La0.6Sr0.4Co0.2Fe0.8O3 Perovskite: A Stable Anode Catalyst for Direct Methane Solid Oxide Fuel Cells
Abstract
:1. Introduction

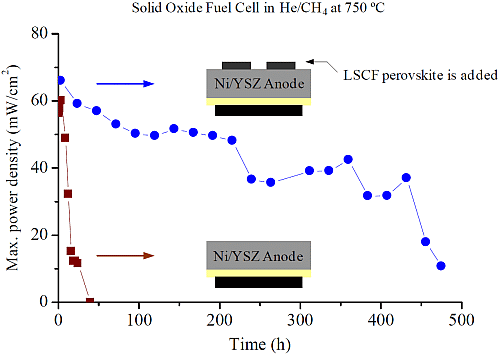
2. Results and Discussion








3. Experimental Section
3.1. Synthesis of LSCF Perovskite

3.2. Fuel Cell Fabrication
3.3. Fuel Cell Testing and Characterization

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Teraoka, Y.; Zhang, H.M.; Okamoto, K.; Yamazoe, N. Mixed ionic-electronic conductivity of La1−xSrxCo1−yFeyO3−δ perovskite-type oxides. Mater. Res. Bull. 1988, 23, 51–58. [Google Scholar] [CrossRef]
- Teraoka, Y.; Nobunaga, T.; Okamoto, K.; Miura, N.; Yamazoe, N. Influence of constituent metal cations in substituted LaCoO3 on mixed conductivity and oxygen permeability. Solid State Ionics 1991, 48, 207–212. [Google Scholar] [CrossRef]
- Stevenson, J.W.; Armstrong, T.R.; Carneim, R.D.; Pederson, L.R.; Weber, W.J. Electrochemical properties of mixed conducting perovskites La1−xMxCo1−yFeyO3−δ (M = Sr, Ba, Ca). J. Electrochem. Soc. 1996, 143, 2722–2729. [Google Scholar]
- Teraoka, Y.; Honbe, Y.; Ishii, J.; Furukawa, H.; Moriguchi, I. Catalytic effects in oxygen permeation through mixed-conductive LSCF perovskite membranes. Solid State Ionics 2002, 152–153, 681–687. [Google Scholar]
- Li, N.; Boréave, A.; Deloume, J.-P.; Gaillard, F. Catalytic combustion of toluene over a Sr and Fe substituted LaCoO3 perovskite. Solid State Ionics 2008, 179, 1396–1400. [Google Scholar] [CrossRef]
- Ten Elshof, J.E.; Bouwmeester, H.J.M.; Verweij, H. Oxidative coupling of methane in a mixed-conducting perovskite membrane reactor. Appl. Catal. A 1995, 130, 195–212. [Google Scholar]
- Taheri, Z.; Seyed-Matin, N.; Safekordi, A.A.; Nazari, K.; Pashne, S.Z. A comparative kinetic study on the oxidative coupling of methane over LSCF perovskite-type catalyst. Appl. Catal. A 2009, 354, 143–152. [Google Scholar] [CrossRef]
- Joshi, A.V.; Krist, K.; Liu, M.; Shen, Y.; Virkar, A.V. Mixed ionic-electronic conducting composites for oxygen separation and electrocatalysis. U.S. Patent US5616223A, 1 April 1997. [Google Scholar]
- Simner, S.P.; Anderson, M.D.; Engelhard, M.H.; Stevenson, J.W. Degradation mechanisms of La – Sr – Co – Fe – O3 SOFC cathodes. Electrochem. Solid-State Lett. 2006, 9, A478–A481. [Google Scholar] [CrossRef]
- Gostovic, D.; Smith, J.R.; Kundinger, D.P.; Jones, K.S.; Wachsman, E.D. Three-dimensional reconstruction of porous LSCF cathodes. Electrochem. Solid-State Lett. 2007, 10, B214–B217. [Google Scholar] [CrossRef]
- Fisher, J.C., II.; Chuang, S.S.C. Investigating the CH4 reaction pathway on a novel LSCF anode catalyst in the SOFC. Catal. Commun. 2009, 10, 772–776. [Google Scholar] [CrossRef]
- Lai, B.-K.; Kerman, K.; Ramanathan, S. Nanostructured La0.6Sr0.4Co0.8Fe0.2O3/Y0.08Zr0.92O1.96/La0.6Sr0.4Co0.8Fe0.2O3 (LSCF/YSZ/LSCF) symmetric thin film solid oxide fuel cells. J. Power Sources 2011, 196, 1826–1832. [Google Scholar]
- Pena, M.; Fierro, J. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Ghouse, M.; Al-Yousef, Y.; Al-Musa, A.; Al-Otaibi, M.F. Preparation of La0.6Sr0.4Co0.2Fe0.8O3 nanoceramic cathode powders for solid oxide fuel cell (SOFC) application. Int. J. Hydrogen Energy 2010, 35, 9411–9419. [Google Scholar]
- Pechini Maggio, P. Method of preparing lead and alkaline earth titanates and niobates and coating method using the same to form a capacitor. U.S. Patent US3330697A, 11 July 1967. [Google Scholar]
- Leea, S.; Songa, H.S.; Hyuna, S.H.; Kimb, J.; Moona, J. Interlayer-free nanostructured La0.58Sr0.4Co0.2Fe0.8O3-cathode on scandium stabilized zirconia electrolyte for intermediate-temperature solid oxide fuel cells. J. Power Sources 2009, 187, 74–79. [Google Scholar] [CrossRef]
- Leng, Y.; Chan, S.; Liu, Q. Development of LSCF-GDC composite cathodes for low-temperature solid oxide fuel cells with thin film GDC electrolyte. Int. J. Hydrogen Energy 2008, 33, 3808–3817. [Google Scholar] [CrossRef]
- Petric, A.; Huang, P.; Tietz, F. Evaluation of La–Sr–Co–Fe–O perovskites for solid oxide fuel cells and gas separation membranes. Solid State Ionics 2000, 135, 719–725. [Google Scholar] [CrossRef]
- Beckel, D.; Muecke, U.P.; Gyger, T.; Florey, G.; Infortuna, A.; Gauckler, L.J. Electrochemical performance of LSCF based thin film cathodes prepared by spray pyrolysis. Solid State Ionics 2007, 178, 407–415. [Google Scholar]
- Popa, M.; Frantti, J.; Kakihana, M. Lanthanum ferrite LaFeO3+d nanopowders obtained by the polymerizable complex method. Solid State Ionics 2002, 154, 437–445. [Google Scholar]
- Popa, M.; Kakihana, M. Synthesis of lanthanum cobaltite (LaCoO3) by the polymerizable complex route. Solid State Ionics 2002, 151, 251–257. [Google Scholar] [CrossRef]
- Chuang, S.S. Catalysis of solid oxide fuel cells. In Catalysis; Spivey, J.J., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2005; Volume 18, pp. 187–188. [Google Scholar]
- Takeguchi, T.; Kani, Y.; Yano, T.; Kikuchi, R.; Eguchi, K.; Tsujimoto, K.; Uchida, Y.; Ueno, A.; Omoshiki, K.; Aizawa, M. Study on steam reforming of CH4 and C2 hydrocarbons and carbon deposition on Ni-YSZ cermets. J. Power Sources 2002, 112, 588–595. [Google Scholar] [CrossRef]
- Li, X.; Lee, J.-P.; Blinn, K.S.; Chen, D.; Yoo, S.; Kang, B.; Bottomley, L.A.; El-Sayed, M.A.; Park, S.; Liu, M. High-temperature surface enhanced raman spectroscopy for in situ study of solid oxide fuel cell materials. Energy Environ. Sci. 2014, 7, 306–310. [Google Scholar]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic steam reforming of methane, methanol, and ethanol over Ni/YSZ: The possible use of these fuels in internal reforming SOFC. J. Power Sources 2007, 163, 943–951. [Google Scholar] [CrossRef]
- Kendall, K.; Finnerty, C.; Saunders, G.; Chung, J. Effects of dilution on methane entering an SOFC anode. J. Power Sources 2002, 106, 323–327. [Google Scholar]
- Pillai, M.; Lin, Y.; Zhu, H.; Kee, R.J.; Barnett, S.A. Stability and coking of direct-methane solid oxide fuel cells: Effect of CO2 and air additions. J. Power Sources 2010, 195, 271–279. [Google Scholar]
- Lee, S.-I.; Vohs, J.M.; Gorte, R.J. A study of SOFC anodes based on Cu–Ni and Cu–Co bimetallics in CeO2 YSZ. J. Electrochem. Soc. 2004, 151, A1319–A1323. [Google Scholar] [CrossRef]
- Zhu, H.; Colclasure, A.M.; Kee, R.J.; Lin, Y.; Barnett, S.A. Anode barrier layers for tubular solid-oxide fuel cells with methane fuel streams. J. Power Sources 2006, 161, 413–419. [Google Scholar]
- Lin, Y.; Zhan, Z.; Barnett, S.A. Improving the stability of direct-methane solid oxide fuel cells using anode barrier layers. J. Power Sources 2006, 158, 1313–1316. [Google Scholar] [CrossRef]
- Lu, Z.; Hardy, J.; Templeton, J.; Stevenson, J. New insights in the polarization resistance of anode-supported solid oxide fuel cells with La0.6Sr0.4Co0.2Fe0.8O3 cathodes. J. Power Sources 2011, 196, 39–45. [Google Scholar] [CrossRef]
- Marinha, D.; Dessemond, L.; Cronin, J.S.; Wilson, J.R.; Barnett, S.A.; Djurado, E. Microstructural 3D reconstruction and performance evaluation of LSCF cathodes obtained by electrostatic spray deposition. Chem. Mater. 2011, 23, 5340–5348. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, M.; Nie, L.; Liu, M. Fabrication and characterization of functionally-graded LSCF cathodes by tape casting. Int. J. Hydrogen Energy 2013, 38, 1082–1087. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, M.; Yang, L.; Liu, M. LSM-infiltrated LSCF cathodes for solid oxide fuel cells. J. Energy Chem. 2013, 22, 555–559. [Google Scholar] [CrossRef]
- Marrero-López, D.; Romero, R.; Martín, F.; Ramos-Barrado, J.R. Effect of the deposition temperature on the electrochemical properties of La0.6Sr0.4Co0.8Fe0.2O3−δ cathode prepared by conventional spray-pyrolysis. J. Power Sources 2014, 255, 308–317. [Google Scholar] [CrossRef]
- Zhao, F.; Virkar, A.V. Dependence of polarization in anode-supported solid oxide fuel cells on various cell parameters. J. Power Sources 2005, 141, 79–95. [Google Scholar] [CrossRef]
- Rismanchian, A.; Mirzababaei, J.; Chuang, S.S.C. Electroless plated Cu–Ni anode catalyst for natural gas solid oxide fuel cells. Catal. Today submitted for publication. 2014. [Google Scholar]
- Koh, J.-H.; Yoo, Y.-S.; Park, J.-W.; Lim, H.C. Carbon deposition and cell performance of Ni-YSZ anode support SOFC with methane fuel. Solid State Ionics 2002, 149, 157–166. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, Z.; Liu, J.; Barnett, S.A. Direct operation of solid oxide fuel cells with methane fuel. Solid State Ionics 2005, 176, 1827–1835. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, S.P.; Tok, A.I.Y.; Luo, L. GDC-impregnated Ni anodes for direct utilization of methane in solid oxide fuel cells. J. Power Sources 2006, 159, 68–72. [Google Scholar] [CrossRef]
- Zhou, X.-D.; Pederson, L.R.; Templeton, J.W.; Stevenson, J.W. Electrochemical performance and stability of the cathode for solid oxide fuel cells: I. Cross validation of polarization measurements by impedance spectroscopy and current-potential sweep. J. Electrochem. Soc. 2010, 157, B220–B227. [Google Scholar] [CrossRef]
- Kim, H.; Lu, C.; Worrell, W.; Vohs, J.; Gorte, R. Cu-Ni cermet anodes for direct oxidation of methane in solid-oxide fuel cells. J. Electrochem. Soc. 2002, 149, A247–A250. [Google Scholar] [CrossRef]
- Gong, Y.; Qin, C.; Huang, K. Can silver be a reliable current collector for electrochemical tests? ECS Electrochem. Lett. 2013, 2, F4–F7. [Google Scholar] [CrossRef]
- McIntosh, S.; Gorte, R.J. Direct hydrocarbon solid oxide fuel cells. Chem. Rev. 2004, 104, 4845–4866. [Google Scholar] [CrossRef]
- Zhang, J.; Munroe, P.; Young, D.J. Microprocesses in nickel accompanying metal dusting. Acta Mater. 2008, 56, 68–77. [Google Scholar] [CrossRef]
- Alzate-Restrepo, V.; Hill, J.M. Carbon deposition on Ni/YSZ anodes exposed to CO/H2 feeds. J. Power Sources 2010, 195, 1344–1351. [Google Scholar] [CrossRef]
- Pomfret, M.B.; Owrutsky, J.C.; Walker, R.A. In situ studies of fuel oxidation in solid oxide fuel cells. Anal. Chem. 2007, 79, 2367–2372. [Google Scholar] [CrossRef]
- Hornés, A.; Gamarra, D.; Munuera, G.; Fuerte, A.; Valenzuela, R.X.; Escudero, M.J.; Daza, L.; Conesa, J.C.; Bera, P.; Martínez-Arias, A. Structural, catalytic/redox and electrical characterization of systems combining Cu–Ni with CeO2 or Ce1−xMxO2−δ (M = Gd or Tb) for direct methane oxidation. J. Power Sources 2009, 192, 70–77. [Google Scholar] [CrossRef]
- Blinn, K.S.; Abernathy, H.; Li, X.; Liu, M.; Bottomley, L.A.; Liu, M. Raman spectroscopic monitoring of carbon deposition on hydrocarbon-fed solid oxide fuel cell anodes. Energy Environ. Sci. 2012, 5, 7913–7917. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.H.; Bae, J.; Yoon, C.W.; Nam, S.W. In situ analyses of carbon dissolution into Ni-YSZ anode materials. J. Phys. Chem. C 2012, 116, 13281–13288. [Google Scholar] [CrossRef]
- Huang, T.J.; Huang, M.C.; Chen, W.J.; Chou, C.L. Oscillation of electrical current during direct methane oxidation over Ni-added LSCF–GDC anode of solid oxide fuel cells. Chem. Eng. J. 2009, 153, 164–169. [Google Scholar]
- Huang, T.-J.; Wang, C.-H. Methane decomposition and self de-coking over gadolinia-doped ceria-supported Ni catalysts. Chem. Eng. J. 2007, 132, 97–103. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L. Properties and Applications of Perovskite-Type Oxides; Marcel Dekker: New York, NY, USA, 1993. [Google Scholar]
- Arakawa, T.; Ohara, N.; Shiokawa, J. Reduction of perovskite oxide LnCoO3 (Ln = La–Eu) in a hydrogen atmosphere. J. Mater. Sci. 1986, 21, 1824–1827. [Google Scholar] [CrossRef]
- Lago, R.; Bini, G.; Peña, M.A.; Fierro, J.L.G. Partial oxidation of methane to synthesis gas using LnCoO3 perovskites as catalyst precursors. J. Catal. 1997, 167, 198–209. [Google Scholar] [CrossRef]
- McCarty, J.G.; Wise, H. Perovskite catalysts for methane combustion. Catal. Today 1990, 8, 231–248. [Google Scholar] [CrossRef]
- Anderson, H.U. Review of p-type doped perovskite materials for SOFC and other applications. Solid State Ionics 1992, 52, 33–41. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Nakamura, T.; Misono, M.; Yoneda, Y. Catalytic properties of perovskite-type mixed oxides, La1−xSrxCoO3. Bull. Chem. Soc. Jpn. 1982, 55, 394–399. [Google Scholar] [CrossRef]
- Arai, H.; Yamada, T.; Eguchi, K.; Seiyama, T. Catalytic combustion of methane over various perovskite-type oxides. Appl. Catal. 1986, 26, 265–276. [Google Scholar] [CrossRef]
- Tsiakaras, P.; Athanasiou, C.; Marnellos, G.; Stoukides, M.; Ten Elshof, J.; Bouwmeester, H. Methane activation on a La0.6Sr0.4Co0.8Fe0.2O3 perovskite-catalytic and electrocatalytic results. Appl. Catal. A 1998, 169, 249–261. [Google Scholar]
- Hardy, J.S.; Templeton, J.W.; Edwards, D.J.; Lu, Z.; Stevenson, J.W. Lattice expansion of LSCF-6428 cathodes measured by in situ XRD during SOFC operation. J. Power Sources 2012, 198, 76–82. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Dixon, A.G.; Ma, Y.H.; Moser, W.R.; Pascucci, M.R. Dense perovskite, La1−xA'xFe1−yCoyO3−δ (A' = Ba, Sr, Ca), membrane synthesis, applications, and characterization. J. Am. Ceram. Soc. 1998, 81, 1437–1444. [Google Scholar]
- Kulkarni, A.; Ciacchi, F.T.; Giddey, S.; Munnings, C.; Badwal, S.P.S.; Kimpton, J.A.; Fini, D. Mixed ionic electronic conducting perovskite anode for direct carbon fuel cells. Int. J. Hydrogen Energy 2012, 37, 19092–19102. [Google Scholar] [CrossRef]
- Crespin, M.; Hall, W.K. The surface chemistry of some perovskite oxides. J. Catal. 1981, 69, 359–370. [Google Scholar] [CrossRef]
- Fierro, J.L.G.; Peña, M.A.; González Tejuca, L. An XPS and reduction study of PrCoO3. J. Mater. Sci. 1988, 23, 1018–1023. [Google Scholar] [CrossRef]
- Oliván, A.M.O.; Peña, M.A.; Tejuca, L.G.; Tascón, J.M.D. A comparative study of the interactions of NO and CO with LaCrO3. J. Mol. Catal. 1988, 45, 355–363. [Google Scholar] [CrossRef]
- Norton, T.T.; Ortiz-Landeros, J.; Lin, Y.S. Stability of La–Sr–Co–Fe oxide-carbonate dual-phase membranes for carbon dioxide separation at high temperatures. Ind. Eng. Chem. Res. 2014, 53, 2432–2440. [Google Scholar] [CrossRef]
- Platon, C.E.; Thomson, W.J. A comparison of LSCF-6428 and Bys for the oxidative conversion of methane and ethane. Ind. Eng. Chem. Res. 2002, 41, 6637–6641. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mirzababaei, J.; Chuang, S.S.C. La0.6Sr0.4Co0.2Fe0.8O3 Perovskite: A Stable Anode Catalyst for Direct Methane Solid Oxide Fuel Cells. Catalysts 2014, 4, 146-161. https://doi.org/10.3390/catal4020146
Mirzababaei J, Chuang SSC. La0.6Sr0.4Co0.2Fe0.8O3 Perovskite: A Stable Anode Catalyst for Direct Methane Solid Oxide Fuel Cells. Catalysts. 2014; 4(2):146-161. https://doi.org/10.3390/catal4020146
Chicago/Turabian StyleMirzababaei, Jelvehnaz, and Steven S. C. Chuang. 2014. "La0.6Sr0.4Co0.2Fe0.8O3 Perovskite: A Stable Anode Catalyst for Direct Methane Solid Oxide Fuel Cells" Catalysts 4, no. 2: 146-161. https://doi.org/10.3390/catal4020146