Abatement of VOCs with Alternate Adsorption and Plasma-Assisted Regeneration: A Review
Abstract
:1. Introduction
2. Sequential Treatment (Adsorption Followed by NTP Oxidation)


| Adsorption | Regeneration/NTP Oxidation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target pollutant | Catalyst/Adsorbent | Specific surface area (m2/g) | Carrier gas and flow rate (mL/min) | Concen-tration (ppm) | Plasma reactor type | Carrier Gas and flow rate (mL/min) | Pdis (W) SIE (J/L) | Time t (min) | Maximum removal efficiency (%) | Mineralization rate m (%) CO2 yield c (%) | Ec (kWh/m3) | Ref. | |
| Acetone (C3H6O) | TiO2 | 38 ± 3 | dry air 1000 | 180 | DBD/IPC | dry air 1000 | 0.33 W | t1 = 103 t2 = 30 | 27 | m = 12 c = 11 | - | [47] | |
| DBD/IPC + TPD | 91 a | m = 52a | |||||||||||
| Benzene | Ag/HZSM-5 | 334 | 80% N2 + 20% O2 (50% RH) 600 | 4.7 | DBD/IPC | O2 60 | 4.7 W | t1 = 840 t2 = 24 | ~100 | c = 99.8 | 3.7 × 10−3 | [40] | |
| Toluene | Ag/HZSM-5 | - | Air 3000 | 3 | link tooth wheel cylin-der/PPC | synthetic air (40 ± 5% RH) 1000 | - | - | 62 a | c > 90 | 2.2 × 10−3 | [43] | |
| Mn/HZSM-5 | 69 | ||||||||||||
| Ce/HZSM-5 | 56 a | ||||||||||||
| Ce-Mn/HZSM-5 | 93 | ||||||||||||
| Ag-Mn/HZSM-5 | 70 a | c = 99.9 | |||||||||||
| Formalde-hyde | Ag(3.6 wt.%)-Cu(2.1 wt.%)/HZSM-5 | 229 | 80% N2 + 20% O2 (50% RH) 300 | 6.8 | DBD/IPC | O2 60 | 2.3 W | t1 = 690 t2 = 10 | 100 | c > 99 | 1.9 × 10−3 | [39] | |
| Isopropanol | TiO2 | 38 ± 3 | Air (50% RH) 1000 | 163 | DBD/IPC | air (50% RH) 1000 | - | t1 = 1 30 t2 ≈ 60 | 91 | - | - | [35] | |
| Benzene | HZSM-5 | 328 | 80% N2 + 20% O2 (50% RH) 600 | 4.7 | DBD/IPC | O2 60 | 4.7 W | t1 = 60 t2 = 9 | 100 | c = 89 a | - | [42] | |
| Ag(0.8 wt.%)/HZSM-5 | 334 | 100 | c = 100 | ||||||||||
| Ag(1.9 wt.%)/HZSM-5 | 329 | 92 a | c = 100 | ||||||||||
| Ag(4.2 wt.%)/HZSM-5 | 306 | 63 | c = 100 | ||||||||||
| Benzene | Ag(1 wt.%)/TiO2 | ≤68 | air (50% PP) 4000–5000 | 200 | DBD/IPC | O2 5000–8000 | 169 J/L | - | 100 a | - | - | [29] | |
| Ag(4 wt.%)/TiO2 | Air (60% PP) | 136 J/L | 75 a | c = 80 a | |||||||||
| 0.5%Ag/ɣ-Al2O3 | ≤210 | 160 J/L | 90 a | c = 75 a | |||||||||
| H-Y zeolite | ≤520 | Air (80% PP)10000 | 140 J/L | 100 | c ~64 a | ||||||||
| Ag(2 wt.%)/H-Y zeolite | 160 J/L | 100 | c ~73 a | ||||||||||
| Formaldehyde | mineral granulate (MP 5) | - | N2 176 | 99 | DBD/IPC | N2 57 | 2.2 W | t1 = 250 t2 = 4 | 94 | m = 38.7 b c = 26.5 b | - | [48] | |
| Acetaldehyde (CH3CHO) | α-Al2O3 | 14 | 95% N2 + 5% O2 100 | 1000 | corona discharge/IPC | 95% N2 + 5% O2 100 | 1 W | - | 25 | - | - | [49] | |
| Toluene | ɣ-Al2O3 | 237 | N2 2500 | - | DBD/IPC | O2 2500 | 88 W | t1 = 190 t2 = 70 | 71.4 | - | 41.2 J/µmol | [46] | |
| Isopropanol | Mn xOy | 16 ± 2 | dry air 1000 | 165 | DBD/IPC | dry air 1000 | 0.82 ± 0.02 W | t1 = 6 t2 = 30 | 66 | m = 56 c = 44 | - | [50] | |
| DBD/IPC + TPD | 96 | m = 84 c = 72 | |||||||||||
| DBD/PPC | 0.42 ± 0.02 W | 41 | m = 27 c = 21 | ||||||||||
| DBD/PPC + TPD | 84 | m = 57 c = 51 | |||||||||||
| Isopropanol | TiO2 | 45 | dry air 750 | 98 | DBD/PPC | dry air | 68.2 mW | t1 = 130 t2 = 70 | 24 | m = 2 c = 1.7 b | - | [51] | |
| Acetone | 175 | 72 mW | m = 6 | ||||||||||
| Acetaldehyde | fibrous activated carbon textile | 26.32 b | air 100 | 200 | ac-neon transformer | - | 6 J/cm2 | - | 100 | - | - | [22] | |
| Toluene | zeolites | - | air 150000 | 10–120 | DBD/IPC | air 150,000 | 89 W | - | 90–39 | - | 2.6–13 g/kWh | [52] | |
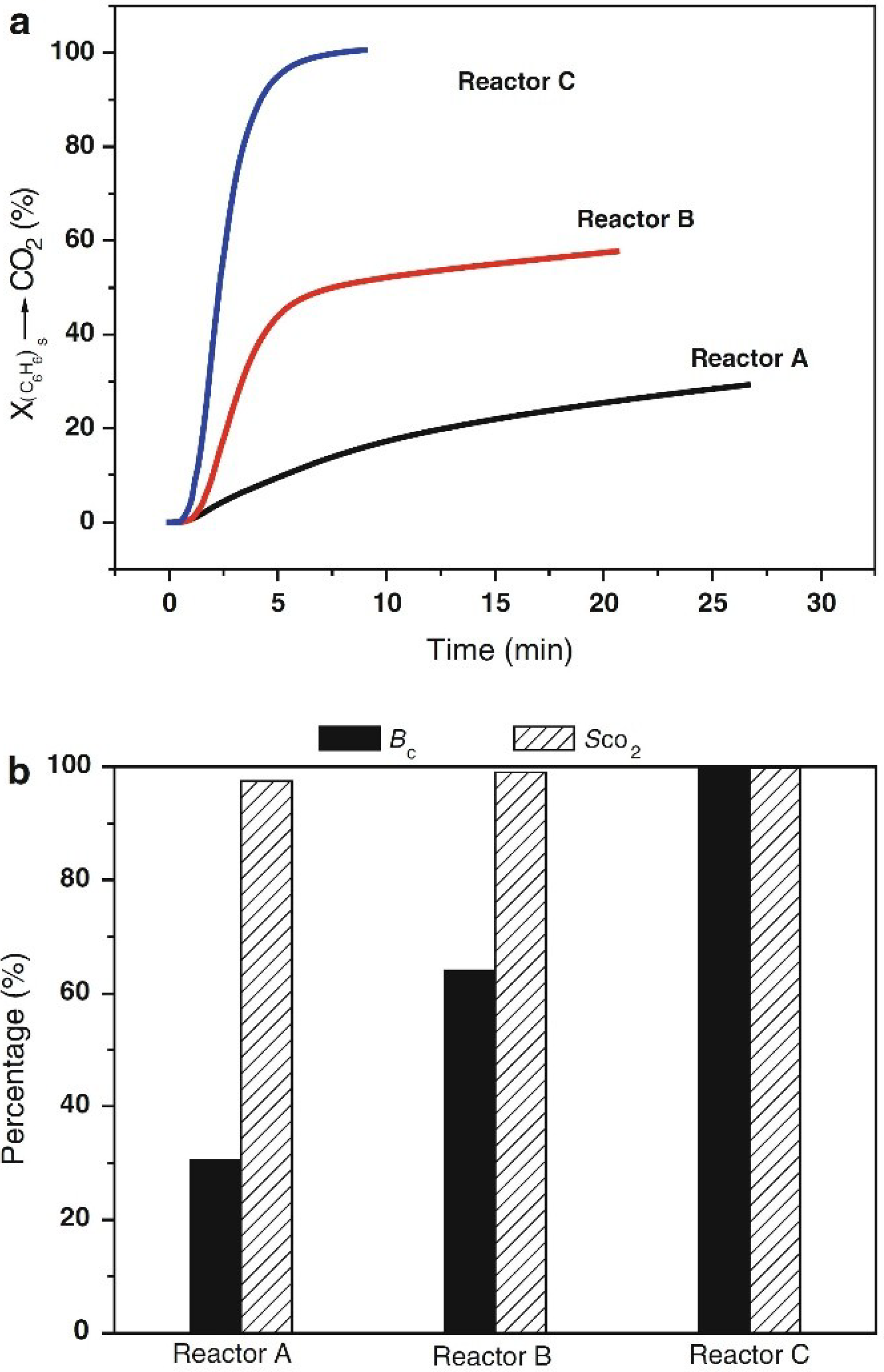

3. Critical Process Parameters for VOC Adsorption
3.1. Physical Properties of Sorbent/Catalyst
3.2. Nature of VOCs

3.3. Relative Humidity

3.4. Temperature
4. Critical Process Parameters for Regeneration
4.1. Flow Rate

4.2. Temperature
4.3. Discharge Power


4.4. Relative Humidity

4.5. O2 Content/O2 Partial Pressure

4.6 Number of Cycles

5. Conclusions
Author Contributions
Conflicts of Interest
References
- Pal, R.; Kim, K.-H.; Hong, Y.-J.; Jeon, E.-C. The Pollution Status of Atmospheric Carbonyls in a Highly Industrialized Area. J. Hazard. Mater. 2008, 153, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Sarigiannis, D.A.; Karakitsios, S.P.; Gotti, A.; Liakos, I.L.; Katsoyiannis, A. Exposure to Major Volatile Organic Compounds and Carbonyls in European Indoor Environments and Associated Health Risk. Environ. Int. 2011, 37, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R. Atmospheric Chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Vineis, P.; Forastiere, F.; Hoek, G.; Lipsett, M. Outdoor Air Pollution and Lung Cancer: Recent Epidemiologic Evidence. Int. J. Cancer 2004, 111, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Tollefsen, P.; Rypdal, K.; Torvanger, A.; Rive, N. Air Pollution Policies in Europe: Efficiency Gains From Integrating Climate Effects with Damage Costs to Health and Crops. Environ. Sci. Policy 2009, 12, 870–881. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ramaanathan, K.; Lawless, P.A.; Ensor, D.S.; Newsome, J.R.; Plaks, N.; Ramsey, G.H. Control of Volatile Organic Compounds by an AC Energized Eerroelectric Pellet Reactor and a Pulsed Corona Reactor. IEEE Trans. Ind. Appl. 1992, 28, 528–534. [Google Scholar] [CrossRef]
- Nunez, C.M.; Ramsey, G.H.; Ponder, W.H.; Abbott, J.H.; Hamel, L.E.; Kariher, P.H. Corona Destruction: An Innovative Control Technology for VOCs and Air Toxics. Air Waste 1993, 43, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Sudnick, J.J.; Corwin, D.L. VOC Control Techniques. Hazard. Waste Hazard. 1994, 11, 129–143. [Google Scholar] [CrossRef]
- Kim, H.H.; Ogata, A.; Futamura, S. Complete Oxidation of Volatile Organic Compounds (VOCs) Using Plasma-Diven Catalysis and Oxygen Plasma. Int. J. Plas. Environ. Sci. Tech. 2007, 4–8. [Google Scholar]
- Keller, R.A.; Dyer, J.A. Abating Halogenated VOCs. Chem. Eng. 1998, 105, 100–105. [Google Scholar]
- Dyer, J.A.; Mulholland, K. Toxic Air Emissions What is the Full Cost to Your Business? Chem. Eng. 1994, 101, 4–8. [Google Scholar]
- Urashima, K.; Chang, J.S. Removal of Volatile Organic Compounds from Air Streams and Industrial Flue Gases by Non-thermal Plasma Technology. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 602–614. [Google Scholar] [CrossRef]
- Chang, J.S. Next Generation Integrated Electrostatic Gas Cleaning Systems. J. Electrostat. 2003, 57, 273–291. [Google Scholar] [CrossRef]
- Dan, Y.; Gao, D.S.; Yu, G.; Shen, X.L.; Gu, F. Investigation of the Treatment of Particulate Matter from Gasoline Engine Exhaust Esing Non-thermal Plasma. J. Hazard. Mater. 2005, 127, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Low Temperature Plasma-based Ssterilization: Overview and State-of-the-art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Chang, C.L.; Lin, T.S. Decomposition of Toluene and Acetone in Packed Dielectric Barrier Discharge Reactors. Plasma Chem. Plasma Process. 2005, 25, 227–243. [Google Scholar] [CrossRef]
- Lee, H.M.; Chang, M.B. Gas-phase Removal of Acetaldehyde Via Packed-bed Dielectric Barrier Discharge Reactor. Plasma Chem. Plasma Process. 2001, 21, 329–343. [Google Scholar] [CrossRef]
- Okubo, M.; Yamamoto, T.; Kuroki, T.; Fukumoto, H. Electric Air Cleaner Composed of Nonthermal Plasma Reactor and Electrostatic Precipitator. IEEE Trans. Ind. Appl. 2001, 37, 1505–1511. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Mora, M.; Jimenez-Sanchidrian, C.; Romero-Salguero, F.J.; de Geyter, N.; Leys, C.; Morent, R. TCE Abatement with a Plasma-catalytic Combined System Using MnO2 as Catalyst. Appl. Catal. B 2014, 156, 94–100. [Google Scholar] [CrossRef]
- Nguyen Dinh, M.T.; Giraudon, J.M.; Lamonier, J.F.; Vandenbroucke, A.; de Geyter, N.; Leys, C.; Morent, R. Plasma-catalysis of Low TCE Concentration in air Using LaMnO3+δ as Catalyst. Appl. Catal. B 2014, 147, 904–911. [Google Scholar] [CrossRef]
- Assadi, A.A.; Palau, J.; Bouzaza, A.; Penya-Roja, J.; Martinez-Soriac, V.; Wolbert, D. Abatement of 3-methylbutanal and Trimethylamine with Combined Plasma and Photocatalysis in a Continuous Planar Reactor. J. Photoch. Photobiol. A 2014, 282, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ohshima, T.; Kondo, T.; Kitajima, N.; Sato, M. Adsorption and Plasma Decomposition of Gaseous Acetaldehyde on Fibrous Activated Carbon. IEEE Trans. Ind. Appl. 2010, 46, 23–28. [Google Scholar] [CrossRef]
- Kim, H.H.; Ogata, A.; Futamura, S. Effect of Different Catalysts on the Decomposition of VOCs Using Flow-type Plasma-driven Catalysis. IEEE Trans. Plasma Sci. 2006, 34, 984–995. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Minh Tuan Nguyen, D.; Giraudon, J.-M.; Morent, R.; De Geyter, N.; Lamonier, J.-F.; Leys, C. Qualitative By-product Identification of Plasma-assisted TCE Abatement by Mass Spectrometry and Fourier-transform Infrared Spectroscopy. Plasma Chem. Plasma Process. 2011, 31, 707–718. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Leys, C.; Van Langenhove, H. Combining Non-thermal Plasma with Heterogeneous Catalysis in Waste Gas Treatment: A Review. Appl. Catal. B 2008, 78, 324–333. [Google Scholar] [CrossRef]
- Roland, U.; Holzer, F.; Kopinke, E.D. Combination of Non-thermal Plasma and Heterogeneous Catalysis for Oxidation of Volatile Organic Compounds Part 2. Ozone Decomposition and Deactivation of ɣ-Al2O3. Appl. Catal. B 2005, 58, 217–226. [Google Scholar] [CrossRef]
- Subrahmanyam, C. Catalytic Non-thermal Plasma Reactor for Total Oxidation of Volatile Organic Compounds. Indian J. Chem. A 2009, 48, 1062–1068. [Google Scholar]
- Zhu, T.; Li, J.; Liang, W.J.; Jin, Y.Q. Synergistic Effect of Catalyst for Oxidation Removal of Toluene. J. Hazard. Mater. 2009, 165, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Ogata, A.; Futamura, S. Oxygen Partial Pressure-dependent Behavior of Various Catalysts for the Total Oxidation of VOCs Using Cycled System of Adsorption and Oxygen Plasma. Appl. Catal. B 2008, 79, 356–367. [Google Scholar] [CrossRef]
- Malik, M.A.; Minamitani, Y.; Schoenbach, K.H. Comparison of Catalytic Activity of Aluminum Oxide and Silica Gel for Decomposition of Volatile Organic Compounds (VOCs) in a Plasmacatalytic Reactor. IEEE Trans. Plasma Sci. 2005, 33, 50–56. [Google Scholar] [CrossRef]
- Ogata, A.; Yamanouchi, K.; Mizuno, K.; Kushiyama, S.; Yamamoto, T. Oxidation of Dilute Benzene in an Alumina Hybrid Plasma Reactor at Atmospheric Pressure. Plasma Chem. Plasma Process. 1999, 19, 383–394. [Google Scholar] [CrossRef]
- Song, Y.H.; Kim, S.J.; Choi, K.I.; Yamamoto, T. Effects of Adsorption and Temperature on a Nonthermal Plasma Process for Removing VOCs. J. Electrostat. 2002, 55, 189–201. [Google Scholar] [CrossRef]
- Martin, L.; Ognier, S.; Gasthauer, E.; Cavadias, S.; Dresvin, S.; Amouroux, J. Destruction of Highly Diluted Volatile Organic Components (VOCs) in air by Dielectric Barrier Discharge and Mineral Bed Adsorption. Energy Fuel. 2008, 22, 576–582. [Google Scholar] [CrossRef]
- Inoue, K.; Okano, H.; Yamagata, Y.; Muraoka, K.; Teraoka, Y. PerformanceTtests of Newly Developed Adsorption/plasma Combined System for Decomposition of Volatile Organic Compounds Under Continuous Flow Condition. J. Environ. Sci. China 2011, 23, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Sivachandiran, L.; Thevenet, F.; Gravejat, P.; Rousseau, A. Isopropanol Saturated TiO2 Surface Regeneration by Non-thermal Plasma: Influence of Air Relative Humidity. Chem. Eng. J. 2013, 214, 17–26. [Google Scholar] [CrossRef]
- Ogata, A.; Ito, D.; Mizuno, K.; Kushiyama, S.; Yamamoto, T. Removal of Dilute Benzene Using a Zeolte-hybrid Plasma Reactor. IEEE Trans. Ind. Appl. 2001, 37, 959–964. [Google Scholar] [CrossRef]
- Thevenet, F.; Sivachandiran, L.; Guaitella, O.; Barakat, C.; Rousseau, A. Plasma-catalyst Coupling for Volatile Organic Compound Removal and Indoor Air Treatment: A Review. J. Phys. D 2014, 47, 224011–224024. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Thevenet, F.; Rousseau, A. Isopropanol Removal Using MnxOy Packed Bed Non-thermal Plasma Reactor: Comparison between Continuous Treatment and Sequential Sorption/regeneration. Chem. Eng. J. 2015, 270, 327–335. [Google Scholar] [CrossRef]
- Zhao, D.Z.; Li, X.S.; Shi, C.; Fan, H.Y.; Zhu, A.M. Low-concentration Formaldehyde Removal from Air Using a Cycled Storage–discharge (CSD) Plasma Catalytic Process. Chem. Eng. Sci. 2011, 66, 3922–3929. [Google Scholar] [CrossRef]
- Fan, H.Y.; Shi, C.; Li, X.S.; Zhao, D.Z.; Xu, Y. High-efficiency Plasma Catalytic Removal of Dilute Benzene from Air. J. Phys. D 2009, 42, 225105–225109. [Google Scholar] [CrossRef]
- Ding, H.X.; Zhu, A.M.; Yang, X.F.; Li, C.H.; Xu, Y. Removal of Formaldehyde from Gas Streams via Packed-bed Dielectric Barrier Discharge Plasmas. J. Phys. D 2005, 38, 4160–4167. [Google Scholar] [CrossRef]
- Fan, H.-Y.; Li, X.-S.; Shi, C.; Zhao, D.-Z.; Liu, J.-L.; Liu, Y.-X.; Zhu, A.-M. Plasma Catalytic Oxidation of Stored Benzene in a Cycled Storage-discharge (CSD) Process: Catalysts, Reactors and Operation Conditions. Plasma Chem. Plasma Process. 2011, 31, 799–810. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, T.; Fan, X. Removal of Gas Phase Low-concentration Toluene by Intermittent Use of Adsorption and Non-thermal Plasma Regeneration. In Proceedings of 21st International Symposium on Plasma Chemistry (ISPC 21), Queensland, Australia, 4–9 August 2013.
- Takahashi, A.; Yang, F.H.; Yang, R.T. Aromatics/aliphatics Separation by Adsorption: New Sorbents for Selective Aromatics Adsorption by π-complexation. Ind. Eng. Chem. Res. 2000, 39, 3856–3867. [Google Scholar] [CrossRef]
- Qu, Z.; Bu, Y.; Qin, Y.; Wang, Y.; Fu, Q. The Improved Reactivity of Manganese Catalysts by Ag in Catalytic Oxidation of Toluene. Appl. Catal. B 2013, 132, 353–362. [Google Scholar] [CrossRef]
- Mok, Y.S.; Kim, D.H. Treatment of Toluene by Using Adsorption and Nonthermal Plasma Oxidation Process. Curr. Appl. Phys. 2011, 11, S58–S62. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Thevenet, F.; Rousseau, A. Non-thermal Plasma Assisted Regeneration of Acetone Adsorbed TiO2 Surface. Plasma Chem. Plasma Process. 2013, 33, 855–871. [Google Scholar] [CrossRef]
- Saulich, K.; Mueller, S. Removal of Formaldehyde by Adsorption and Plasma Treatment of Mineral Adsorbent. J. Phys. D 2013, 46, 045201–045208. [Google Scholar] [CrossRef]
- Klett, C.; Duten, X.; Tieng, S.; Touchard, S.; Jestin, P.; Hassouni, K.; Vega-González, A. Acetaldehyde Removal Using an Atmospheric Non-thermal Plasma Combined with a Packed Bed: Role of the Adsorption Process. J. Hazard. Mater. 2014, 279, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Sivachandiran, L.; Thevenet, F.; Rousseau, A. Regeneration of Isopropyl Alcohol Saturated MnxOy Surface: Comparison of Thermal, Ozonolysis and Non-thermal Plasma Treatments. Chem. Eng. J. 2014, 246, 184–195. [Google Scholar] [CrossRef]
- Barakat, C.; Gravejat, P.; Guaitella, O.; Thevenet, F.; Rousseau, A. Oxidation of Isopropanol and Acetone Adsorbed on TIO2 Under Plasma Generated Ozone Flow: Gas Phase and Adsorbed Species Monitoring. Appl. Catal. B 2014, 147, 302–313. [Google Scholar] [CrossRef]
- Yamagata, Y.; Niho, K.; Inoue, K.; Okano, H.; Muraoka, K. Decomposition of Volatile Organic Compounds at Low Concentrations Using Combination of Densification by Zeolite Adsorption and Dielectric Barrier Discharge. Jpn. J. Appl. Phys. 2006, 45, 8251–8254. [Google Scholar] [CrossRef]
- Kim, H.H.; Tsubota, S.; Date, M.; Ogata, A.; Futamura, S. Catalyst Regeneration and Activity Enhancement of Au/TiO2 by Atmospheric Pressure Nonthermal Plasma. Appl. Catal. A 2007, 329, 93–98. [Google Scholar] [CrossRef]
- Arsac, F.; Bianchi, D.; Chovelon, J.M.; Ferronato, C.; Herrmann, J.M. Experimental Microkinetic Approach of the Photocatalytic Oxidation of Isopropyl Alcohol on TiO2. Part 1. Surface Elementary Steps Involving Gaseous and Adsorbed C3HxO species. J. .Phys. Chem. A 2006, 110, 4202–4212. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.A.; Widegren, J.A.; Falconer, J.L. Transient Studies of 2-propanol Photocatalytic Oxidation on Titania. J. Catal. 1995, 157, 611–625. [Google Scholar] [CrossRef]
- Xu, W.Z.; Raftery, D.; Francisco, J.S. Effect of Irradiation Sources and Oxygen Concentration on the Photocatalytic Oxidation of 2-propanol and Acetone Studied by in situ FTIR. J. Phys. Chem. B 2003, 107, 4537–4544. [Google Scholar] [CrossRef]
- Yamamoto, T.; Asada, S.; Iida, T.; Ehara, Y. Novel NOx and VOC Treatment Using Concentration and Plasma Decomposition. IEEE Trans. Ind. Appl. 2011, 47, 2235–2240. [Google Scholar] [CrossRef]
- Dang, X.; Huang, J.; Cao, L.; Zhou, Y. Plasma-catalytic Oxidation of Adsorbed Toluene with Gas Circulation. Catal. Commun. 2013, 40, 116–119. [Google Scholar] [CrossRef]
- Kuroki, T.; Hirai, K.; Matsuoka, S.; Kim, J.Y.; Okubo, M. Oxidation System of Adsorbed VOCs on Adsorbent Using Nonthermal Plasma Flow. IEEE Trans. Ind. Appl. 2011, 47, 1916–1921. [Google Scholar] [CrossRef]
- Kuroki, T.; Hirai, K.; Kawabata, R.; Okubo, M.; Yamamoto, T. Decomposition of Adsorbed Xylene on Adsorbents Using Nonthermal Plasma with Gas Circulation. IEEE Trans. Ind. Appl. 2010, 46, 672–679. [Google Scholar] [CrossRef]
- Inoue, K.; Furuki, K.; Okano, H.; Yamagata, Y.; Muraoka, K. A New Decomposition System for Volatile Organic Compounds Using Combinations of Dielectric Barrier Discharges with Zeolite Honeycomb Sheets. Electron. Eng. Jpn. 2009, 168, 1–10. [Google Scholar] [CrossRef]
- Das, D.; Gaur, V.; Verma, N. Removal of Volatile Organic Compound by Activated Carbon Fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Kim, K.-J.; Ahn, H.-G. The Effect of Pore Structure of Zeolite on the Adsorption of VOCs and their Desorption Properties by Microwave Heating. Microporous Mesoporous Mater. 2012, 152, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Chiang, Y.C.; Chiang, P.C.; Chang, E.E. Effects of Surface Characteristics of Activated Carbons on VOC Adsorption. J. Environ. Eng. 2001, 127, 54–62. [Google Scholar] [CrossRef]
- Meininghaus, C.K.W.; Prins, R. Sorption of Volatile Organic Compounds on Hydrophobic Zeolites. Microporous Mesoporous Mater. 2000, 35–36, 349–365. [Google Scholar] [CrossRef]
- Dubinin, M.M. Fundamentals of the Theory of Adsorption in Micropores of Carbon Adsorbents—Characteristics of their Adsorption Properties and Microporous Structures. Pure Appl. Chem. 1989, 61, 1841–1843. [Google Scholar] [CrossRef]
- Mangun, C.L.; Daley, M.A.; Braatz, R.D.; Economy, J. Effect of Pore Size on Adsorption of Hydrocarbons in Phenolic-based Activated Carbon Fibers. Carbon 1998, 36, 123–129. [Google Scholar] [CrossRef]
- Huang, M.C.; Chou, C.H.; Teng, H.S. Pore-size Effects on Activated-carbon Capacities for Volatile Organic Compound Adsorption. AICHE J. 2002, 48, 1804–1810. [Google Scholar] [CrossRef]
- Brodu, N.; Zaitan, H.; Manero, M.H.; Pic, J.S. Removal of Volatile Organic Compounds by Heterogeneous Ozonation on Microporous Synthetic Alumina Silicate. Water Sci. Technol. 2012, 66, 2020–2026. [Google Scholar] [CrossRef] [PubMed]
- Lillo-Rodenas, M.A.; Cazorla-Amoros, D.; Linares-Solano, A. Behaviour of Activated Carbons with Different Pore Size Distributions and Surface Oxygen Groups for Benzene and Toluene Adsorption at Low Concentrations. Carbon 2005, 43, 1758–1767. [Google Scholar] [CrossRef]
- Lillo-Rodenas, M.A.; Cazorla-Amoros, D.; Linares-Solano, A. Benzene and Toluene Adsorption at Low Concentration on Activated Carbon Fibres. Adsorption 2011, 17, 473–481. [Google Scholar] [CrossRef]
- Li, L.; Sun, Z.; Li, H.; Keener, T.C. Effects of Activated Carbon Surface Properties on the Adsorption of Volatile Organic Compounds. J. Air Waste Manage. Assoc. 2012, 62, 1196–1202. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Q.; Xue, F.; Lin, D. Adsorption of Volatile Organic Compounds by Metal-organic Frameworks MILL-101: Influence of Molecular Size and Shape. J. Hazard. Mater. 2011, 195, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, T.; Fujioka, T.; Okubo, M.; Yamamoto, T. Toluene Concentration Using Honeycomb Nonthermal Plasma Desorption. Thin Solid Films 2007, 515, 4272–4277. [Google Scholar] [CrossRef]
- Gundry, P.M.; Tompkins, F.C. Chemisorption of Gases on Metals. Q. Rev. Chem. Soc. 1960, 14, 257–291. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Chaing, P.C.; Huang, C.P. Effects of Pore Structure and Temperature on VOC Adsorption on Activated Carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface-area and Porosity, 2nd edition. Text. Res. J. 1984, 54, 792–792. [Google Scholar]
- Magne, L.; Pasquiers, S. Lif Spectroscopy Applied to the Study of Non-thermal Plasmas for Atmospheric Pollutant Abatement. C. R. Phys. 2005, 6, 908–917. [Google Scholar] [CrossRef]
- Pringle, K.J.; Whitehead, J.C.; Wilman, J.J.; Wu, J.H. The Chemistry of Methane Remediation by a Non-thermal Atmospheric Pressure Plasma. Plasma Chem. Plasma Process. 2004, 24, 421–434. [Google Scholar] [CrossRef]
- Ogata, A.; Mizuno, K.; Kushiyama, S.; Yamamoto, T. Methane Decomposition in a Barium Titanate Packed-bed Nonthermal Plasma Reactor. Plasma Chem. Plasma Process. 1998, 18, 363–373. [Google Scholar] [CrossRef]
- Mok, Y.S.; Lee, S.B.; Oh, J.H.; Ra, K.S.; Sung, B.H. Abatement of Trichloromethane by Using Nonthermal Plasma Reactors. Plasma Chem. Plasma Process. 2008, 28, 663–676. [Google Scholar] [CrossRef]
- Kim, H.H.; Ogata, A. Nonthermal Plasma Activates Catalyst: From Current Understanding and Future Prospects. Eur. Phys. J. 2011, 55, 13806–13818. [Google Scholar]
- Qu, G.; Liang, D.; Qu, D.; Huang, Y.; Li, J. Comparison between Dielectric Barrier Discharge Plasma and Ozone Regenerations of Activated Carbon Exhausted with Pentachlorophenol. Plasma Sci. Technol. 2014, 16, 608–613. [Google Scholar] [CrossRef]
- Kuroki, T.; Fujioka, T.; Kawabata, R.; Okubo, M.; Yamamoto, T. Regeneration of Honeycomb Zeolite by Nonthermal Plasma Desorption of Toluene. IEEE Trans. Ind. Appl. 2009, 45, 10–15. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, S.; Vandenbroucke, A.M.; Leys, C.; De Geyter, N.; Morent, R. Abatement of VOCs with Alternate Adsorption and Plasma-Assisted Regeneration: A Review. Catalysts 2015, 5, 718-746. https://doi.org/10.3390/catal5020718
Sultana S, Vandenbroucke AM, Leys C, De Geyter N, Morent R. Abatement of VOCs with Alternate Adsorption and Plasma-Assisted Regeneration: A Review. Catalysts. 2015; 5(2):718-746. https://doi.org/10.3390/catal5020718
Chicago/Turabian StyleSultana, Sharmin, Arne M. Vandenbroucke, Christophe Leys, Nathalie De Geyter, and Rino Morent. 2015. "Abatement of VOCs with Alternate Adsorption and Plasma-Assisted Regeneration: A Review" Catalysts 5, no. 2: 718-746. https://doi.org/10.3390/catal5020718







