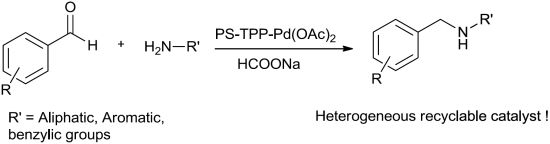
Polymer Supported Triphenylphosphine-Palladium Acetate Complex PS-TPP-Pd(OAc)2 as a Heterogeneous and Reusable Catalyst for Indirect Reductive Amination of Aldehydes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Catalyst Loading
| Entry | Catalyst conc. mol-% | (%) Yield by G.C. |
|---|---|---|
| 1 | 2.5 | 19 |
| 2 | 5 | 65 |
| 3 | 10 | 74 |
| 4 | 15 | 74 |
2.2. Effect of Various Solvents on the Reaction
| Entry | Solvent | (%) Yield by G.C. |
|---|---|---|
| 1 | Toluene | Traces |
| 2 | Ethanol | 47 |
| 3 | Dioxane | 18 |
| 4 | DMF | 74 |
| 5 | Acetontrile | 12 |
2.3. Effect of Temperature on the Reaction
| Entry | Temp °C | (%) Yield by G.C. |
|---|---|---|
| 1 | 40 | 58 |
| 2 | 50 | 64 |
| 3 | 60 | 71 |
| 4 | 70 | 72 |
| 5 | 85 | 74 |
| 6 | 95 | 69 |
2.4. Effect of Time on Reaction Yields
| Entry | Time (h) | (%) Yield by G.C. |
|---|---|---|
| 1 | 2 | 56 |
| 2 | 3 | 64 |
| 3 | 4 | 70 |
| 4 | 5 | 73 |
| 5 | 6 | 74 |
| 6 | 7 | 72 |
2.5. Preparation and Evaluation of PS-TPP-Pd(OAc)2 Complex Catalyst Varying Ratio of Pd(OAc)2 to PS-TPP
| Entry | Pd/P Ratio | Pd(OAc)2 (mol-%) | PS-TPP (mol-%) | Color changes | (%) Yield by G.C. |
|---|---|---|---|---|---|
| 1 | 1:1 | 10 | 10 | Black | 23 |
| 2 | 1:2 | 10 | 20 | Black | 45 |
| 3 | 1:3 | 10 | 30 | Brown-black | 65 |
| 4 | 1:4 | 10 | 40 | Yellow | 74 |
| 5 | 1: 5 | 10 | 50 | Yellow | 74 |
| 6 b | 1:4 | 10 | --- | Yellow | 75 |
2.6. Effect of Formic Acid Salts in Reduction of Amine
| Entry | Hydrogen donor | Hydrogen donor (mmol) | (%) Yield by G.C. |
|---|---|---|---|
| 1 | HCOONa | 1.5 | 67 |
| 2 | HCOONa | 2 | 71 |
| 3 | HCOONa | 2.5 | 73 |
| 4 | HCOONa | 3 | 74 |
| 5 | HCOOK | 3 | 59 |
| 6 | HCOONH4 | 3 | 21 |
2.7. Reductive Amination of Aldehydes Using Sodium Formate
| Entry | Aldehyde | Amine | Product | %Yield b |
|---|---|---|---|---|
| 1 |  |  |  | 74 |
| 2 |  |  |  | 84 |
| 3 |  |  |  | 56 |
| 4 |  |  |  | 85 |
| 5 |  |  |  | 76 |
| 6 |  |  |  | 82 |
| 7 |  |  |  | 84 |
| 8 |  |  |  | 77 |
| 9 |  |  |  | 68 |
| 10 |  |  |  | 65 |
| 11 |  |  |  | 70 |
| 12 |  |  |  | 58 |
| 13 |  |  |  | 61 |
2.8. Recycle Study of Catalyst
| Entry | Catalyst recycle number | Yield (%) b |
|---|---|---|
| 1 | 0 | 74 |
| 2 | 1 | 71 |
| 3 | 2 | 68 |
| 4 | 3 | 66 |
| 5 | 4 | 65 |
3. Experimental Section
3.1. General
3.1.1. Preparation of Polymer Supported Triphenyphosphane Palldium Acetate PS-TPP-Pd(OAc)2 Complex [15,16,17,18,19]
3.1.2. Stage No. 1, Preparation of Imine
3.1.3. Stage No. 2, Reduction of Imine
3.1.4. Recovery and Recycle of PS-TPP-Pd(OAc)2 Catalyst
3.1.5. Characterization Data of Products
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haddenham, D.; Pasumansky, L.; DeSoto, J.; Eagon, S.; Singaram, B. Reductions of aliphatic and aromatic nitriles to primary amines with diisopropylaminoborane. J. Org. Chem. 2009, 74, 1964–1970. [Google Scholar] [CrossRef]
- Abdel-Majid, A.F.; Carson, K.G.; Harris, B.D.; Maryanoff, C.A.; Shah, R.D. Reductive amination of aldehydes and ketones with Sodium Triacetoborohydride. J. Org. Chem. 1996, 61, 3849–3862. [Google Scholar]
- Borch, R.F.; Bernstein, M.D.; Durst, H.D. Reductive amination reduction with use of Sodium Cyano-borohydride. J. Am. Chem. Soc. 1971, 93, 2897–2904. [Google Scholar]
- Malkov, A.V.; Figlus, M.; Stoncius, S.; Kocovsky, P. Assymetric reduction of ketimines with trichlorosilanes catalyzed by N-methylvaline derived lewis-basic formamide with enantio selectivitiy at room temperature. J. Org. Chem. 2007, 72, 1315–1325. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, M.; Wu, P.; Wei, S.; Sun, J. L-Piperaizine-2-carboxylic acid derived N-Formamide, highly enantioselective Lewis basic catalyst for hydrosilylation of imines with trichlorosilane. Org. Lett. 2006, 8, 3045–3048. [Google Scholar]
- Wang, Z.; Ye, X.; Wei, S.; Wu, P.; Zhang, A.; Sun, J. L-Picolinic acid derived N-formamide, highly efficient and enantioselective Lewis basic organocatalyst for mild reduction of various N-aryl imines with trichlorosilane. Org. Lett. 2006, 8, 999–1001. [Google Scholar] [CrossRef]
- Blackwell, J.M.; Sonmer, E.R.; Scoccitti, T.; Piers, W.E. Various benzaldimines and ketimines hydrosilated effectively with PMe2SiH employing B(C6F5)3 as a catalyst. Org. Lett. 2000, 2, 3921–3923. [Google Scholar] [CrossRef]
- Li, G.; Liang, Y.; Antilla, J.C. α-imino esters derived from aryl and alkyl esters could be reduced to corresponding α-amino esters in excellent yields, using 5 mol% of chiral phosphoric acid. J. Am. Chem. Soc. 2007, 129, 5830–5831. [Google Scholar]
- Misumi, Y.; Seino, H.; Mizobe, Y. A benzene dithiolate Ru(III) complex catalyzes hydrogenation of imines under ambient temperature and pressure. J. Am. Chem. Soc. 2009, 131, 14636–14637. [Google Scholar]
- Cho, B.T.; Kang, S.K. A simple and convenient procedure for reductive amination of aldehydes and ketones using sodium borohydride as reducing agent and boric acid, PTSA H2O, benzoic acid as activator under solvent free conditions. Tetrahedron 2005, 61, 5725–5734. [Google Scholar] [CrossRef]
- Knettle, B.W.; Flowers, R.A. The combination of HMPA and SmBr2 in THF is powerful reductant in reducing ketamines and alkyl chlorides at room temperature. Org. Lett. 2001, 3, 2321–2324. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Zhou, G.; Guo, W. The organic reductant 1-Acetyl-2,3-dimethylimidazolidine is able to reduce a series of aromatic, aliphatic and α,β-unsaturated aldehydes as well as imines in high yields. Synlett 2008, 225–228. [Google Scholar]
- Khedkar, V.; Tillak, A.; Beller, M. Aryl oxotitanium complex as catalyst in intramolecular hydroamination of terminal alkynes. Branched imines are obtained in good yields with various primary aromatic and aliphatic amines. Org. Lett. 2003, 5, 4767–4770. [Google Scholar]
- Basu, B.; Jha, S.; Bhuiyan, M.M.H.; Das, P. Simple protocol for direct reductive amination of aldehydes and ketones using potassium formate and catalytic palladium acetate. Synlett 2003, 4, 555–557. [Google Scholar]
- Pittman, C.U.; Wuu, S.I.; Jacobson, S.E. 1,3-Butadiene oligomerization catalyzed by polymer-attached palladium complexes. Comparison with homogeneous catalysis. J. Catal. 1976, 44, 87–100. [Google Scholar] [CrossRef]
- Andersson, C.M.; Karabelas, K.; Hallberg, A.; Andersson, C. Palladium/phosphinated polystyrene as a catalyst in the Heck arylation. A comparative study. J. Org. Chem. 1985, 50, 3891–3895. [Google Scholar]
- Terasawa, M.; Kaneda, K.; Imanaka, T.; Teranishi, S. Study of hydrogenation of olefins catalyzed by polymer-bound palladium (II) complexes. J. Catal. 1978, 51, 406–421. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Chen, B.; Weatherford, D. New strategies in using macromolecular catalysts in organic synthesis. J. Mol. Catal. 1992, 74, 409–419. [Google Scholar] [CrossRef]
- Wagh, Y.S.; Tambade, P.J.; Sawant, D.N.; Bhanage, B.M. Allylic amination of internal alkynes with aromatic and aliphatic amines using polymer-supported triphenylphosphane-palladium complex as a heterogeneous and recyclable catalyst. Eur. J. Org. Chem. 2010, 5071–5076. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ekbote, S.S.; Gadge, S.T.; Bhanage, B.M. Polymer Supported Triphenylphosphine-Palladium Acetate Complex PS-TPP-Pd(OAc)2 as a Heterogeneous and Reusable Catalyst for Indirect Reductive Amination of Aldehydes. Catalysts 2014, 4, 289-298. https://doi.org/10.3390/catal4030289
Ekbote SS, Gadge ST, Bhanage BM. Polymer Supported Triphenylphosphine-Palladium Acetate Complex PS-TPP-Pd(OAc)2 as a Heterogeneous and Reusable Catalyst for Indirect Reductive Amination of Aldehydes. Catalysts. 2014; 4(3):289-298. https://doi.org/10.3390/catal4030289
Chicago/Turabian StyleEkbote, Sunil S., Sandip T. Gadge, and Bhalchandra M. Bhanage. 2014. "Polymer Supported Triphenylphosphine-Palladium Acetate Complex PS-TPP-Pd(OAc)2 as a Heterogeneous and Reusable Catalyst for Indirect Reductive Amination of Aldehydes" Catalysts 4, no. 3: 289-298. https://doi.org/10.3390/catal4030289