Recent Development of Pd-Based Electrocatalysts for Proton Exchange Membrane Fuel Cells
Abstract
:1. Introduction

2. Pd Nanostructures
2.1. The 0-D Pd Strucutres

2.2. The 1-D Pd Structures

2.3. The 3-D Pd Structures
2.4. Hollow or Core-Shell Structures

2.5. Conclusions and Perspective Discussions
3. Pd-Based Catalysts for the Direct Formic Acid Fuel Cells (DFAFCs)
| Catalysts | EASA/m2 g−1 | Peak Potential/V RHE | Peak Current Density/mA mg−1Pd | Conditions H2SO4/Formic Acid/Scan Rate | References |
|---|---|---|---|---|---|
| Pt@Pd/C | 156.5 | 0.277 | 3900 | 0.5 M/2 M/10 mV s−1 | [48] |
| Pt/C | 198 | 0.687 | / | 0.5 M/2 M/10 mV s−1 | [48] |
| Pd/C | 208.2 | 0.417 | 1200 | 0.5 M/2 M/10 mV s−1 | [48] |
| butylphenyl-stabilized Pd | 122 | 0.38 | 3390 | 0.1 M/0.1 M/100 mV s−1 | [49] |
| Pd black | 33.6 | 0.46 | 750 | 0.1 M/0.1 M/100 mV s−1 | [49] |
| butylphenyl-stabilized Pd | 122 | 0.38 | 3390 | 0.1 M/0.1 M/100 mV s−1 | [49] |
| PdPt/C | 49 | / | 2500 | 0.5 M/0.5 M/50 mV s−1 | [50] |
| Pd/C | / | / | 2400 | 0.1 M/0.5 M/20 mV s−1 | [51] |
| Pd/C | 107.2 | / | 1426.5 | 0.5 M/0.5 M/50 mV s−1 | [52] |
| PdSn/C | / | / | 1420 | 0.5 M/0.5 M/20 mV s−1 | [53] |
| Pd/C | / | / | 610 | 0.5 M/0.5 M/20 mV s−1 | [53] |
| Pd/CMRT | 67.27 | / | 1140 at 0.44 V * | 0.5 M/0.5 M/50 mV s−1 | [54] |
| Pd/RT | 41.47 | / | 670 at 0.44 V * | 0.5 M/0.5 M/50 mV s−1 | [54] |
| Pd/C | 63.72 | / | 440 at 0.44 V * | 0.5 M/0.5 M/50 mV s−1 | [54] |
| Pd/HPMo-PDDA-MWCNT | / | / | 945 | 0.5 M/0.5 M/20 mV s−1 | [55] |
| Pd/AO-MWCNTs | / | / | 554 | 0.5 M/0.5 M/20 mV s−1 | [55] |
| Pd/C | / | / | 373 | 0.5 M/0.5 M/20 mV s−1 | [55] |
| Pd57Ni43 | / | / | 830 | 0.5 M/0.5 M/50 mV s−1 | [23] |
| Pd/C | / | / | 700 | 0.5 M/0.5 M/50 mV s−1 | [23] |
| Pd-PANI | / | / | 822 | 0.5 M/0.2 M/100 mV s−1 | [56] |
| PdAu | 90 | / | 800 | 0.5 M/0.5 M/20 mV s−1 | [57] |
| Pd/C | 43 | / | 250 | 0.5 M/0.5 M/20 mV s−1 | [57] |
| Pd/graphene | 72.72 | / | 446.3 | 0.5 M/0.5 M/50 mV s−1 | [58] |
| Pd/C | 31.85 | / | 191.9 | 0.5 M/0.5 M/50 mV s−1 | [58] |
| Pd/graphene | / | 0.39 | 300 | 0.5 M/0.5 M/50 mV s−1 | [59] |
| Pd/C | / | / | 193 | 0.5 M/0.5 M/50 mV s−1 | [59] |
| Pd networks | / | / | 275.4 | 0.5 M/0.5 M/50 mV s−1 | [31] |
| Nanoporous Pd | 23 | / | 262 | 0.5 M/0.5 M/10 mV s−1 | [60] |
| Pd/CNT | / | 0.44 | 200 at 0.27 V * | 0.5 M/0.5 M/50 mV s−1 | [61] |
| Pt/CNT | / | 0.92 | 30 at 0.27 V * | 0.5 M/0.5 M/50 mV s−1 | [61] |
| Pt Pd/CNT | / | 0.64 | 50 at 0.27 V * | 0.5 M/0.5 M/50 mV s−1 | [61] |
| Pt1Pd3/CNT | / | 0.64 | 125 at 0.27 V * | 0.5 M/0.5 M/50 mV s−1 | [61] |
| PdSn/C | 64.7 | / | 170.2 | 0.5 M/1 M/10 mV s−1 | [62] |
| Pd/C | 39.8 | / | 102.3 | 0.5 M/1 M/10 mV s−1 | [62] |
| Pd black | / | / | 56.5 | 0.5 M/0.5 M/50 mV s−1 | [31] |
| Pd/untreated-MWCNT | 46.2 | 0.517 | 159 mA cm−2 | 0.5 M/0.5 M/50 mV s−1 with glutamate | [63] |
| Pd/acid-oxidized MWCNT | 35.7 | / | 108 mA cm−2 | 0.5 M/0.5 M/50 mV s−1 | [63] |
| Pd/untreated-MWCNT | 21.4 | / | 72 mA cm−2 | 0.5 M/0.5 M/50 mV s−1 without glutamate | [63] |
| PdNi/C | / | 0.247 | 105.1 mA cm−2 | 0.5 M/1 M/10 mV s−1 | [64] |
| Pd/C | / | 0.287 | 72.9 mA cm−2 | 0.5 M/1 M/10 mV s−1 | [64] |
| PdCo/MWCNTs | / | 0.28 | 107 mA cm−2 | 0.5 M/0.1 M/20 mV s−1 | [46] |
| Pd/MWCNTs | / | 0.32 | 35.6 mA cm−2 | 0.5 M/0.1 M/20 mV s−1 | [46] |
| Pd/XC-72 | / | 0.38 | 24.8 mA cm−2 | 0.5 M/0.1 M/20 mV s−1 | [46] |
| Pd0.9Pt0.1/C | 83 | 0.35 | 87.5 mA cm−2 | 0.5 M/0.5 M/50 mV s−1 | [45] |
| Pt/C | 85.6 | 0.52 | 15.1 mA cm−2 | 0.5 M/0.5 M/50 mV s−1 | [45] |
| Pd/C | 89.7 | 0.48 | 42.5 mA cm−2 | 0.5 M/0.5 M/50 mV s−1 | [45] |
| Pd-B/C | 87.6 | / | 65.4 mA cm−2 | 0.5 M/0.5 M/50 mV s−1 | [65] |
| Pd/C | 90 | / | 36.0 mA cm−2 | 0.5 M/0.5 M/50 mV s−1 | [65] |
| Pd–Au/C | / | 0.37 | 18.6 mA cm−2 | 0.5 M/0.5 M | [66] |
| Pd/C | / | 0.48 | 12.4 mA cm−2 | 0.5 M/0.5 M | [66] |
| Pd/phen-MWCNTs | 37.6 | / | / | 0.5 M/1 M/50 mV s−1 | [67] |
| Pd/AO-MWCNTs | 15.7 | / | / | 0.5 M/1 M/50 mV s−1 | [67] |
| Pd/graphene | 44 | 0.367 | / | 1 M/1 M/10 mV s−1 | [68] |
| Pd/Vulcan C | 35 | 0.377 | / | 1 M/1 M/10 mV s−1 | [68] |
3.1. Pd Supported on Carbon Materials
3.1.1. Pd on Carbon Powders
3.1.2. Pd alloys on Carbon Powders
3.1.3. Pd Supported on Carbon Nanotubes
3.1.4. Pd Alloys Supported on Carbon Nanotubes
3.1.5. Pd Supported on Graphene
3.2. Pd Supported on Oxides
3.3. Pd Supported on other Supporting Materials
3.4. Unsupported Pd

3.5. Single Fuel Cell Characterization
3.5.1. Single Fuel Cell Performance
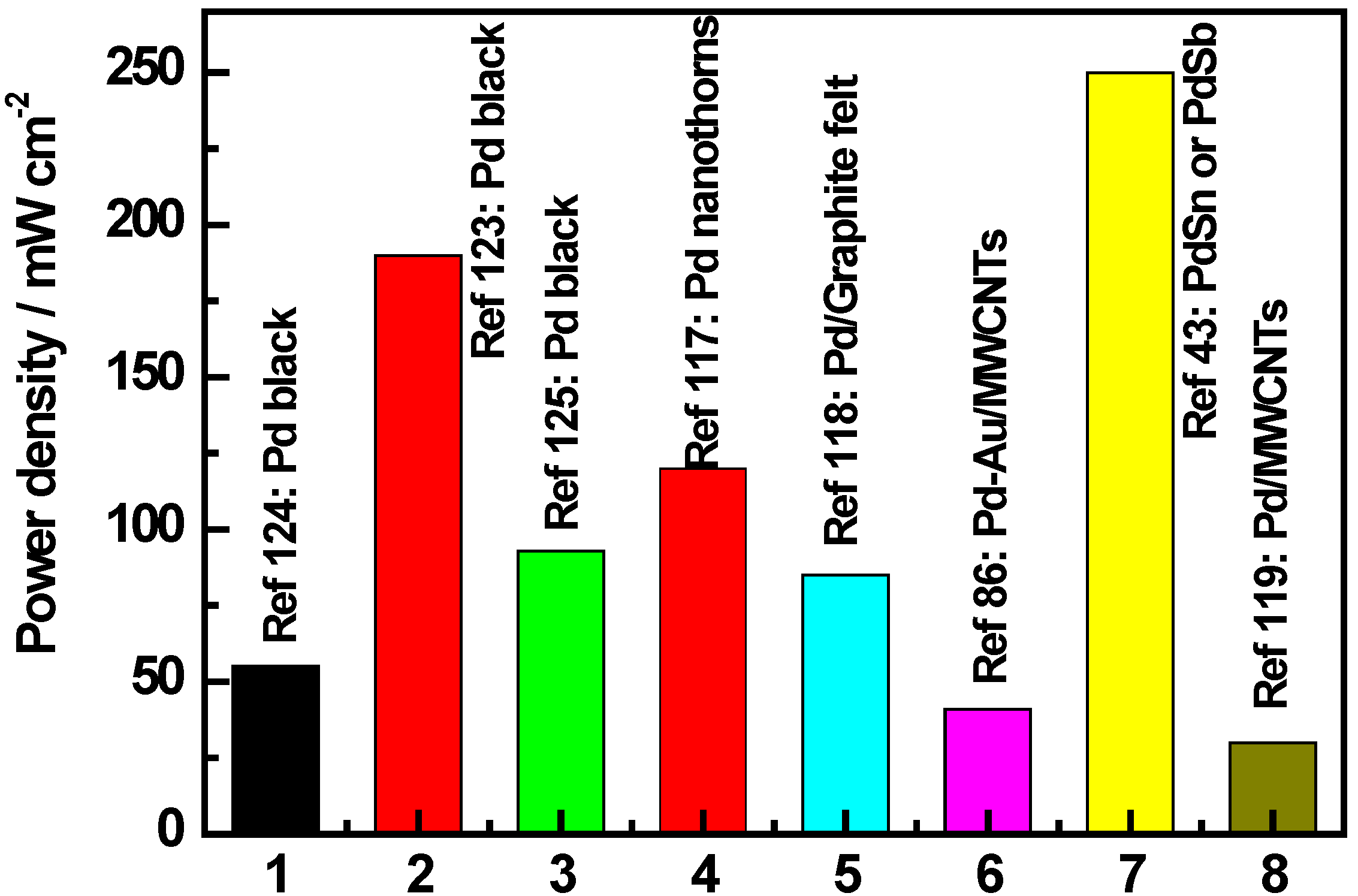
3.5.2. Deactivation Mechanism and Reactivation
4. Pd-Based Catalysts for the Direct Ethanol Fuel Cells (DEFCs)
| Catalysts | SA1/mA cm−2 | MA2/A g−1Pd | OP3/V RHE | PP4/V RHE | Conditions Ethanol/KOH/Scan Rate | References |
|---|---|---|---|---|---|---|
| Dendritic Pd | 17 | / | −0.353 | −0.003 | 1 M/1 M/20 mV s−1 | [28] |
| Pd/CNTs | / | 800 | −0.353 | 0.007 | 0.5 M/0.5 M/50 mV s−1 | [35] |
| Pd shell/Au core | 0.890 | / | −0.582 | −0.15 | 1 M/1 M/50 mV s−1 | [38] |
| Pd/HCHs | 42 | 2200 | −0.352 | −0.072 | 2 M/1 M/50 mV s−1 | [130] |
| PdNPs/CFCNT | 16 | / | −0.24 | 0.14 | 1 M/1 M/60 mV s−1 | [136] |
| Pd–In2O3/CNTs | 61 | / | −0.347 | −0.022 | 1 M/0.5 M/50 mV s−1 | [137] |
| Pd/CNFs | 74.5 | / | −0.541 | −0.021 | 1 M/1 M/50 mV s−1 | [138] |
| Pd/CNFs | 66.1 | 1187 | −0.511 | −0.057 | 1 M/1 M/50 mV s−1 | [139] |
| Pd/CNFs | 80 | 1400 | −0.46 | −0.04 | 1 M/1 M/50 mV s−1 | [140] |
| Pd-Ni/CNFs | 200 | / | −0.602 | −0.072 | 1 M/1 M/10 mV s−1 | [141] |
| Pd/SnO2-GNS | 46.1 | / | −0.403 | 0.227 | 0.25 M/0.25 M/50 mV s−1 | [142] |
| Pd-PANI | / | 1300 | −0.46 | 0.07 | 1 M/0.5 M/100 mV s−1 | [56] |
| Pd/polyamide 6 | 70 | / | / | −0.072 | 0.5 M/1.5 M/50 mV s−1 | [143] |
| Pd/Nickel foam | 107.7 | / | −0.442 | 0.078 | 1 M/1 M/50 mV s−1 | [144] |
| Pd-TiN | 2.87 | 59.2 | −0.474 | 0.022 | 0.5 M/1 M/20 mV s−1 | [145] |
| Pd-NiO/MgO@C | 69.3 | / | −0.602 | −0.052 | 1 M/1 M/10 mV s−1 | [146] |
| Ru@PtPd/C | / | 3600 | −0.402 | −0.052 | 1 M/1 M/30 mV s−1 | [134] |
| Pd particles | 151 | / | −0.548 | / | 1 M/1 M/50 mV s−1 | [147] |
| Nanoporous Pd | 90.64 | 227.7 | −0.403 | 0 | 0.5 M/1 M/10 mV s−1 | [148] |
| Pd NWs | / | 7.96 * | / | −0.01 | 0.5 M/1 M/50 mV s−1 | [149] |
| Pd NWs | / | 2.16 * | −0.566 | −0.068 | 1 M/1 M/50 mV s−1 | [150] |
| Pd/Au NWA | 199 | / | −0.402 | 0.138 | 1 M/1 M/50 mV s−1 | [151] |
| PdPt | 34 | 478 | −0.523 | 0.077 | 1 M/1 M/50 mV s−1 | [152] |
| Pd-Sn/C | 121.59 | −0.46 | 0.135 | 3 M/0.5 M/50 mV s−1 | [153] | |
| Pd-Ru-Sn/C | 65 | / | −0.536 | 0.097 | 3 M/0.5 M/50 mV s−1 | [153] |
| Pd91Sn9/C | 130 | / | −0.205 | 0.347 | 1 M/1 M/50 mV s−1 | [154] |
| Pd7 Ir/C | 103 | / | −0.584 | 0.008 | 1 M/1 M/50 mV s−1 | [155] |
| Pd-Ni | 6 | / | / | −0.03 | 0.5 M/1 M/50mV s−1 | [156] |
| Pd40 Ni60 | 180 | / | −0.712 | 0.098 | 1 M/1 M/50mV s−1 | [157] |
| Pd-Ni/CNF | 199.8 | / | −0.602 | −0.0.072 | 1 M/1 M/10mV s−1 | [131] |
| Pd-Ni-Zn/C | 78.5 | 3600 | −0.423 | 0.057 | 10 wt%/2 M/50mV s−1 | [135] |
| Pd-Ni-Zn-P/C | 108.7 | 3030 | −0.373 | 0.097 | 10wt%/2 M/50 mV s−1 | [135] |
| Pd- Ag/C | 3.7** | / | −0.602 | 0.018 | 1 M/1 M/50mV s−1 | [158] |
| Pd-Ag film | 5 ** | / | 0.02 | 1 M/1 M/20 mV s−1 | [159] | |
| Pd–Pb/C | 4.25 ** | / | −0.452 | −0.0.012 | 1 M/1 M/20 mV s−1 | [160] |
| PdAu/C | 165 | / | −0.402 | 0.0273 | 1 M/1 M/50 mV s−1 | [132] |
| Pd4Au/C | / | / | 0.5 | 0.88 | 1 M/0.25 M/10 mV s−1 | [161] |
| Pd2.5Sn/C | / | / | 0.55 | 0.86 | 1 M/0.25 M/10 mV s−1 | [161] |
| PdAu nanowire | 83.7 | / | −0.472 | −0.001 | 1 M/1 M/50 mV s−1 | [133] |
| Pd@Au/C | / | 800 | −0.41 | 0.04 | 1 M/1 M/50 mV s−1 | [162] |
4.1. Pd Supported on Carbon Materials
4.1.1. Pd Supported on Carbon Spheres

4.1.2. Pd Supported on Carbon Nanotubes
4.1.3. Pd Supported on Carbon Nanofibers
4.1.4. Pd Supported on Graphene
4.2. Pd Supported on Non-Carbon Supports
4.2.1. Pd Supported on Conducting Polymers
4.2.2. Pd Supported on Zeolite
4.2.3. Pd Supported on Metal Supports
4.2.4. Pd Supported on Nitrides
4.3. Performance of Pd Novel Nano Structures
4.3.1. Core-Shell Structure
4.3.2. Other Structures
4.4. Pd Alloys
4.4.1. PdNi Alloys
4.4.2. PdAu Alloys
4.5. Single Fuel Cell Characterizations

5. Pd-Based Catalysts for the Oxygen Reduction Reaction (ORR)
| Catalysts | Mass Activity/mA mg−1Pd | Onset Potential/V vs. RHE | Conditions Solution **/Scan Rate/Rotating Speed | References |
|---|---|---|---|---|
| Pd/C | / | 0.87 | A/5 mV s−1/1600 rpm | [195] |
| Pt/C (JM) | / | 1.05 | A/5 mV s−1/1600 rpm | [195] |
| Pd–PPy/C | / | 0.82 (NHE) | A/5 mV s−1/1600 rpm | [196] |
| 40% E-TEK Pt/C | / | 0.98 (NHE) | A/5 mV s−1/1600 rpm | [196] |
| Pd/Vulcan XC-72R | 47 at 0.7 V * | 0.82 | A/5 mV s−1/1600 rpm | [197] |
| 20% E-TEK Pt/C | 0.92 | A/5 mV s−1/1600 rpm | [197] | |
| E-Tek 20% Pd/C | 23 at 0.7 V * | 0.77 | A/5 mV s−1/1600 rpm | [197] |
| PdFe-WC/C | / | 0.91 | A/10 mV s−1/1600 rpm | [198] |
| Pt50Au50/CexC | 4.1 at 0.7 V * | / | A/5 mV s−1/1600 rpm | [180] |
| PdCoMo/CDX975 | 4.1 at 0.7 V * | 0.915 | A/5 mV s−1/1600 rpm | [199] |
| Pd/CDX975 | 1.6 at 0.7 V * | 0.84 | A/5 mV s−1/1600 rpm | [199] |
| Pd100−xWx | / | 0.85 | A/5 mV s−1/1600 rpm | [200] |
| Pd | / | 0.7 | A/5 mV s−1/1600 rpm | [200] |
| PdPt/C | / | 0.98 | A/5 mV s−1/1600 rpm | [201] |
| Pt rich-core Pd rich-shell | 9 at 0.85 V * (catalyst) | / | A/5 mV s−1/1600 rpm | [202] |
| JM20 Pt/C | 5.8 at 0.85 V * (catalyst) | / | A/5 mV s−1/1600 rpm | [202] |
| Pd/Au | 0.34 at 0.8 V * | / | A/10 mV s−1 | [203] |
| 1.1 at 0.8 V * | / | B/10 mV s−1 | [203] | |
| Pd/MWCNTs | / | 0.76 (SHE) | B/10 mV s−1 | [91] |
| PdCu/C | 23 at 0.9 V * | 0.96 | B/10 mV s−1/1600 rpm | [204] |
| Pd–Cu film | / | 0.86 | B/5 mV s−1/1000 rpm | [205] |
| Pd–Co/C | 3.6 at 0.7 V * | / | B/5 mV s−1/1600 rpm | [206] |
| Pt–Pd/C | / | 0.92 | B/5 mV s−1/1600 rpm | [207] |
| Pt–Pd/C | 114.87 at 0.9 V * (Pt) | 1.05 | B/5 mV s−1/1600 rpm | [208] |
| Pt/C | 73.87 at 0.9 V * (Pt) | 1.0 | B/5 mV s−1/1600 rpm | [208] |
| PdFe@PdPt/C | 1.92 at 0.8 V * (Pt) | / | B/20 mV s−1/1600 rpm | [209] |
| 1.2 at 0.8 V * (Pt) | / | B/20 mV s−1/1600 rpm | [209] | |
| PdCo@PdPt/C | 65 at 0.9 V * (Pt) | / | B/20 mV s−1/1600 rpm | [210] |
| Pt/C | 14 at 0.9 V * (Pt) | / | B/20 mV s−1/1600 rpm | [210] |
| PdFe Nanorods | 284 at 0.85 V * | / | B/10 mV s−1/1600 rpm | [211] |
| Pt/C | 265 at 0.85 V * (Pt) | / | B/10 mV s−1/1600 rpm | [211] |
| Pt/Pd/Pd3Fe | 1.8 at 0.9 V * | / | B/20 mV s−1/1600 rpm | [212] |
| Pt(111) | 0.8 at 0.9 V * | / | B/20 mV s−1/1600 rpm | [212] |
| Pd/SnO2–KB | 0.75 at 0.8 V * | 0.88 | B/5 mV s−1/900 rpm | [64] |
| Pd/KB | 0.28 at 0.8 V * | 0.86 | B/5 mV s−1/900 rpm | [213] |
| Tanaka Pt/C | / | 0.95 | B/5 mV s−1/900 rpm | [213] |
| Pd/GNS | 280 at 0.9 V * | 1.06 | C/10 mV s−1/1600 rpm | [188] |
| Pt/GNS | 110 at 0.9 V * | 1.0 | C/10 mV s−1/1600 rpm | [188] |
| Pd@MnO2/C | 450 at 0.9 V * | 1.02 | D/10 mV s−1/2500 rpm | [214] |
| Pd black | 180 at 0.9 V * | 1.07 | D/10 mV s−1/2500 rpm | [214] |
5.1. Pd Supported on Carbon Materials
5.1.1. Pd on Carbon Powder
5.1.2. Pd Supported on Carbon Nanotubes
5.1.3. Pd Supported on Graphene
5.2. Pd Supported on Oxides
5.3. Pd Alloys
5.3.1. PdFe Alloys
5.3.2. PdCu Alloys
5.3.3. PdAg Alloys
5.3.4. PdAu Alloys
5.3.5. PdCo Alloys
5.3.6. PdPt Alloys

5.4. Novel Nanostructures
5.4.1. Core-Shell Structures
5.4.2. Unsupported Pd
6. Conclusions and Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grove, W.R. XXIV. On voltaic series and the combination of gases by platinum. Philos. Mag. Ser. 1839, 14, 127–130. [Google Scholar]
- Soszko, M.; Lukaszewski, M.; Mianowska, Z.; Czerwinski, A. Electrochemical characterization of the surface and methanol electrooxidation on Pt–Rh–Pd ternary alloys. J. Power Sources 2011, 196, 3513–3522. [Google Scholar] [CrossRef]
- Shao, M. Palladium-based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J. Power Sources 2011, 196, 2433–2444. [Google Scholar] [CrossRef]
- Palladium Chart—Last 5 years. Available online: http://www.kitco.com/charts/popup/pd1825nyb.html (accessed on 1 May 2015).
- Bianchini, C.; Shen, P.K. Palladium-Based Electrocatalysts for Alcohol Oxidation in Half Cells and in Direct Alcohol Fuel Cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Mazumder, V.; Lee, Y.; Sun, S.H. Recent Development of Active Nanoparticle Catalysts for Fuel Cell Reactions. Adv. Funct. Mater. 2010, 20, 1224–1231. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Morozan, A.; Jousselme, B.; Palacin, S. Low-platinum and platinum-free catalysts for the oxygen reduction reaction at fuel cell cathodes. Energy Environ. Sci. 2011, 4, 1238–1254. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, M.; Ma, L.; Liang, L.; Liu, C.P.; Liao, J.H.; Lu, T.H.; Xing, W. Recent advances in catalysts for direct methanol fuel cells. Energy Environ. Sci. 2011, 4, 2736–2753. [Google Scholar] [CrossRef]
- Adams, B.D.; Chen, A.C. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Tian, N.; Li, J.T.; Broadwell, I.; Sun, S.G. Nanomaterials of high surface energy with exceptional properties in catalysis and energy storage. Chem. Soc. Rev. 2011, 40, 4167–4185. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Zhou, Z.Y.; Yu, N.F.; Wang, L.Y.; Sun, S.G. Direct Electrodeposition of Tetrahexahedral Pd Nanocrystals with High-Index Facets and High Catalytic Activity for Ethanol Electrooxidation. J. Am. Chem. Soc. 2010, 132, 7580–7581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Tian, N.; Huang, Z.Z.; Chen, D.J.; Sun, S.G. Nanoparticle catalysts with high energy surfaces and enhanced activity synthesized by electrochemical method. Faraday Discuss. 2008, 140, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.M.; Min, Q.H.; Shi, J.J.; Jiang, L.P.; Zhang, J.R.; Hou, W.H.; Zhu, J.J. Morphology-Controlled Synthesis of Palladium Nanostructures by Sonoelectrochemical Method and Their Application in Direct Alcohol Oxidation. J. Phys. Chem. C 2009, 113, 1267–1273. [Google Scholar] [CrossRef]
- Ding, H.; Shi, X.Z.; Shen, C.M.; Hui, C.; Xu, Z.C.; Li, C.; Tian, Y.A.; Wang, D.K.; Gao, H.J. Synthesis of monodisperse palladium nanocubes and their catalytic activity for methanol electrooxidation. Chin. Phys. B 2010, 19. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, H.J.; Ma, D.; Bao, X.H. Porous Palladium Nanoflowers that Have Enhanced Methanol Electro-Oxidation Activity. J. Phys. Chem. C 2009, 113, 1001–1005. [Google Scholar] [CrossRef]
- Meng, H.; Sun, S.; Masse, J.P.; Dodelet, J.P. Electrosynthesis of Pd Single-Crystal Nanothorns and Their Application in the Oxidation of Formic Acid. Chem. Mater. 2008, 20, 6998–7002. [Google Scholar] [CrossRef]
- Meng, H.; Wang, C.X.; Shen, P.K.; Wu, G. Palladium thorn clusters as catalysts for electrooxidation of formic acid. Energy Environ. Sci. 2011, 4, 1522–1526. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.Y.; Sun, S.G. Electrochemical preparation of Pd nanorods with high-index facets. Chem. Commun. 2009, 1502–1504. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Viswanath, B.; Barai, K.; Ravishankar, N.; Munichandraiah, N. High-Surface Step Density on Dendritic Pd Leads to Exceptional Catalytic Activity for Formic Acid Oxidation. ACS Appl. Mater. Interfaces 2010, 2, 2965–2969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, Y.; Lu, S.F.; Jia, L.C.; Shen, P.K.; Jiang, S.P. Significant promotion effect of carbon nanotubes on the electrocatalytic activity of supported Pd NPs for ethanol oxidation reaction of fuel cells: The role of inner tubes. Chem. Commun. 2014, 50, 13732–13734. [Google Scholar] [CrossRef] [PubMed]
- Du, C.Y.; Chen, M.; Wang, W.G.; Yin, G.P. Nanoporous PdNi Alloy Nanowires As Highly Active Catalysts for the Electro-Oxidation of Formic Acid. ACS Appl. Mater. Interfaces 2011, 3, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Lim, M.A.; Kang, S.W.; Park, I.; Han, S.W. Facile synthesis of noble metal nanotubes by using ZnO nanowires as sacrificial scaffolds and their electrocatalytic properties. Chem. Commun. 2011, 47, 6299–6301. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Guo, S.J.; Dong, S.J.; Wang, E.K. Ultrathin Pd nanowire as a highly active electrode material for sensitive and selective detection of ascorbic acid. Biosens. Bioelectron. 2010, 26, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Jena, B.K.; Sahu, S.C.; Satpati, B.; Sahu, R.K.; Behera, D.; Mohanty, S. A facile approach for morphosynthesis of Pd nanoelectrocatalysts. Chem. Commun. 2011, 47, 3796–3798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, W.Q.; Zhang, H.M.; Du, Y.K.; Yang, P.; Wang, C.Y.; Xu, J.K. Facile template-free synthesis of pine needle-like Pd micro/nano-leaves and their associated electro-catalytic activities toward oxidation of formic acid. Nanoscale Res. Lett. 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Fujita, T.; Inoue, A.; Sakurai, T.; Chen, M.W. Electrochemical synthesis of palladium nanostructures with controllable morphology. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.X.; Guo, S.J.; Zhu, C.Z.; Dong, S.J.; Wang, E.K. Twenty Second Synthesis of Pd Nanourchins with High Electrochemical Activity through an Electrochemical Route. Langmuir 2010, 26, 17816–17820. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; E, Y.; Yuan, J.M.; Luo, X.Y.; Yang, Y.; Fan, L.Z. Electrosynthesis of Pd/Au hollow cone-like microstructures for electrocatalytic formic acid oxidation. Electrochim. Acta 2011, 56, 6237–6244. [Google Scholar] [CrossRef]
- Ye, L.; Wang, Y.; Chen, X.Y.; Yue, B.; Tsang, S.C.; He, H.Y. Three-dimensionally ordered mesoporous Pd networks templated by a silica super crystal and their application in formic acid electrooxidation. Chem. Commun. 2011, 47, 7389–7391. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Cerritos, R.; Fuentes-Ramirez, R.; Cuevas-Muniz, F.M.; Ledesma-Garcia, J.; Arriaga, L.G. Performance and stability of Pd nanostructures in an alkaline direct ethanol fuel cell. J. Power Sources 2014, 269, 370–378. [Google Scholar] [CrossRef]
- Choi, S.I.; Shao, M.H.; Lu, N.; Ruditskiy, A.; Peng, H.C.; Park, J.; Guerrero, S.; Wang, J.G.; Kim, M.J.; Xia, Y.N. Synthesis and Characterization of Pd@Pt–Ni Core-Shell Octahedra with High Activity toward Oxygen Reduction. ACS Nano 2014, 8, 10363–10371. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Kim, S.K.; Lee, S.C.; Choi, J.; Nahm, K.S.; Yoo, S.J.; Kim, P. One-step synthesis of carbon-supported Pd@Pt/C core-shell nanoparticles as oxygen reduction electrocatalysts and their enhanced activity and stability. Nanoscale 2014, 6, 4038–4042. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Zhao, B.; Guo, C.L.; Sun, Y.J.; Shi, Y.; Yang, H.B.; Li, Z.A. Carbon nanotube/raspberry hollow Pd nanosphere hybrids for methanol, ethanol, and formic acid electro-oxidation in alkaline media. J. Colloid Interface Sci. 2010, 351, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.Y.; Yang, L.; Li, L.; Lv, J.; Wang, K.; Zhang, J. A Facile Preparation of Hollow Palladium Nanosphere Catalysts for Direct Formic Acid Fuel Cell. J. Phys. Chem. C 2009, 113, 10568–10573. [Google Scholar] [CrossRef]
- Fang, P.P.; Duan, S.; Lin, X.D.; Anema, J.R.; Li, J.F.; Buriez, O.; Ding, Y.; Fan, F.R.; Wu, D.Y.; Ren, B.; et al. Tailoring Au-core Pd-shell Pt-cluster nanoparticles for enhanced electrocatalytic activity. Chem. Sci. 2011, 2, 531–539. [Google Scholar] [CrossRef]
- Ksar, F.; Ramos, L.; Keita, B.; Nadjo, L.; Beaunier, P.; Remita, H. Bimetallic Palladium-Gold Nanostructures: Application in Ethanol Oxidation. Chem. Mater. 2009, 21, 3677–3683. [Google Scholar] [CrossRef]
- Hsu, C.J.; Huang, C.W.; Hao, Y.W.; Liu, F.Q. Au/Pd core-shell nanoparticles with varied hollow Au cores for enhanced formic acid oxidation. Nanoscale Res. Lett. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kang, S.W.; Choi, K.W.; Choi, S.W.; Han, S.W.; Im, S.H.; Park, O.O. Au@Pd nanostructures with tunable morphologies and sizes and their enhanced electrocatalytic activity. Crystengcomm 2013, 15, 7113–7120. [Google Scholar] [CrossRef]
- Miao, F.J.; Tao, B.R.; Sun, L.; Liu, T.; You, J.C.; Wang, L.W.; Chu, P.K. Preparation and characterization of novel nickel-palladium electrodes supported by silicon microchannel plates for direct methanol fuel cells. J. Power Sources 2010, 195, 146–150. [Google Scholar] [CrossRef]
- Mikolajczuk, A.; Borodzinski, A.; Kedzierzawski, P.; Stobinski, L.; Mierzwa, B.; Dziura, R. Deactivation of carbon supported palladium catalyst in direct formic acid fuel cell. Appl. Surface Sci. 2011, 257, 8211–8214. [Google Scholar] [CrossRef]
- Haan, J.L.; Stafford, K.M.; Masel, R.I. Effects of the Addition of Antimony, Tin, and Lead to Palladium Catalyst Formulations for the Direct Formic Acid Fuel Cell. J. Phys. Chem. C 2010, 114, 11665–11672. [Google Scholar] [CrossRef]
- Xu, W.F.; Gao, Y.; Lu, T.H.; Tang, Y.W.; Wu, B. Kinetic Study of Formic Acid Oxidation on Highly Dispersed Carbon Supported Pd-TiO2 Electrocatalyst. Catal. Lett. 2009, 130, 312–317. [Google Scholar] [CrossRef]
- Zhang, H.X.; Wang, C.; Wang, J.Y.; Zhai, J.J.; Cai, W.B. Carbon-Supported Pd-Pt Nanoalloy with Low Pt Content and Superior Catalysis for Formic Acid Electro-oxidation. J. Phys. Chem. C 2010, 114, 6446–6451. [Google Scholar] [CrossRef]
- Morales-Acosta, D.; Ledesma-Garcia, J.; Godinez, L.A.; Rodriguez, H.G.; Alvarez-Contreras, L.; Arriaga, L.G. Development of Pd and Pd–Co catalysts supported on multi-walled carbon nanotubes for formic acid oxidation. J. Power Sources 2010, 195, 461–465. [Google Scholar] [CrossRef]
- Du, C.Y.; Chen, M.; Wang, W.G.; Yin, G.P.; Shi, P.F. Electrodeposited PdNi2 alloy with novelly enhanced catalytic activity for electrooxidation of formic acid. Electrochem. Commun. 2010, 12, 843–846. [Google Scholar] [CrossRef]
- Wu, Y.N.; Liao, S.J.; Su, Y.L.; Zeng, J.F.; Dang, D. Enhancement of anodic oxidation of formic acid on palladium decorated Pt/C catalyst. J. Power Sources 2010, 195, 6459–6462. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Kang, X.W.; Song, Y.; Chen, S.W. Butylphenyl-functionalized palladium nanoparticles as effective catalysts for the electrooxidation of formic acid. Chem. Commun. 2011, 47, 6075–6077. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Wang, M.E.; Zhou, D.D.; Xia, Y.Y. Structural design and facile synthesis of a highly efficient catalyst for formic acid electrooxidation. Phys. Chem. Chem. Phys. 2011, 13, 13594–13597. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.J.; Chen, X.M.; Liu, C.P.; Lu, T.H.; Liao, J.H.; Liang, L.A.; Xing, W. Promoting effect of vanadium ions on the anodic Pd/C catalyst for direct formic acid fuel cell application. Electrochim. Acta 2010, 55, 9132–9136. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, M.N.; Ma, J.; Tang, Y.W.; Chen, Y.; Lu, T.H. Highly dispersed carbon-supported Pd nanoparticles catalyst synthesized by novel precipitation-reduction method for formic acid electrooxidation. Electrochim. Acta 2011, 56, 4696–4702. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Ge, J.J.; Ma, L.A.; Liao, J.H.; Lu, T.H.; Xing, W. Highly Active Carbon-supported PdSn Catalysts for Formic Acid Electrooxidation. Fuel Cells 2009, 9, 114–120. [Google Scholar] [CrossRef]
- Wang, X.M.; Wang, J.; Zou, Q.Q.; Xia, Y.Y. Pd nanoparticles supported on carbon-modified rutile TiO2 as a highly efficient catalyst for formic acid electrooxidation. Electrochim. Acta 2011, 56, 1646–1651. [Google Scholar] [CrossRef]
- Cui, Z.M.; Kulesza, P.J.; Li, C.M.; Xing, W.; Jiang, S.P. Pd nanoparticles supported on HPMo-PDDA-MWCNT and their activity for formic acid oxidation reaction of fuel cells. Int. J. Hydrogen Energy 2011, 36, 8508–8517. [Google Scholar] [CrossRef]
- Pandey, R.K.; Lakshminarayanan, V. Electro-Oxidation of Formic Acid, Methanol, and Ethanol on Electrodeposited Pd-Polyaniline Nanofiber Films in Acidic and Alkaline Medium. J. Phys. Chem. C 2009, 113, 21596–21603. [Google Scholar] [CrossRef]
- Park, I.S.; Lee, K.S.; Yoo, S.J.; Cho, Y.H.; Sung, Y.E. Electrocatalytic properties of Pd clusters on Au nanoparticles in formic acid electro-oxidation. Electrochim. Acta 2010, 55, 4339–4345. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, J.; Wang, L.; Tian, C.G.; Jiang, B.J.; Fu, H.G. Fabrication of a palladium nanoparticle/graphene nanosheet hybrid via sacrifice of a copper template and its application in catalytic oxidation of formic acid. Chem. Commun. 2011, 47, 2014–2016. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, C.G.; Wang, L.; Fu, H.G. An effective strategy for small-sized and highly-dispersed palladium nanoparticles supported on graphene with excellent performance for formic acid oxidation. J. Mater. Chem. 2011, 21, 3384–3390. [Google Scholar] [CrossRef]
- Wang, X.G.; Wang, W.M.; Qi, Z.; Zhao, C.C.; Ji, H.; Zhang, Z.H. High catalytic activity of ultrafine nanoporous palladium for electro-oxidation of methanol, ethanol, and formic acid. Electrochem. Commun. 2009, 11, 1896–1899. [Google Scholar] [CrossRef]
- Winjobi, O.; Zhang, Z.Y.; Liang, C.H.; Li, W.Z. Carbon nanotube supported platinum-palladium nanoparticles for formic acid oxidation. Electrochim. Acta 2010, 55, 4217–4221. [Google Scholar] [CrossRef]
- Tu, D.D.; Wu, B.; Wang, B.X.; Deng, C.; Gao, Y. A highly active carbon-supported PdSn catalyst for formic acid electrooxidation. Appl. Catal. B 2011, 103, 163–168. [Google Scholar]
- Hu, C.G.; Bai, Z.Y.; Yang, L.; Lv, J.; Wang, K.; Guo, Y.M.; Cao, Y.X.; Zhou, J.G. Preparation of high performance Pd catalysts supported on untreated multi-walled carbon nanotubes for formic acid oxidation. Electrochim. Acta 2010, 55, 6036–6041. [Google Scholar] [CrossRef]
- Gao, Y.W.; Wang, G.; Wu, B.; Deng, C.; Gao, Y. Highly active carbon-supported PdNi catalyst for formic acid electrooxidation. J. Appl. Electrochem. 2011, 41, 1–6. [Google Scholar] [CrossRef]
- Wang, J.Y.; Kang, Y.Y.; Yang, H.; Cai, W.B. Boron-Doped Palladium Nanoparticles on Carbon Black as a Superior Catalyst for Formic Acid Electro-oxidation. J. Phys. Chem. C 2009, 113, 8366–8372. [Google Scholar] [CrossRef]
- Zhang, G.J.; Wang, Y.E.; Wang, X.; Chen, Y.; Zhou, Y.M.; Tang, Y.W.; Lu, L.D.; Bao, J.C.; Lu, T.H. Preparation of Pd-Au/C catalysts with different alloying degree and their electrocatalytic performance for formic acid oxidation. Appl. Catal. B 2011, 102, 614–619. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Guo, Y.M.; Yang, L.; Li, L.; Li, W.J.; Xu, P.L.; Hu, C.G.; Wang, K. Highly dispersed Pd nanoparticles supported on 1,10-phenanthroline-functionalized multi-walled carbon nanotubes for electrooxidation of formic acid. J. Power Sources 2011, 196, 6232–6237. [Google Scholar] [CrossRef]
- Bong, S.; Uhm, S.; Kim, Y.R.; Lee, J.; Kim, H. Graphene Supported Pd Electrocatalysts for Formic Acid Oxidation. Electrocatalysis 2010, 1, 139–143. [Google Scholar] [CrossRef]
- Hu, G.Z.; Nitze, F.; Barzegar, H.R.; Sharifi, T.; Mikolajczuk, A.; Tai, C.W.; Borodzinski, A.; Wagberg, T. Palladium nanocrystals supported on helical carbon nanofibers for highly efficient electro-oxidation of formic acid, methanol and ethanol in alkaline electrolytes. J. Power Sources 2012, 209, 236–242. [Google Scholar] [CrossRef]
- Nitze, F.; Mazurkiewicz, M.; Malolepszy, A.; Mikolajczuk, A.; Kedzierzawski, P.; Tai, C.W.; Hu, G.Z.; Kurzydlowski, K.J.; Stobinski, L.; Borodzinski, A.; et al. Synthesis of palladium nanoparticles decorated helical carbon nanofiber as highly active anodic catalyst for direct formic acid fuel cells. Electrochim. Acta 2012, 63, 323–328. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Yin, Q.Y.; Lu, G.P.; Yang, H.F.; Zhu, X.; Kong, D.S.; You, J.M. Enhanced catalytic performance of Pd catalyst for formic acid electrooxidation in ionic liquid aqueous solution. J. Power Sources 2014, 272, 606–613. [Google Scholar] [CrossRef]
- Cheng, N.C.; Lv, H.F.; Wang, W.; Mu, S.C.; Pan, M.; Marken, F. An ambient aqueous synthesis for highly dispersed and active Pd/C catalyst for formic acid electro-oxidation. J. Power Sources 2010, 195, 7246–7249. [Google Scholar] [CrossRef]
- Suo, Y.; Hsing, I.M. Size-controlled synthesis and impedance-based mechanistic understanding of Pd/C nanoparticles for formic acid oxidation. Electrochim. Acta 2009, 55, 210–217. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kwak, D.H.; Lee, Y.W.; Lee, S.; Park, K.W. Synthesis of cubic PtPd alloy nanoparticles as anode electrocatalysts for mehtanol and formic acid oxidation reactions. Phys. Chem. Chem. Phys. 2015, 17, 8642–8648. [Google Scholar] [CrossRef] [PubMed]
- Baranova, E.A.; Miles, N.; Mercier, P.H.J.; le Page, Y.; Patarachao, B. Formic acid electro-oxidation on carbon supported PdxPt1−x (0 ≤ x ≤ 1) nanoparticles synthesized via modified polyol method. Electrochim. Acta 2010, 55, 8182–8188. [Google Scholar] [CrossRef]
- Matin, M.A.; Jang, J.H.; Lee, E.; Kwon, Y.U. Sonochemical synthesis of Pt-doped Pd nanoparticles with enhanced electrocatalytic activity for formic acid oxidation reaction. J. Appl. Electrochem. 2012, 42, 827–832. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhang, X.H. Carbon-supported PdSn nanoparticles as catalysts for formic acid oxidation. Electrochem. Commun. 2009, 11, 1667–1670. [Google Scholar] [CrossRef]
- Celorrio, V.; de Oca, M.G.M.; Plana, D.; Moliner, R.; Lazaro, M.J.; Fermin, D.J. Effect of Carbon Supports on Electrocatalytic Reactivity of Au-Pd Core-Shell Nanoparticles. J. Phys. Chem. C 2012, 116, 6275–6282. [Google Scholar] [CrossRef]
- Celorrio, V.; de Oca, M.G.M.; Plana, D.; Moliner, R.; Fermin, D.J.; Lazaro, M.J. Electrochemical performance of Pd and Au-Pd core-shell nanoparticles on surface tailored carbon black as catalyst support. Int. J. Hydrogen Energy 2012, 37, 7152–7160. [Google Scholar] [CrossRef]
- Wang, H.; Ge, X.B. Facile Fabrication of Porous Pd-Au Bimetallic Nanostructures for Electrocatalysis. Electroanalysis 2012, 24, 911–916. [Google Scholar] [CrossRef]
- Shi, R.R.; Wang, J.S.; Cheng, N.C.; Sun, X.L.; Zhang, L.; Zhang, J.J.; Wang, L.C. Electrocatalytic activity and stability of carbon nanotubes-supported Pt-on-Au, Pd-on-Au, Pt-on-Pd-on-Au, Pt-on-Pd, and Pd-on-Pt catalysts for methanol oxidation reaction. Electrochim. Acta 2014, 148, 1–7. [Google Scholar] [CrossRef]
- Li, G.Q.; Feng, L.G.; Chang, J.F.; Wickman, B.; Gronbeck, H.; Liu, C.P.; Xing, W. Activity of Platinum/Carbon and Palladium/Carbon Catalysts Promoted by Ni2P in Direct Ethanol Fuel Cells. Chemsuschem 2014, 7, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Koenigsmann, C.; Adzic, R.R.; Wong, S.S. Probing Ultrathin One-Dimensional Pd–Ni Nanostructures As Oxygen Reduction Reaction Catalysts. ACS Catal. 2014, 4, 2544–2555. [Google Scholar] [CrossRef]
- Yu, X.W.; Pickup, P.G. Novel Pd-Pb/C bimetallic catalysts for direct formic acid fuel cells. J. Power Sources 2009, 192, 279–284. [Google Scholar] [CrossRef]
- Feng, L.G.; Yang, J.; Hu, Y.; Zhu, J.B.; Liu, C.P.; Xing, W. Electrocatalytic properties of PdCeOx/C anodic catalyst for formic acid electrooxidation. Int. J. Hydrogen Energy 2012, 37, 4812–4818. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhang, X.H.; Tay, S.W. Nanostructured PdRu/C catalysts for formic acid oxidation. J. Solid State Electrochem. 2012, 16, 545–550. [Google Scholar] [CrossRef]
- Arikan, T.; Kannan, A.M.; Kadirgan, F. Binary Pt–Pd and ternary Pt–Pd–Ru nanoelectrocatalysts for direct methanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 2900–2907. [Google Scholar] [CrossRef]
- Awasthi, R.; Singh, R.N. Graphene-supported Pd–Ru nanoparticles with superior methanol electrooxidation activity. Carbon 2013, 51, 282–289. [Google Scholar] [CrossRef]
- Qin, Y.H.; Jia, Y.B.; Jiang, Y.; Niu, D.F.; Zhang, X.S.; Zhou, X.G.; Niu, L.; Yuan, W.K. Controllable synthesis of carbon nanofiber supported Pd catalyst for formic acid electrooxidation. Int. J. Hydrogen Energy 2012, 37, 7373–7377. [Google Scholar] [CrossRef]
- Li, Y.H.; Xu, Q.Z.; Li, Q.Y.; Wang, H.Q.; Huang, Y.G.; Xu, C.W. Pd deposited on MWCNTs modified carbon fiber paper as high-efficient electrocatalyst for ethanol electrooxidation. Electrochim. Acta 2014, 147, 151–156. [Google Scholar] [CrossRef]
- Chakraborty, S.; Raj, C.R. Electrocatalytic performance of carbon nanotube-supported palladium particles in the oxidation of formic acid and the reduction of oxygen. Carbon 2010, 48, 3242–3249. [Google Scholar] [CrossRef]
- Liu, J.; Liu, R.; Yuan, C.L.; Wei, X.P.; Yin, J.L.; Wang, G.L.; Cao, D.X. Pd-Co/MWCNTs Catalyst for Electrooxidation of Hydrazine in Alkaline Solution. Fuel Cells 2013, 13, 903–909. [Google Scholar] [CrossRef]
- Selvaraj, V.; Grace, A.N.; Alagar, M. Electrocatalytic oxidation of formic acid and formaldehyde on nanoparticle decorated single walled carbon nanotubes. J. Colloid Interface Sci. 2009, 333, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Limpattayanate, S.; Hunsom, M. Electrocatalytic activity of Pt-Pd electrocatalysts for the oxygen reduction reaction in proton exchange membrane fuel cells: Effect of supports. Renew. Energy 2014, 63, 205–211. [Google Scholar] [CrossRef]
- Chen, C.H.; Liou, W.J.; Lin, H.M.; Wu, S.H.; Mikolajczuk, A.; Stobinski, L.; Borodzinski, A.; Kedzierzawski, P.; Kurzydlowski, K. Carbon nanotube-supported bimetallic palladium-gold electrocatalysts for electro-oxidation of formic acid. Phys. Status Solidi Appl. Mater. Sci. 2010, 207, 1160–1165. [Google Scholar] [CrossRef]
- Chen, C.H.; Liou, W.J.; Lin, H.M.; Wu, S.H.; Borodzinski, A.; Stobinski, L.; Kedzierzawski, P. Palladium and Palladium Gold Catalysts Supported on MWCNTs for Electrooxidation of Formic Acid. Fuel Cells 2010, 10, 227–233. [Google Scholar] [CrossRef]
- Mikolajczuk, A.; Borodzinski, A.; Stobinski, L.; Kedzierzawski, P.; Lesiak, B.; Laszlo, K.; Jozsef, T.; Lin, H.M. Study of Pd-Au/MWCNTs formic acid electrooxidation catalysts. Phys. Status Solidi B 2010, 247, 2717–2721. [Google Scholar] [CrossRef]
- Qin, Y.H.; Jiang, Y.; Niu, D.F.; Zhang, X.S.; Zhou, X.G.; Niu, L.; Yuan, W.K. Carbon nanofiber supported bimetallic PdAu nanoparticles for formic acid electrooxidation. J. Power Sources 2012, 215, 130–134. [Google Scholar] [CrossRef]
- Wang, X.G.; Tang, B.; Huang, X.B.; Ma, Y.; Zhang, Z.H. High activity of novel nanoporous Pd-Au catalyst for methanol electro-oxidation in alkaline media. J. Alloys Compd. 2013, 565, 120–126. [Google Scholar] [CrossRef]
- Takenaka, S.; Tsukamoto, T.; Matsune, H.; Kishida, M. Carbon nanotube-supported Pd–Co catalysts covered with silica layers as active and stable cathode catalysts for polymer electrolyte fuel cells. Catal. Sci. Technol. 2013, 3, 2723–2731. [Google Scholar] [CrossRef]
- Yang, S.D.; Shen, C.M.; Lu, X.J.; Tong, H.; Zhu, J.J.; Zhang, X.G.; Gao, H.J. Preparation and electrochemistry of graphene nanosheets-multiwalled carbon nanotubes hybrid nanomaterials as Pd electrocatalyst support for formic acid oxidation. Electrochim. Acta 2012, 62, 242–249. [Google Scholar] [CrossRef]
- Qu, K.G.; Wu, L.; Ren, J.S.; Qu, X.G. Natural DNA-Modified Graphene/Pd Nanoparticles as Highly Active Catalyst for Formic Acid Electro-Oxidation and for the Suzuki Reaction. ACS Appl. Mater. Interfaces 2012, 4, 5001–5009. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hao, H.; Huang, T.; Yu, A.S. Electrodeposited Pd–MoOx catalysts with enhanced catalytic activity for formic acid electrooxidation. Electrochim. Acta 2012, 76, 292–299. [Google Scholar] [CrossRef]
- Lu, H.T.; Fan, Y.; Huang, P.; Xu, D.L. SnO2 nanospheres supported Pd catalyst with enhanced performance for formic acid oxidation. J. Power Sources 2012, 215, 48–52. [Google Scholar] [CrossRef]
- Kim, I.T.; Choi, M.; Lee, H.K.; Shim, J. Characterization of methanol-tolerant Pd–WO3 and Pd–SnO2 electrocatalysts for the oxygen reduction reaction in direct methanol fuel cells. J. Ind. Eng. Chem. 2013, 19, 813–818. [Google Scholar] [CrossRef]
- Maheswari, S.; Sridhar, P.; Pitchumani, S. Pd–TiO2/C as a methanol tolerant catalyst for oxygen reduction reaction in alkaline medium. Electrochem. Commun. 2013, 26, 97–100. [Google Scholar] [CrossRef]
- Matos, J.; Borodzinski, A.; Zychora, A.M.; Kedzierzawski, P.; Mierzwa, B.; Juchniewicz, K.; Mazurkiewicz, M.; Hernandez-Garrido, J.C. Direct formic acid fuel cells on Pd catalysts supported on hybrid TiO2–C materials. Appl. Catal. B 2015, 163, 167–178. [Google Scholar] [CrossRef]
- Wang, X.M.; Xia, Y.Y. The influence of the crystal structure of TiO2 support material on Pd catalysts for formic acid electrooxidation. Electrochim. Acta 2010, 55, 851–856. [Google Scholar] [CrossRef]
- Liao, M.Y.; Hu, Q.; Zheng, J.B.; Li, Y.H.; Zhou, H.; Zhong, C.J.; Chen, B.H. Pd decorated Fe/C nanocatalyst for formic acid electrooxidation. Electrochim. Acta 2013, 111, 504–509. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Yang, L.; Guo, Y.M.; Zheng, Z.; Hu, C.G.; Xu, P.L. High-efficiency palladium catalysts supported on ppy-modified C-60 for formic acid oxidation. Chem. Commun. 2011, 47, 1752–1754. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.Q.; Jia, H.T.; Wei, S.Y.; Guo, Z.H. Electrocatalysis of Sandwich-Structured Pd/Polypyrrole/Pd Composites toward Formic Acid Oxidation. Ind. Eng. Chem. Res. 2011, 50, 7077–7082. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, J.B.; Liang, L.; Liu, C.P.; Liao, J.H.; Xing, W. Enhanced electroactivity of Pd nanocrystals supported on H3PMo12O40/carbon for formic acid electrooxidation. J. Power Sources 2012, 210, 392–396. [Google Scholar] [CrossRef]
- Qin, Y.H.; Yue, J.; Yang, H.H.; Zhang, X.S.; Zhou, X.G.; Niu, L.; Yuan, W.K. Synthesis of highly dispersed and active palladium/carbon nanofiber catalyst for formic acid electrooxidation. J. Power Sources 2011, 196, 4609–4612. [Google Scholar] [CrossRef]
- Cheng, N.C.; Webster, R.A.; Pan, M.; Mu, S.C.; Rassaei, L.; Tsang, S.C.; Marken, F. One-step growth of 3–5 nm diameter palladium electrocatalyst in a carbon nanoparticle-chitosan host and characterization for formic acid oxidation. Electrochim. Acta 2010, 55, 6601–6610. [Google Scholar] [CrossRef]
- Zhang, B.A.; Ye, D.D.; Li, J.; Zhu, X.; Liao, Q. Electrodeposition of Pd catalyst layer on graphite rod electrodes for direct formic acid oxidation. J. Power Sources 2012, 214, 277–284. [Google Scholar] [CrossRef]
- Liu, S.L.; Han, M.; Shi, Y.; Zhang, C.Z.; Chen, Y.; Bao, J.C.; Dai, Z.H. Gram-Scale Synthesis of Multipod Pd Nanocrystals by a Simple Solid-Liquid Phase Reaction and Their Remarkable Electrocatalytic Properties. Eur. J. Inorg. Chem. 2012, 2012, 3740–3746. [Google Scholar] [CrossRef]
- Xu, C.X.; Liu, Y.Q.; Wang, J.P.; Geng, H.R.; Qiu, H.J. Nanoporous PdCu alloy for formic acid electro-oxidation. J. Power Sources 2012, 199, 124–131. [Google Scholar] [CrossRef]
- Meng, H.; Xie, F.Y.; Chen, J.; Shen, P.K. Electrodeposited palladium nanostructure as novel anode for direct formic acid fuel cell. J. Mater. Chem. 2011, 21, 11352–11358. [Google Scholar] [CrossRef]
- Cheng, T.T.; Gyenge, E.L. Novel catalyst-support interaction for direct formic acid fuel cell anodes: Pd electrodeposition on surface-modified graphite felt. J. Appl. Electrochem. 2009, 39, 1925–1938. [Google Scholar] [CrossRef]
- Mikolajczuk, A.; Borodzinski, A.; Stobinski, L.; Kedzierzawski, P.; Lesiak, B.; Kover, L.; Toth, J.; Lin, H.M. Physicochemical characterization of the Pd/MWCNTs catalysts for fuel cell applications. Phys. Status Solidi B 2010, 247, 3063–3067. [Google Scholar] [CrossRef]
- Morales-Acosta, D.; Rodriguez, H.; Godinez, L.A.; Arriaga, L.G. Performance increase of microfluidic formic acid fuel cell using Pd/MWCNTs as catalyst. J. Power Sources 2010, 195, 1862–1865. [Google Scholar] [CrossRef]
- Yu, X.W.; Pickup, P.G. Screening of PdM and PtM catalysts in a multi-anode direct formic acid fuel cell. J. Appl. Electrochem. 2011, 41, 589–597. [Google Scholar] [CrossRef]
- Yan, Z.X.; Xie, J.M.; Shen, P.K.; Zhang, M.M.; Zhang, Y.; Chen, M. Pd supported on 2–4 nm MoC particles with reduced particle size, synergistic effect and high stability for ethanol oxidation. Electrochim. Acta 2013, 108, 644–650. [Google Scholar] [CrossRef]
- Jung, W.S.; Han, J.; Yoon, S.P.; Nam, S.W.; Lim, T.H.; Hong, S.A. Performance degradation of direct formic acid fuel cell incorporating a Pd anode catalyst. J. Power Sources 2011, 196, 4573–4578. [Google Scholar] [CrossRef]
- Ren, M.J.; Kang, Y.Y.; He, W.; Zou, Z.Q.; Xue, X.Z.; Akins, D.L.; Yang, H.; Feng, S.L. Origin of performance degradation of palladium-based direct formic acid fuel cells. Appl. Catal. B 2011, 104, 49–53. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.G.; Ye, J.L.; Zou, Z.G.; Ye, J.H.; Gu, J.; Yu, T.; Yang, A.D. Poisoning and regeneration of Pd catalyst in direct formic acid fuel cell. Electrochim. Acta 2010, 55, 5024–5027. [Google Scholar] [CrossRef]
- Yu, X.W.; Pickup, P.G. Deactivation/reactivation of a Pd/C catalyst in a direct formic acid fuel cell (DFAFC): Use of array membrane electrode assemblies. J. Power Sources 2009, 187, 493–499. [Google Scholar] [CrossRef]
- Chen, Y.X.; Lavacchi, A.; Chen, S.P.; di Benedetto, F.; Bevilacqua, M.; Bianchini, C.; Fornasiero, P.; Innocenti, M.; Marelli, M.; Oberhauser, W.; et al. Electrochemical Milling and Faceting: Size Reduction and Catalytic Activation of Palladium Nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 8500–8504. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.M.; Rao, Z.X.; Yuan, L.Z.; Jiang, L.H.; Sun, G.Q.; Ruan, J.M.; Zhou, Z.C.; Sang, S.B. Carbon supported PdO with improved activity and stability for oxygen reduction reaction in alkaline solution. Electrochim. Acta 2014, 150, 157–166. [Google Scholar] [CrossRef]
- Yan, Z.X.; Hu, Z.F.; Chen, C.; Meng, H.; Shen, P.K.; Ji, H.B.; Meng, Y.Z. Hollow carbon hemispheres supported palladium electrocatalyst at improved performance for alcohol oxidation. J. Power Sources 2010, 195, 7146–7151. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Scott, K. Performance of carbon nanofiber supported Pd–Ni catalysts for electro-oxidation of ethanol in alkaline medium. J. Power Sources 2010, 195, 5246–5251. [Google Scholar] [CrossRef]
- Xu, J.B.; Zhao, T.S.; Shen, S.Y.; Li, Y.S. Stabilization of the palladium electrocatalyst with alloyed gold for ethanol oxidation. Int. J. Hydrogen Energy 2010, 35, 6490–6500. [Google Scholar] [CrossRef]
- Cheng, F.L.; Dai, X.C.; Wang, H.; Jiang, S.P.; Zhang, M.; Xu, C.W. Synergistic effect of Pd–Au bimetallic surfaces in Au-covered Pd nanowires studied for ethanol oxidation. Electrochim. Acta 2010, 55, 2295–2298. [Google Scholar] [CrossRef]
- Gao, H.L.; Liao, S.J.; Liang, Z.X.; Liang, H.G.; Luoa, F. Anodic oxidation of ethanol on core-shell structured Ru@PtPd/C catalyst in alkaline media. J. Power Sources 2011, 196, 6138–6143. [Google Scholar] [CrossRef]
- Bambagioni, V.; Bianchini, C.; Filippi, J.; OberhauserIal, W.; Marchionni, A.; Vizza, F.; Psaro, R.; Sordelli, L.; Foresti, M.L.; Innocenti, M. Ethanol Oxidation on Electrocatalysts Obtained by Spontaneous Deposition of Palladium onto Nickel-Zinc Materials. Chemsuschem 2009, 2, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Lin, Z.J.; Jia, T.T.; Cai, Z.M.; Huang, X.L.; Jiang, Y.Q.; Chen, X.; Chen, G.N. A facile synthesis of palladium nanoparticles supported on functional carbon nanotubes and its novel catalysis for ethanol electrooxidation. Anal. Chim. Acta 2009, 650, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.F.; Zhu, C.Z.; Du, D.; Lin, Y.H. Facile One-Step Synthesis of Three-Dimensional Pd–Ag Bimetallic Alloy Networks and Their Electrocatalytic Activity toward Ethanol Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 13842–13848. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.J.; Wisitruangsakul, N.; Feng, J.J.; Luo, J.; Fang, K.M.; Wang, A.J. Biomolecule-assisted synthesis of porous PtPd alloyed nanoflowers supported on reduced graphene oxide with highly electrocatalytic performance for ethanol oxidation and oxygen reduction. Electrochim. Acta 2015, 160, 100–107. [Google Scholar]
- Wang, A.L.; He, X.J.; Lu, X.F.; Xu, H.; Tong, Y.X.; Li, G.R. Palladium-Cobalt Nanotube Arrays Supported on Carbon Fiber Cloth as High-Performance Flexible Electrocatalysts for Ethanol Oxidation. Angew. Chem. Int. Ed. 2015, 54, 3669–3673. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.W.; Wang, J.; Zhang, G.H.; Fan, X.B.; Zhang, G.L.; Zhang, F.B.; Li, Y. Deoxyribonucleic acid-directed growth of well dispersed nickel-palladium-platinum nanoclusters on graphene as an efficient catalyst for ethanol electrooxidation. J. Power Sources 2015, 278, 43–49. [Google Scholar] [CrossRef]
- Peng, C.; Hu, Y.L.; Liu, M.R.; Zheng, Y.X. Hollow raspberry-like PdAg alloy nanospheres: High electrocatalytic activity for ethanol oxidation in alkaline media. J. Power Sources 2015, 278, 69–75. [Google Scholar] [CrossRef]
- Singh, R.N.; Awasthi, R. Graphene support for enhanced electrocatalytic activity of Pd for alcohol oxidation. Catal. Sci. Technol. 2011, 1, 778–783. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.Z.; Schempf, A.; Ding, Y.; Lei, Y. Free-Standing Palladium/Polyamide 6 Nanofibers for Electrooxidation of Alcohols in Alkaline Medium. J. Phys. Chem. C 2009, 113, 16174–16180. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhao, Y.Q.; Xu, C.L.; Zhao, D.D.; Xu, M.W.; Su, Z.X.; Li, H.L. Improved performance of Pd electrocatalyst supported on three-dimensional nickel foam for direct ethanol fuel cells. J. Power Sources 2010, 195, 6496–6499. [Google Scholar] [CrossRef]
- Thotiyl, M.M.O.; Kumar, T.R.; Sampath, S. Pd Supported on Titanium Nitride for Efficient Ethanol Oxidation. J. Phys. Chem. C 2010, 114, 17934–17941. [Google Scholar] [CrossRef]
- Mahendiran, C.; Maiyalagan, T.; Scott, K.; Gedanken, A. Synthesis of a carbon-coated NiO/MgO core/shell nanocomposite as a Pd electro-catalyst support for ethanol oxidation. Mater. Chem. Phys. 2011, 128, 341–347. [Google Scholar] [CrossRef]
- Yi, Q.F.; Niu, F.J.; Sun, L.Z. Fabrication of novel porous Pd particles and their electroactivity towards ethanol oxidation in alkaline media. Fuel 2011, 90, 2617–2623. [Google Scholar] [CrossRef]
- Wang, X.G.; Wang, W.M.; Qi, Z.; Zhao, C.C.; Ji, H.; Zhang, Z.H. Fabrication, microstructure and electrocatalytic property of novel nanoporous palladium composites. J. Alloys Compd. 2010, 508, 463–470. [Google Scholar] [CrossRef]
- Liu, R.; Liu, J.F.; Jiang, G.B. Use of Triton X-114 as a weak capping agent for one-pot aqueous phase synthesis of ultrathin noble metal nanowires and a primary study of their electrocatalytic activity. Chem. Commun. 2010, 46, 7010–7012. [Google Scholar] [CrossRef] [PubMed]
- Ksar, F.; Surendran, G.; Ramos, L.; Keita, B.; Nadjo, L.; Prouzet, E.; Beaunier, P.; Hagege, A.; Audonnet, F.; Remita, H. Palladium Nanowires Synthesized in Hexagonal Mesophases: Application in Ethanol Electrooxidation. Chem. Mater. 2009, 21, 1612–1617. [Google Scholar] [CrossRef]
- Cherevko, S.; Xing, X.L.; Chung, C.H. Pt and Pd decorated Au nanowires: Extremely high activity of ethanol oxidation in alkaline media. Electrochim. Acta 2011, 56, 5771–5775. [Google Scholar] [CrossRef]
- Lin, S.C.; Chen, J.Y.; Hsieh, Y.F.; Wu, P.W. A facile route to prepare PdPt alloys for ethanol electro-oxidation in alkaline electrolyte. Mater. Lett. 2011, 65, 215–218. [Google Scholar] [CrossRef]
- Modibedi, R.M.; Masombuka, T.; Mathe, M.K. Carbon supported Pd-Sn and Pd–Ru–Sn nanocatalysts for ethanol electro-oxidation in alkaline medium. Int. J. Hydrogen Energy 2011, 36, 4664–4672. [Google Scholar] [CrossRef]
- Jou, L.H.; Chang, J.K.; Whang, T.J.; Sun, I.W. Electrodeposition of Palladium-Tin Alloys from 1-Ethyl-3-methylimidazolium Chloride-Tetrafluoroborate Ionic Liquid for Ethanol Electro-Oxidation. J. Electrochem. Soc. 2010, 157, D443–D449. [Google Scholar] [CrossRef]
- Shen, S.Y.; Zhao, T.S.; Xu, J.B. Carbon-supported bimetallic Pdlr catalysts for ethanol oxidation in alkaline media. Electrochim. Acta 2010, 55, 9179–9184. [Google Scholar] [CrossRef]
- Qiu, C.C.; Shang, R.; Xie, Y.F.; Bu, Y.R.; Li, C.Y.; Ma, H.Y. Electrocatalytic activity of bimetallic Pd-Ni thin films towards the oxidation of methanol and ethanol. Mater. Chem. Phys. 2010, 120, 323–330. [Google Scholar] [CrossRef]
- Qi, Z.; Geng, H.R.; Wang, X.G.; Zhao, C.C.; Ji, H.; Zhang, C.; Xu, J.L.; Zhang, Z.H. Novel nanocrystalline PdNi alloy catalyst for methanol and ethanol electro-oxidation in alkaline media. J. Power Sources 2011, 196, 5823–5828. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Law, H.M.; Nguyen, H.T.; Kristian, N.; Wang, S.Y.; Chan, S.H.; Wang, X. Enhancement effect of Ag for Pd/C towards the ethanol electro-oxidation in alkaline media. Appl. Catal. B 2009, 91, 507–515. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Rego, R.; Fernandes, L.S.; Tavares, P.B. Evaluation of the catalytic activity of Pd–Ag alloys on ethanol oxidation and oxygen reduction reactions in alkaline medium. J. Power Sources 2011, 196, 6092–6098. [Google Scholar] [CrossRef]
- Wang, Y.; Nguyen, T.S.; Liu, X.W.; Wang, X. Novel palladium-lead (Pd–Pb/C) bimetallic catalysts for electrooxidation of ethanol in alkaline media. J. Power Sources 2010, 195, 2619–2622. [Google Scholar] [CrossRef]
- He, Q.G.; Chen, W.; Mukerjee, S.; Chen, S.W.; Laufek, F. Carbon-supported PdM (M = Au and Sn) nanocatalysts for the electrooxidation of ethanol in high pH media. J. Power Sources 2009, 187, 298–304. [Google Scholar] [CrossRef]
- Zhu, L.D.; Zhao, T.S.; Xu, J.B.; Liang, Z.X. Preparation and characterization of carbon-supported sub-monolayer palladium decorated gold nanoparticles for the electro-oxidation of ethanol in alkaline media. J. Power Sources 2009, 187, 80–84. [Google Scholar] [CrossRef]
- Hu, Z.F.; Yan, Z.X.; Shen, P.K.; Zhong, C.J. Nano-architectures of ordered hollow carbon spheres filled with carbon webs by template-free controllable synthesis. Nanotechnology 2012, 23, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.X.; Meng, H.; Shen, P.K.; Meng, Y.Z.; Ji, H.B. Effect of the templates on the synthesis of hollow carbon materials as electrocatalyst supports for direct alcohol fuel cells. Int. J. Hydrogen Energy 2012, 37, 4728–4736. [Google Scholar] [CrossRef]
- Yan, Z.X.; Meng, H.; Shi, L.; Li, Z.H.; Shen, P.K. Synthesis of mesoporous hollow carbon hemispheres as highly efficient Pd electrocatalyst support for ethanol oxidation. Electrochem. Commun. 2010, 12, 689–692. [Google Scholar] [CrossRef]
- Cerritos, R.C.; Guerra-Balcazar, M.; Ramirez, R.F.; Ledesma-Garcia, J.; Arriaga, L.G. Morphological Effect of Pd Catalyst on Ethanol Electro-Oxidation Reaction. Materials 2012, 5, 1686–1697. [Google Scholar] [CrossRef]
- Ding, K.Q.; Yang, G.K. HCl-assisted pyrolysis of PdCl2 to immobilize palladium nanoparticles on multi-walled carbon nanotubes. Mater. Chem. Phys. 2010, 123, 498–501. [Google Scholar] [CrossRef]
- Ding, K.Q.; Yang, G.K. Using RTILs of EMIBF4 as “water” to prepare palladium nanoparticles onto MWCNTs by pyrolysis of PdCl2. Electrochim. Acta 2010, 55, 2319–2324. [Google Scholar] [CrossRef]
- Chu, D.B.; Wang, J.; Wang, S.X.; Zha, L.W.; He, J.G.; Hou, Y.Y.; Yan, Y.X.; Lin, H.S.; Tian, Z.W. High activity of Pd–In2O3/CNTs electrocatalyst for electro-oxidation of ethanol. Catal. Commun. 2009, 10, 955–958. [Google Scholar] [CrossRef]
- Qin, Y.H.; Yang, H.H.; Zhang, X.S.; Li, P.; Ma, C.A. Effect of carbon nanofibers microstructure on electrocatalytic activities of Pd electrocatalysts for ethanol oxidation in alkaline medium. Int. J. Hydrogen Energy 2010, 35, 7667–7674. [Google Scholar] [CrossRef]
- Qin, Y.H.; Li, H.C.; Yang, H.H.; Zhang, X.S.; Zhou, X.G.; Niu, L.; Yuan, W.K. Effect of electrode fabrication methods on the electrode performance for ethanol oxidation. J. Power Sources 2011, 196, 159–163. [Google Scholar] [CrossRef]
- Qin, Y.H.; Yang, H.H.; Zhang, X.S.; Li, P.; Zhou, X.G.; Niu, L.; Yuan, W.K. Electrophoretic deposition of network-like carbon nanofibers as a palladium catalyst support for ethanol oxidation in alkaline media. Carbon 2010, 48, 3323–3329. [Google Scholar] [CrossRef]
- Wen, Z.L.; Yang, S.D.; Liang, Y.Y.; He, W.; Tong, H.; Hao, L.A.; Zhang, X.G.; Song, Q.J. The improved electrocatalytic activity of palladium/graphene nanosheets towards ethanol oxidation by tin oxide. Electrochim. Acta 2010, 56, 139–144. [Google Scholar] [CrossRef]
- Chen, X.M.; Wu, G.H.; Chen, J.M.; Chen, X.; Xie, Z.X.; Wang, X.R. Synthesis of “Clean” and Well-Dispersive Pd Nanoparticles with Excellent Electrocatalytic Property on Graphene Oxide. J. Am. Chem. Soc. 2011, 133, 3693–3695. [Google Scholar] [CrossRef] [PubMed]
- El-Shafei, A.A.; Elhafeez, A.M.A.; Mostafa, H.A. Ethanol oxidation at metal-zeolite-modified electrodes in alkaline medium. Part 2: Palladium-zeolite-modified graphite electrode. J. Solid State Electrochem. 2010, 14, 185–190. [Google Scholar] [CrossRef]
- Hasan, M.; Newcomb, S.B.; Rohan, J.F.; Razeeb, K.M. Ni nanowire supported 3D flower-like Pd nanostructures as an efficient electrocatalyst for electrooxidation of ethanol in alkaline media. J. Power Sources 2012, 218, 148–156. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhang, J.; Yang, S.C.; Ding, B.J.; Song, X.P. Au@Pd Core-Shell Nanobricks with Concave Structures and Their Catalysis of Ethanol Oxidation. Chemsuschem 2013, 6, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Cheng, D.J.; Qiu, X.G.; Cao, D.P. Synthesis of Cu@Pd core-shell nanowires with enhanced activity and stability for formic acid oxidation. Electrochim. Acta 2014, 143, 44–48. [Google Scholar] [CrossRef]
- Li, C.L.; Su, Y.; Lv, X.Y.; Shi, H.J.; Yang, X.G.; Wang, Y.J. Enhanced ethanol electrooxidation of hollow Pd nanospheres prepared by galvanic exchange reactions. Mater. Lett. 2012, 69, 92–95. [Google Scholar] [CrossRef]
- Wu, H.X.; Li, H.J.; Zhai, Y.J.; Xu, X.L.; Jin, Y.D. Facile Synthesis of Free-Standing Pd-Based Nanomembranes with Enhanced Catalytic Performance for Methanol/Ethanol Oxidation. Adv. Mater. 2012, 24, 1594–1597. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, Z.; Sen, F.; Sen, S.; Gokagac, G. The preparation and characterization of nano-sized Pt-Pd/C catalysts and comparison of their superior catalytic activities for methanol and ethanol oxidation. J. Mater. Sci. 2012, 47, 8134–8144. [Google Scholar] [CrossRef]
- Shen, S.Y.; Zhao, T.S.; Xu, J.B.; Li, Y.S. Synthesis of PdNi catalysts for the oxidation of ethanol in alkaline direct ethanol fuel cells. J. Power Sources 2010, 195, 1001–1006. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Guo, S.J.; Dong, S.J. PdM (M = Pt, Au) Bimetallic Alloy Nanowires with Enhanced Electrocatalytic Activity for Electro-oxidation of Small Molecules. Adv. Mater. 2012, 24, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, J.; Lewera, A. Electrooxidation of ethanol on carbon-supported Pt–Pd nanoparticles. J. Power Sources 2012, 205, 264–271. [Google Scholar] [CrossRef]
- Lv, J.J.; Zheng, J.N.; Zhang, H.B.; Lin, M.; Wang, A.J.; Chen, J.R.; Feng, J.J. Simple synthesis of platinum-palladium nanoflowers on reduced graphene oxide and their enhanced catalytic activity for oxygen reduction reaction. J. Power Sources 2014, 269, 136–143. [Google Scholar] [CrossRef]
- Ding, L.X.; Wang, A.L.; Ou, Y.N.; Li, Q.; Guo, R.; Zhao, W.X.; Tong, Y.X.; Li, G.R. Hierarchical Pd–Sn Alloy Nanosheet Dendrites: An Economical and Highly Active Catalyst for Ethanol Electrooxidation. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Assumpcao, M.; da Silva, S.G.; de Souza, R.F.B.; Buzzo, G.S.; Spinace, E.V.; Santos, M.C.; Neto, A.O.; Silva, J.C.M. Investigation of PdIr/C electrocatalysts as anode on the performance of direct ammonia fuel cell. J. Power Sources 2014, 268, 129–136. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Yang, Y.H.; Wang, X. Ethanol electro-oxidation activity of Nb-doped-TiO2 supported PdAg catalysts in alkaline media. Appl. Catal. B 2012, 113, 261–270. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Xu, C.X. Nanoporous PdTi Alloys as Non-Platinum Oxygen-Reduction Reaction Electrocatalysts with Enhanced Activity and Durability. Chemsuschem 2013, 6, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Zhao, T.S. A high-performance integrated electrode for anion-exchange membrane direct ethanol fuel cells. Int. J. Hydrogen Energy 2011, 36, 7707–7713. [Google Scholar] [CrossRef]
- Shen, S.Y.; Zhao, T.S.; Xu, J.B.; Li, Y.S. High performance of a carbon supported ternary PdIrNi catalyst for ethanol electro-oxidation in anion-exchange membrane direct ethanol fuel cells. Energy Environ. Sci. 2011, 4, 1428–1433. [Google Scholar] [CrossRef]
- Antolini, E.; Colmati, F.; Gonzalez, E.R. Ethanol oxidation on carbon supported (PtSn)(alloy)/SnO2 and (PtSnPd)(alloy)/SnO2 catalysts with a fixed Pt/SnO2 atomic ratio: Effect of the alloy phase characteristics. J. Power Sources 2009, 193, 555–561. [Google Scholar] [CrossRef]
- Bambagioni, V.; Bianchini, C.; Marchionni, A.; Filippi, J.; Vizza, F.; Teddy, J.; Serp, P.; Zhiani, M. Pd and Pt-Ru anode electrocatalysts supported on multi-walled carbon nanotubes and their use in passive and active direct alcohol fuel cells with an anion-exchange membrane (alcohol = methanol, ethanol, glycerol). J. Power Sources 2009, 190, 241–251. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; More, K.L.; Sun, K.; Wu, Z.L.; Li, W.Z. Preparation and Characterization of PdFe Nanoleaves as Electrocatalysts for Oxygen Reduction Reaction. Chem. Mater. 2011, 23, 1570–1577. [Google Scholar] [CrossRef]
- Tang, Y.F.; Zhang, H.M.; Zhong, H.X.; Ma, Y.W. A facile synthesis of Pd/C cathode electrocatalyst for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 725–731. [Google Scholar] [CrossRef]
- Jeyabharathi, C.; Venkateshkumar, P.; Mathiyarasu, J.; Phani, K.L.N. Carbon-Supported Palladium-Polypyrrole Nanocomposite for Oxygen Reduction and Its Tolerance to Methanol. J. Electrochem. Soc. 2010, 157, B1740–B1745. [Google Scholar] [CrossRef]
- Kumar, S.M.S.; Herrero, J.S.; Irusta, S.; Scott, K. The effect of pretreatment of Vulcan XC-72R carbon on morphology and electrochemical oxygen reduction kinetics of supported Pd nano-particle in acidic electrolyte. J. Electroanal. Chem. 2010, 647, 211–221. [Google Scholar] [CrossRef]
- Yin, S.B.; Cai, M.; Wang, C.X.; Shen, P.K. Tungsten carbide promoted Pd–Fe as alcohol-tolerant electrocatalysts for oxygen reduction reactions. Energy Environ. Sci. 2011, 4, 558–563. [Google Scholar] [CrossRef]
- Rao, C.V.; Viswanathan, B. Carbon supported Pd–Co–Mo alloy as an alternative to Pt for oxygen reduction in direct ethanol fuel cells. Electrochim. Acta 2010, 55, 3002–3007. [Google Scholar] [CrossRef]
- Sarkar, A.; Murugan, A.V.; Manthiram, A. Low cost Pd-W nanoalloy electrocatalysts for oxygen reduction reaction in fuel cells. J. Mater. Chem. 2009, 19, 159–165. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Shao, Z.G.; Qin, X.P.; Chen, X.G.; Wei, Z.D.; Yi, B.L. Durability study of Pt–Pd/C as PEMFC cathode catalyst. Int. J. Hydrogen Energy 2010, 35, 1719–1726. [Google Scholar] [CrossRef]
- Chang, S.H.; Su, W.N.; Yeh, M.H.; Pan, C.J.; Yu, K.L.; Liu, D.G.; Lee, J.F.; Hwang, B.J. Structural and Electronic Effects of Carbon-Supported PtxPd1−x Nanoparticles on the Electrocatalytic Activity of the Oxygen-Reduction Reaction and on Methanol Tolerance. Chem. Eur. J. 2010, 16, 11064–11071. [Google Scholar] [CrossRef] [PubMed]
- Sarapuu, A.; Kasikov, A.; Wong, N.; Lucas, C.A.; Sedghi, G.; Nichols, R.J.; Tammeveski, K. Electroreduction of oxygen on gold-supported nanostructured palladium films in acid solutions. Electrochim. Acta 2010, 55, 6768–6774. [Google Scholar] [CrossRef]
- Kariuki, N.N.; Wang, X.P.; Mawdsley, J.R.; Ferrandon, M.S.; Niyogi, S.G.; Vaughey, J.T.; Myers, D.J. Colloidal Synthesis and Characterization of Carbon-Supported Pd–Cu Nanoparticle Oxygen Reduction Electrocatalysts. Chem. Mater. 2010, 22, 4144–4152. [Google Scholar] [CrossRef]
- Fouda-Onana, F.; Bah, S.; Savadogo, O. Palladium-copper alloys as catalysts for the oxygen reduction reaction in an acidic media I: Correlation between the ORR kinetic parameters and intrinsic physical properties of the alloys. J. Electroanal. Chem. 2009, 636, 1–9. [Google Scholar] [CrossRef]
- Wei, Y.C.; Liu, C.W.; Chang, Y.W.; Lai, C.M.; Lim, P.Y.; Tsai, L.D.; Wang, K.W. The structure-activity relationship of Pd–Co/C electrocatalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2010, 35, 1864–1871. [Google Scholar] [CrossRef]
- Ohashi, M.; Beard, K.D.; Ma, S.G.; Blom, D.A.; St-Pierre, J.; van Zee, J.W.; Monnier, J.R. Electrochemical and structural characterization of carbon-supported Pt–Pd bimetallic electrocatalysts prepared by electroless deposition. Electrochim. Acta 2010, 55, 7376–7384. [Google Scholar] [CrossRef]
- Peng, Z.M.; Yang, H. Synthesis and Oxygen Reduction Electrocatalytic Property of Pt-on-Pd Bimetallic Heteronanostructures. J. Am. Chem. Soc. 2009, 131, 7542–7543. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Zhou, W.J.; Cheng, C.H.; Lee, J.Y.; Liu, Z.L. Pt-Decorated PdFe Nanoparticles as Methanol-Tolerant Oxygen Reduction Electrocatalyst. ACS Appl. Mater. Interfaces 2010, 2, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, C.H.; Zhou, W.; Lee, J.Y.; Liu, Z. Methanol-Tolerant Heterogeneous PdCo@PdPt/C Electrocatalyst for the Oxygen Reduction Reaction. Fuel Cells 2010, 10, 907–913. [Google Scholar] [CrossRef]
- Li, W.Z.; Haldar, P. Supportless PdFe nanorods as highly active electrocatalyst for proton exchange membrane fuel cell. Electrochem. Commun. 2009, 11, 1195–1198. [Google Scholar] [CrossRef]
- Zhou, W.P.; Yang, X.F.; Vukmirovic, M.B.; Koel, B.E.; Jiao, J.; Peng, G.W.; Mavrikakis, M.; Adzic, R.R. Improving Electrocatalysts for O2 Reduction by Fine-Tuning the Pt-Support Interaction: Pt Monolayer on the Surfaces of a Pd3Fe(111) Single-Crystal Alloy. J. Am. Chem. Soc. 2009, 131, 12755–12762. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.A.; Kwon, K.; Pak, C.; Chang, H. The oxygen reduction electrocatalytic activity of intermetallic compound of palladium-tin supported on tin oxide-carbon composite. Catal. Today 2011, 164, 176–180. [Google Scholar] [CrossRef]
- Sun, W.; Hsu, A.; Chen, R.R. Palladium-coated manganese dioxide catalysts for oxygen reduction reaction in alkaline media. J. Power Sources 2011, 196, 4491–4498. [Google Scholar] [CrossRef]
- Arroyo-Ramirez, L.; Rodriguez, D.; Otano, W.; Cabrera, C.R. Palladium Nanoshell Catalysts Synthesis on Highly Ordered Pyrolytic Graphite for Oxygen Reduction Reaction. Acs Appl. Mater. Interfaces 2012, 4, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Jukk, K.; Alexeyeva, N.; Johans, C.; Kontturi, K.; Tammeveski, K. Oxygen reduction on Pd nanoparticle/multi-walled carbon nanotube composites. J. Electroanal. Chem. 2012, 666, 67–75. [Google Scholar] [CrossRef]
- Kim, Y.S.; Shin, J.Y.; Chun, Y.S.; Lee, C.; Lee, S.G. Anion-Dependent Electrocatalytic Activity of Supported Palladium Catalysts onto Imidazolium Salt-Functionalized Carbon Nanotubes in Oxygen Reduction Reaction. Bull. Korean Chem. Soc. 2011, 32, 3209–3210. [Google Scholar] [CrossRef]
- Seo, M.H.; Choi, S.M.; Kim, H.J.; Kim, W.B. The graphene-supported Pd and Pt catalysts for highly active oxygen reduction reaction in an alkaline condition. Electrochem. Commun. 2011, 13, 182–185. [Google Scholar] [CrossRef]
- Kim, D.; Ahmed, M.S.; Jeon, S. Different length linkages of graphene modified with metal nanoparticles for oxygen reduction in acidic media. J. Mater. Chem. 2012, 22, 16353–16360. [Google Scholar] [CrossRef]
- Gotoh, K.; Kawabata, K.; Fujii, E.; Morishige, K.; Kinumoto, T.; Miyazaki, Y.; Ishida, H. The use of graphite oxide to produce mesoporous carbon supporting Pt, Ru, or Pd nanoparticles. Carbon 2009, 47, 2120–2124. [Google Scholar] [CrossRef] [Green Version]
- Du, S.F.; Lu, Y.X.; Steinberger-Wilckens, R. PtPd nanowire arrays supported on reduced graphene oxide as advanced electrocatalysts for methanol oxidation. Carbon 2014, 79, 346–353. [Google Scholar] [CrossRef]
- Huang, S.Y.; Ganesan, P.; Popov, B.N. Electrocatalytic Activity and Stability of Titania-Supported Platinum-Palladium Electrocatalysts for Polymer Electrolyte Membrane Fuel Cell. ACS Catal. 2012, 2, 825–831. [Google Scholar] [CrossRef]
- Bae, S.J.; Nahm, K.S.; Kim, P. Electroreduction of oxygen on Pd catalysts supported on Ti-modified carbon. Curr. Appl. Phys. 2012, 12, 1476–1480. [Google Scholar] [CrossRef]
- Ramanathan, M.; Ramani, V.; Prakash, J. Kinetics of the oxygen reduction reaction on Pd3M (M = Cu, Ni, Fe) electrocatalysts synthesized at elevated annealing temperatures. Electrochim. Acta 2012, 75, 254–261. [Google Scholar] [CrossRef]
- Wen, M.; Zhou, B.; Fang, H.; Wu, Q.S.; Chen, S.P. Novel-Phase Structural High-Efficiency Anode Catalyst for Methanol Fuel Cells: α-(NiCu)3Pd Nanoalloy. J. Phys. Chem. C 2014, 118, 26713–26720. [Google Scholar] [CrossRef]
- Lee, K.R.; Jung, Y.; Woo, S.I. Combinatorial Screening of Highly Active Pd Binary Catalysts for Electrochemical Oxygen Reduction. ACS Comb. Sci. 2012, 14, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Shen, P.K. The beneficial effect of the addition of tungsten carbides to Pt catalysts on the oxygen electroreduction. Chem. Commun. 2005. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Shen, P.K. Tungsten carbide nanocrystal promoted Pt/C electrocatalysts for oxygen reduction. J. Phys. Chem. B 2005, 109, 22705–22709. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Shen, P.K.; Wu, M.; Wei, Z.D.; Meng, H. A study of oxygen reduction on improved Pt–WC/C electrocatalysts. J. Power Sources 2006, 162, 173–176. [Google Scholar] [CrossRef]
- Cui, G.F.; Shen, P.K.; Meng, H.; Zhao, J.; Wu, G. Tungsten carbide as supports for Pt electrocatalysts with improved CO tolerance in methanol oxidation. J. Power Sources 2011, 196, 6125–6130. [Google Scholar] [CrossRef]
- He, G.Q.; Yan, Z.X.; Ma, X.M.; Meng, H.; Shen, P.K.; Wang, C.X. A universal method to synthesize nanoscale carbides as electrocatalyst supports towards oxygen reduction reaction. Nanoscale 2011, 3, 3578–3582. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, F.; Wu, K.; Lu, Z.Y.; Chen, Y.; Zhou, Y.M.; Tang, Y.W.; Lu, T.H. Carbon supported Palladium-Iron nanoparticles with uniform alloy structure as methanol-tolerant electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2012, 37, 2993–3000. [Google Scholar] [CrossRef]
- Han, B.H.; Xu, C.X. Nanoporous PdFe alloy as highly active and durable electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2014, 39, 18247–18255. [Google Scholar] [CrossRef]
- Gobal, F.; Arab, R. A preliminary study of the electro-catalytic reduction of oxygen on Cu–Pd alloys in alkaline solution. J. Electroanal. Chem. 2010, 647, 66–73. [Google Scholar] [CrossRef]
- Yang, R.Z.; Bian, W.Y.; Strasser, P.; Toney, M.F. Dealloyed PdCu3 thin film electrocatalysts for oxygen reduction reaction. J. Power Sources 2013, 222, 169–176. [Google Scholar] [CrossRef]
- You, D.J.; Jin, S.A.; Lee, K.H.; Pak, C.; Choi, K.H.; Chang, H. Improvement of activity for oxygen reduction reaction by decoration of Ir on PdCu/C catalyst. Catal. Today 2012, 185, 138–142. [Google Scholar] [CrossRef]
- Xiong, L.; Huang, Y.X.; Liu, X.W.; Sheng, G.P.; Li, W.W.; Yu, H.Q. Three-dimensional bimetallic Pd-Cu nanodendrites with superior electrochemical performance for oxygen reduction reaction. Electrochim. Acta 2013, 89, 24–28. [Google Scholar] [CrossRef]
- Lee, C.L.; Chiou, H.P.; Chang, K.C.; Huang, C.H. Carbon nanotubes-supported colloidal Ag–Pd nanoparticles as electrocatalysts toward oxygen reduction reaction in alkaline electrolyte. Int. J. Hydrogen Energy 2011, 36, 2759–2764. [Google Scholar] [CrossRef]
- Godinez-Garcia, A.; Perez-Robles, J.F.; Martinez-Tejada, H.V.; Solorza-Feria, O. Characterization and electrocatalytic properties of sonochemical synthesized PdAg nanoparticles. Mater. Chem. Phys. 2012, 134, 1013–1019. [Google Scholar] [CrossRef]
- Xu, L.; Luo, Z.M.; Fan, Z.X.; Zhang, X.; Tan, C.L.; Li, H.; Zhang, H.; Xue, C. Triangular Ag–Pd alloy nanoprisms: Rational synthesis with high-efficiency for electrocatalytic oxygen reduction. Nanoscale 2014, 6, 11738–11743. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.B.; Zhao, T.S.; Li, Y.S.; Yang, W.W. Synthesis and characterization of the Au-modified Pd cathode catalyst for alkaline direct ethanol fuel cells. Int. J. Hydrogen Energy 2010, 35, 9693–9700. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, J.H.; Jeong, I.K.; Choi, J.K.; Kim, Y.T. Phase change of bimetallic PdCo electrocatalysts caused by different heat-treatment temperatures: Effect on oxygen reduction reaction activity. J. Catal. 2012, 290, 65–78. [Google Scholar] [CrossRef]
- Son, D.N.; Takahashi, K. Selectivity of Palladium-Cobalt Surface Alloy toward Oxygen Reduction Reaction. J. Phys. Chem. C 2012, 116, 6200–6207. [Google Scholar] [CrossRef]
- Ren, Y.B.; Zhang, S.C.; Fang, H.; Wei, X.; Yang, P.H. Investigation of Co3O4 nanorods supported Pd anode catalyst for methanol oxidation in alkaline solution. J. Energy Chem. 2014, 23, 801–808. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Manthiram, A. Factors influencing the electrocatalytic activity of Pd100−xCox (0 ≤ x ≤ 50) nanoalloys for oxygen reduction reaction in fuel cells. Appl. Catal. B-Environ. 2009, 90, 184–194. [Google Scholar] [CrossRef]
- Oishi, K.; Savadogo, O. Electrochemical investigation of Pd-Co thin films binary alloy for the oxygen reduction reaction in acid medium. J. Electroanal. Chem. 2013, 703, 108–116. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Abdelkareem, M.A.; Shin, G.; Kim, H.Y. Pd-doped Co nanofibers immobilized on a chemically stable metallic bipolar plate as novel strategy for direct formic acid fuel cells. Int. J. Hydrogen Energy 2013, 38, 7438–7447. [Google Scholar] [CrossRef]
- Tominaka, S.; Hayashi, T.; Nakamura, Y.; Osaka, T. Mesoporous PdCo sponge-like nanostructure synthesized by electrodeposition and dealloying for oxygen reduction reaction. J. Mater. Chem. 2010, 20, 7175–7182. [Google Scholar] [CrossRef]
- Serov, A.; Nedoseykina, T.; Shvachko, O.; Kwak, C. Effect of precursor nature on the performance of palladium-cobalt electrocatalysts for direct methanol fuel cells. J. Power Sources 2010, 195, 175–180. [Google Scholar] [CrossRef]
- Alia, S.M.; Jensen, K.O.; Pivovar, B.S.; Yan, Y.S. Platinum-Coated Palladium Nanotubes as Oxygen Reduction Reaction Electrocatalysts. ACS Catal. 2012, 2, 858–863. [Google Scholar] [CrossRef]
- Slanac, D.A.; Li, L.; Mayoral, A.; Yacaman, M.J.; Manthiram, A.; Stevenson, K.J.; Johnston, K.P. Atomic resolution structural insights into PdPt nanoparticle-carbon interactions for the design of highly active and stable electrocatalysts. Electrochim. Acta 2012, 64, 35–45. [Google Scholar] [CrossRef]
- Chen, X.T.; Jiang, Y.Y.; Sun, J.Z.; Jin, C.H.; Zhang, Z.H. Highly active nanoporous Pt-based alloy as anode and cathode catalyst for direct methanol fuel cells. J. Power Sources 2014, 267, 212–218. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.J.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.M.; Zhu, Y.M.; Xia, Y.N. Pd–Pt Bimetallic Nanodendrites with High Activity for Oxygen Reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.F.; Gao, F.M.; Yu, S.X.; Li, Z.P.; Zhao, Y.F. Surfactant-free synthesis of highly methanol-tolerant, polyhedral Pd–Pt nanocrystallines for oxygen reduction reaction. J. Power Sources 2013, 239, 374–381. [Google Scholar] [CrossRef]
- Limpattayanate, S.; Hunsom, M. Effect of supports on activity and stability of Pt–Pd catalysts for oxygen reduction reaction in proton exchange membrane fuel cells. J. Solid State Electrochem. 2013, 17, 1221–1231. [Google Scholar] [CrossRef]
- Thanasilp, S.; Hunsom, M. Effect of Pt: Pd atomic ratio in Pt–Pd/C electrocatalyst-coated membrane on the electrocatalytic activity of ORR in PEM fuel cells. Renew. Energy 2011, 36, 1795–1801. [Google Scholar] [CrossRef]
- Ficicilar, B.; Bayrakceken, A.; Eroglu, I. Effect of Pd loading in Pd-Pt bimetallic catalysts doped into hollow core mesoporous shell carbon on performance of proton exchange membrane fuel cells. J. Power Sources 2009, 193, 17–23. [Google Scholar] [CrossRef]
- Ang, S.Y.; Walsh, D.A. Palladium-vanadium alloy electrocatalysts for oxygen reduction: Effect of heat treatment on electrocatalytic activity and stability. Appl. Catal. B 2010, 98, 49–56. [Google Scholar] [CrossRef]
- Li, B.; Prakash, J. Oxygen reduction reaction on carbon supported Palladium-Nickel alloys in alkaline media. Electrochem. Commun. 2009, 11, 1162–1165. [Google Scholar] [CrossRef]
- Zhao, J.; Sarkar, A.; Manthiram, A. Synthesis and characterization of Pd–Ni nanoalloy electrocatalysts for oxygen reduction reaction in fuel cells. Electrochim. Acta 2010, 55, 1756–1765. [Google Scholar] [CrossRef]
- Miah, M.R.; Masud, J.; Ohsaka, T. Kinetics of oxygen reduction reaction at electrochemically fabricated tin-palladium bimetallic electrocatalyst in acidic media. Electrochim. Acta 2010, 56, 285–290. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.E.; Momma, T.; Osaka, T. Synthesis of Pd-Sn nanoparticles by ultrasonic irradiation and their electrocatalytic activity for oxygen reduction. Electrochim. Acta 2009, 54, 3412–3418. [Google Scholar] [CrossRef]
- Karan, H.I.; Sasaki, K.; Kuttiyiel, K.; Farberow, C.A.; Mavrikakis, M.; Adzic, R.R. Catalytic Activity of Platinum Mono layer on Iridium and Rhenium Alloy Nanoparticles for the Oxygen Reduction Reaction. ACS Catal. 2012, 2, 817–824. [Google Scholar] [CrossRef]
- Xiao, L.; Zhuang, L.; Liu, Y.; Lu, J.T.; Abruna, H.D. Activating Pd by Morphology Tailoring for Oxygen Reduction. J. Am. Chem. Soc. 2009, 131, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Nakamura, M.; Maki, N.; Hoshi, N. Active Sites for the Oxygen Reduction Reaction on the Low and High Index Planes of Palladium. J. Phys. Chem. C 2009, 113, 12625–12628. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Alexeyeva, N.; Tammeveski, K.; Solla-Gullon, J.; Feliu, J.M. Electrochemical reduction of oxygen on palladium nanocubes in acid and alkaline solutions. Electrochim. Acta 2012, 59, 329–335. [Google Scholar] [CrossRef]
- Lee, C.L.; Chiou, H.P. Methanol-tolerant Pd nanocubes for catalyzing oxygen reduction reaction in H2SO4 electrolyte. Appl. Catal. B 2012, 117, 204–211. [Google Scholar] [CrossRef]
- Wang, D.L.; Xin, H.L.; Wang, H.S.; Yu, Y.C.; Rus, E.; Muller, D.A.; DiSalvo, F.J.; Abruna, H.D. Facile Synthesis of Carbon-Supported Pd–Co Core-Shell Nanoparticles as Oxygen Reduction Electrocatalysts and Their Enhanced Activity and Stability with Monolayer Pt Decoration. Chem. Mater. 2012, 24, 2274–2281. [Google Scholar] [CrossRef]
- Jang, J.H.; Pak, C.; Kwon, Y.U. Ultrasound-assisted polyol synthesis and electrocatalytic characterization of PdxCo alloy and core-shell nanoparticles. J. Power Sources 2012, 201, 179–183. [Google Scholar] [CrossRef]
- Koenigsmann, C.; Sutter, E.; Adzic, R.R.; Wong, S.S. Size- and Composition-Dependent Enhancement of Electrocatalytic Oxygen Reduction Performance in Ultrathin Palladium-Gold (Pd1−xAux) Nanowires. J. Phys. Chem. C 2012, 116, 15297–15306. [Google Scholar] [CrossRef]
- Wang, J.X.; Inada, H.; Wu, L.J.; Zhu, Y.M.; Choi, Y.M.; Liu, P.; Zhou, W.P.; Adzic, R.R. Oxygen Reduction on Well-Defined Core-Shell Nanocatalysts: Particle Size, Facet, and Pt Shell Thickness Effects. J. Am. Chem. Soc. 2009, 131, 17298–17302. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Naohara, H.; Cai, Y.; Choi, Y.M.; Liu, P.; Vukmirovic, M.B.; Wang, J.X.; Adzic, R.R. Core-Protected Platinum Monolayer Shell High-Stability Electrocatalysts for Fuel-Cell Cathodes. Angew. Chem.-Int. Ed. 2010, 49, 8602–8607. [Google Scholar] [CrossRef] [PubMed]
- Di Noto, V.; Negro, E. Synthesis, characterization and electrochemical performance of tri-metal Pt-free carbon nitride electrocatalysts for the oxygen reduction reaction. Electrochim. Acta 2010, 55, 1407–1418. [Google Scholar] [CrossRef]
- Rego, R.; Oliveira, M.C.; Alcaide, F.; Alvarez, G. Development of a carbon paper-supported Pd catalyst for PEMFC application. Int. J. Hydrogen Energy 2012, 37, 7192–7199. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, H.; Zeng, D.; Xie, F. Recent Development of Pd-Based Electrocatalysts for Proton Exchange Membrane Fuel Cells. Catalysts 2015, 5, 1221-1274. https://doi.org/10.3390/catal5031221
Meng H, Zeng D, Xie F. Recent Development of Pd-Based Electrocatalysts for Proton Exchange Membrane Fuel Cells. Catalysts. 2015; 5(3):1221-1274. https://doi.org/10.3390/catal5031221
Chicago/Turabian StyleMeng, Hui, Dongrong Zeng, and Fangyan Xie. 2015. "Recent Development of Pd-Based Electrocatalysts for Proton Exchange Membrane Fuel Cells" Catalysts 5, no. 3: 1221-1274. https://doi.org/10.3390/catal5031221





