; Pantaleo, G.; Venezia, A.M.; Liotta, L.F. Mesoporous Silica Based Gold Catalysts: Novel Synthesis and Application in Catalytic Oxidation of CO and Volatile Organic Compounds (VOCs). Catalysts 2013, 3, 774-793. https://doi.org/10.3390/catal3040774
Wu H, Pantaleo G, Venezia AM, Liotta LF. Mesoporous Silica Based Gold Catalysts: Novel Synthesis and Application in Catalytic Oxidation of CO and Volatile Organic Compounds (VOCs). Catalysts. 2013; 3(4):774-793. https://doi.org/10.3390/catal3040774
Chicago/Turabian StyleWu, Hongjing, Giuseppe Pantaleo, Anna M. Venezia, and Leonarda F. Liotta. 2013. "Mesoporous Silica Based Gold Catalysts: Novel Synthesis and Application in Catalytic Oxidation of CO and Volatile Organic Compounds (VOCs)" Catalysts 3, no. 4: 774-793. https://doi.org/10.3390/catal3040774






